Abstract
Sargassum muticum is an invasive species to the coasts of the British Isles, mainland Europe and North America, with negative ecological and socioeconomic impacts. Pelagic Sargassum inundations on the beaches of the Caribbean have also been causing adverse health, ecological and economic effects. The finding of commercial uses of these biomasses may alleviate the costs of removal and control. Both pelagic Sargassum and S. muticum could be low-cost biosorbents for removing aqueous cationic dyes but may not be suitable for anionic substances without modification. This study found that a Sargassum biomass could remove up to 93% of methylene blue and that the species, concentration and treatment (CaCl2) were all statistically highly significant factors (p < 0.001) in its removal.
1. Introduction
Sargassum is a broad genus or family of brown seaweed with over 300 species [1]. Three species of Sargassum have recently been causing environmental and economic issues [2,3,4]. S. muticum, which is native to the northwest Pacific region [5], appeared on the shores of Europe in the 1970s, displacing native species and having negative environmental impacts and is classified in many European countries as an invasives species [6,7,8]. Pelagic Sargassum, particularly Sargassum fluitans and S. natans, is a tremendous ecological resource floating in the open ocean [4,9,10,11]. Small quantities arriving on the beach give environmental benefits, such as dune stabilisation [3,4,9,11,12]. Nevertheless, beaches across the Caribbean and the Gulf of Mexico have experienced massive inundations of pelagic Sargassum since 2011, negatively impacting the environment, human health and the local economies [3,13,14,15,16,17,18,19,20,21]. Removing and disposing of Sargassum is costly [3,9,11,22,23] and applications that generate revenue are being researched [3,4,22].
The living and dead cells of seaweeds can effectively remove heavy metals and other pollutants from wastewater [24,25,26,27]. Both S. muticum and pelagic Sargassum were suggested as biosorbents [2,9,26,28,29]. Synthetic dyes are found in the wastewater streams of many industries and can be highly resistant to conventional biological wastewater treatments, leaving behind highly coloured and toxic effluents [30,31]. Methylene blue (MB) is a cationic dye that is commonly used in the textile industry. Ingestion may cause respiratory issues, nausea, jaundice and skin irritation [32]. Some initial studies have shown that both S. muticum and pelagic Sargassum can effectively remove synthetic dyes, such as Methylene Blue [28,33]. However, these studies used freshwater washed and pretreated Sargassum with H2O2, CaCl2, HCl and formaldehyde to improve the absorption capacity via chemical modification, including protonation and chemical crosslinking [28,33]. Enhanced biosorption capacity is attributed to the protonation of cell wall components and improved stability by the Ca2+ ions of alginate molecules via the formation of the characteristic alginate arrangement, known as the egg-box structure.
Azo dyes, which are synthetic colours containing an azo group (-N=N-), are the most used dyes (60–70%) in industrial applications, including printing, textiles, tanning, packaging and, to a rapidly reducing extent, in the food industry [34,35,36]. Congo Red (CR) is an anionic dye that is often used in experiments as a typical example of one of the hundreds of potential azo dyes [34,35,36]. Brilliant Blue R (BB), which is a disulfonated triphenylmethane dye, is an anionic dye that is widely used in biotechnology. It was effectively removed using a fungal biosorbent (A. tubingensis), with the highest removal efficiency at lower pH values (pH 2) where the nitrogen-containing functional groups of the fungus are positively charged, allowing for electrostatic interaction with the OH− groups of the dye [37].
This study examined the biosorption of three dyes (MB, CR and BB) using two types of Sargassum with a minimum of pretreatment (S. muticum freshwater rinsed and freeze-dried and mixed pelagic Sargassum drained and freeze-dried). This research also studied the effect of CaCl2 pretreatment of Sargassum on the biosorption of MB.
2. Materials and Methods
2.1. Sample Collection and Pretreatment
Sargassum muticum was collected in Broadstairs, Kent (UK) (54.3602° N, 1.4320° E), in Spring 2020. The seaweed was rinsed with deionised water and frozen at −20 °C, then freeze-dried for 72 h (ScanVac, Coolsafe, Laboscene freeze drier running at −50 °C). Mixed sargassum samples (Sargassum fluitans, Sargassum natans I, Sargassum natans VIII) were collected from Shark Bay, South Caicos, the Turks and Caicos Islands 55 (21.491° N, 71.503° W), between September 2020 and May 2021. The mixed samples were frozen at −40 °C. (Harvest Right HRFD-PMed-SS, Salt Lake City, Utah USA). Samples were frozen to −40 °C. A vacuum established <66 Pa in the chamber. During the drying phase, trays were warmed to 52 °C at <66 Pa for 26 h. At the end of this process, samples were double-bagged and shipped via air to the University of Greenwich, UK. Seaweed (2.5 g) was incubated in a CaCl2 solution (0.2 mol L−1), pH 5.0, for 24 h while stirring. The biomass was then filtered (70 mm qualitative filter paper) and washed twice with deionised water to remove excess calcium. The treated biomass was then dried in an oven at 60 °C for 24 h.
2.2. Methylene Blue, Brilliant Blue and Congo Red Dye Solutions
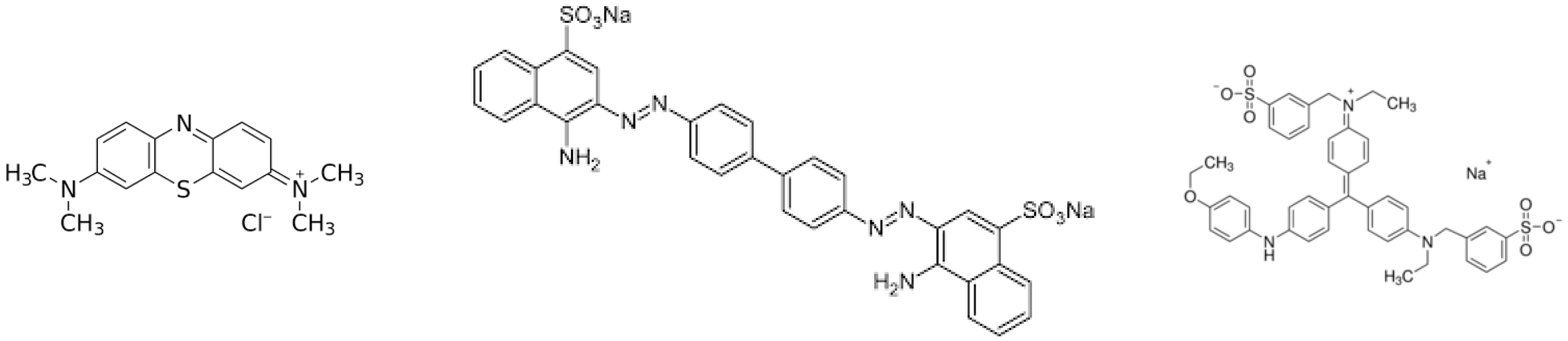
Methylene blue (C16H18ClN3S·3H2O), brilliant blue (C45H44N3NaO7S2) and congo red (C32H22N6Na2O6S2) were purchased from Sigma Aldrich (Gillingham, UK). The chemical structures are shown in Figure 1. Different stock solutions were prepared from a 1000 mg L−1 stock solution. Solutions for calibration curves were created by diluting the stock solution (0–312.5 mg L−1).
