In Vitro Activity of Caffeic Acid Phenethyl Ester against Different Oral Microorganisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. CAPE Preparation
2.2. Microorganism
2.3. CAPE Antibacterial Effectiveness against Tested Organisms
2.4. Minimum Inhibitory Concentration (MIC) Test
2.5. Minimum Bactericidal Concentration (MBC) Test
2.6. MBC Values for Bacterial Count throughout Various Periods
2.7. Well Diffusion Test
2.8. Statistical Analysis
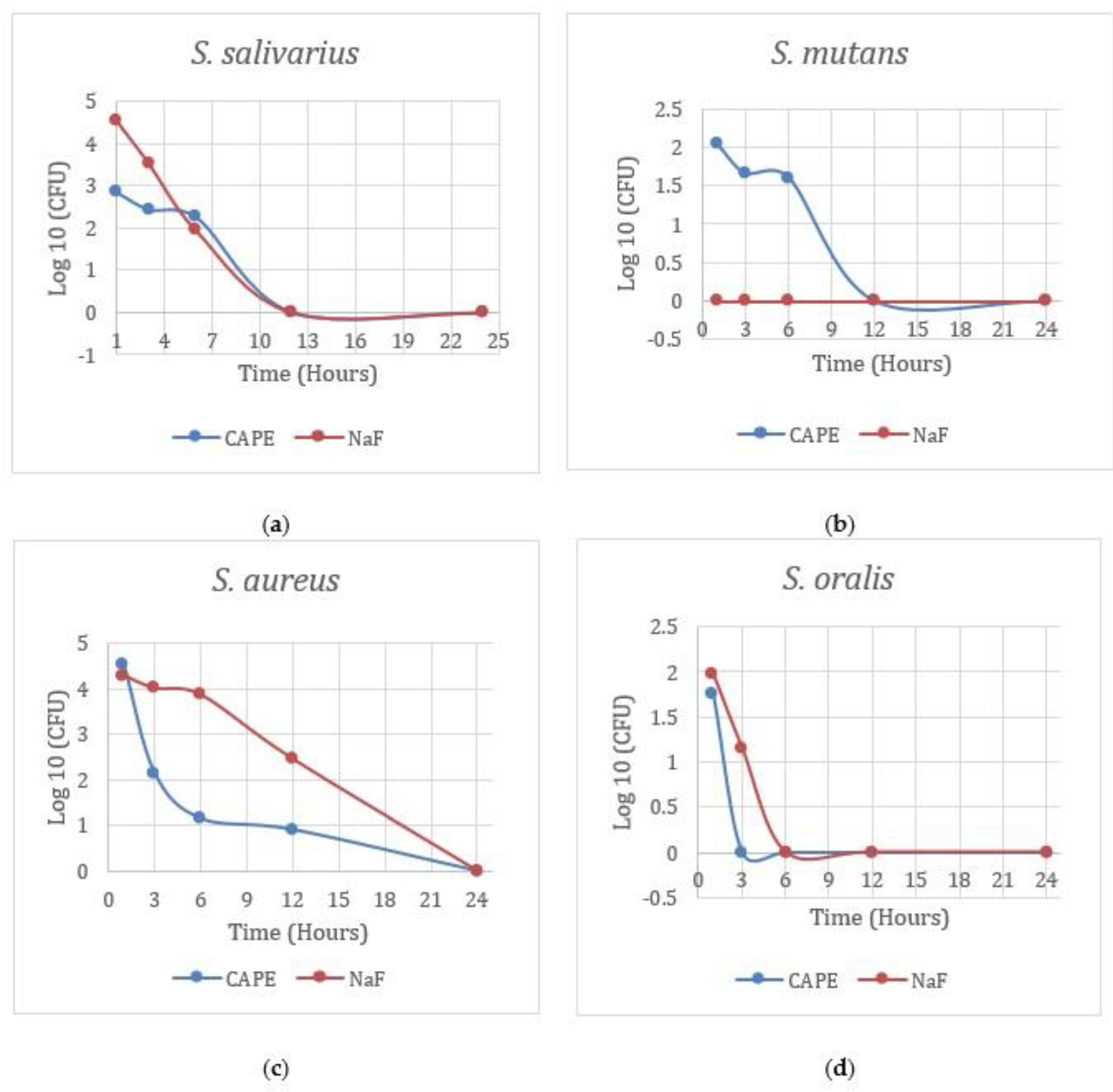
3. Results
4. Discussion
5. Conclusions
6. Further Perspectives
7. Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moynihan, P. Sugars and Dental Caries: Evidence for Setting a Recommended Threshold for Intake. Adv. Nutr. 2016, 7, 149–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, S.; Liu, J.; Lo, E.C.; Chu, C.H. Dental caries status of Bulang preschool children in Southwest China. BMC Oral Health 2014, 14, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prakasha Shrutha, S.; Vinit, G.B.; Giri, K.Y.; Alam, S. Feeding practices and early childhood caries: A cross-sectional study of preschool children in kanpur district, India. ISRN Dent 2013, 2013, 275193. [Google Scholar] [CrossRef] [PubMed]
- Thekiso, M.; Yengopal, V.; Rudolph, M.J.; Bhayat, A. Caries status among children in the West Rand District of Gauteng Province, South Africa. SADJ 2012, 67, 318–320. [Google Scholar]
- Pitts, N.B.; Boyles, J.; Nugent, Z.J.; Thomas, N.; Pine, C.M. The dental caries experience of 5-year-old children in Great Britain (2005/6). Surveys co-ordinated by the British Association for the study of community dentistry. Community Dent Health 2007, 24, 59–63. [Google Scholar]
- Congiu, G.; Campus, G.; Sale, S.; Spano, G.; Cagetti, M.G.; Lugliè, P.F. Early childhood caries and associated determinants: A cross-sectional study on Italian preschool children. J. Public Health Dent 2014, 74, 147–152. [Google Scholar] [CrossRef]
- Conrads, G.; About, I. Pathophysiology of Dental Caries. Monogr. Oral Sci. 2018, 27, 1–10. [Google Scholar] [CrossRef]
