Advanced Oxidation Pretreatment for Biological Treatment of Reclaimer Wastewater Containing High Concentration N-methyldiethanolamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Wastewater Characteristics
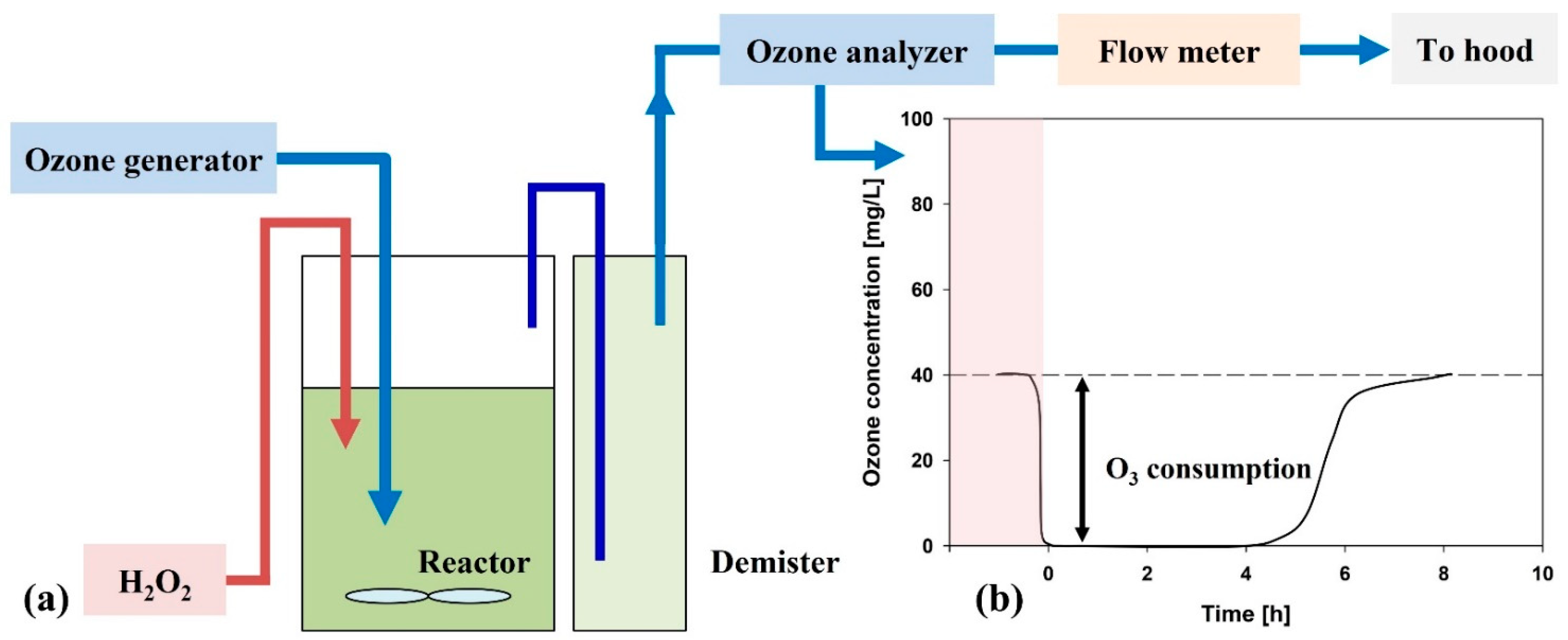
2.2. Advanced Oxidation Pretreatment Tests
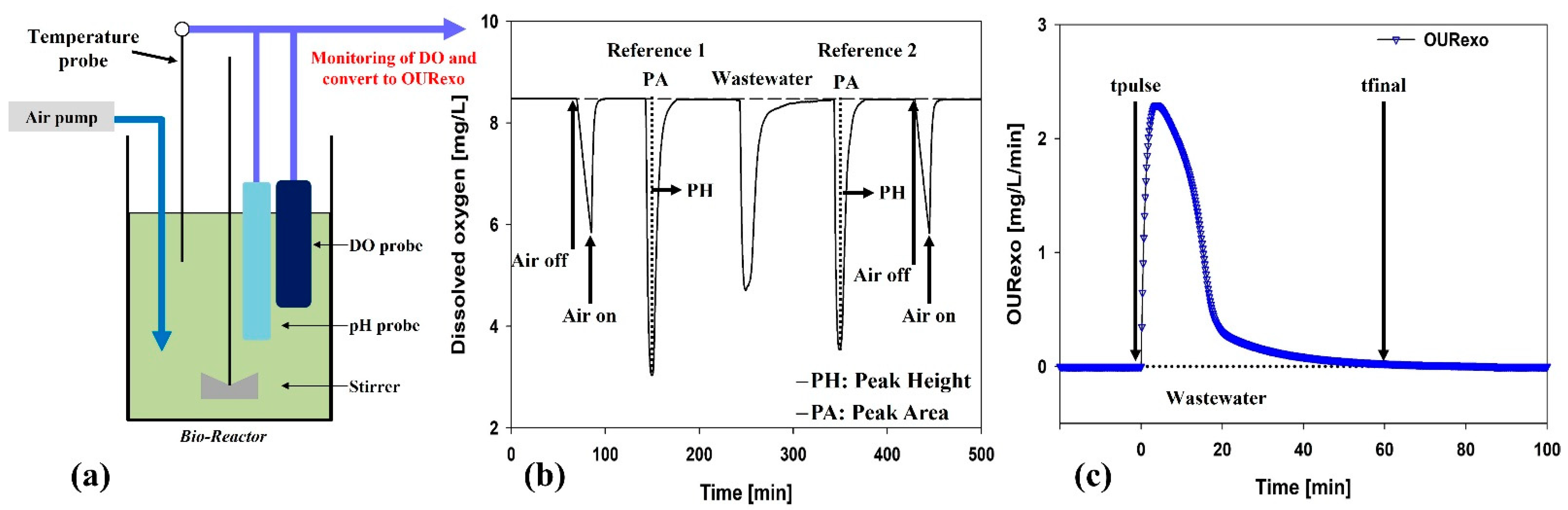
2.3. Respirometer Experiments
2.4. Analysis
3. Results and Discussion
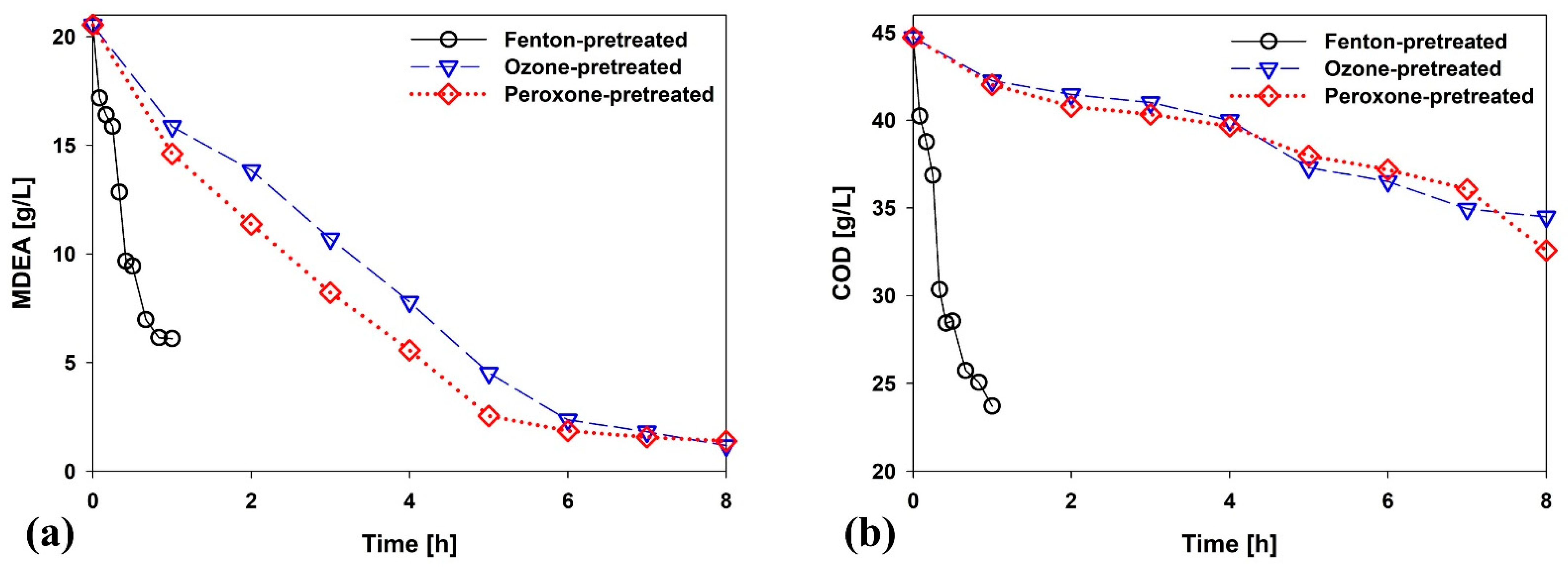
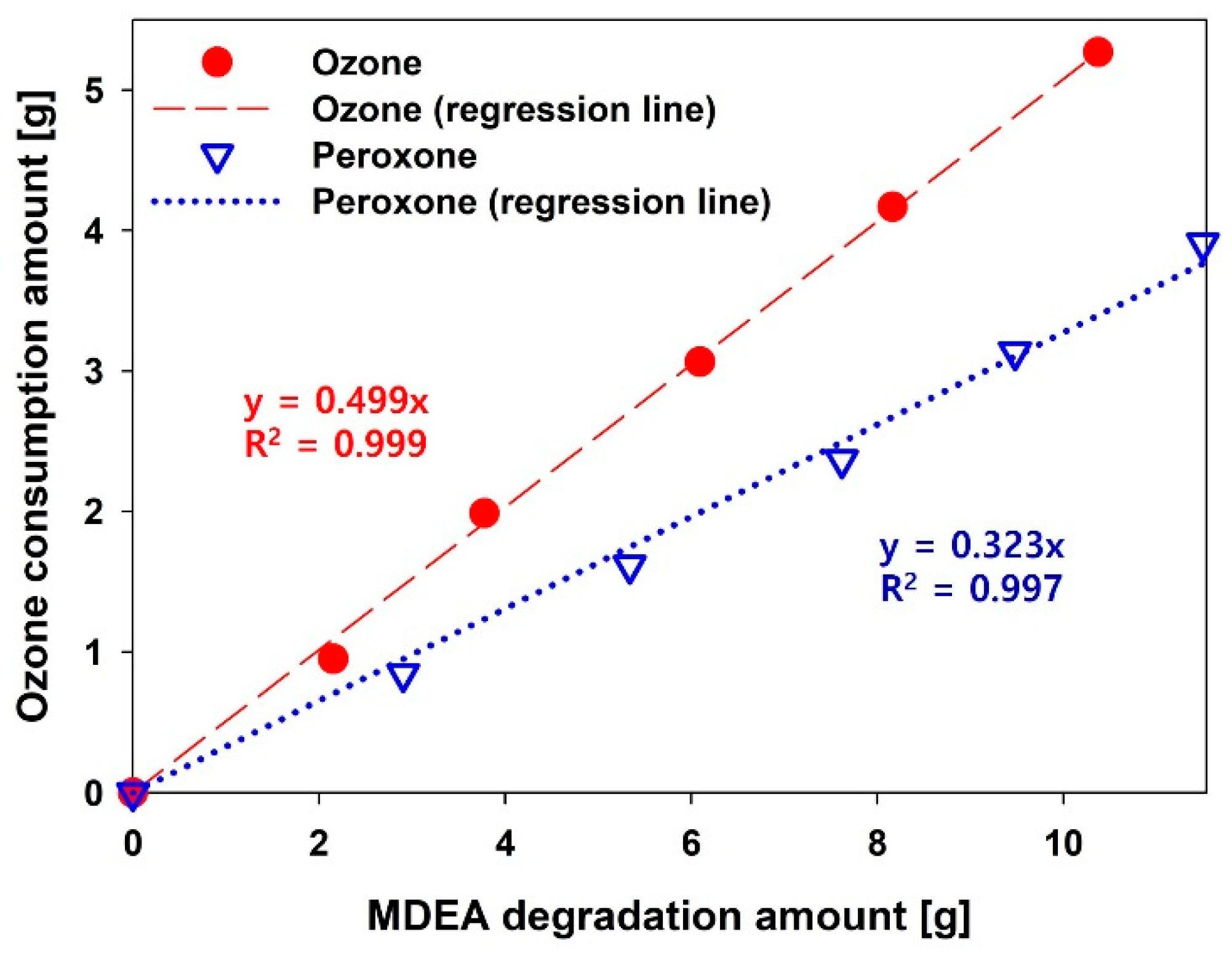
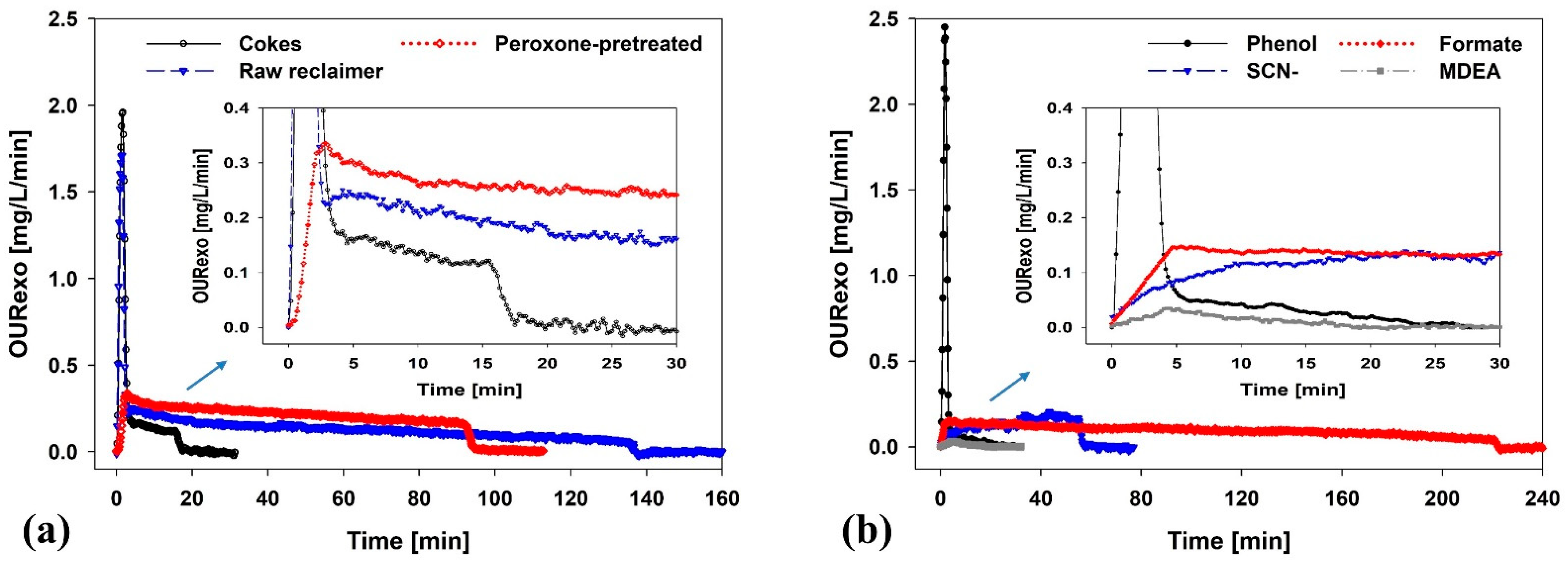
3.1. Performance of AOP Pretreatment
3.2. Effect of Peroxone-Pretreated Wastewater on BWTP
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fürhacker, M.; Pressl, A.; Allabashi, R. Aerobic biodegradability of methyldiethanolamine (MDEA) used in natural gas sweetening plants in batch tests and continuous flow experiments. Chemosphere 2003, 52, 1743–1748. [Google Scholar] [CrossRef]
- Omar, A.A.; Ramli, R.M.; Faizura, P.N.; Khamaruddin, M. Fenton oxidation of natural gas plant wastewater. Can. J. Chem. Eng. 2010, 1, 1–6. [Google Scholar]
- Duran-Moreno, A.; García-Gonzalez, S.A.; Gutierrez-Lara, M.R.; Rigas, F.; Ramírez-Zamora, R.M. Assessment of Fenton’s reagent and ozonation as pre-treatments for increasing the biodegradability of aqueous diethanolamine solutions from an oil refinery gas sweetening process. J. Hazard. Mater. 2011, 186, 1652–1659. [Google Scholar] [CrossRef]
