Part 2: Stabilization/Containment of Radiological Particle Contamination to Enhance First Responder, Early Phase Worker, and Public Safety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Description of Stabilization Materials
2.2. Experimental Procedure
2.2.1. Preparation of Simulated Fallout Material (SFM) Containing Radiostrontim (Sr-85)
2.2.2. Preparation of Surfaces for Testing
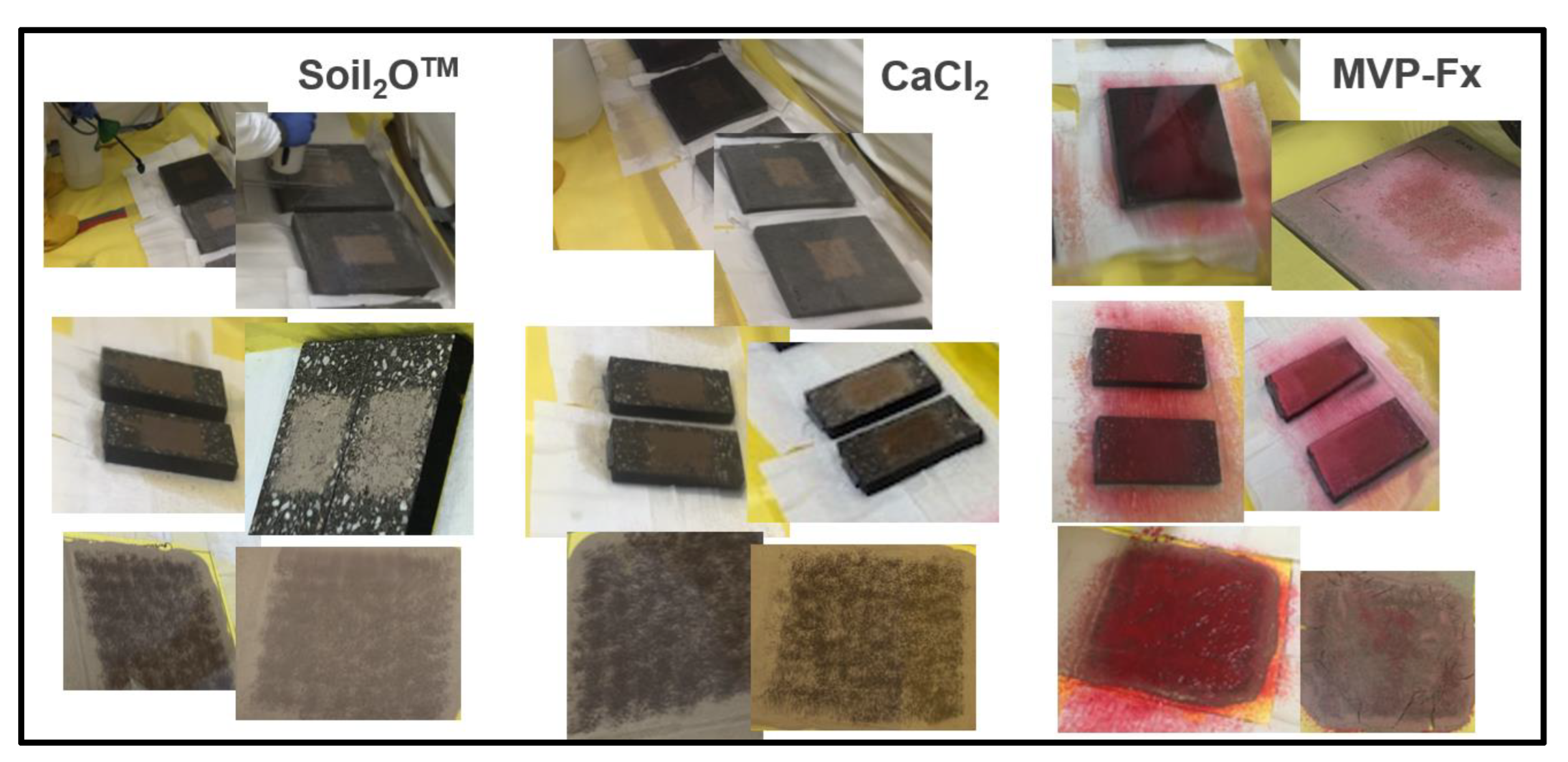
2.2.3. Preparation of Stabilization Technologies
2.2.4. Radiological Contamination and Stabilization of Surfaces
2.2.5. Measurement of Sr-85 Activity
2.2.6. Evaluation of Stabilization Technologies
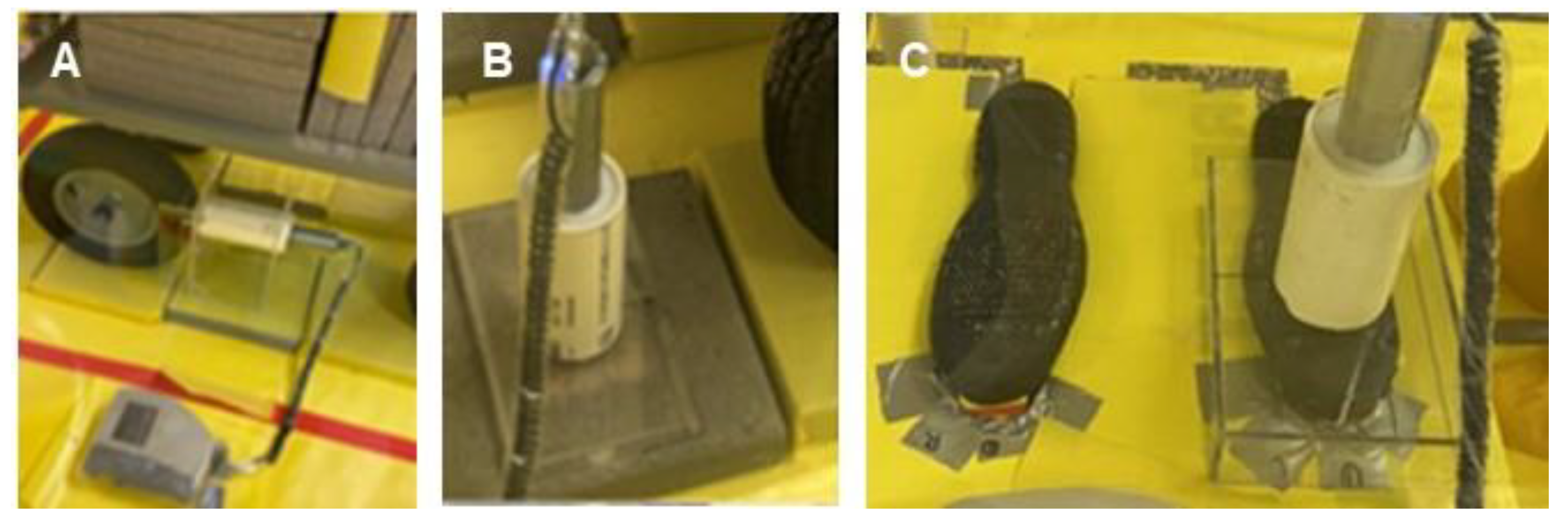
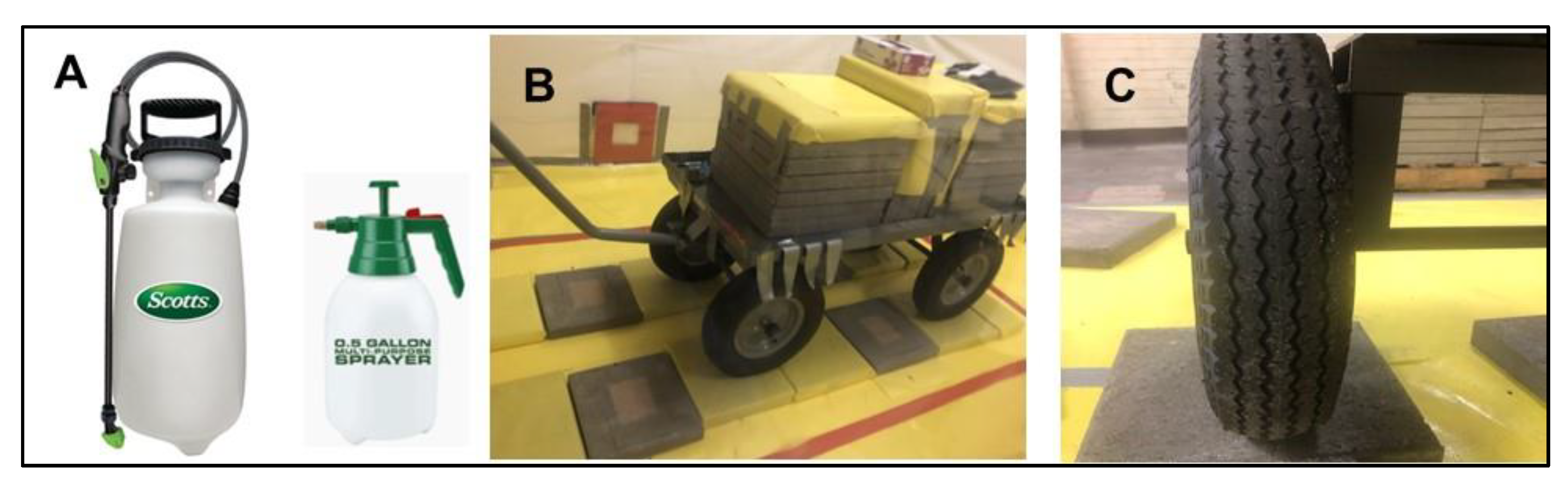
2.2.7. Simulated Vehicle Experiments
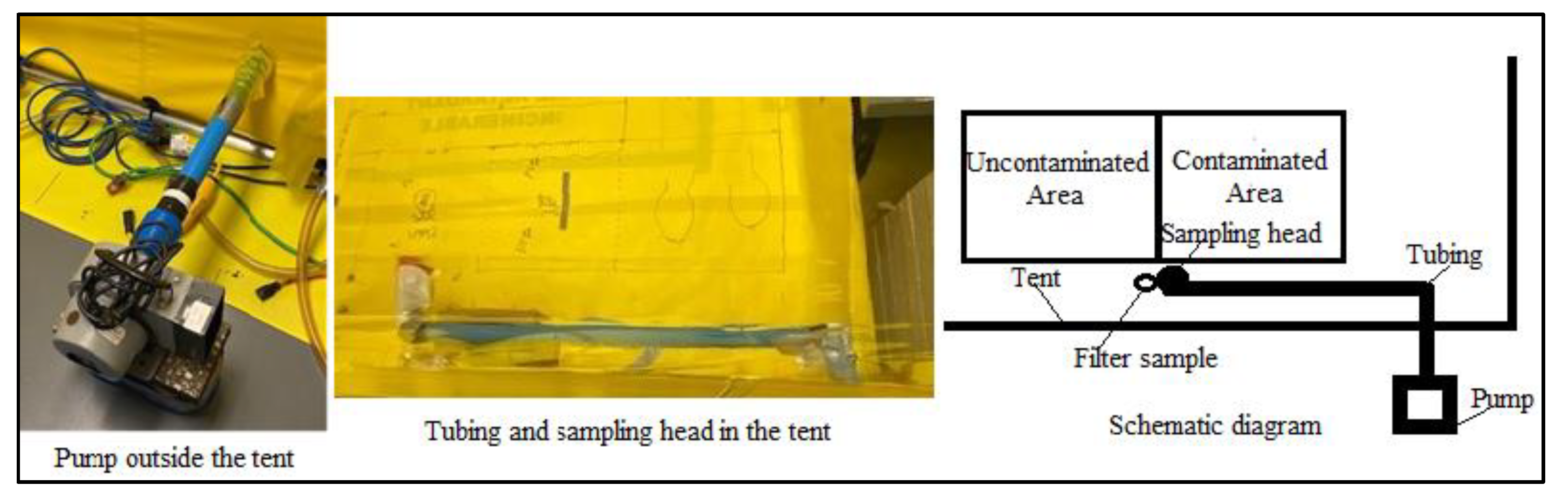
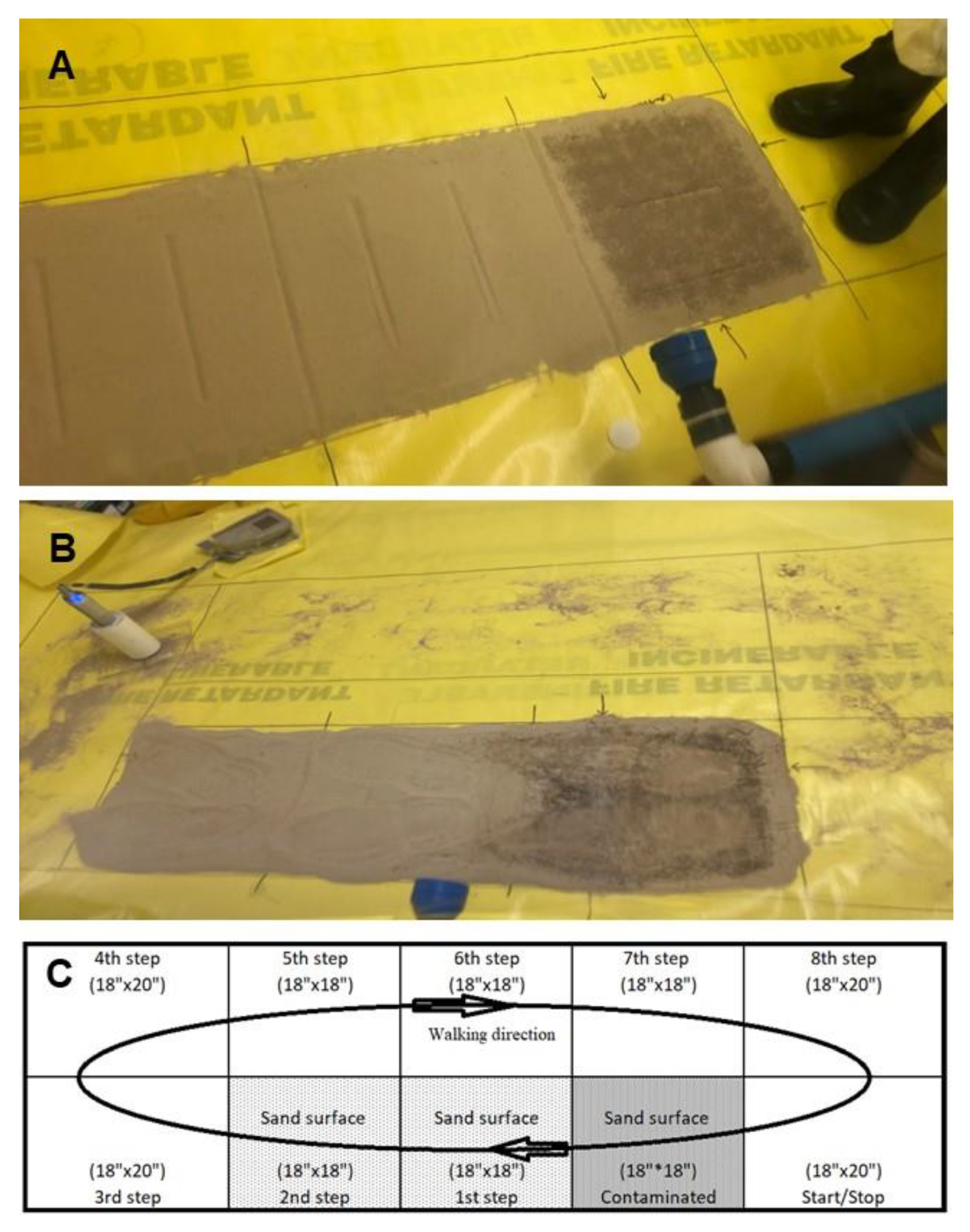
2.2.8. Straight-Line Walking Experiments
2.2.9. Circuit Path Walking Experiments
2.2.10. Calculation of Percent Removal, Percent Transfer, and Percent Residual
3. Results and Discussion
