1. Introduction
Therapeutic options now available for hepatocellular carcinoma (HCC) include surgical resection, liver transplant, arterial chemoembolization, and systemic therapy. Unfortunately, all these efforts have not changed the fact that HCC is still one of the most common causes of cancer-related death worldwide. Furthermore, current therapies cannot fully meet clinical needs, especially in late detection and advanced diseases, which so far represent most HCC cases. Compared with traditional clinical methods, nanotechnology has the advantages of high individual selectivity, active targeting ability, and integration of diagnosis and treatment. However, the high-potential nanomedicine still has complex problems to understand and address. It has been realized, for example, that the advantage of long circulation time and passive accumulation of nanosystems covered by a hydrophilic shell by the EPR effect can be lost the moment the nanoparticle is inside the tumor microenvironment; in proximity to the tumoral cell surface, the uptake is hampered by the high hydrophilicity and hydration state. This is the so-called PEG dilemma [
1]. Stealth and effective uptake are two faces of this “dilemma” involving PEG as well as other hydrophilic shell-forming polymers [
2] on nanoparticles. An exciting approach to this problem is to use cleavable PEG groups [
3]. With this strategy, PEG helps to achieve a long circulation time; however, the layer sheds after extravasation and increases the chance of the cellular uptake, uncovering the less encumbered, less hydrophilic core.
A better approach to circumvent the low cellular uptake of PEG-conjugated nanoparticles is to install specific moieties at the distal end of the hydrophilic polymer chain to switch the passive targeting (mediated by the EPR effect) to an active targeting approach, mediated by the specific recognition through a hyper-expressed protein on the target cell surface [
4]. However, even if the system is, in theory, apparently fixed, a second underestimated dilemma appears after the ligand is installed. Indeed, the ligand itself can trigger unwanted interactions of the nanocarriers with biological components, face the risk of enzymatic degradation and potential immune response, and even promote RES uptake, shortening the circulation to unproductive levels. The consequences null the advantage of having both the hydrophilic shell covering the system and the active targeting agent.
A specific liver-directed drug delivery system is proposed in the present paper. The new material is a structural analogue to one of the most studied copolymers in the field of nanotechnology for biomedical application: PEG-PLA. Otherwise, the block copolymer is here obtained by the condensation between a phenylboronic acid ended methoxy polyethylene glycol (MeO-PEG
2000-PBA) and a galactose capped PLA of two different molecular weights, 1–5 KDa (PLA-Gal
1–5K) and 5–10 KDa (PLA-Gal
5–10K). The resulting amphiphilic co-polymer, MeO-PEG
2000-PBGal-PLA, is designed to be: (1) able to assemble in an aqueous environment forming nanoparticles/micelles; (2) pH adaptive, as the boronic ester formation is reversible and the equilibrium can be moved towards a de-shielded system in acidic environment. In the tumoral environment, where the pH is lower compared to blood and normal tissues, this would correspond to a selective exposition of Gal ligands in proximity of the target [
5]. Moreover, because Gal is recognized by the asialoglycoprotein receptor (ASGPR), which is hyper-expressed on hepatocytes, its anchoring on the surface of nanocarriers has already paved the way for the efficient liver active-targeting approach [
6]. Contrarily to what has been reported before, our approach would allow a new ligand presentation strategy in which Gal is together structural element for the stimulus-responsive PEG de-shielding as well as the targeting moiety.
2. Materials and Methods
2.1. Materials
6-Azido-6-deoxy-D-galactose (Gal-N3), N,N’-dicicloesilcarbodiimmide (DCC), CuBr, CuSO4, 4-(Carboxymethyl)phenylboronic acid pinacol ester (PBAPin), dimethylaminopyridine DMAP, Triethylamine (TEA), Tetrahydrofuran inhibitor-free, for HPLC, ≥99.9% (THF), dimethylsulphoxyde (DMSO), N,N-Dimethylformamide anhydrous, 99.8% (DMF), Poly(D,L-lactide) (Mn: 5000–10,000) Acid endcap (PLA5–10K), Poly(D,L-lactide) (Mn: 5000–10,000) Acid endcap (PLA1–5K), Polystyrene (low molecular) Standard ReadyCal Set M(p) 250–70,000, P2O5, molecular sieves (MS) 4 Å beads 8–12 mesh, DOWEX 50WX8 were purchased from Merk Life Science S.r.l. (Milano, Italy). Solvents were dried over activated MS 4 Å before use. All the reagents were dried under reduced pressure over P2O5 for at least 24 h before use.