- Harimurti, S.; Omar, A.A.; Rahmah, A.U.; Murugesan, T. The degradation mechanism of wastewater containing MDEA using UV/H2O2 advanced oxidation process. In Proceedings of the National Postgraduate Conference, Kuala Lumpur, Malaysia, 19–20 September 2011; pp. 1–5. [Google Scholar]
- Rodríguez, N.; Mussati, S.; Scenna, N. Optimization of post-combustion CO2 process using DEA–MDEA mixtures. Chem. Eng. Res. Des. 2011, 89, 1763–1773. [Google Scholar] [CrossRef]
- Dumée, L.; Scholes, C.; Stevens, G.; Kentish, S. Purification of aqueous amine solvents used in post combustion CO2 capture: A review. Int. J. Greenh. Gas Con. 2012, 10, 443–455. [Google Scholar] [CrossRef]
- Dutta, B.K.; Harimurti, S.; Ariff, I.F.M.; Chakrabarti, S.; Vione, D. Degradation of diethanolamine by Fenton’s reagent combined with biological post-treatment. Desalin. Water Treat. 2010, 19, 286–293. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tian, K.; Xiong, X.; Ren, H. Treatment of overhaul wastewater containing N-methyldiethanolamine (MDEA) through modified Fe-C microelectrolysis-configured ozonation: Investigation on process optimization and degradation mechanisms. J. Hazard. Mater. 2019, 369, 655–664. [Google Scholar] [CrossRef]