3.1. Simulated Vehicle Experiments
3.1.1. Percent Removal from Contaminated Surfaces
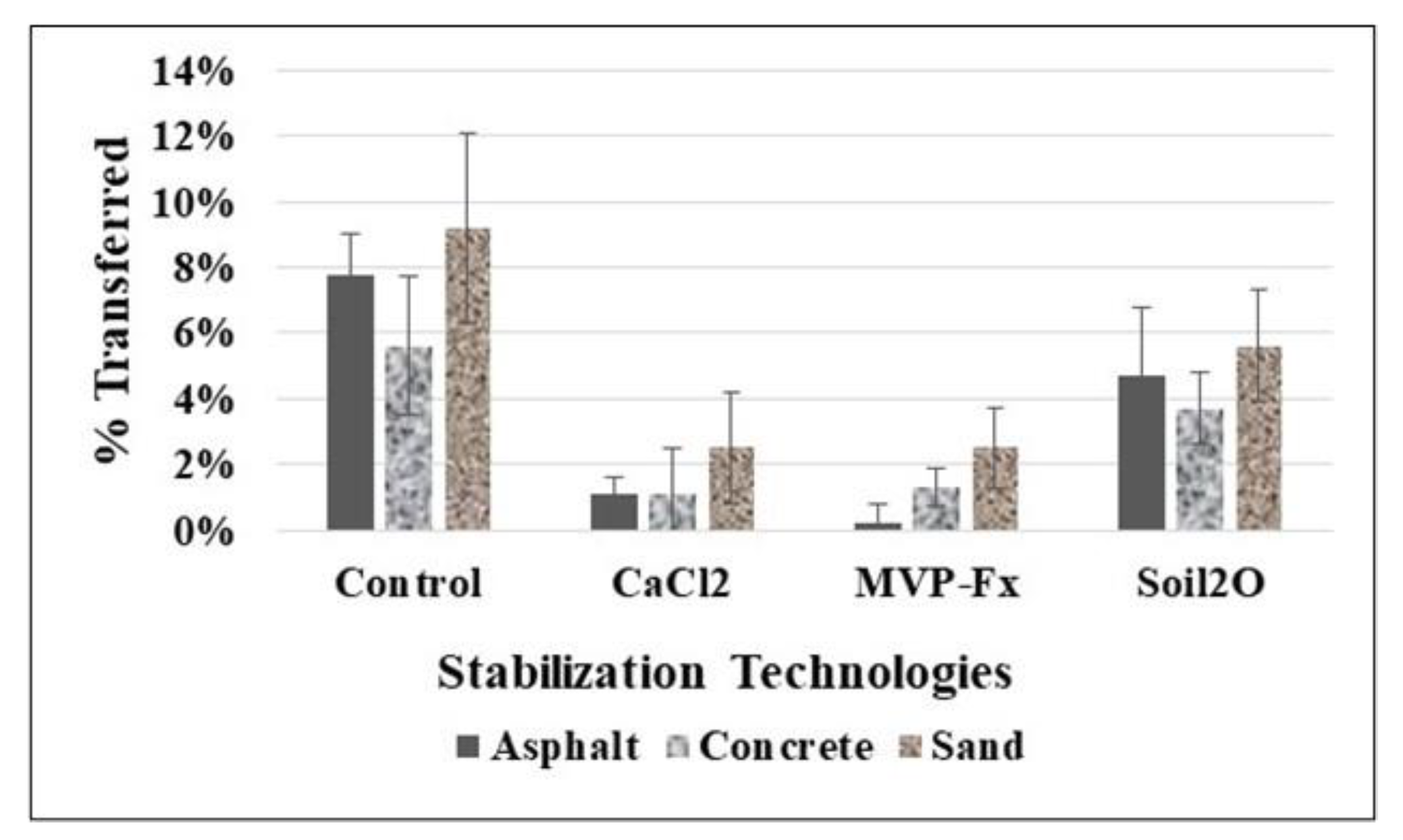
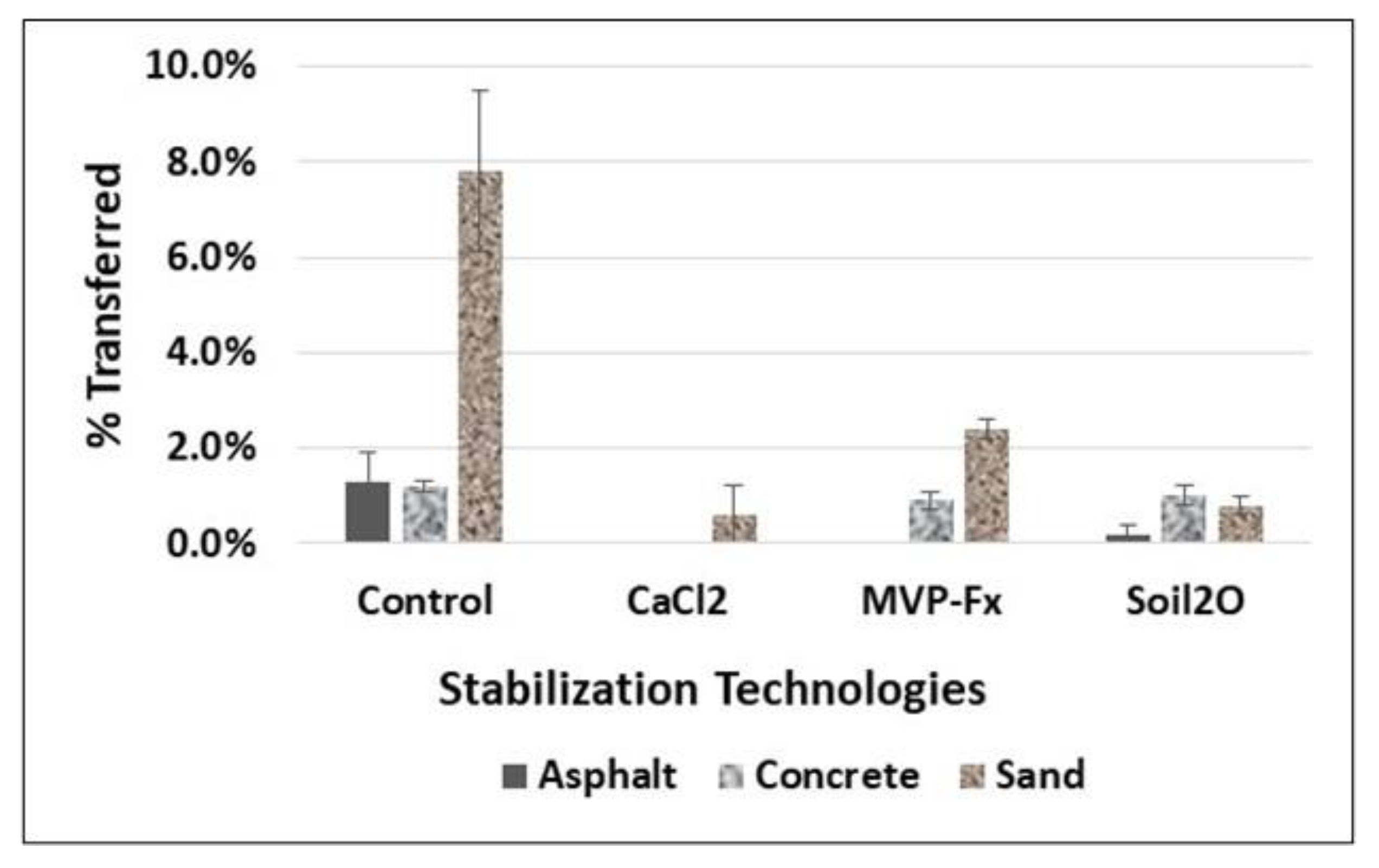
3.1.2. Percent Transfer to Uncontaminated Surfaces
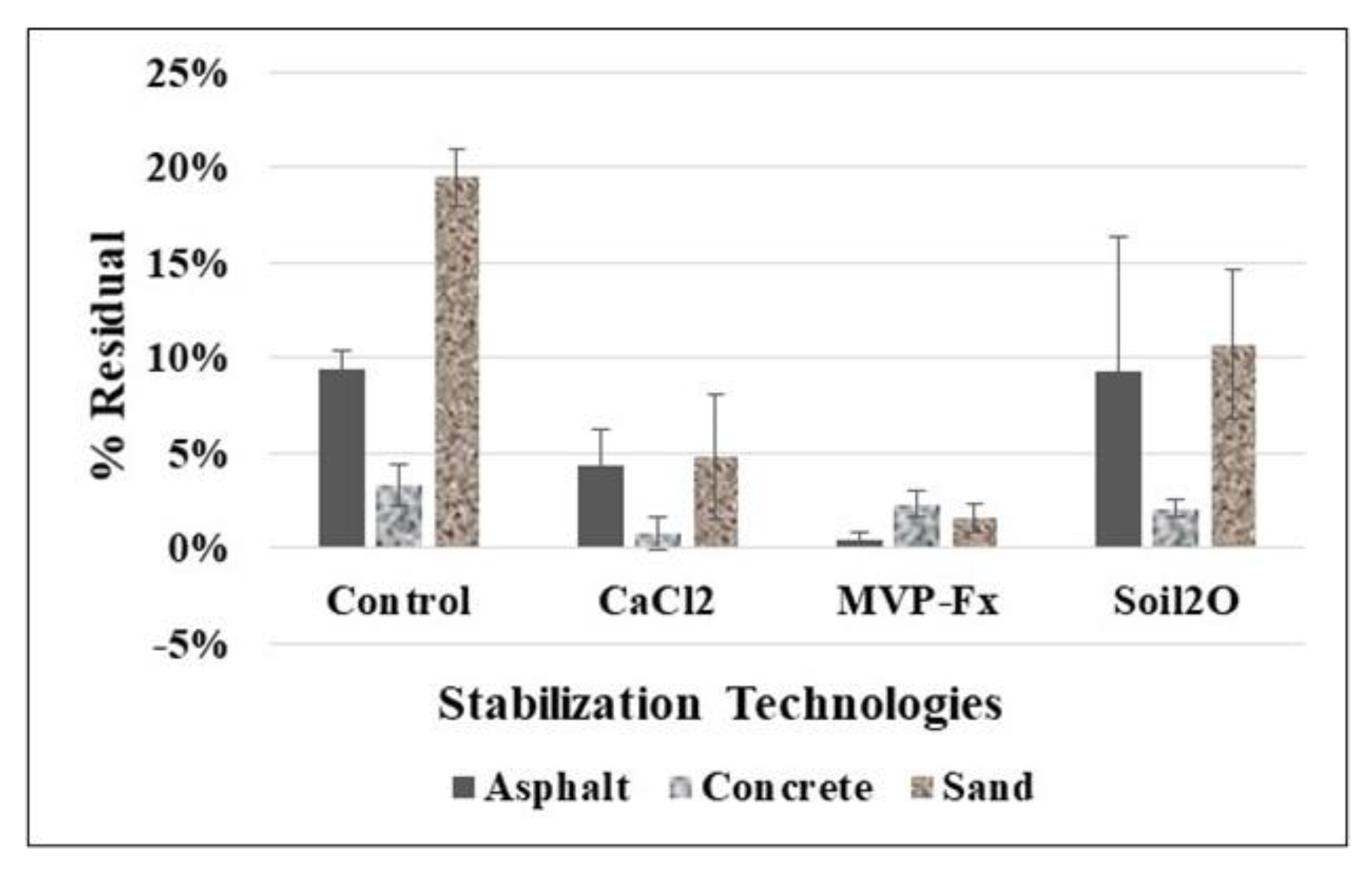
3.1.3. Percent Residual Activity on Simulated Vehicle Tires
3.1.4. Simulated Vehicle Experimental Observations and Conclusions
3.2. Simulated Foot Traffic Experiments
3.2.1. Percent Removal from Contaminated Surfaces for Straight-Line Walking
3.2.2. Percent Transfer to Uncontaminated Surfaces for Straight-Line Walking
3.3. Circuit Walking Path Results
3.3.1. Percent Residual Activity on Boots from Circuit Walking
3.3.2. Resuspension during Circuit Walking
3.3.3. Foot Traffic Experimental Observations and Conclusions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Disclaimer
References
