Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Samples
2.3. Accelerated Browning Test
2.4. HPLC Determination of Individual Phenolic Compounds
2.5. Determination of Total Phenols (TP), Flavanols (TF), Hydroxycinnamic Contents (HC), Free Sulfhydryl Groups, and Antioxidant Activity (AA)
2.6. Statistical Analysis
3. Results and Discussion
3.1. Browning Rates
3.2. SO2 Content
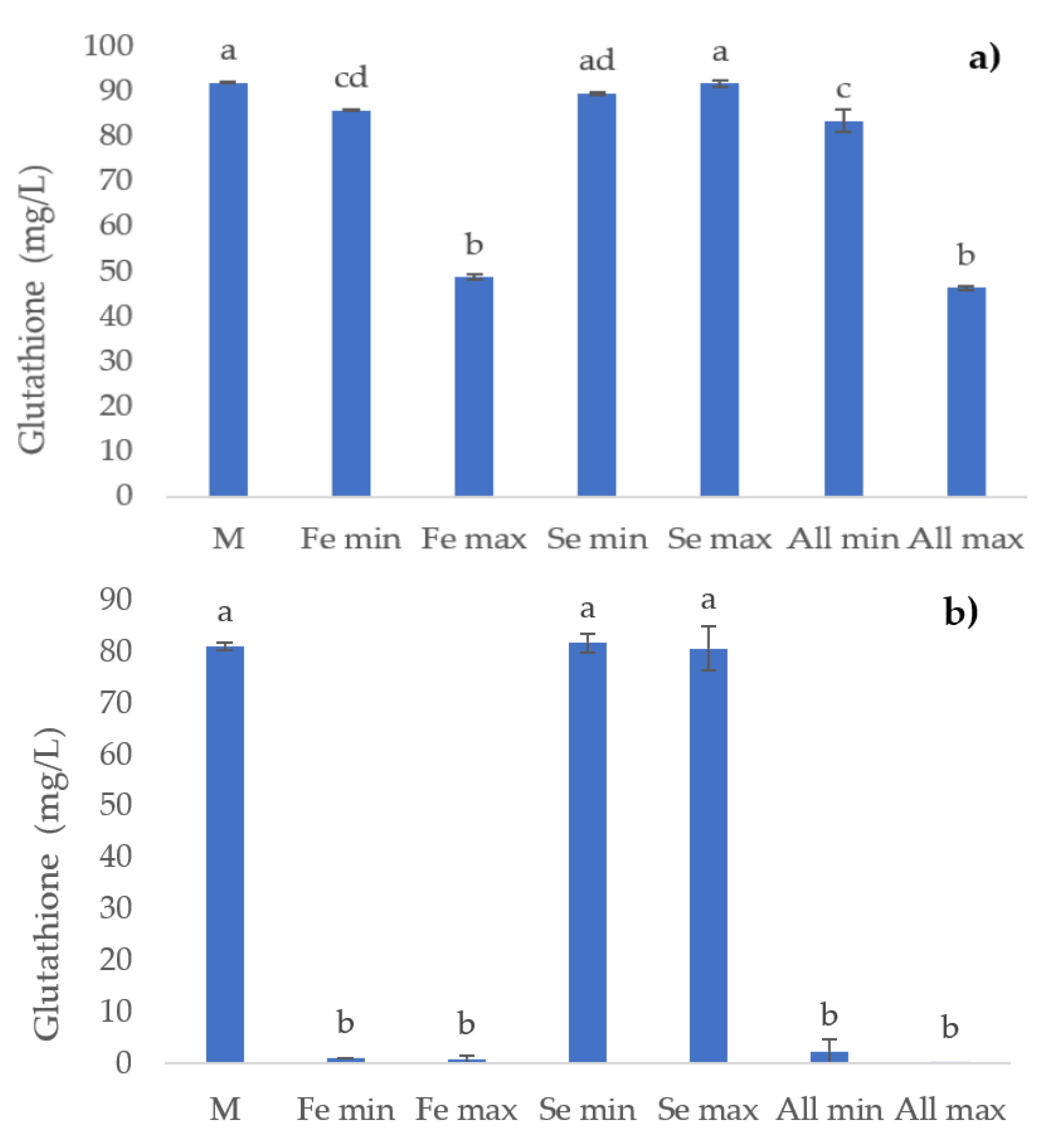
3.3. Free -SH Groups
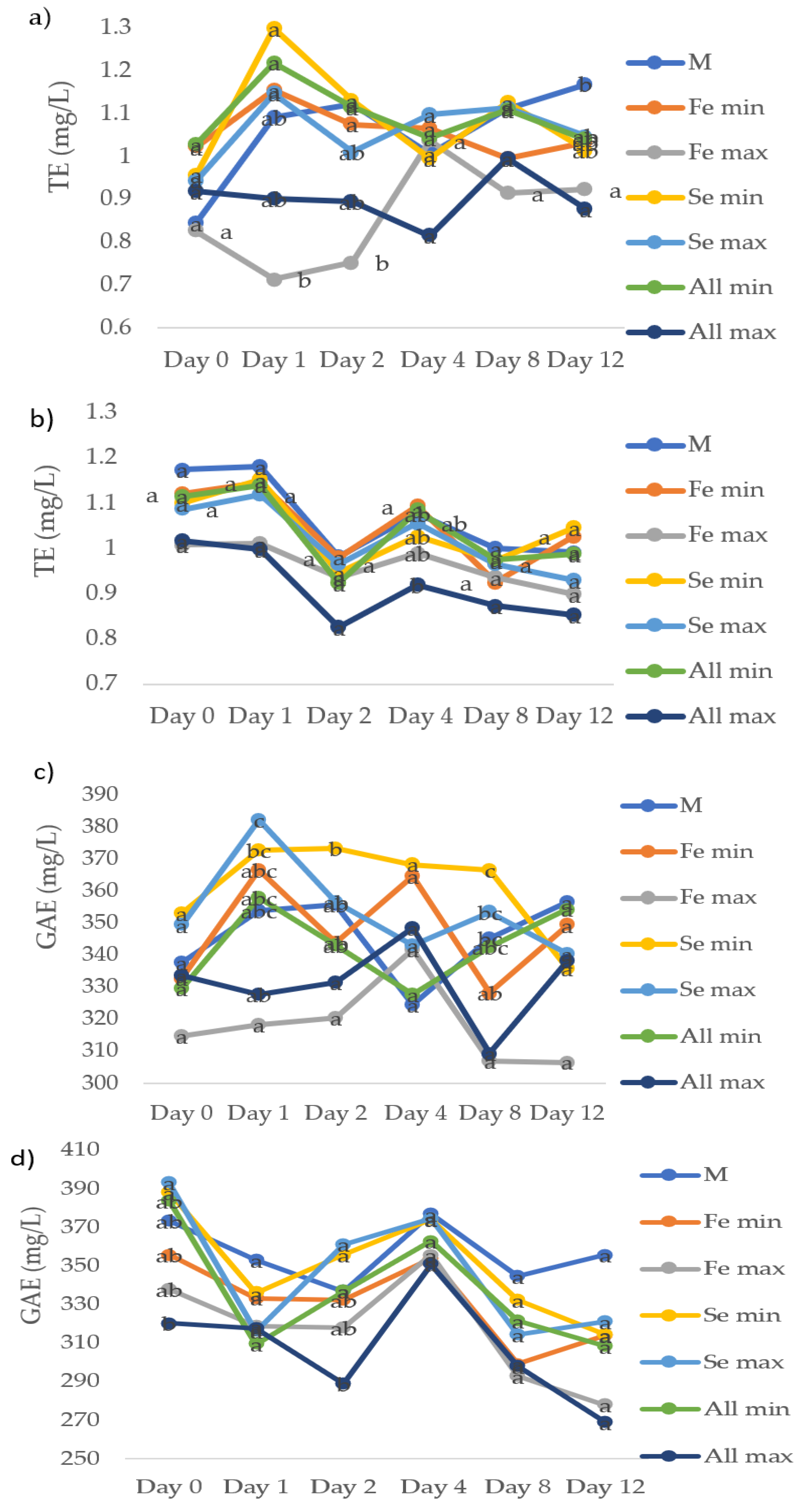
3.4. Antioxidant Activity, Total Phenols
3.5. Total Flavanol and Hydroxycinnamic Content
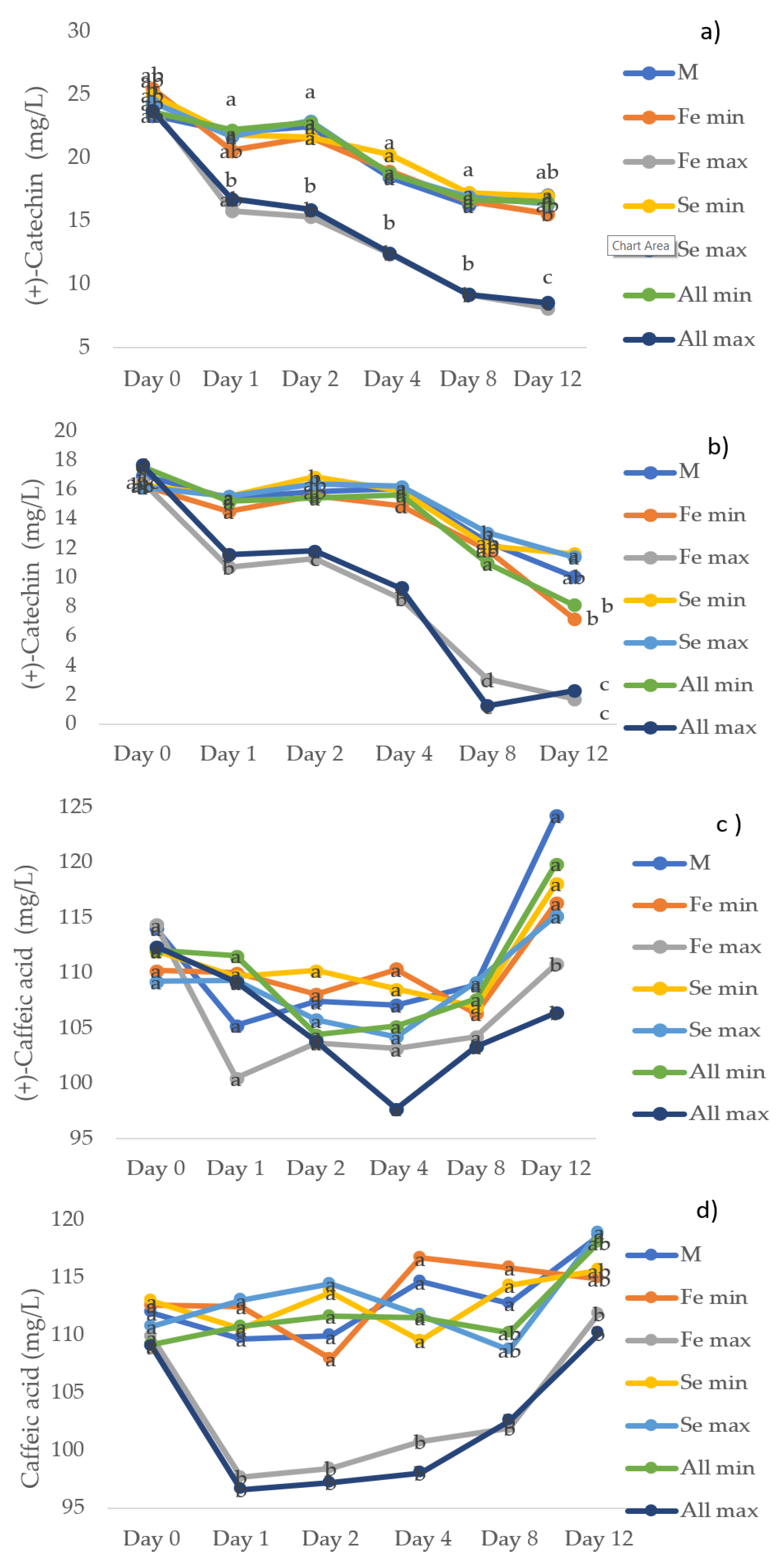
3.6. Individual Polyphenolic Compounds Determined by HPLC
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ricci, A.; Parpinello, G.P.; Versari, A. Modelling the evolution of oxidative browning during storage of white wines: Effects of packaging and closures. Int. J. Food Sci. Technol. 2017, 52, 472–479. [Google Scholar] [CrossRef]
- Silva Ferreira, A.C.; Hogg, T.; Guedes de Pinho, P. Identification of key odorants related to the typical aroma of oxidation-spoiled white wines. J. Agric. Food Chem. 2003, 51, 1377–1381. [Google Scholar] [CrossRef] [PubMed]
- El Hosry, L.; Auezova, L.; Sakr, A.; Hajj-Moussa, E. Browning susceptibility of white wine and antioxidant effect of glutathione. Int. J. Food Sci. Technol. 2009, 44, 2459–2463. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Protecting juice from oxidation. In Handbook of Enology: The Microbiology of Wine and Vinifications, 2nd ed.; John Wiley & Sons, Ltd.: Chichester, UK, 2006; Volume 1, pp. 417–421. [Google Scholar]
- Oliveira, C.M.; Silva Ferreira, A.C.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Benucci, I.; Antonacci, D.; Di Luccia, A.; Esti, M. HPLC-DAD-MS/MS characterization of phenolic compounds in white wine stored without added sulfite. Food Res. Int. 2014, 66, 207–215. [Google Scholar] [CrossRef]
- Pati, S.; Crupi, P.; Savastano, M.L.; Benucci, I.; Esti, M. Evolution of phenolic and volatile compounds during bottle storage of a white wine without added sulfite. J. Sci. Food Agric. 2020, 100, 775–784. [Google Scholar] [CrossRef] [PubMed]