- Sexton, A.; Fisher, K.; Beitler, C.; Dombrowski, K.; Youngerman, J.; Rochelle, G.; Chen, E.; Nielsen, P.; Davison, J.; Singh, P. Evaluation of amine reclaimer operation and waste disposal from post-combustion CO2 capture. In Proceedings of the Laurance Reid Gas Conditioning Conference, Norman, OK, USA, 21–24 February 2016; pp. 21–24. [Google Scholar]
- Mare, M.; Waldner, G.; Bauer, R.; Jacobs, H.; Broekaert, J. Degradation of nitrogen containing organic compounds by combined photocatalysis and ozonation. Chemosphere 1999, 38, 2013–2027. [Google Scholar] [CrossRef]
- Parisheva, Z.; Demirev, A. Ozonization of ethanolamine in aqueous medium. Water Res. 2000, 34, 1340–1344. [Google Scholar] [CrossRef]
- Pietsch, J.; Sacher, F.; Schmidt, W.; Brauch, H.-J. Polar nitrogen compounds and their behaviour in the drinking water treatment process. Water Res. 2001, 35, 3537–3544. [Google Scholar] [CrossRef]
- Jagadevan, S.; Graham, N.J.; Thompson, I.P. Treatment of waste metalworking fluid by a hybrid ozone-biological process. J. Hazard. Mater. 