- Kaminski, M.D.; Lee, S.D.; Magnuson, M. Wide-area decontamination in an urban environment after radiological dispersion: A review and perspectives. J. Hazard. Mater. 2016, 305, 67–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amato, F.; Querol, X.; Johansson, C.; Nagl, C.; Alastuey, A. A review on the effectiveness of street sweeping, washing and dust suppressants as urban PM control methods. Sci. Total Environ. 2010, 408, 3070–3084. [Google Scholar] [CrossRef]
- Gulia, S.; Goyal, P.; Goyal, S.; Kumar, R. Re-suspension of road dust: Contribution, assessment and control through dust suppressants—A review. Int. J. Environ. Sci. Technol. 2019, 16, 1717–1728. [Google Scholar] [CrossRef]
- Norman, M.; Johansson, C. Studies of some measures to reduce road dust emissions from paved roads in Scandinavia. Atmos. Environ. 2006, 40, 6154–6164. [Google Scholar] [CrossRef]
- Saito, H.; Sutton, M.; Zhao, P.; Lee, S.D.; Magnuson, M. Review of technologies for preventing secondary transport of soluble and particulate radiological contamination from roadways, roadside vegetation, and adjacent soils. Environ. Adv. 2020, 1, 100003. [Google Scholar] [CrossRef]