- Es-Safi, N.-E.; Le Guernevé, C.; Fulcrand, H.; Cheynier, V.; Moutounet, M. Xanthylium salts formation involved in wine colour changes. Int. J. Food Sci. Technol. 2000, 35, 63–74. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of Wine Phenolics: A Critical Evaluation and Hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-Mercaptohexanol, Hydrogen Sulfide, and Methyl Mercaptan during Bottle Storage of Sauvignon blanc Wines. Effect of Glutathione, Copper, Oxygen Exposure, and Closure-Derived Oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]
- Singleton, V.L.; Kramlinga, T.E. Browning of White Wines and an Accelerated Test for Browning Capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar]
- Gonzales Cartagena, L.; Perez-Zuniga, F.J.; Bravo Abad, F. Interactions of Some Environmental and Chemical Parameters Affecting the Colour Attributes of Wine. Am. J. Enol. Vitic. 1994, 45, 43–48. [Google Scholar]
- Sioumis, N.; Kallithraka, S.; Makris, D.P.; Kefalas, P. Kinetics of browning onset in white wines: Influence of principal redox-active polyphenols and impact on the reducing capacity. Food Chem. 2006, 94, 98–104. [Google Scholar] [CrossRef]
- Boulton, R.B.; Singleton, V.L.; Bisson, L.F.; Kunkee, R.E. Oxidation and Browning. In Principles and Practices of Winemaking; Springer: New York, NY, USA, 2013; Volume 10E, pp. 406–414. [Google Scholar]
- Scrimgeour, N.; Nordestgaard, S.; Lloyd, N.D.R.; Wilkes, E.N. Exploring the effect of elevated storage temperature on wine composition. Aust. J. Grape Wine Res. 2015, 21, 713–722. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Jourdes, M.; Riu-Aumatell, M.; Buxaderas, S.; Teissedre, P.-L.; López-Tamames, E. Kinetics of Browning, Phenolics, and 5-Hydroxymethylfurfural in Commercial Sparkling Wines. J. Agric. Food Chem. 2014, 62, 1159–1166. [Google Scholar] [CrossRef]
- Witkowska, A.M.; Zujko, M.E.; Borawska, M.H.; Socha, K. Antioxidant Properties and Selenium Content of Wines. Pol. J. Environ. Stud. 2006, 15, 208–211. [Google Scholar]