2013, 244–245, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Harimurti, S.; Ur Rahmah, A.; Aziz Omar, A.; Murugesan, T. UV/H2O2 Process for Removal of Total Organic Carbon from Refinery Effluent: Screening of Influence Factors Using Response Surface Methodology; Advanced Materials Research; Trans Tech Publ: Kapellweg, Switzerland, 2014; pp. 168–177. [Google Scholar]
- von Gunten, U. Ozonation of drinking water: Part I. Oxidation kinetics and product formation. Water Res. 2003, 37, 1443–1467. [Google Scholar] [CrossRef]
- Jang, H.H.; Seo, G.T.; Jeong, D.W. Investigation of Oxidation Methods for Waste Soy Sauce Treatment. Int. J. Environ. Res. 2017, 14, 1190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, K.; Mohseni, M.; Taghipour, F. Application of ultraviolet light-emitting diodes (UV-LEDs) for water disinfection: A review. Water Res. 2016, 94, 341–349. [Google Scholar] [PubMed]
- Pacholak, A.; Burlaga, N.; Frankowski, R.; Zgola-Grzeskowiak, A.; Kaczorek, E. Azole fungicides: (Bio)degradation, transformation products and toxicity elucidation. Sci. Total Environ. 2022, 802, 149917. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Dai, H.; Tan, C.; Pan, Q.; Hu, F.; Peng, X. Photo-Fenton degradation of tetracycline over Z-scheme Fe-g-C3N4/Bi2WO6 heterojunctions: Mechanism insight, degradation pathways and DFT calculation. Appl. Catal. B Environ. 2022, 310, 121326. [Google Scholar] [CrossRef]