- Xu, L.; Pei, Z. Preparation and optimization of a novel dust suppressant for construction sites. J. Mater. Civ. Eng. 2017, 29, 04017051. [Google Scholar] [CrossRef]
- Zhan, Q.; Qian, C.; Yi, H. Microbial-induced mineralization and cementation of fugitive dust and engineering application. Constr. Build. Mater. 2016, 121, 437–444. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, Y.; Zhao, X.; Cao, L.; Tong, R. Effectiveness of road dust suppressants: Insights from particulate matter-related health damage. Environ. Geochem. Health 2021, 43, 4139–4162. [Google Scholar] [CrossRef] [PubMed]
- Parvej, S.; Naik, D.L.; Sajid, H.U.; Kiran, R.; Huang, Y.; Thanki, N. Fugitive Dust Suppression in Unpaved Roads: State of the Art Research Review. Sustainability 2021, 13, 2399. [Google Scholar] [CrossRef]
- Tsogt, B.; Oh, S.-Y. Preparations and application of dust suppressants from biomass-based materials. J. Air Waste Manag. Assoc. 2021, 71, 1386–1396. [Google Scholar] [CrossRef] [PubMed]
- Stallworth, A.M.; Chase, E.H.; McDevitt, B.; Marak, K.K.; Freedman, M.A.; Wilson, R.T.; Burgos, W.D.; Warner, N.R. Efficacy of oil and gas produced water as a dust suppressant. Sci. Total Environ. 2021, 799, 149347. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhao, B.; Lin, H.; Shuang, H.; Kong, X.; Yang, E. Review and prospects of surfactant-enhanced spray dust suppression: Mechanisms and effectiveness. Process Saf. Environ. Prot. 2021, 154, 410–424. [Google Scholar] [CrossRef]
- USEPA. Wide Area Stabilization of Radiological Particulate Contamination. EPA/600/R-616/067. 2017. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=335602 (accessed on 9 April 2022).
- USEPA. Technical Report for the Demonstration of Wide Area Radiological Decontamination and Mitigation Technologies for Building Structures and Vehicles. EPA/600/R-616/019. 2016. Available online: https://cfpub.epa.gov/si/si_public_record_report.cfm?Lab=NHSRC&dirEntryId=312072 (accessed on 9 April 2022).