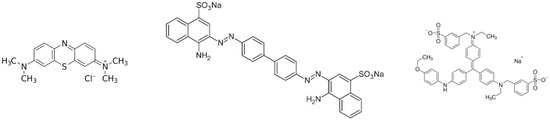
Figure 1.
Chemical structures of methylene blue, congo red and brilliant blue R.
2.3. Biosorption Study
Experiments using different amounts of biomass (0.01–0.4 g) and dye solutions of 10 mg L−1 (80 mL) were conducted in Erlenmeyer flasks (250 mL) at room temperature (25 ± 2 °C) while stirring with contact times between 0–120 min. Samples were centrifuged at 5000 rpm for 2 min. All experiments were carried out in triplicates. The supernatant was analysed using a UV spectrophotometer (Jenway 6305, Fisher Scientific, Loughborough, UK) at wavelengths of 664 nm (MB), 554 nm (BB) and 497 nm (CR) to determine the dye concentrations. The removal efficiency (η) was calculated using the initial and residual MB concentrations as follows:
where C0 is the initial concentration of the dye in solution and Ce is the equilibrium concentration of dye.
The equilibrium biosorption capacity (Qe) was calculated according to Equation (2).
where Qe (mg g−1) is the amount of dye adsorbed by the biomass, C0 and Ce (mg L−1) are the initial and equilibrium concentration of the dye solution, and m (g) is the amount of dried biomass.
A first-order kinetic equation was fitted to the MB adsorption by Sargassum (Equation (3)) [38] using the nonlinear regression function for parameter estimation in IBM SPSS Statistics (v27) (IBM Corp, Armonk, NY, USA).
where q(t) is the amount of dye adsorbed at time t, qe is the amount of dye adsorbed at equilibrium and is the adsorption rate constant.
2.4. Statistical Analyses
IBM SPSS Statistics 25 SPSS was used for a three-way ANOVA on the effects of species (Pelagic Sargassum or S. muticum), pretreatment (CaCl2), Sargassum concentration and their interactions on the final concentration of MB. Excel 2021 (Microsoft Corporation 2021)) was used for t-tests to compare the statistical significance of the effects of species (Pelagic Sargassum or S. muticum), pretreatment (CaCl2) and Sargassum concentration on the final concentration of MB.
3. Results
Effect of CaCl2 Treatment on the Biosorption of Methylene Blue
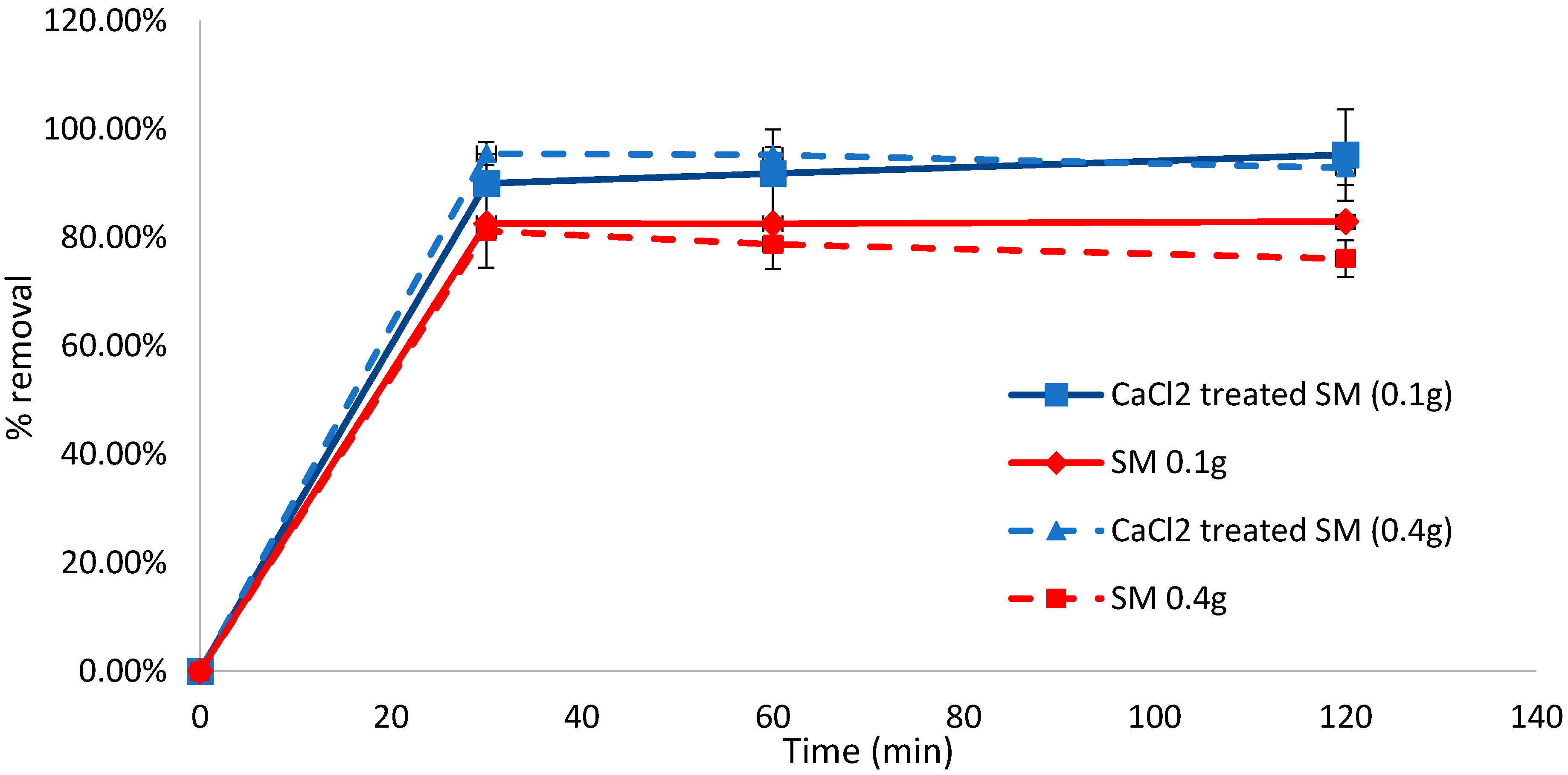
At a biosorbent dose of 0.1 g, removal efficiencies of 89.98% and 82.55% were achieved in the initial 30 min of biosorption for CaCl2-treated and untreated S. muticum, respectively. The difference between the treated and untreated biomass after 30 min was found to be significant (p = 0.003, t-test, unequal variance, two-tailed). When the biosorbent was increased to 0.4 g, the removal efficiency increased to 95.48% within the initial 30 min for CaCl2-treated S. muticum but remained fairly constant at 81.19% for the untreated S. muticum, indicating that the pretreatment may have some effect on the biosorption capacity of methylene blue (Figure 2). For the untreated S. muticum, no significant difference was observed between biosorbent doses of 0.1 g and 0.4 g after 30 min (p = 0.762) and 60 min (p = 0.299); however, after 120 min at 0.4 g, the concentration in the solution increased, indicating desorption of the dye, decreasing the removal efficiency to 76.09% compared to 82.91% at 0.1 g, which was a significant difference (p = 0.048).