- LuIs, H.S.; Luis, L.S.; Bernardo, M. study of the effect of an essential oil and a delmopinol mouth rinse on dental plaque bacteria. Indian J. Dent. Res. 2016, 27, 648–651. [Google Scholar] [CrossRef]
- Iyer, M.; Gujjari, A.K.; Gowda, V.; Angadi, S. Antifungal response of oral-associated candidal reference strains (American Type Culture Collection) by supercritical fluid extract of nutmeg seeds for geriatric denture wearers: An. J. Indian Prosthodont. Soc. 2017, 17, 267–272. [Google Scholar] [CrossRef]
- Fong, H.H. Integration of herbal medicine into modern medical practices: Issues and prospects. Integr. Cancer Ther. 2002, 1, 287–293. [Google Scholar] [CrossRef]
- Dattner, A.M. From medical herbalism to phytotherapy in dermatology: Back to the future. Dermatol. Ther. 2003, 16, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Anjum, S.I.; Ullah, A.; Khan, K.A.; Attaullah, M.; Khan, H.; Ali, H.; Bashir, M.A.; Tahir, M.; Ansari, M.J.; Ghramh, H.A.; et al. Composition and functional properties of propolis (bee glue): A review. Saudi J. Biol. Sci. 2019, 26, 1695–1703. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Bamunuarachchi, N.I.; Tabassum, N.; Kim, Y.M. Caffeic Acid and Its Derivatives: Antimicrobial Drugs toward Microbial Pathogens. J. Agric. Food Chem. 2021, 69, 2979–3004. [Google Scholar] [CrossRef] [PubMed]
- Muhammad Abdul Kadar, N.N.; Ahmad, F.; Teoh, S.L.; Yahaya, M.F. Caffeic Acid on Metabolic Syndrome: A Review. Molecules 2021, 26, 5490. [Google Scholar] [CrossRef]
- Al-Khalifa, K.S.; AlSheikh, R.; Al-Hariri, M.T.; El-Sayyad, H.; Alqurashi, M.S.; Ali, S.; Bugshan, A.S. Evaluation of the Antimicrobial Effect of Thymoquinone against Different Dental Pathogens: An In Vitro Study. Molecules 2021, 26, 6451. [Google Scholar] [CrossRef]
- Niederstebruch, N.; Sixt, D.; Benda, B.I.; Banboye, N. A suitable blood agar containing human blood especially for the use in laboratories of developing countries. J. Infect. Dev. Ctries. 2017, 11, 399–406. [Google Scholar] [CrossRef] [Green Version]
- CLSI Document M07-A9; Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. Approved Standard—Ninth Edition; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012.