2.2. Nuclear Magnetic Resonance Experiments
Spectra were acquired on a Bruker AVANCE instrument operating at 400 MHz. Additionally, 10 mg of the sample were dissolved in 600 μL of DMSO-d6. Spectra were acquired with 32 scans. To establish a chemical shift scale, residual solvent signal of DMSO-d6 was used as reference (-CHD2 1:2:3:2:1 quintet at 2.5 ppm). NMR data were visualized, processed, and analyzed by Mestrenova software (Mestrelab Research, Santiago de Compostela, Spain).
2.3. Gel Permeation Chromatography
The GPC system used consisted of a pump (LC-20AD, Shimadzu, Kyoto, Japan), a degassing unit (DGU-20A3R, Shimadzu, Kyoto, Japan), two in-series GPC columns packed with styrene-divinylbenzene copolymer gel (500 Å and 10
3 Å fixed pore columns (Phenogel, from Phenomenex srl, Milano, Italy)) preceded by a guard column and a refractive index detector (RID-10A, Shimadzu, Kyoto, Japan). GPC conditions were as follows: 2.5 mg/mL as sample concentration, 100 µL of injection volume, and 0.7 mL/min flow rate at room temperature. The elution solvent was pure, not stabilized THF, LC-GC grade. Samples were filtered through 0.45 µm PTFE syringe filters before injection. Data acquisition was carried out using Lab Solutions Lite software (Shimadzu, Kyoto, Japan). Detailed information about processing and calibration is reported in
Figure S7.
2.4. Synthesis and Characterization of MeO-PEG-PBA
To a solution of PBAPin (0.5 mmol) and DMAP (0.75 mmol) in anhydrous CHCl3 (4 mL) under nitrogen atmosphere, a DCC solution in the same solvent (0.75 mmol, 1 mL) was slowly added dropwise. After 1.5 h, a solution of MeO-PEG2000 in CHCl3 (0.25 mmoles, 1 mL) was added dropwise, and stirring was continued under nitrogen atmosphere at room temperature for 24 h. The dicyclohexylurea formed was filtered off by 0.45 µm PTFE syringe filter, and the clear yellowish solution was dropped into an excess of cold EtOEt under stirring. The formed solid was isolated by centrifugation (ALC PK120 centrifuge, 1129 rcf, 10 min, r.t.), redissolved in CHCl3, and re-precipitated in EtOEt one more time. After the removal of organic solvents by evaporation under reduced pressure, an off-white, waxy solid (yield 89%) was obtained.
Protocol of deprotection of pinacol boronic esterwith TFA: solid MeO-PEG2000-PBAPin was dissolved in CH2Cl2 at a concentration of 64 mg/mL and added with 630 µL of TFA. After 4 to 24 h stirring, the mixture was poured into an excess of cold EtOEt, and the obtained solid was recovered by centrifugation. The yield was 85% of the initial weight.
Protocol of deprotection of pinacol boronic esterwith NaIO4: MeO-PEG2000-PBAPin was dissolved in a mixture H2O/THF 1:4 at a concentration of 240 mg/mL and added to NaIO4 (1.2 eq). The solution turned immediately pink, transiently, and reverted to pale yellow after few minutes. After 80 min, HCl 1 M (1.2 eq) was added, and the mixture was left to stir for other 20 min. After this time, the mixture was diluted with DCM and dried with NaSO4. The clear organic solution was separated by filtration and concentrated down to a final volume of about 1 mL. The concentrated mixture was then dropped in an excess (40 mL) of cold diethyl ether for precipitation of the polymers. The solid was separated by centrifugation and washed with the same solvent 4 times. Then, the residual solvent was removed from the isolated product by evaporation under reduced pressure.