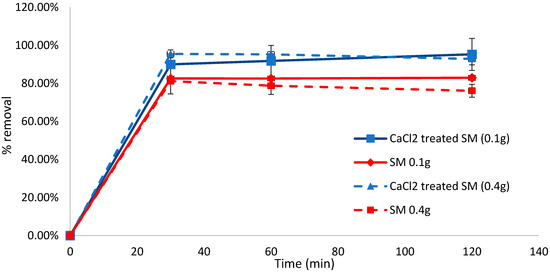
Figure 2.
Biosorption of methylene blue over 120 min using CaCl2-pretreated or untreated Sargassum muticum (SM) (0.1 and 0.4 g). Standard deviation n = 3.
The three-way ANOVA showed that species, Sargassum concentration and treatment (CaCl2) were statistically highly significant factors (p < 0.001) in the MB removal. The interactions between the species and Sargassum concentration (p < 0.05) and Sargassum concentration and CaCl2 (p < 0.001) were also statistically significant factors in the removal of MB.
To describe the adsorption of MB on Sargassum, a first-order kinetic model was fitted with a high coefficient of determination (Table 1). It was generally seen that the amount of dye adsorbed at equilibrium was higher in CaCl2-treated seaweed compared to untreated seaweed, with a maximum difference of 17.29% between the 0.4 g CaCl2-treated and untreated mixed Sargassum. For the CaCl2-treated seaweed samples (0.1 g biomass), the adsorption constant differed by only 0.008 between S. muticum and mixed Sargassum. There was a relatively higher difference in the adsorption constants between using 0.1 g or 0.4 g of CaCl2-treated mixed Sargassum (difference of 0.066). The decrease in adsorption over time limited the fitting of this model for 0.4 g CaCl2-treated S. muticum.

Table 1.
Kinetic parameters qe is the amount of dye adsorbed at equilibrium, is the adsorption rate constant and R2 is the coefficient of determination between calculated and average experimental results.
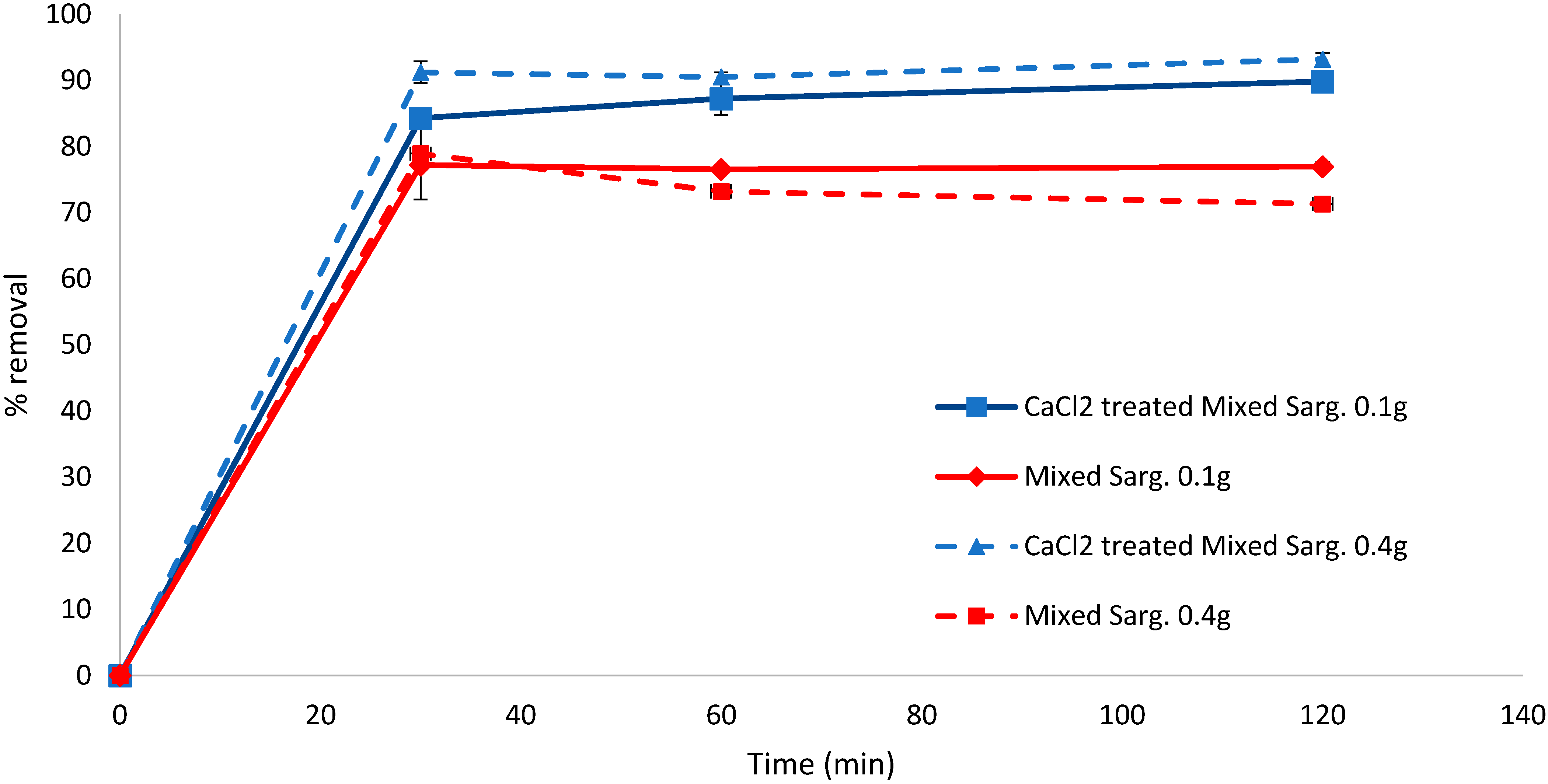
Pretreatment of the mixed pelagic Sargassum (S. fluitans, S. natans I, S. natans VIII) biomass also showed better biosorption capacity when the biomass was treated; after 120 min, CaCl2-treated samples had removed 89.81% (0.1 g biomass) and 93.20% (0.4 g biomass) of the dye compared to 77.19% and 71.32% for the equivalent untreated biomass quantities (Figure 3). The difference between the treated and untreated biomass was significant at all times analysed (30 min, p = 0.011, 60 min p = 0.018, 120 min p = 0.0009).