- Frías, S.; Díaz, C.; Conde, J.E.; Pérez Trujillo, J.P. Selenium and mercury concentrations in sweet and dry bottled wines from the Canary Islands, Spain. Food Addit. Contam. 2003, 20, 237–240. [Google Scholar] [CrossRef]
- Battin, E.E.; Brumaghim, J.L. Antioxidant Activity of Sulfur and Selenium: A Review of Reactive Oxygen Species Scavenging, Glutathione Peroxidase, and Metal-Binding Antioxidant Mechanisms. Cell Biochem. Biophys. 2009, 55, 1–23. [Google Scholar] [CrossRef]
- Assuncao, M.; Martins, L.L.; Mourato, M.P.; Baleiras-Couto, M.M. Effect of selenium on growth and antioxidant enzyme activities of wine related yeasts. World J. Microbiol. Biotechnol. 2015, 31, 1899–1906. [Google Scholar] [CrossRef]
- Talbi, W.; Ghazouani, T.; Braconi, D.; Ben Abdallah, R.; Raboudi, T.; Santucci, A.; Fattouch, S. Effects of selenium on oxidative damage and antioxidant enzymes of eukaryotic cells: Wine Saccharomyces cerevisiae. J. Appl. Microbiol. 2018, 126, 555–566. [Google Scholar] [CrossRef]
- Fontanella, M.C.; D’Amato, R.; Regni, L.; Proietti, P.; Beone, G.M.; Businelli, D. Selenium speciation profiles in biofortified sangiovese wine. J. Trace Elem. Med. Biol. 2017, 43, 87–92. [Google Scholar] [CrossRef] [PubMed]
- Patrianakou, M.; Roussis, I.G. Decrease of Wine Volatile Aroma Esters by Oxidation. S. Afr. J. Enol. Vitic. 2013, 34, 241–245. [Google Scholar] [CrossRef]
- Voltea, S.; Karabagias, I.K.; Roussis, I.G. Use of Fe (II) and H2O2 along with heating for the estimation of the browning susceptibility of white wine. J. Appl. Sci. 2022. submitted. [Google Scholar]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Salacha, M.-I.; Kallithraka, S.; Tzourou, I. Browning of white wines: Correlation with antioxidant characteristics, total polyphenolic composition and flavanol content. Int. J. Food Sci. Technol. 2008, 43, 1073–1077. [Google Scholar] [CrossRef]
- Robinson, A.L.; Mueller, M.; Heymann, H.; Ebeler, S.E.; Boss, P.K.; Solomon, P.S.; Trengove, R.D. Effect of simulated shipping conditions on sensory attributes and volatile composition of commercial white and red wines. Am. J. Enol. Vitic. 2010, 61, 337–347. Available online: https://www.ajevonline.org/content/61/3/337 (accessed on 5 April 2022).