Protocol of deprotection of pinacol boronic esterwith acidic resin: MeO-PEG2000-PBAPin was dissolved in a mixture THF/H2O 10:1 at a concentration of 50 mg/mL. The same weight amount of DOWEX 50WX8 was then added, and the mixture was left to stir at room temperature for 24 h. The solution was then isolated by centrifugation (3000 rpm, 10 min), and the resin was washed with 2 mL di of THF. The collected supernatants were treated with anhydrous MgSO4, filtered, concentrated down to 1 mL under reduced pressure, and added to 40 mL of Et2O. The solid formed was collected by centrifugation and washed two times with 40 mL of the same solvent. The white solid was dried under gentle nitrogen flux. Yield 84% of the initial weight. 1H NMR (400 MHz, DMSO-d6) δ 7.05 (m, 2H), 6.69 (m, 2H), 4.50 (m, 2H), 4.12 (m, 2H), 3.68 (m, 2H), 3.51 (s, 230H), and 3.24 (s, 3H). ATR FT-IR (cm−1) 3471, 2884, 2860, 2741, 2696, 2166, 1964, 1736, 1615, 1519, 1456, 1360, 1340, 1279, 1240, 1145, 1103, 1060, 956, 946, 841, and 656.
2.5. Synthesis and Characterization of Poly-d,l-lactide Alkyne Via Amide Bond Formation
Propargylamine was conjugated to PLA of various lengths through amide formation in the presence of EDC and NHS. The general procedure was as follows: PLA-COOH (1 eq) was dissolved in DMSO (250 mg/mL); then, EDC and NHS (2 eq, 0.3 mmol/mL) were added under stirring at room temperature. Propargylamine (2 eq) was then added, and the reaction mixture stirred for another 24 h. The crude product was obtained by precipitation in double distilled water, and it was purified by exhaustive washings with the same solvent. A white powder was recovered by freeze-drying. Yield 89 ± 1%. 1H NMR (400 MHz, DMSO-d6) δ 8.48 (m, 1H), 5.17 (tq, J = 19.6, 6.1 Hz, 1H*PD), 4.20 (d, J = 6.6 Hz, 1H), 3.87 (dt, J = 5.7, 2.8 Hz, 2H), 2.15 (s, 1H), and 1.67–0.83 (m, 3H*PD). ATR FT-IR (cm−1) 3507, 3411, 2995, 2945, 2884, 2638, 1748, 1634, 1526, 1452, 1381, 1364, 1318, 1269, 1185, 1129, 1083, 1052, 956, 864, 748, and 693.
2.6. CuAAC Procedure for the Functionalization of Alkyne Ended PLA with Gal-N3
An amount of 100 mg of PLA-alk and 2 eq of Gal-N3 were dissolved in 0.767 mL of anhydrous DMF. The mixture was bubbled with nitrogen for half hour, and then 2 eq of ascorbic acid was added under nitrogen. After few minutes of further bubbling with nitrogen, 1 eq of CuBr was added, and the mixture was left to stir under nitrogen at room temperature overnight. The pure product was obtained by precipitation in double distilled water and washings in the same solvent. The solid, pure product was obtained as a white powder after freeze drying of the slurry. Yield 71 ± 4%. 1H NMR (400 MHz, DMSO-d6) δ 8.53 (s), 7.85 (d, J = 6.4 Hz), 6.59 (dd, J = 6.8, 2.0 Hz), 6.20 (d, J = 5.0 Hz), 5.59 (m), 5.49 (m), 5.17 (m), 4.74 (m), 4.60 (d, J = 5.2 Hz), 4.45 (m), 4.32 (dt, J = 14.4, 7.2 Hz), 4.19 (dt, J = 13.3, 6.8 Hz), 3.86 (t, J = 6.3 Hz), and 1.46 (m). ATR FT-IR (cm−1) 3492, 2996, 2946, 1748, 1676, 1531, 1452, 1381, 1365, 1269, 1186, 1129, 1083, 1052, 952, 865, 748, 697, and 668.