- Silva, F.R.G.; Matias, T.M.S.; Souza, L.I.O.; Matos-Rocha, T.J.; Fonseca, S.A.; Mousinho, K.C.; Santos, A.F. Phytochemical screening and in vitro antibacterial, antifungal, antioxidant and antitumor activities of the red propolis Alagoas. Braz. J. Biol. 2019, 79, 452–459. [Google Scholar] [CrossRef]
- Shakoor, S.; Fasih, N.; Jabeen, K.; Jamil, B. Rothia dentocariosa endocarditis with mitral valve prolapse: Case report and brief review. Infection 2011, 39, 177–179. [Google Scholar] [CrossRef]
- Kojima, A.; Nomura, R.; Naka, S.; Okawa, R.; Ooshima, T.; Nakano, K. Aggravation of inflammatory bowel diseases by oral streptococci. Oral Dis. 2014, 20, 359–366. [Google Scholar] [CrossRef]
- Haraszthy, V.I.; Reynolds, H.S.; Sreenivasan, P.K.; Subramanyam, R.; Cummins, D.; Zambon, J.J. Media- and method-dependent variations in minimal inhibitory concentrations of antiplaque agents on oral bacteria. Lett. Appl. Microbiol. 2006, 43, 256–261. [Google Scholar] [CrossRef]
- Galeotti, F.; Maccari, F.; Fachini, A.; Volpi, N. Chemical Composition and Antioxidant Activity of Propolis Prepared in Different Forms and in Different Solvents Useful for Finished Products. Foods 2018, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuropatnicki, A.K.; Szliszka, E.; Krol, W. Historical aspects of propolis research in modern times. Evid Based Complement Altern. Med. 2013, 2013, 964149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews, J.M. Determination of minimum inhibitory concentrations. J. Antimicrob. Chemother. 2001, 48 (Suppl. 1), 5–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duailibe, S.A.; Gonçalves, A.G.; Ahid, F.J. Effect of a propolis extract on Streptococcus mutans counts in vivo. J. Appl. Oral. Sci. 2007, 15, 420–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nazeri, R.; Ghaiour, M.; Abbasi, S. Evaluation of Antibacterial Effect of Propolis and its Application in Mouthwash Production. Front. Dent. 2019, 16, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, G.L.S.; Bezerra, L.M.D.; Ribeiro, I.L.A.; Morais Júnior, R.C.D.; Castro, R.D. Susceptibility of cariogenic microorganisms to phytoconstituents. Braz. J. Biol. 2018, 78, 691–696. [Google Scholar] [CrossRef] [Green Version]
- Awawdeh, L.; Al-Beitawi, M.; Hammad, M. Effectiveness of propolis and calcium hydroxide as a short-term intracanal medicament against Enterococcus faecalis: A laboratory study. Aust. Endod. J. 2009, 35, 52–58. [Google Scholar] [CrossRef]
- Akca, A.E.; Akca, G.; Topçu, F.T.; Macit, E.; Pikdöken, L.; Özgen, I. The Comparative Evaluation of the Antimicrobial Effect of Propolis with Chlorhexidine against Oral Pathogens: An In Vitro Study. Biomed. Res. Int. 2016, 2016, 3627463. [Google Scholar] [CrossRef] [Green Version]
- Choo, S.W.; Mohammed, W.K.; Mutha, N.V.R.; Rostami, N.; Ahmed, H.; Krasnogor, N.; Tan, G.Y.A.; Jakubovics, N.S. Transcriptomic Responses to Coaggregation between Streptococcus gordonii and Streptococcus oralis. Appl. Environ. Microbiol. 2021, 87, e0155821. [Google Scholar] [CrossRef]