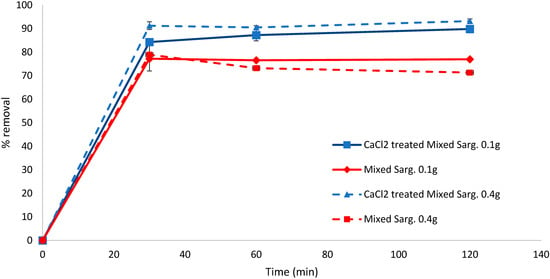
Figure 3.
Biosorption of methylene blue over 120 min using CaCl2-pretreated or untreated mixed Sargassum biomass (0.1 and 0.4 g). Standard deviation n = 3.
At low additions of pelagic Sargassum (0.1 g), the treated (p < 0.05) and untreated (p < 0.01) samples removed statistically more significant amounts than S. muticum. However, there was no significant statistical difference (p > 0.05) in MB removal between pelagic Sargassum and S. muticum at 0.4 g, even though the adsorption rate constant was considerably lower for CaCl2-treated S. muticum (k = 0.099) compared to the treated pelagic Sargassum (k = 0.165).
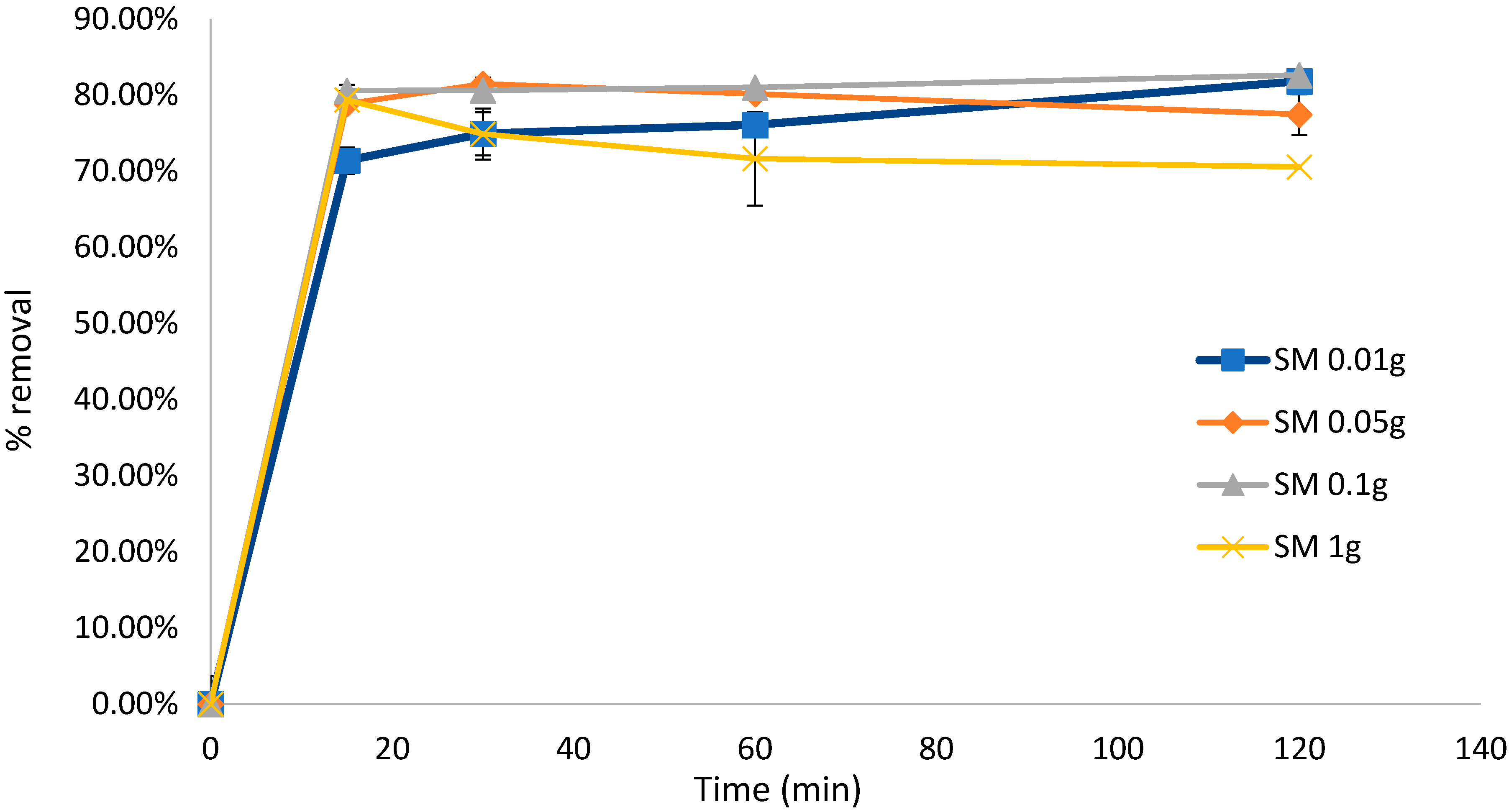
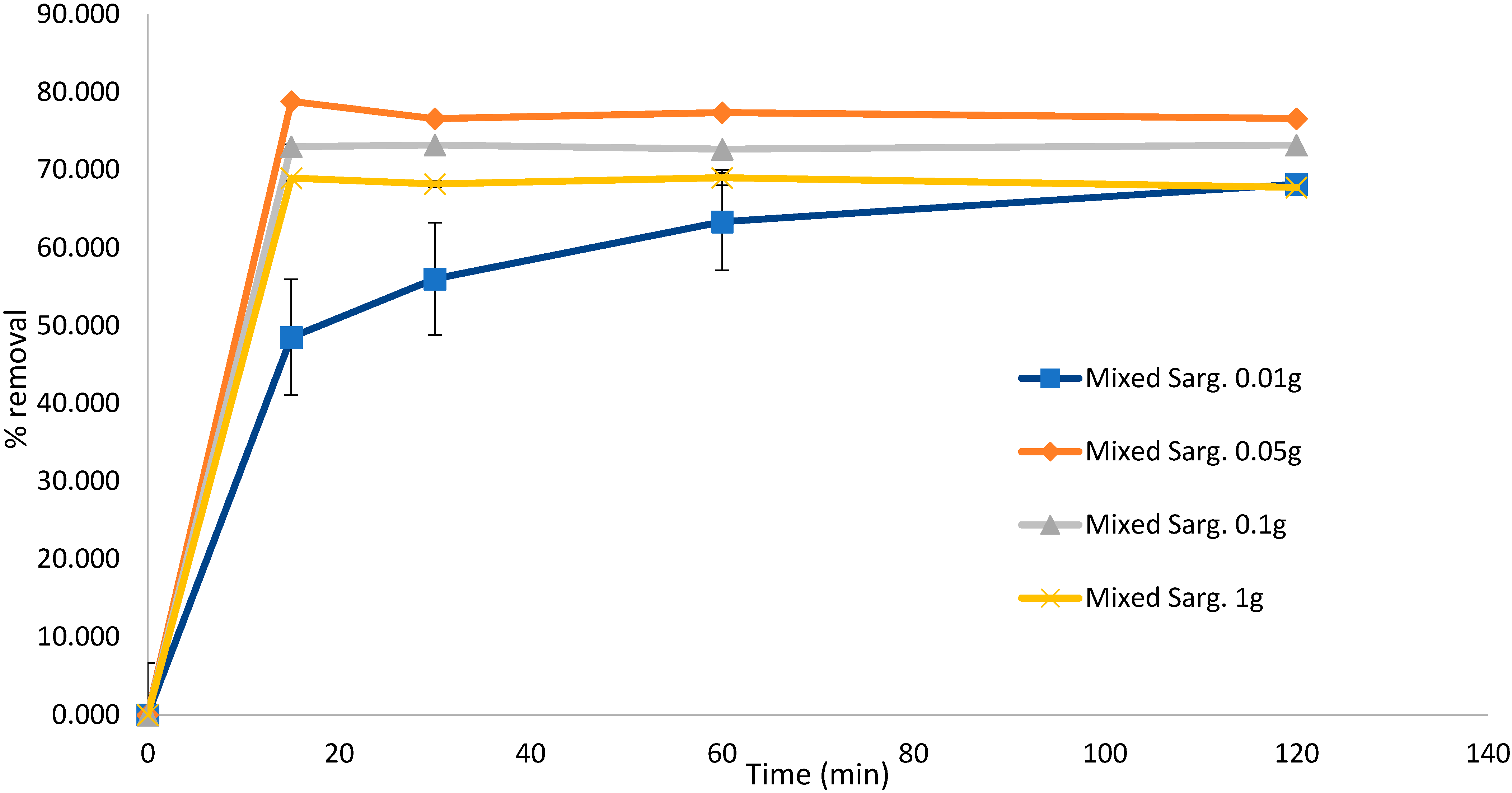
To test the effect of the biosorbent dose on the biosorption efficiency, the experiment for untreated S. muticum was repeated, confirming that a higher biosorbent dose resulted in significant desorption of the dye solution after 120 min. This experiment also confirmed that biosorption occurred within the initial 15 min of contact (Figure 4) and that Sargassum biomass as low as 0.05 g was equally efficient in removing MB in the initial 15 min compared to higher dosages and down to 0.01 g if left for longer than 120 min. Similar results were obtained with the mixed Sargassum biomass (Figure 5) with 0.05 g biosorbent in 10 mg L−1 (80 mL) of dye, indicating optimal conditions.
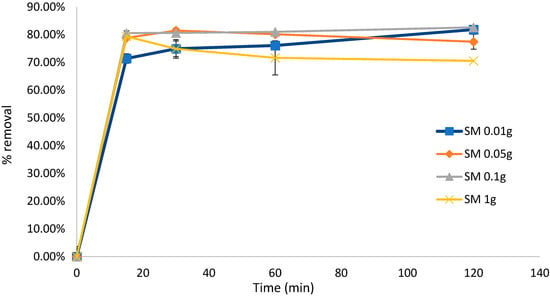
Figure 4.
Biosorption of methylene blue over 120 min using Sargassum muticum (SM) at different concentrations. Concentration of dye remained constant at 10 mg L−1 (80 mL). Standard deviation n = 3.
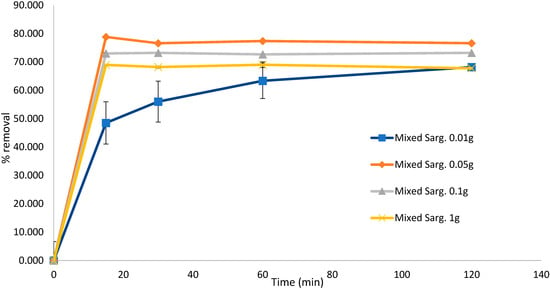
Figure 5.
Biosorption of methylene blue over 120 min using mixed Sargassum spp. at different concentrations. Concentration of dye remained constant at 10 mg L−1 (80 mL). Standard deviation n = 3.