- Zhu, X.; Tian, J.; Liu, R.; Chen, L. Optimization of Fenton and electro-Fenton oxidation of biologically treated coking wastewater using response surface methodology. Sep. Purif. Technol. 2011, 81, 444–450. [Google Scholar] [CrossRef]
- Sun, K.; Yuan, D.; Liu, Y.; Song, Y.; Sun, Z.; Liu, R. Study on the efficiency and mechanism of Direct Red 80 dye by conventional ozonation and peroxone (O3/H2O2) treatment. Sep. Sci. Technol. 2019, 55, 3175–3183. [Google Scholar] [CrossRef]
- Riano, B.; Coca, M.; Garcia-Gonzalez, M.C. Evaluation of Fenton method and ozone-based processes for colour and organic matter removal from biologically pre-treated swine manure. Chemosphere 2014, 117, 193–199. [Google Scholar] [CrossRef]
- Bokare, A.D.; Choi, W. Review of iron-free Fenton-like systems for activating H2O2 in advanced oxidation processes. J. Hazard. Mater. 2014, 275, 121–135. [Google Scholar] [CrossRef]
- Kim, Y.M.; Cho, H.U.; Lee, D.S.; Park, C.; Park, D.; Park, J.M. Response of nitrifying bacterial communities to the increased thiocyanate concentration in pre-denitrification process. Bioresour. Technol. 2011, 102, 913–922. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Mao, X.H. Degradation of Phenol-containing Wastewater Using an Improved Electro-Fenton Process. Int. J. Electrochem. Sci. 2012, 7, 4078–4088. [Google Scholar]