2.7. Determination of Alizarin Red S (ARS) Molar Extinction Coefficient (ɛ)
Solutions at different concentrations of ARS (ranging from 2.05 × 10
−4 mmol/mL to 4.1 × 10
−5 mmol/mL) were prepared by dilution of a stock solution at a concentration of 2.05 × 10
−4 M in an aqueous medium containing 0.5% DMSO at 2 different pH, 7.4 (phosphate buffer 0.1 M) and pH 10 (bicarbonate buffer 0.2 M). The UV-Vis spectra (200–600 nm) of these solutions were acquired using a Thermo Fischer Evolution 201 spectrophotometer, using a quartz cuvette with an optical path of 10 mm. The ɛ at 511 nm for ARS at pH 7.4 and 546 nm for ARS at pH 10 was determined as the slope of the line abs vs. concentration accordingly with Lambert Beer law [
7].
2.8. Binding Experiments
Binding experiments were conducted by using a method reported by Gennari et al. [
8]. 150 µL of a solution of ARS (final ARS concentration = 10.2 × 10
−2 mM) were mixed with 150 µL of a solution of MeO-PEG
2000-PBA, at various concentrations, to obtain molar ratios MeO-PEG
2000-PBA/ARS ranging from 0 to 100 at pH 7.4 and ranging from 0 to 2 at pH 10. The solutions were incubated overnight at room temperature, and then the UV-Vis spectra were recorded between 200 and 600 nm. Analytical methods used for the calculation of binding constants are reported in
Supporting Information section.
2.9. Nanoprecipitation at PH 10
An amount of 10 mg of MeO-PEG2000-PBA was dissolved in 0.5 mL of DMSO. The solution was added to 0.5 mL of a solution of PLA-Gal (2 eq) in the same solvent. The resulting mixture was then dropped in 15 mL of carbonate/bicarbonate buffer at pH 10. After 2 h, samples were dialyzed against bidistilled water (RC membrane 3.5 KDa MWCO) for 24 h. The content of the dialysis was then sonicated for 15 min, filtered through a 0.45 µm syringe filter, and freeze-dried.
3. Results and Discussion
3.1. pH-Responsive PEG De-Shielding and Gal Presentation Strategy: The Plan
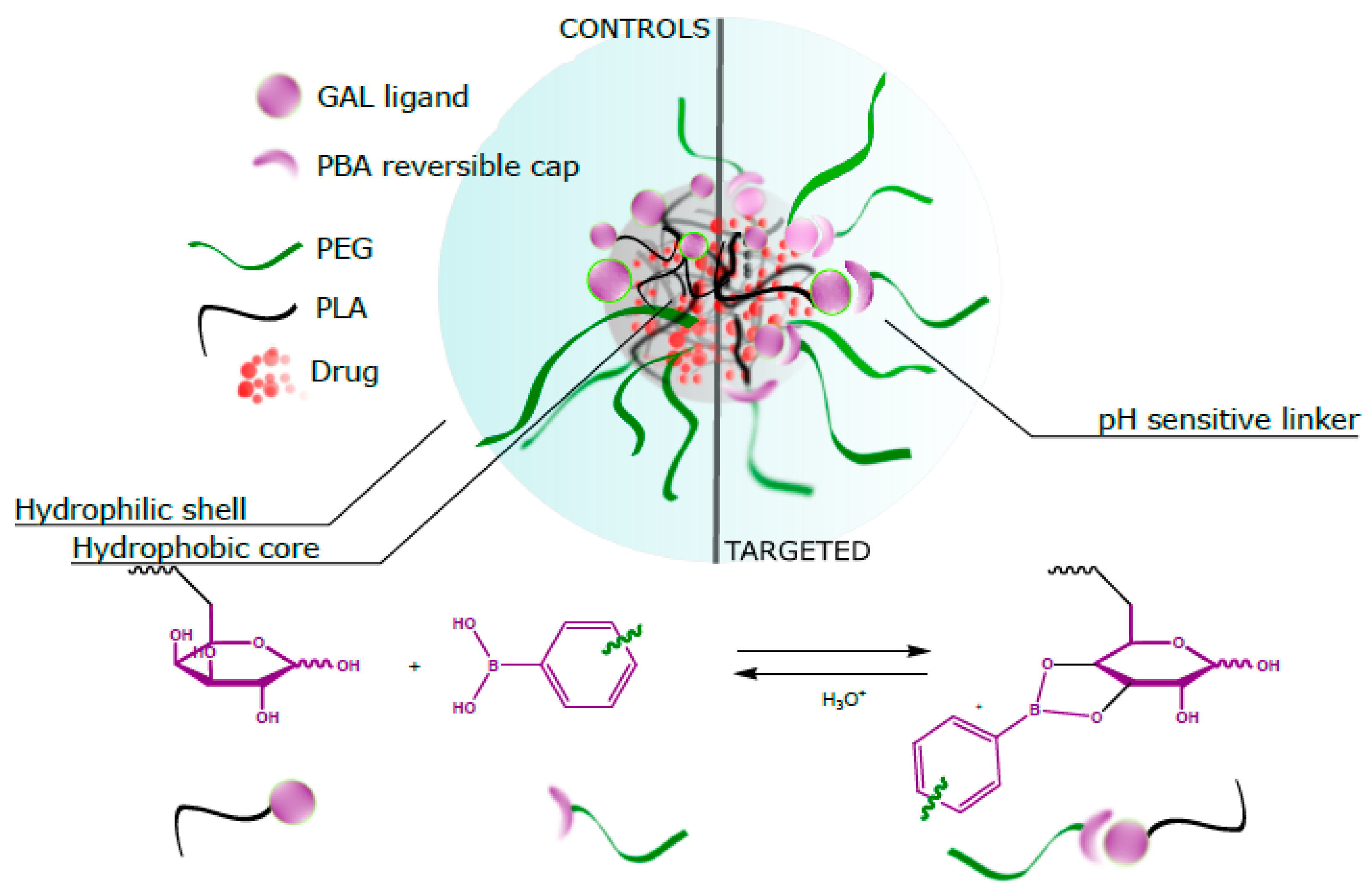
The research aims to find optimized design elements to fulfill multiple requirements for a new smart PLA-PEG drug delivery system: (1) hepatocarcinoma targeting through galactosylation; (2) overcoming the “PEG dilemma” to prolong nanosystems blood circulation and to enhance their tumor accumulation exploiting the pH-controlled ligand presentation; (3) minimizing the structural heterogeneity of the components of the final nanosystem by anchoring the de-shieldable PEG on the targeting ligand (Gal is thus reversible anchor and hidden targeting moiety).