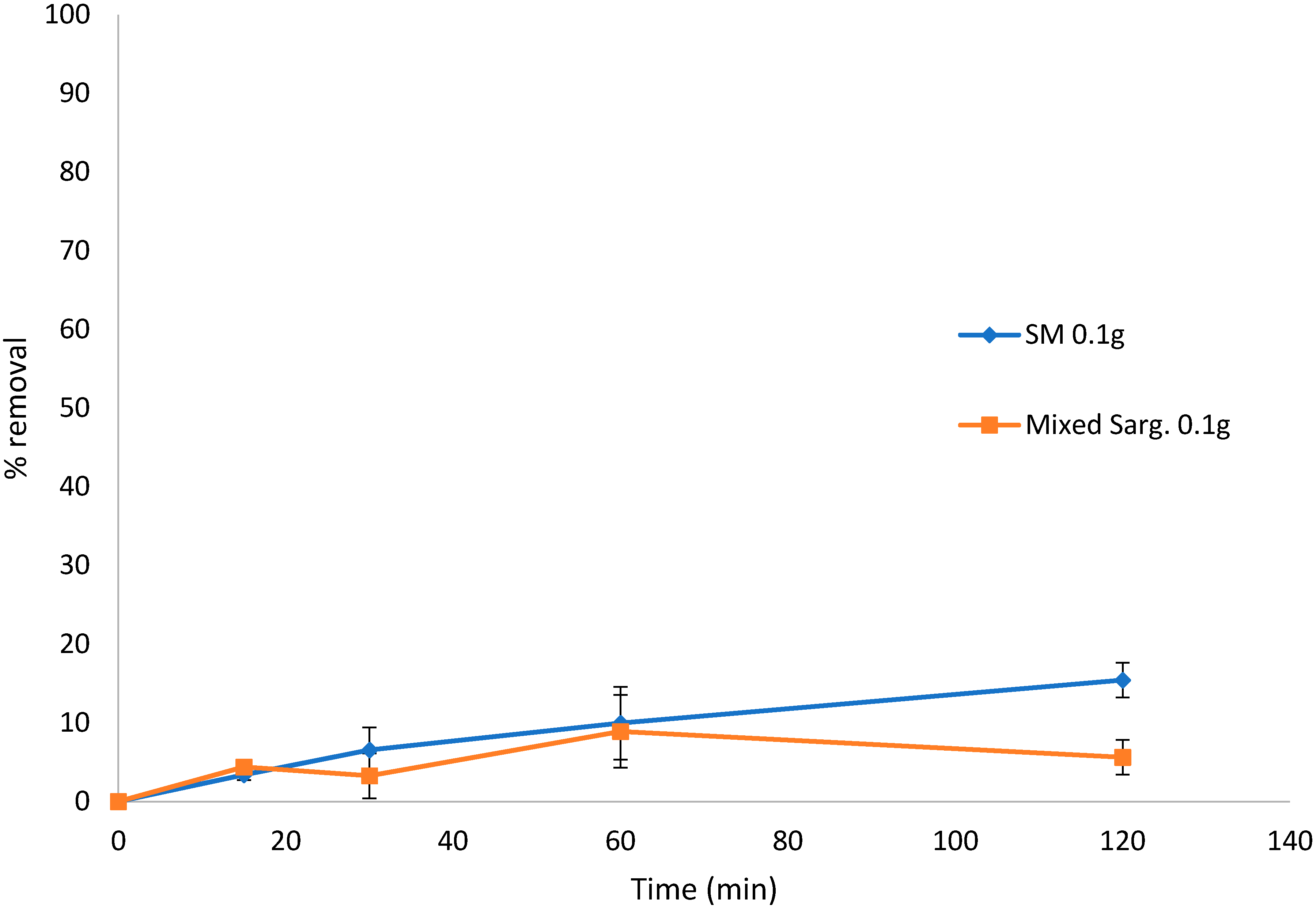
There was no removal of BB from the solution when using S. muticum at three concentrations studied (<16.80%). However, there was some slight removal of BB by the mixed Sargassum biomass (<26.99%) (results not shown). Likewise, the removal of congo red from the solution by either S. muticum or the mixed Sargassum samples showed little biosorption (<16% after 120 min) (Figure 6).
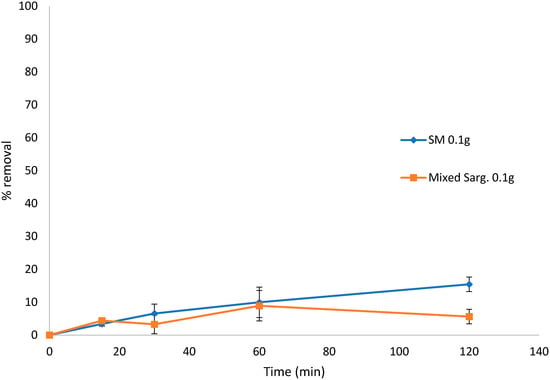
Figure 6.
Biosorption of congo red over 120 min by Sargassum muticum (SM) and mixed Sargassum spp. in 0.1 g and 10 mg L−1 (80 mL) dye solution. Standard deviation n = 3.
4. Discussion
Methylene blue (MB) is a cationic thiazine dye that is largely used as a model of cationic dyes for adsorption studies. Algae were shown to be a low-cost and efficient alternative biomaterial to remove dyes due to the functional groups present. FTIR analysis of the brown seaweed, S. muticum, ascribed effective biosorption to the cell wall structure containing functional groups such as amino, hydroxyl, carboxyl and sulphate, which can act as binding sites via electrostatic attraction, ion exchange and complexation [39]. Both anionic dyes investigated showed no or little affinity for the Sargassum biomasses. In addition to the interactions mentioned above, hydrophobic attractions, chemical bonding, hydrogen bonds and physical adsorption interactions are also likely to occur between a biosorbent and dyes. Hence, the ionic charge of the dye will directly affect the adsorption capacity of the biomass.
Chemical characterisation of many Sargassum species showed that metal content (in particular arsenic) severely hampers the prospects of using Sargassum species for food or feed. However, this seaweed’s high metal sorption ability could offer a feasible and economical approach for removing industrial heavy-metal-bearing wastewaters that require efficient and cost-effective treatment [40]. Other non-conventional low-cost adsorbents were reviewed by Rafatullah et al. [41]. The effect of biosorption dosage on methylene blue dye removal was investigated (0.01−1 g) using an 80 mL dye solution (10 mg L−1, room temperature).