- Niu, Y.; Wang, K.; Zheng, S.; Wang, Y.; Ren, Q.; Li, H.; Ding, L.; Li, W.; Zhang, L. Antibacterial Effect of Caffeic Acid Phenethyl Ester on Cariogenic Bacteria and Streptococcus mutans Biofilms. Antimicrob. Agents Chemother. 2020, 64, E00251-20. [Google Scholar] [CrossRef]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filogônio, C.e.F.; Soares, R.V.; Horta, M.C.; Penido, C.V.; Cruz, R.e.A. Effect of vegetable oil (Brazil nut oil) and mineral oil (liquid petrolatum) on dental biofilm control. Braz. Oral. Res. 2011, 25, 556–561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lobo, P.L.; Fonteles, C.S.; Marques, L.A.; Jamacaru, F.V.; Fonseca, S.G.; de Carvalho, C.B.; de Moraes, M.E. The efficacy of three formulations of Lippia sidoides Cham. essential oil in the reduction of salivary Streptococcus mutans in children with caries: A randomized, double-blind, controlled study. Phytomedicine 2014, 21, 1043–1047. [Google Scholar] [CrossRef] [PubMed]
- Marya, C.M.; Oberoi, S.S.; Nagpal, R.; Dhingra, C. Comparison of Antimicrobial Efficacy of Brazilian Propolis With Chlorhexidine and Sodium Fluoride Against Common Oral Pathogens: An In Vitro Study. Jundishapur J. Nat. Pharm. Prod. 2015, 10, e19069. [Google Scholar] [CrossRef]
| Concentration (mg/mL) | Inhibition Zone (Mean and SD in mm) | |||
|---|---|---|---|---|
| S. mutans | S. aureus | S. salivarius | S. oralis | |
| 12.5 | 19 ± 1.4 | 31 ± 0.3 | 26 ± 1.2 | 25 ± 0.9 |
| 25 | 26 ± 1.5 | 31 ± 1.1 | 26 ± 0.8 | 27 ± 1.0 |
| 50 | 26 ± 0.9 | 32 ± 0.8 | 27 ± 0.9 | 27 ± 1.1 |
| 75 | 27 ± 0.7 | 32 ± 0.4 | 27 ± 0.8 | 29 ± 0.8 |
| 100 | 28 ± 0.8 | 37 ± 0.3 | 31 ± 1.0 | 30 ± 1.1 |
| NaF (+ve control) | 31 ± 0.9 | 16 ± 1.1 | 12 ± 1.1 | 19 ± 1.2 |
| Microorganisms | CAPE | NaF | ||
|---|---|---|---|---|
| MIC | MBC | MIC | MBC | |
| S. mutans | 1.0 | 1.5 | 1.86 | >3.0 |
| S. salivarius | 3.0 | 4.0 | 0.93 | >4.0 |
| S. oralis | 1.0 | 1.5 | 0.72 | >2.0 |
| S. aureus | 3.0 | 4.0 | 0.74 | >2.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
AlSheikh, R.; Albagieh, H.N.; Abdouh, I.; Zaki, H.; Alzahrani, A.M.; Halawany, H.S.; Al-Khalifa, K.S. In Vitro Activity of Caffeic Acid Phenethyl Ester against Different Oral Microorganisms. Appl. Sci. 2022, 12, 3959. https://doi.org/10.3390/app12083959
AlSheikh R, Albagieh HN, Abdouh I, Zaki H, Alzahrani AM, Halawany HS, Al-Khalifa KS. In Vitro Activity of Caffeic Acid Phenethyl Ester against Different Oral Microorganisms. Applied Sciences. 2022; 12(8):3959. https://doi.org/10.3390/app12083959
Chicago/Turabian StyleAlSheikh, Rasha, Hamad N. Albagieh, Ismail Abdouh, Hattan Zaki, Amal M. Alzahrani, Hassan S. Halawany, and Khalifa S. Al-Khalifa. 2022. "In Vitro Activity of Caffeic Acid Phenethyl Ester against Different Oral Microorganisms" Applied Sciences 12, no. 8: 3959. https://doi.org/10.3390/app12083959
APA StyleAlSheikh, R., Albagieh, H. N., Abdouh, I., Zaki, H., Alzahrani, A. M., Halawany, H. S., & Al-Khalifa, K. S. (2022). In Vitro Activity of Caffeic Acid Phenethyl Ester against Different Oral Microorganisms. Applied Sciences, 12(8), 3959. https://doi.org/10.3390/app12083959