- Walther, A.K.; Durner, D.; Fischer, U. Impact of temperature during bulk shipping on the chemical composition and sensory profile of a Chardonnay wine. Am. J. Enol. Vitic. 2018, 69, 247–257. [Google Scholar] [CrossRef]
- International Organization of Vine and Wine (OIV). Compendium of International Methods of Wine and Must Analysis; OIV: Paris, France, 2020; Volume 1, 770p, Available online: https://www.oiv.int/public/medias/7372/oiv-compendium-volume-1-2020.pdf (accessed on 15 January 2022).
- Kyraleou, M.; Kallithraka, S.; Chira, K.; Tzanakouli, E.; Ligas, I.; Kotseridis, Y. Differentiation of Wines Treated with Wood Chips Based on Their Phenolic Content, Volatile Composition, and Sensory Parameters. J. Food Sci. 2015, 80, 2701–2710. [Google Scholar] [CrossRef]
- Arnous, A.; Makris, D.P.; Kefalas, P. Effect of principal polyphenolic components in relation to antioxidant characteristics of aged red wines. J. Agric. Food Chem. 2001, 49, 5736–5742. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Comp. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Kontogeorgos, N.; Roussis, I.G. Total free sulphydryls of several white and red wines. S. Afr. J. Enol. Vitic. 2014, 35, 125–127. [Google Scholar] [CrossRef][Green Version]
- Psarra, E.; Makris, D.P.; Kallithraka, S.; Kefalas, P. Evaluation of the antiradical and reducing properties of selected Greek white wines: Correlation with polyphenolic composition. J. Sci. Food Agric. 2002, 82, 1014–1020. [Google Scholar] [CrossRef]
- Guo, A.; Kontoudakis, N.; Scollary, G.R.; Clark, A.C. Production and Isomeric Distribution of Xanthylium Cation Pigments and Their Precursors in Wine-like Conditions: Impact of Cu(II), Fe(II), Fe(III), Mn(II), Zn(II), and Al(III). J. Agric. Food Chem. 2017, 65, 2414–2425. [Google Scholar] [CrossRef]
- Kanavouras, A.; Coutelieris, F.A.; Karanika, E.; Kotseridis, Y.; Kallithraka, S. Colour change of bottled white wines as a quality indicator. OENO One 2020, 54, 543–551. [Google Scholar] [CrossRef]
- Celotti, E.; Lazaridis, G.; Figelj, J.; Scutaru, Y.; Natolino, A. Comparison of a Rapid Light-Induced and Forced Test to Study the Oxidative Stability of White Wines. Molecules 2022, 27, 326. [Google Scholar] [CrossRef]