Figure 1 gives a schematic representation of the designed system.
With this aim, we designed a synthetic plan where two short routes, one starting from MeO-PEG
2000 and the other from acid endcap P(D,L)LA, converge towards a two block copolymer kept together by a boronic acid galactosyl ester, as summarized in
Scheme 1.
3.2. Functionalization of the Hydrophilic Block: MeO-PEG2000-PBA
The MeO-PEG
2000 was directly esterified with 4-(Carboxymethyl)phenylboronic acid pinacol ester to obtain MeO-PEG
2000-PBAPin through Steglich esterification [
9], involving DCC and DMAP as catalyst. The pinacol moiety served as a protection and was successively cleaved to give the phenylboronic acid-terminated PEG, MeO-PEG
2000-PBA. The synthesis was settled up starting from a procedure previously described by Cao et al. [
5] for the functionalization of PEG. It is worth noting that we found that when the esterification with PBAPin was carried out in CH
2Cl
2 and without the elimination of oxygen from the mixture, the
1H NMR of the purified product in DMSO-
d6 showed no signal of the methyl protons of the pinacol group. The
1H NMR spectrum gave evidence of singlet signal at around 9 ppm instead. The peak had a 1:4 ratio with the protons of the aromatic group of PBA. This observation is compatible with the formation of a phenol derivative of PEG coming from oxidative cleavage of the boronic acid (
Scheme 2).
Only when the solvent was switched to anhydrous CHCl3 and the MeO-PEG2000 solution was bubbled with nitrogen before the addition of reagents (previously dried under vacuum over P2O5) was the product MeO-PEG2000-PBAPin obtained. By comparing the signal due to the methylene group of the esterified end of the isolated product at 4.1 ppm, with the signal of the methoxy group in the 1H NMR spectrum, it was determined that PBApin was installed in the 53.2 ± 9.1% of the chains.
Three different deprotection methods for the removal of cyclic boronate esters were attempted: (1) TFA-mediated acidic hydrolysis; (2) sodium periodate (NaIO4)-mediated cleavage; and (3) cationic-resin-mediated hydrolysis.