Optimal biosorption dosages can be used to predict the overall cost of biomass per unit of the dye solution to be treated. The optimal biosorbent dose for either S. muticum or mixed Sargassum species was 0.05 g/80 mL, corresponding to Qe values of 157 mg g−1 (120 min) for S. muticum and 115 mg g−1 (120 min) for the mixed Sargassum sample. Likewise, contact times over 120 min were investigated, indicating that biosorption (>75% for mixed Sargassum and >78% for S. muticum) occurred in the initial 15 min. The maximum adsorption capacity of MB on S. muticum at the optimal pH was reported to be 279 mg g−1 [28]. The pH was not adjusted in this study, which could have caused the lower values obtained in this study. Chemical treatment with CaCl2 improved the adsorption capacity, which is in line with those previously reported for other types of biomaterial [42].
5. Conclusions
Both pelagic Sargassum and S. muticum, either untreated or treated with CaCl2, were effective in the biosorption of MB from solution. However, Sargassum did not remove the anionic dyes, namely, CR and BB. Brown seaweed’s primary cell wall polysaccharides are negatively charged [43,44] and algal cells are typically negatively charged [45,46,47]. This negative surface charge may make Sargassum suitable for removing cationic pollutants but may not be suitable for anionic substances without modification. Still, the results are encouraging, offering some potential benefits for commercial purposes for this brown, invasive seaweed as a low-cost adsorbent. Future work could include investigations into the number of cycles the Sargassum samples will be effective for MB biosorption.