- Vanrolleghem, P.A.; Kong, Z.; Rombouts, G.; Verstraete, W. An on-line respirographic biosensor for the characterization of load and toxicity of wastewaters. J. Chem. Technol. Biotechnol. 1994, 59, 321–333. [Google Scholar] [CrossRef]
- Spanjers, H.; Vanrolleghem, P.A. Respirometry. In Experimental Methods in Wastewatertreatment; van Loosdrecht, M.C., Nielsen, P.H., Lopez–Vazquez, C.M., Brdjanovic, D., Eds.; IWA: London, UK, 2016; pp. 133–176. [Google Scholar]
- Vasiliadou, I.A.; Molina, R.; Martinez, F.; Melero, J.A.; Stathopoulou, P.M.; Tsiamis, G. Toxicity assessment of pharmaceutical compounds on mixed culture from activated sludge using respirometric technique: The role of microbial community structure. Sci. Total Environ. 2018, 630, 809–819. [Google Scholar] [CrossRef] [PubMed]
- Premanoch, P.; Saensing, P. Influence of Temperature and pH on Short-term Biochemical Oxygen Demand (BODst) Estimation. Thai Environ. Eng. J. 2019, 33, 77–83. [Google Scholar]
- C-MAC Co., Ltd. Homepage. Available online: http://www.c-mac.net/m_eng/index.html (accessed on 9 April 2022).