- Raveh-Amit, H.; Sharon, A.; Katra, I.; Stilman, T.; Serre, S.; Archer, J.; Magnuson, M. Limiting Wind-Induced Resuspension of Radioactively Contaminated Particles to Enhance First Responder, Early Phase Worker and Public Safety—Part 1. Appl. Sci. 2022, 12, 2463. [Google Scholar] [CrossRef]
- IWTSD. Irregular Warfare Technical Support Directorate. 2022. Available online: https://www.cttso.gov (accessed on 9 April 2022).
- Clark, D.E.; Cobbin, W.C. Removal Effectiveness of Simulated Dry Fallout from Paved Areas by Motorized and Vacuumized Street Sweepers; Naval Radiological Defense Lab: San Francisco, CA, USA, 1963; Available online: https://apps.dtic.mil/sti/pdfs/AD0456495.pdf (accessed on 9 April 2022).
- Wiltshire, L.L.; Owen, L.W. Three Tests of Firehosing Technique and Equipment for the Removal of Fallout from Asphalt Streets and Roofing Materials; Naval Radiological Defense Lab: San Francisco, CA, USA, 1966; Available online: https://apps.dtic.mil/sti/pdfs/AD0640491.pdf (accessed on 9 April 2022).
- NRC. Appendix B to Part 20—Annual Limits on Intake (ALIs) and Derived Air Concentrations (DACs) of Radionuclides for Occupational Exposure; Effluent Concentrations; Concentrations for Release to Sewerage. 2021. Available online: https://www.nrc.gov/reading-rm/doc-collections/cfr/part020/part020-appb.html (accessed on 9 April 2022).
- Alam, M.S.; Hyde, B.; Duffy, P.; McNabola, A. Analysing the Co-Benefits of transport fleet and fuel policies in reducing PM2.5 and CO2 emissions. J. Clean. Prod. 2018, 172, 623–634. [Google Scholar] [CrossRef]
- Piscitello, A.; Bianco, C.; Casasso, A.; Sethi, R. Non-exhaust traffic emissions: Sources, characterization, and mitigation measures. Sci. Total Environ. 2021, 766, 144440. [Google Scholar] [CrossRef] [PubMed]






| Particle Size Analysis | Wetted | Nonwetted |
|---|---|---|
| Clay (%) | 24.00 | 28.00 |
| Silt (%) | 59.90 | 56.70 |
| Sand (%) | 16.10 | 15.30 |
| Organic Matter (%) | 0.43 | 0.25 |
| Fine Gravel (%) | 0 | 0 |
| Sand Fractions (%) | ||
| 1 mm | 0.10 | 0.00 |
| 0.25 mm | 1.10 | 0.00 |
| 0.15 mm | 0.60 | 0.20 |
| 0.05 mm | 14.30 | 15.10 |
| Particle Size Analysis | Israel Soil Samples | Experimental Bulk Soil | ||
|---|---|---|---|---|
| Ze’elim | Rotem | Ohio Mulch | Garick | |
| Clay (%) | 4 | 2 | 1.5 | 2 |
| Silt (%) | 4 | 1.2 | 1.6 | 0.8 |
| Sand (%) | 92 | 96.8 | 74.4 | 97.2 |
| Organic Matter (%) | 0.08 | 0 | 0.87 | 0.05 |
| Fine Gravel (%) | 0 | 0 | 22.5 | 0 |
| Sand Fractions (%) | ||||