Author Contributions
Conceptualisation B.V.N.; methodology, B.V.N., S.M., J.D.A. and R.M.A.; data analysis, J.J.M., S.M. and B.V.N.; investigation, J.D.A., R.M.A., S.M. and M.M.A.F.; writing—original draft preparation, B.V.N. and J.J.M.; writing—review and editing, B.V.N., J.J.M. and S.M.; supervision, B.V.N., S.M. and M.M.A.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The mixed Sargassum samples were collected by Heidi Hertler as part of the DEFRA Darwin Plus project (grant number DPR7P\100059).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hardouin, K.; Bedoux, G.; Burlot, A.-S.; Nyvall-Collén, P.; Bourgougnon, N. Chapter Ten—Enzymatic Recovery of Metabolites from Seaweeds: Potential Applications. In Advances in Botanical Research; Bourgougnon, N., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 71, pp. 279–320. [Google Scholar]
- Milledge, J.J.; Nielsen, B.V.; Bailey, D. High-value products from macroalgae: The potential uses of the invasive brown seaweed, Sargassum muticum. Rev. Environ. Sci. Biotechnol. 2015, 15, 67–88. [Google Scholar] [CrossRef]
- Milledge, J.J.; Harvey, P. Golden Tides: Problem or Golden Opportunity? The Valorisation of Sargassum from Beach Inundations. J. Mar. Sci. Eng. 2016, 4, 60. [Google Scholar] [CrossRef]
- Oxenford, H.A.; Cox, S.-A.; van Tussenbroek, B.I.; Desrochers, A. Challenges of Turning the Sargassum Crisis into Gold: Current Constraints and Implications for the Caribbean. Phycology 2021, 1, 27–48. [Google Scholar] [CrossRef]
- Edwards, M.; Hanniffy, D.; Heesch, S.; Hernández-Kantun, J.; Queguineur, B.; Ratcliff, J.; Soler-Vila, A.; Wan, A. Microalgae Fact-Sheets; NUI: Galway, Ireland, 2014. [Google Scholar]
- Davison, D.M. Sargassum Muticum in Scotland 2008: A Review of Information, Issues and Implications; Scottish Natural Heritage: Inverness, Scotland, 2009. [Google Scholar]
- Gibson, C.E. Northern Ireland State of the Seas Report; Agri-Food and Biosciences Institute: Belfast, Ireland, 2011. [Google Scholar]
- del Río, P.G.; Flórez-Fernández, N.; Álvarez-Viñas, M.; Torres, M.D.; Romaní, A.; Domínguez, H.; Garrote, G. Evaluation of sustainable technologies for the processing of Sargassum muticum: Cascade biorefinery schemes. Green Chem. 2021, 23, 7001–7015. [Google Scholar] [CrossRef]
- Desrochers, A.; Cox, S.-A.; Oxenford, H.A.; van Tussenbroek, B. Sargassum Uses Guide: A Resource for Caribbean Researchers, Entrepreneurs and Policy Makers; Report Prepared for the Climate Change Adaptation in the Eastern Caribbean Fisheries Sector (CC4FISH) Project of the Food and Agriculture Organization (FAO) and the Global Environment Facility (GEF); Centre for Resource Management and Environmental Studies (CERMES), University of the West Indies, Cave Hill Campus: Bridgetown, Barbados, 2020. [Google Scholar]
- Oxenford, H.A. Sargassum Moss: Ecological aspects and source of influx. In Proceedings of the Sargassum Symposium, UWI, Cave Hill, Barbados, 17 August 2015. [Google Scholar]
- Laffoley, A.; Roe, H.S.J.; Angel, M.V.; Ardron, J.; Bates, N.R.; Boyd, L.L.; Brooke, S.; Buck, K.N.; Carlson, C.A.; Causey, B.; et al. The Protection and Management of the SARGASSO Sea: The Golden Floating Rainforest of the Atlantic Ocean: Summary Science and Supporting Evidence Case; Sargassu Sea Alliance: Bermuda, 2011; p. 44. [Google Scholar]
- Williams, A.; Feagin, R. Sargassum as a Natural Solution to Enhance Dune Plant Growth. Environ. Manag. 2010, 46, 738–747. [Google Scholar] [CrossRef]
- Smetacek, V.; Zingone, A. Green and golden seaweed tides on the rise. Nature 2013, 504, 84–88. [Google Scholar] [CrossRef] [Green Version]
- van Tussenbroek, B.I.; Hernandez Arana, H.A.; Rodriguez-Martinez, R.E.; Espinoza-Avalos, J.; Canizales-Flores, H.M.; Gonzalez-Godoy, C.E.; Barba-Santos, M.G.; Vega-Zepeda, A.; Collado-Vides, L. Severe impacts of brown tides caused by Sargassum spp. on near-shore Caribbean seagrass communities. Mar. Pollut. Bull. 2017, 122, 272–281. [Google Scholar] [CrossRef]
- Burrowes, R.; Wabnitz, C.; Eyzaguirre, J. The Great Sargassum Disaster of 2018. Available online: https://essa.com/the-great-sargassum-disaster-of-2018/ (accessed on 25 March 2019).
- Langin, K. Seaweed masses assault Caribbean islands. Science 2018, 360, 1157–1158. [Google Scholar] [CrossRef]
- Thompson, T.M.; Young, B.R.; Baroutian, S. Pelagic Sargassum for energy and fertiliser production in the Caribbean: A case study on Barbados. Renew. Sustain. Energy Rev. 2020, 118, 109564. [Google Scholar] [CrossRef]
- Hendy, I.W.; Woolford, K.; Vincent-Piper, A.; Burt, O.; Schaefer, M.; Cragg, S.M.; Sanchez-Navarro, P.; Ragazzola, F. Climate-driven golden tides are reshaping coastal communities in Quintana Roo, Mexico. Clim. Chang. Ecol. 2021, 2, 100033. [Google Scholar] [CrossRef]
- Bartlett, D.; Elmer, F. The Impact of Sargassum Inundations on the Turks and Caicos Islands. Phycology 2021, 1, 83–104. [Google Scholar] [CrossRef]
- Resiere, D.; Valentino, R.; Nevière, R.; Banydeen, R.; Gueye, P.; Florentin, J.; Cabié, A.; Lebrun, T.; Mégarbane, B.; Guerrier, G.; et al. Sargassum seaweed on Caribbean islands: An international public health concern. Lancet 2018, 392, 2691. [Google Scholar] [CrossRef] [Green Version]
- Resiere, D.; Mehdaoui, H.; Florentin, J.; Gueye, P.; Lebrun, T.; Blateau, A.; Viguier, J.; Valentino, R.; Brouste, Y.; Kallel, H.; et al. Sargassum seaweed health menace in the Caribbean: Clinical characteristics of a population exposed to hydrogen sulfide during the 2018 massive stranding. Clin. Toxicol. 2020, 59, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Chávez, V.; Uribe-Martínez, A.; Cuevas, E.; Rodríguez-Martínez, R.E.; van Tussenbroek, B.I.; Francisco, V.; Estévez, M.; Celis, L.B.; Monroy-Velázquez, L.V.; Leal-Bautista, R.; et al. Massive Influx of Pelagic Sargassum spp. on the Coasts of the Mexican Caribbean 2014–2020: Challenges and Opportunities. Water 2020, 12, 2908. [Google Scholar] [CrossRef]