- Boulton, R. A Method for the Assessment of Copigmentation in Red Wines. Am. J. Enol. Vitic. 1996, 47, 346. [Google Scholar]
- Ma, L.; Waterhouse, A.L. Flavanols react preferentially with quinones through an electron transfer reaction, stimulating rather than preventing wine browning. Anal. Chim. Acta 2018, 1039, 162–171. [Google Scholar] [CrossRef]
- Cucciniello, R.; Forino, M.; Picariello, L.; Coppola, F.; Moio, L.; Gambuti, A. How acetaldehyde reacts with low molecular weight phenolics in white and red wines. Eur. Food Res. Technol. 2021, 247, 2935–2944. [Google Scholar] [CrossRef]
- Sartor, S.; Burin, V.M.; Ferreira-Lima, N.E.; Caliari, V.; Bordignon-Luiz, M.T. Polyphenolic Profiling, Browning, and Glutathione Content of Sparkling Wines Produced with Nontraditional Grape Varieties: Indicator of Quality during the Biological Aging. J. Food Sci. 2019, 84, 3546–3554. [Google Scholar] [CrossRef]
- Landrault, N.; Poucheret, P.; Ravel, P.; Gasc, F.; Cros, G.; Teissedre, P.-L. Antioxidant Capacities and Phenolics Levels of French Wines from Different Varieties and Vintages. J. Agric. Food Chem. 2001, 49, 3341–3348. [Google Scholar] [CrossRef]
- De Beer, D.; Joubert, E.; Gelderblom, W.C.A.; Manley, M. Changes in phenolic composition and antioxidant activity of Pinotage, Cabernet Sauvignon, Chardonay and Chenin blanc wines during bottle ageing. S. Afr. J. Enol. Vitic. 2005, 26, 6–15. [Google Scholar]
- Roginsky, V.; de Beer, D.; Harbertson, J.F.; Kilmartin, P.A.; Barsukova, T.; Adams, D.O. The antioxidant activity of Californian red wines does not correlate with wine age. J. Sci. Food Agric. 2006, 86, 834–840. [Google Scholar] [CrossRef]
- Kallithraka, S.; Kotseridis, Y.; Kyraleou, M.; Proxenia, N.; Tsakiris, A.; Karapetrou, G. Analytical phenolic composition and sensory assessment of selected rare Greek cultivars after extended bottle ageing. J. Sci. Food Agric. 2015, 95, 2353. [Google Scholar] [CrossRef] [PubMed]