| 1 mm | 0.1 | 1 | 12.6 | 1.4 |
| 0.25 mm | 28.9 | 87.3 | 59.6 | 74 |
| 0.15 mm | 10.6 | 5.8 | 1.4 | 12.3 |
| 0.05 mm | 52.4 | 2.7 | 0.08 | 9.5 |
| Texture Class | Sand | Sand | Sand | Sand |
| Test Surface | Stabilizaiton Technology | Initial CPS (Average) | Removal (%R) | Transferred (%T) | Residual (%R) |
|---|---|---|---|---|---|
| Asphalt | Control | 1497 | 15 ± 1 | 8 ± 1 | 9 ± 1 |
| CaCl2 | 1089 | 5 ± 4 | 1 ± 1 | 4 ± 2 | |
| MVP-Fx | 1292 | 2 ± 1 | 0.2 ± 0.6 | 0.5 ± 0.4 | |
| Soil2O | 1210 | 10 ± 5 | 5 ± 2 | 9 ± 7 | |
| Concrete | Control | 1773 | 7 ± 3 | 5 ± 2 | 3 ± 1 |
| CaCl2 | 1219 | 0.9 ± 0.7 | 1 ± 1 | 0.8 ± 0.9 | |
| MVP-Fx | 1346 | 3 ± 1 | 1 ± 1 | 2 ± 1 | |
| Soil2O | 1382 | 5 ± 1 | 4 ± 1 | 2 ± 1 | |
| Sand | Control | 1363 | 41 ± 8 | 9 ± 3 | 20 ± 2 |
| CaCl2 | 1303 | 17 ± 4 | 3 ± 2 | 5 ± 3 | |
| MVP-Fx | 1312 | 16 ± 6 | 3 ± 1 | 2 ± 1 | |
| Soil2O | 1325 | 25 ± 2 | 6 ± 2 | 11 ± 4 |
| Surface | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | 0.0172 | <0.0001 | 0.1764 |
| CaCl2 | 0.2535 | 0.1655 | ||
| MVP-Fx | 0.0475 | |||
| Concrete | Control | 0.0255 | 0.0394 | 0.2081 |
| CaCl2 | 0.0133 | 0.0010 | ||
| MVP-Fx | 0.0322 | |||
| Sand | Control | 0.0017 | 0.0023 | 0.0251 |
| CaCl2 | 0.6876 | 0.0101 | ||
| MVP-Fx | 0.0256 |
| Surfaces | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | <0.0001 | <0.0001 | 0.0444 |
| CaCl2 | 0.0802 | 0.0160 | ||
| MVP-Fx | 0.0065 | |||
| Concrete | Control | 0.0139 | 0.0072 | 0.2285 |
| CaCl2 | 0.8527 | 0.0281 | ||
| MVP-Fx | 0.0073 | |||
| Sand | Control | 0.0070 | 0.0055 | 0.0759 |
| CaCl2 | 0.9631 | 0.0411 | ||
| MVP-Fx | 0.0277 |
| Surfaces | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | 0.0036 | <0.0001 | 0.9709 |
| CaCl2 | 0.0267 | 0.2282 | ||
| MVP-Fx | 0.0907 | |||
| Concrete | Control | 0.0089 | 0.1401 | 0.0743 |
| CaCl2 | 0.0388 | 0.0404 | ||
| MVP-Fx | 0.7378 | |||
| Sand | Control | 0.0002 | <0.0001 | 0.0056 |
| CaCl2 | 0.1503 | 0.0601 | ||
| MVP-Fx | 0.0169 |
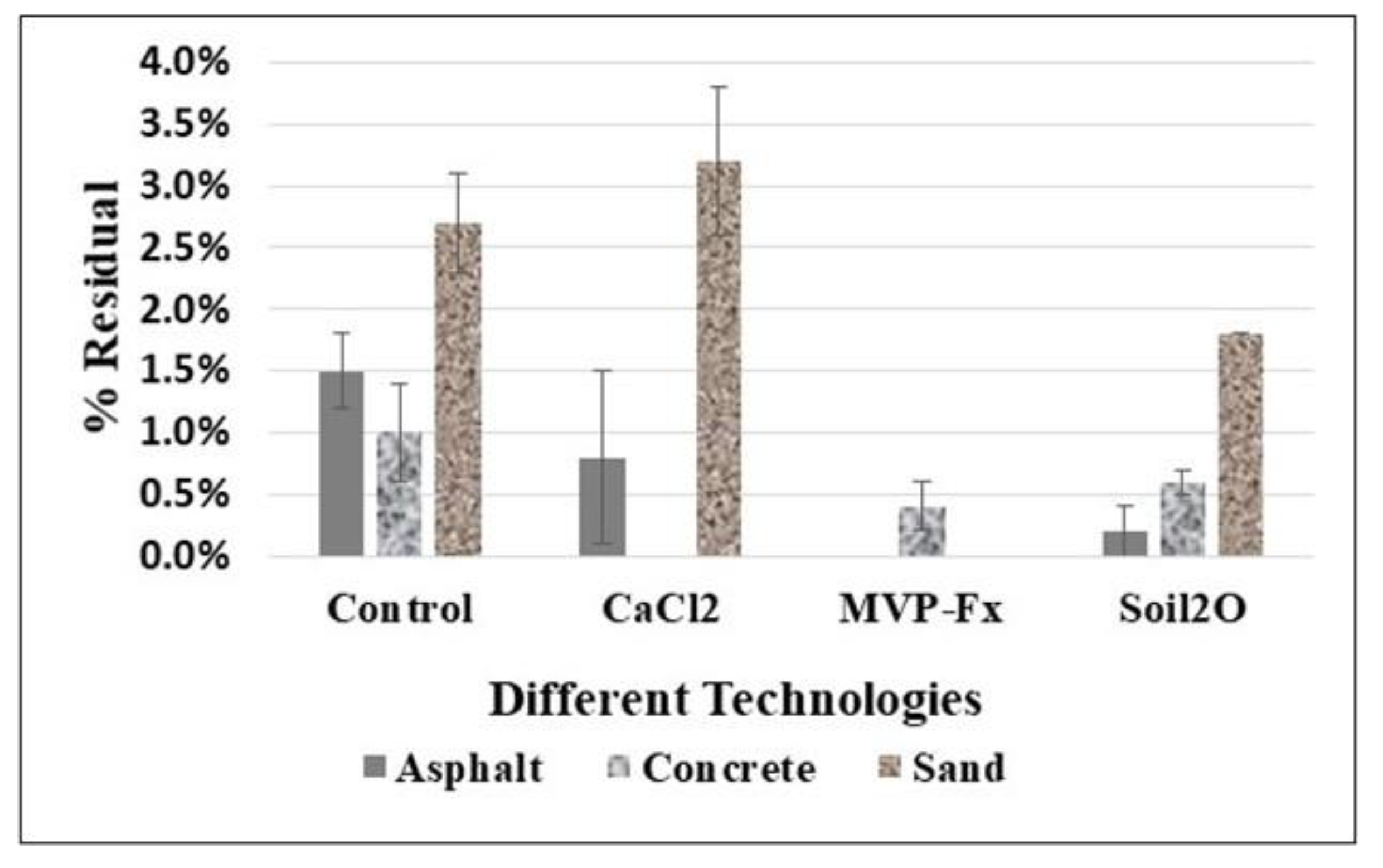
| Test Surface | Stabilizaiton Technology | Initial CPS (Average) | Removal (%R) | Transferred (%T) | Residual (%R) |
|---|---|---|---|---|---|
| Asphalt | Control | 1918 | 4 ± 1 | 1 ± 0.6 | 2 ± 0.3 |
| CaCl2 | 1283 | 1 ± 1 | ND | 0.8 ± 0.7 | |
| MVP-Fx | 1671 | ND | ND | ND | |
| Soil2O | 1688 | 2 ± 1 | 0.2 ± 0.2 | 0.2 ± 0.2 | |
| Concrete | Control | 1869 | 2 ± 1 | 1 ± 0.1 | 1 ± 0.4 |
| CaCl2 | 1794 | 1 ± 1 | ND | ND | |
| MVP-Fx | 1685 | 3 ± 1 | 0.9 ± 0.2 | 0.4 ± 0.2 | |