- Williams, F.E.; Eschen, R.; Harris, A.; Djeddour, D.H.; Pratt, C.F.; Shaw, R.S.; Varia, S.; Lamontagne-Godwin, J.D.; Thomas, S.E.; Murphy, S.T. The Economic Cost of Invasive Non-Native Species on Great Britain; CABI: Wallingford, UK, 2010. [Google Scholar]
- Zeraatkar, A.K.; Ahmadzadeh, H.; Talebi, A.F.; Moheimani, N.R.; McHenry, M.P. Potential use of algae for heavy metal bioremediation, a critical review. J. Environ. Manag. 2016, 181, 817–831. [Google Scholar] [CrossRef]
- Milledge, J.J.; Thompson, E.P.; Sauvêtre, A.; Schroeder, P.; Harvey, P.J. Novel developments in biological technologies for wastewater processing. In Sustainable Water and Wastewater Processing; Galanakis, C.M., Agrafioti, E., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 239–278. [Google Scholar]
- Ungureanu, G.; Santos, S.; Boaventura, R.; Botelho, C. Biosorption of antimony by brown algae S. muticum and A. nodosum. Environ. Eng. Manag. J. 2015, 14, 455–463. [Google Scholar]
- Arumugam, N.; Chelliapan, S.; Kamyab, H.; Thirugnana, S.; Othman, N.; Nasri, N. Treatment of Wastewater Using Seaweed: A Review. Int. J. Environ. Res. Public Health 2018, 15, 2851. [Google Scholar] [CrossRef] [Green Version]
- Rubin, E.; Rodriguez, P.; Herrero, R.; Cremades, J.; Barbara, I.; de Vicente, M.E.S. Removal of Methylene Blue from aqueous solutions using as biosorbent Sargassum muticum: An invasive macroalga in Europe. J. Chem. Technol. Biotechnol. 2005, 80, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Rubin, E.; Rodriguez, P.; Herrero, R.; de Vicente, M.E.S. Biosorption of phenolic compounds by the brown alga Sargassum muticum. J. Chem. Technol. Biotechnol. 2006, 81, 1093–1099. [Google Scholar] [CrossRef]
- Yaseen, D.A.; Scholz, M. Textile dye wastewater characteristics and constituents of synthetic effluents: A critical review. Int. J. Environ. Sci. Technol. 2019, 16, 1193–1226. [Google Scholar] [CrossRef] [Green Version]
- Dutta, S.; Gupta, B.; Srivastava, S.K.; Gupta, A.K. Recent advances on the removal of dyes from wastewater using various adsorbents: A critical review. Mater. Adv. 2021, 2, 4497–4531. [Google Scholar] [CrossRef]
- Alves de Lima, R.O.; Bazo, A.P.; Salvadori, D.M.F.; Rech, C.M.; de Palma Oliveira, D.; de Aragão Umbuzeiro, G. Mutagenic and carcinogenic potential of a textile azo dye processing plant effluent that impacts a drinking water source. Mutat. Res./Genet. Toxicol. Environ. Mutagenesis 2007, 626, 53–60. [Google Scholar] [CrossRef] [PubMed]
- López-Miranda, J.L.; Silva, R.; Molina, G.A.; Esparza, R.; Hernandez-Martinez, A.R.; Hernández-Carteño, J.; Estévez, M. Evaluation of a Dynamic Bioremediation System for the Removal of Metal Ions and Toxic Dyes Using Sargassum spp. J. Mar. Sci. Eng. 2020, 8, 899. [Google Scholar] [CrossRef]
- Benkhaya, S.; M’Rabet, S.; El Harfi, A. Classifications, properties, recent synthesis and applications of azo dyes. Heliyon 2020, 6, e03271. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theng, M.L.; Tan, L.S.; Siaw, W.C. Adsorption of Methylene Blue and Congo Red Dye from Water onto Cassava Leaf Powder. Prog. Energy Environ. 2020, 12, 11–21. [Google Scholar]
- Chudgar, R.J. Azo Dyes. In Kirk-Othmer Encyclopedia of Chemical Technology; Kirk, R.E., Othmer, D.F., Eds.; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Karatay, S.E.; Aksu, Z.; Özeren, İ.; Dönmez, G. Potentiality of newly isolated Aspergillus tubingensis in biosorption of textile dyes: Equilibrium and kinetic modeling. Biomass Conv. Bioref. 2021. [Google Scholar] [CrossRef]
- Revellame, E.D.; Fortela, D.L.; Sharp, W.; Hernandez, R.; Zappi, M.E. Adsorption kinetic modeling using pseudo-first order and pseudo-second order rate laws: A review. Clean. Eng. Technol. 2020, 1, 100032. [Google Scholar] [CrossRef]
- El Atouani, S.; Bentiss, F.; Reani, A.; Zrid, R.; Belattmania, Z.; Pereira, L.; Mortadi, A.; Cherkaoui, O.; Sabour, B. The invasive brown seaweed Sargassum muticum as new resource for alginate in Morocco: Spectroscopic and rheological characterization. Phycol. Res. 2016, 64, 185–193. [Google Scholar] [CrossRef]
- Nielsen, B.V.; Milledge, J.J.; Hertler, H.; Maneein, S.; Al Farid, M.M.; Bartlett, D. Chemical Characterisation of Sargassum Inundation from the Turks and Caicos: Seasonal and Post Stranding Changes. Phycology 2021, 1, 143–162. [Google Scholar] [CrossRef]
- Rafatullah, M.; Sulaiman, O.; Hashim, R.; Ahmad, A. Adsorption of methylene blue on low-cost adsorbents: A review. J. Hazard. Mater. 2010, 177, 70–80. [Google Scholar] [CrossRef]
- Batzias, F.A.; Sidiras, D.K. Dye adsorption by calcium chloride treated beech sawdust in batch and fixed-bed systems. J. Hazard. Mater. 2004, 114, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Kloareg, B.; Badis, Y.; Cock, J.M.; Michel, G. Role and Evolution of the Extracellular Matrix in the Acquisition of Complex Multicellularity in Eukaryotes: A Macroalgal Perspective. Genes 2021, 12, 1059. [Google Scholar] [CrossRef] [PubMed]
- Schulz, A.; Katsen-Globa, A.; Huber, E.J.; Mueller, S.C.; Kreiner, A.; Pütz, N.; Gepp, M.M.; Fischer, B.; Stracke, F.; von Briesen, H.; et al. Poly(amidoamine)-alginate hydrogels: Directing the behavior of mesenchymal stem cells with charged hydrogel surfaces. J. Mater. Sci. Mater. Med. 2018, 29, 105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edzwald, J.K. Algae, bubbles, coagulants, and dissolved air flotation. Water Sci. Technol. 1993, 27, 67–81. [Google Scholar] [CrossRef]
- Moraine, R.; Shelef, G.; Meydan, A.; Levi, A. Algal single cell protein from wastewater-treatment and renovation process. Biotechnol. Bioeng. 1979, 21, 1191–1207. [Google Scholar] [CrossRef]
- Packer, M. Algal capture of carbon dioxide; biomass generation as a tool for greenhouse gas mitigation with reference to New Zealand energy strategy and policy. Energy Policy 2009, 37, 3428–3437. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).