- Castellanos, E.R.; Jofre, V.P.; Fanzone, M.L.; Assof, M.V.; Catania, A.A.; Diaz-Sambueza, A.M.; Heredia, F.J.; Mercado, L.A. Effect of different closure types and storage temperatures on the color and sensory characteristics development of Argentinian Torrontes Riojano white wines aged in bottles. Food Control 2021, 130, 108343. [Google Scholar] [CrossRef]
- Bührle, F.; Gohl, A.; Weber, F. Impact of Xanthylium Derivatives on the Color of White Wine. Molecules 2017, 22, 1376. [Google Scholar] [CrossRef]
- Recamales, A.F.; Sayago, A.; Gonzalez-Miret, M.L.; Hernanz, D. The effect of storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Milat, A.M.; Boban, M.; Teissedre, P.-L.; Šešelja-Perišin, A.; Jurić, D.; Skroza, D.; Generalić-Mekinić, I.; Ljubenkov, I.; Volarević, J.; Rasines-Perea, Z.; et al. Effects of oxidation and browning of macerated white wine on its antioxidant and direct vasodilatory activity. J. Funct. Foods 2019, 59, 138–147. [Google Scholar] [CrossRef]
- Di Lecce, G.; Boselli, E.; D’Ignazi, G.; Frega, N.G. Evolution of phenolics and glutathione in Verdicchio wine obtained with maceration under reductive conditions. LWT-Food Sci. Technol. 2013, 53, 54–60. [Google Scholar] [CrossRef]
- Webber, V.; Dutra, S.V.; Spinelli, F.R.; Carnieli, G.J.; Cardozo, A.; Vanderlinde, R. Effect of glutathione during bottle storage of sparkling wine. Food Chem. 2017, 216, 254–259. [Google Scholar] [CrossRef]
- Serra-Cayuela, A.; Aguilera-Curiel, M.A.; Riu-Aumatell, M.; Buxaderas, S.; López-Tamames, E. Browning during biological aging and commercial storage of Cava sparkling wine and the use of 5-HMF as a quality marker. Food Res. Int. 2013, 53, 226–231. [Google Scholar] [CrossRef]
- Ferreira-Lima, N.E.; Burin, V.M.; Caliari, V.; Bordignon-Luiz, M.T. Impact of Pressing Conditions on the Phenolic Composition, Radical Scavenging Activity and Glutathione Content of Brazilian Vitis vinifera White Wines and Evolution During Bottle Ageing. Food Bioprocess Technol. 2016, 9, 944–957. [Google Scholar] [CrossRef]

| Sample Code | FeII (mM) | H2O2 (mM) | Se (mg/L) |
|---|---|---|---|
| M | _ | _ | _ |
| Fe min | 0.10 | 0.63 | _ |
| Fe max | 0.025 | 0.157 | _ |
| Se min | _ | _ | 1.5 |
| Se max | _ | _ | 3.0 |
| All min | 0.10 | 0.63 | 1.5 |
| All max | 0.025 | 0.157 | 3.0 |