| Soil2O | 1575 | 2 ± 1 | 1 ± 0.2 | 0.6 ± 0.1 | |
| Sand | Control | 3401 | 40 ± 4 | 8 ± 2 | 3.0 ± 0.4 |
| CaCl2 | 2683 | 38.0 ± 0.4 | 0.6 ± 0.6 | 3 ± 1 | |
| MVP-Fx | 2544 | 14.0 ± 0.2 | 2.0 ± 0.2 | 0 ± 0 | |
| Soil2O | 2968 | 24.0 ± 0.9 | 0.8 ± 0.2 | 2 ± 0 |
| Surfaces | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | 0.0024 | <0.0001 | 0.0009 |
| CaCl2 | 0.0366 | 0.5490 | ||
| MVP-Fx | 0.0027 | |||
| Concrete | Control | 0.0429 | 0.5701 | 0.2801 |
| CaCl2 | 0.0230 | 0.3127 | ||
| MVP-Fx | 0.1435 |
| Surfaces | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | 0.0035 | 0.0121 | 0.0108 |
| CaCl2 | 0.7594 | 0.0422 | ||
| MVP-Fx | 0.0010 | |||
| Concrete | Control | <0.0001 | 0.0407 | 0.1677 |
| CaCl2 | <0.0001 | <0.0001 | ||
| MVP-Fx | 0.4502 |
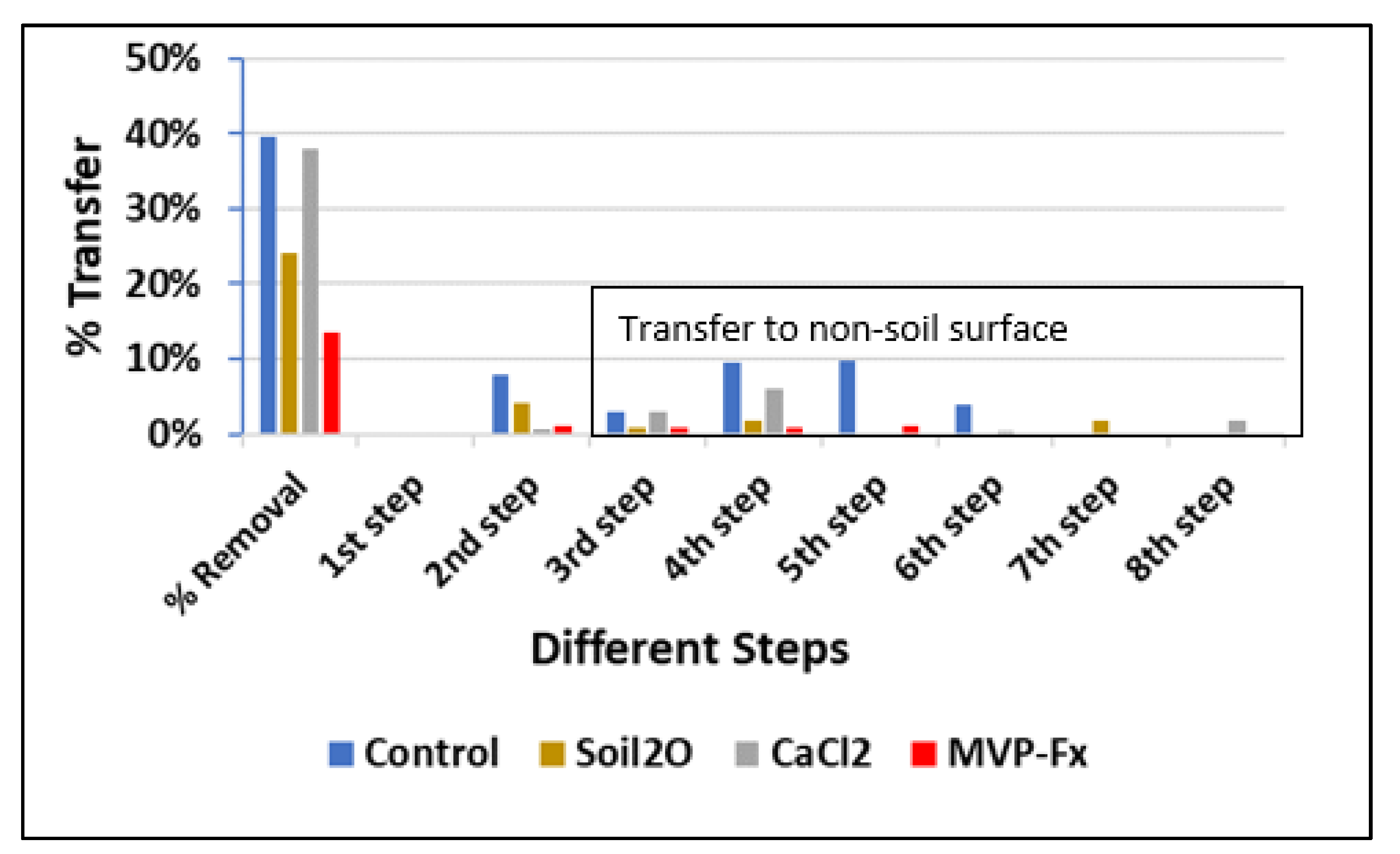
| Step Number | Control | Soil2O | CaCl2 | MVP-Fx |
|---|---|---|---|---|
| 1 | ND 1 | ND | ND | ND |
| 2 | 8% | 4% | 0.6% | 1% |
| 3 | 3% | 0.8% | 3% | 0.8% |
| 4 | 10% | 2% | 6% | 0.9% |
| 5 | 1% | 0.2% | NC | 1% |
| 6 | 4% | ND | 0.3% | ND |
| 7 | ND | 2% | ND | ND |
| 8 | NC 2 | ND | 2% | ND |
| Surfaces | Stab. Tech. | CaCl2 | MVP-Fx | Soil2O |
|---|---|---|---|---|
| Asphalt | Control | 0.1143 | <0.0001 | 0.0004 |
| CaCl2 | 0.0481 | 0.1662 | ||
| MVP-Fx | 0.0023 | |||
| Concrete | Control | 0.0120 | 0.0553 | 0.1460 |
| CaCl2 | 0.0799 | 0.0157 | ||
| MVP-Fx | 0.1769 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Magnuson, M.; Stilman, T.; Serre, S.; Archer, J.; James, R.; Xia, X.; Lawrence, M.; Tamargo, E.; Raveh-Amit, H.; Sharon, A. Part 2: Stabilization/Containment of Radiological Particle Contamination to Enhance First Responder, Early Phase Worker, and Public Safety. Appl. Sci. 2022, 12, 3861. https://doi.org/10.3390/app12083861
Magnuson M, Stilman T, Serre S, Archer J, James R, Xia X, Lawrence M, Tamargo E, Raveh-Amit H, Sharon A. Part 2: Stabilization/Containment of Radiological Particle Contamination to Enhance First Responder, Early Phase Worker, and Public Safety. Applied Sciences. 2022; 12(8):3861. https://doi.org/10.3390/app12083861
Chicago/Turabian StyleMagnuson, Matthew, Terry Stilman, Shannon Serre, John Archer, Ryan James, Xiaoyan Xia, Mitchell Lawrence, Erin Tamargo, Hadas Raveh-Amit, and Avi Sharon. 2022. "Part 2: Stabilization/Containment of Radiological Particle Contamination to Enhance First Responder, Early Phase Worker, and Public Safety" Applied Sciences 12, no. 8: 3861. https://doi.org/10.3390/app12083861