- APHA. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Washington, DC, USA, 1998. [Google Scholar]
- Kishore, K.; Dey, G.; Mukherjee, T. OH radical reactions with ethanolamines: Formation of reducing as well as oxidizing radicals. Res. Chem. Intermediat. 2004, 30, 837–845. [Google Scholar] [CrossRef]
- Zahardis, J.; Geddes, S.; Petrucci, G.A. The ozonolysis of primary aliphatic amines in fine particles. Atmos. Chem. Phys. 2008, 8, 1181–1194. [Google Scholar] [CrossRef] [Green Version]
- Katsoyiannis, I.A.; Canonica, S.; von Gunten, U. Efficiency and energy requirements for the transformation of organic micropollutants by ozone, O3/H2O2 and UV/H2O2. Water Res. 2011, 45, 3811–3822. [Google Scholar] [CrossRef]
- Dochain, D.; Vanrolleghem, P.A.; Van Daele, M. Structural identifiability of biokinetic models of activated sludge respiration. Water Res. 1995, 29, 2571–2578. [Google Scholar] [CrossRef]
- Vanrolleghem, P.A.; Sin, G.; Gernaey, K.V. Transient response of aerobic and anoxic activated sludge activities to sudden substrate concentration changes. Biotechnol. Bioeng. 2004, 86, 277–290. [Google Scholar] [CrossRef]
| Parameter | Reclaimer Wastewater | Cokes Wastewater |
|---|---|---|
| COD (mg/L) | 44,721 | 3967 |
| TN (mg-N/L) | 3064 | 263 |
| NH3 (mg-N/L) | 227 | 47 |
| MDEA (mg/L) | 20,548 | n.d. 1 |
| Formate (mg/L) | 20,420 | n.d. 1 |
| Phenol (mg/L) | 841 | 1024 |
| SCN− (mg/L) | 2479 | 455 |
| pH | 10.0 | 9.0 |
| Parameter | Fenton Pretreated | Ozone Pretreated | Peroxone Pretreated |
|---|---|---|---|
| COD (mg/L) | 23,710 | 34,487 | 32,575 |
| MDEA (mg/L) | 6111 | 1179 | 1382 |
| Formate (mg/L) | 5479 | 20,228 | 17,787 |
| Phenol (mg/L) | - 1 | 10.57 | 10.71 |
| SCN− (mg/L) | - 1 | - 1 | 50.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, G.-T.; Ahn, C.-K.; Lee, M.-W. Advanced Oxidation Pretreatment for Biological Treatment of Reclaimer Wastewater Containing High Concentration N-methyldiethanolamine. Appl. Sci. 2022, 12, 3960. https://doi.org/10.3390/app12083960
Oh G-T, Ahn C-K, Lee M-W. Advanced Oxidation Pretreatment for Biological Treatment of Reclaimer Wastewater Containing High Concentration N-methyldiethanolamine. Applied Sciences. 2022; 12(8):3960. https://doi.org/10.3390/app12083960
Chicago/Turabian StyleOh, Gi-Taek, Chi-Kyu Ahn, and Min-Woo Lee. 2022. "Advanced Oxidation Pretreatment for Biological Treatment of Reclaimer Wastewater Containing High Concentration N-methyldiethanolamine" Applied Sciences 12, no. 8: 3960. https://doi.org/10.3390/app12083960