| Sample Code | k (day−1) × 10−3 35 °C | k (day−1) × 10−3 50 °C | %ΔA420 35 °C | %ΔA420 50 °C |
|---|---|---|---|---|
| M | 1.0 a | 7.5 a | 10.37 a | 97.70 a |
| Fe min | 1.4 b | 15.8 b | 27.76 b | 205.15 b |
| Fe max | 4.7 c | 43.8 c | 162.21 d | 312.04 c |
| Se min | _ | 8.8 a | _ | 107.73 a |
| Se max | _ | 7.7 a | _ | 101.17 a |
| All min | 1.6 b | 16.6 b | 25.10 b | 220.20 b |
| All max | 5.1 c | 45.5 c | 136.8 c | 338.74 c |
| Samples | (+)-Catechin | (−)-Epicatechin | (−)-Epigallocatechin-3-0-Gallate |
|---|---|---|---|
| Samples at 35 °C | |||
| M | 7.34 ab ± 0.01 | 2.56 a ± 0.01 | 1.34 ab ± 0.06 |
| Fe min | 8.42 a ± 0.45 | 2.25 a ± 0.12 | 1.26 ab ± 0.04 |
| Fe max | 7.87 a ± 0.27 | 1.90 ab ± 0.02 | 0.91 b ± 0.01 |
| Se min | 8.84 a ± 0.42 | 2.12 a ± 0.02 | 1.41 a ± 0.01 |
| Se max | 7.80 a ± 0.58 | 2.17 a ± 0.10 | 1.39 a ± 0.03 |
| All min | 8.58 a ± 0.00 | 1.81 ab ± 0.02 | 1.30 a ± 0.03 |
| All max | 5.29 b ± 0.01 | 1.69 b ± 0.06 | 0.88 b ± 0.02 |
| Samples at 50 °C | |||
| M | 7.81 a ± 0.16 | 1.96 a ± 0.01 | 1.24 a ± 0.01 |
| Fe min | 5.30 b ± 0.37 | 1.81 ab ± 0.07 | 1.38 a ± 0.01 |
| Fe max | 5.27 b ± 0.16 | 1.36 b ± 0.03 | 0.71 b ± 0.02 |
| Se min | 7.02 a ± 0.58 | 1.56 ab ± 0.05 | 1.34 a ± 0.01 |
| Se max | 6.88 ab ± 0.02 | 1.56 ab ± 0.01 | 1.29 a ± 0.00 |
| All min | 4.39 c ± 0.03 | 1.34 b ± 0.01 | 1.28 a ± 0.03 |
| All max | 3.59 c ± 0.08 | 1.37 b ± 0.10 | 0.80 b ± 0.02 |
| Samples | Caftaric Acid | Coutaric Acid | Fertaric Acid | Caffeic Acid | Ferulic Acid | p-Coumaric Acid | Gallic Acid |
|---|---|---|---|---|---|---|---|
| Samples at 35 °C * | |||||||
| M | 62.26 ab ± 0.10 | 6.69 b ± 0.60 | 7.86 a ± 0.60 | 2.62 a ± 0.01 | 0.38 a ± 0.00 | 0.34 a ± 0.00 | 4.43 a ± 0.00 |
| Fe min | 60.43 b ±1.10 | 7.07 ab ± 0.52 | 7.68 a ± 0.2 | 3.05 b ± 0.13 | 0.35 ab ± 0.09 | 0.32 a ± 0.01 | 4.13 a ± 0.05 |
| Fe max | 51.78 c ± 0.96 | 6.53 b ± 0.43 | 6.84 b ± 0.26 | 2.64 a ± 0.06 | 0.30 b ± 0.01 | 0.22 b ± 0.02 | 1.93 b ± 0.02 |
| Se min | 63.18 ab ±2.67 | 7.17 ab ± 0.48 | 7.77 a ± 0.15 | 2.82 a ± 0.01 | 0.33 ab ± 0.00 | 0.32 a ± 0.01 | 4.23 a ± 0.11 |
| Se max | 66.17 a ±4.51 | 7.60 a ± 0.01 | 7.83 a ± 0.23 | 2.96 ab ± 0.02 | 0.34 ab ± 0.01 | 0.36 a ± 0.01 | 4.47 a ± 0.02 |
| All min | 64.92 ab ±4.08 | 7.60 b ± 0.12 | 7.25 a ± 0.25 | 3.19 b ± 0.26 | 0.36 ab ± 0.02 | 0.34 a ± 0.02 | 4.35 a ± 0.06 |
| All max | 52.03 c ±1.52 | 6.43 b ± 0.01 | 6.02 b ± 0.14 | 2.90 ab ± 0.16 | 0.26 c ± 0.00 | 0.27 b ± 0.00 | 2.03 b ± 0.02 |
| Samples at 50 °C | |||||||
| M | 64.48 a ±1.23 | 8.61 a ± 0.02 | 7.58 a ± 0.02 | 2.57 ab ± 0.01 | 0.42 a ± 0.00 | 0.50 a ± 0.01 | 4.45 a ± 0.08 |
| Fe min | 63.97 a ±1.42 | 8.27 a ± 0.01 | 7.32 a ± 0.02 | 2.58 ab ± 0.02 | 0.39 ab ± 0.01 | 0.47 ab ± 0.03 | 3.55 b ± 0.10 |
| Fe max | 46.46 b ± 0.31 | 6.89 b ± 0.06 | 5.73 c ± 0.02 | 2.37 b ± 0.08 | 0.26 c ± 0.02 | 0.44 ab ± 0.00 | 2.23 c ± 0.05 |
| Se min | 64.74 a ±1.59 | 8.60 a ± 0.04 | 7.46 a ± 0.08 | 2.51 ab ± 0.03 | 0.45 a ± 0.01 | 0.52 a ± 0.01 | 4.07 a ± 0.01 |
| Se max | 64.16 a ± 1.44 | 8.42 a ± 0.06 | 7.40 a ± 0.09 | 2.63 a ± 0.04 | 0.36 b ± 0.00 | 0.51 a ± 0.00 | 4.14 a ± 0.06 |
| All min | 44.95 b ± 0.15 | 6.66 b ± 0.10 | 6.69 b ± 0.03 | 2.28 b ± 0.03 | 0.27 c ± 0.00 | 0.43 ab ± 0.01 | 3.71 b ± 0.01 |
| All max | 37.85 c ± 0.28 | 7.44 c ± 0.14 | 5.54 c ± 0.02 | 2.71 a ± 0.03 | 0.29 c ± 0.04 | 0.35 b ± 0.003 | 2.11 c ± 0.01 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vlahou, E.; Christofi, S.; Roussis, I.G.; Kallithraka, S. Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition. Appl. Sci. 2022, 12, 3834. https://doi.org/10.3390/app12083834
Vlahou E, Christofi S, Roussis IG, Kallithraka S. Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition. Applied Sciences. 2022; 12(8):3834. https://doi.org/10.3390/app12083834
Chicago/Turabian StyleVlahou, Eftihia, Stefania Christofi, Ioannis G. Roussis, and Stamatina Kallithraka. 2022. "Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition" Applied Sciences 12, no. 8: 3834. https://doi.org/10.3390/app12083834
APA StyleVlahou, E., Christofi, S., Roussis, I. G., & Kallithraka, S. (2022). Browning Development and Antioxidant Compounds in White Wines after Selenium, Iron, and Peroxide Addition. Applied Sciences, 12(8), 3834. https://doi.org/10.3390/app12083834