Method 1 led to a product where the pinacolyl group was still present in at least 50% of the chains. This was ascertained by
1H NMR by dividing the signal at about 1.3 ppm of the methyl protons of pinacolic group with the signals of aromatic protons of phenylboronic moiety. The second transformation tested, mediated by sodium periodate in water/THF and subsequent addition of diluted HCl [
10], was more advantageous than the previous one, both in terms of timing and conditions as well as in terms of the percentage of deprotection obtained. In this case, the
1H NMR spectrum showed the signal at around 1.3 ppm of the pinacol methyl protons only in traces. By analysis of the
1H NMR spectrum and the calculation of pinacol still esterified, it was observed that only a residue of 6% of the phenylboronic groups was still protected, and that PBA was installed in about 60% of the chains in the final product. Although the method was effective, the product was unstable and suffered from oxidative cleavage, probably due to residual traces of NaIO
4. The third method (previously explored by Vrbata and Uchman [
11] but in acetone at 50 °C) led to a deprotection rate up to 75%, resulting in the most advantageous approach in terms of workup and stability of the obtained product. The
1H NMR spectrum of the MeO-PEG
2000-PBA product with signal attribution to the structure and the detail of the characteristic peaks are shown in
Figure 2. It was calculated that, on average, the free PBA content was 16.76 × 10
−3 mmol/mg%.
Interestingly, aromatic proton peak shift was observed after transformation of the tetrahedral anionic protected species to the neutral trigonal free PBA specie (
Figure 2B), indicating the effective deprotection of PBA. Signals of deprotected PBA are already visible in the spectrum of MeO-PEG
2000PBAPin, indicating that traces of MeO-PEG
2000PBA were present in the sample.
ATR FT-IR (please see
Figures S1 and S2 in Supplementary Material) shows characteristic peaks of PEG. In addition, the band at 1736 cm
−1 was ascribed to the vibration absorption of the carboxyl groups of the ester bonds in MeO-PEG-PBA, whereas the band due to B–O asymmetric stretching overlaps with PEG absorption around 1340 cm
−1.
3.3. Functionalization of the Hydrophobic Block: PLA-Gal
Acid end-capped PLA of two different molecular weights (1–5 and 5–10 KDa) was functionalized by introducing an alkyne terminal group. The introduction of a triple bond gives access to a virtually infinite possible post-polymerization functionalization by the use of orthogonal click reactions such as Cu(I)-catalyzed Huisgen 1,3-dipolar cycloaddition of alkynes and azides (CuAAC) [
12], or thiol−yne addition [
13]. Propargylamine was chosen as alkyne donor because of its reactivity but also because the formation of an amide linkage was preferred over the ester with propargyl alcohol which was found to be not sufficiently stable in water (data not shown). A reasonable stability of derivatives is relevant to easily follow the condensation of the two blocks without the interference of the hydrolysis of the end-groups on them. Furthermore, the boiling point of propargylamine is 83 °C, and this allows us to “strip” it off by vacuum, avoiding additional workup procedures to remove potential unreacted traces, which benefits its cost-effectiveness. The reaction involved the use of EDC-HCl and NHS as coupling agents. Despite the use of two molar excess of the reagents compared to PLA, a complete alkynylation of the free acid ends of PLA chains was not achieved in both cases. The degree of functionalization (DD mol%) was estimated by
1H NMR by comparing the integral of the signal of the -C-H next to the terminal hydroxyl group of the PLA chain at 4.2 ppm, with the signal at 3.9 of the methylene protons of the propargylamine and resulted to be equal to 68.9 ± 4.9% and 67% for PLA-Alk
1–5KDa and PLA-Alk
5–10KDa, respectively.
The two propargylated products were used as a substrate for the conjugation of an azide derivative of galactose. The presence of azide in position 6 of the modified sugar makes possible to use it in copper catalyzed [3 + 2] azide-alkyne cycloaddition (CuAAC), attributable to the group of “click” reactions. In our protocol, we used CuBr/ascorbic acid catalytic system in DMF. After overnight incubation at room temperature, a quantitative conversion of the starting alkynes into galactosylate moieties was obtained. This was true for both molecular weight ranges. The products, PLA-Gal
1–5KDa and PLA-Gal
5–10KDa, were characterized by
1H NMR. A representative spectrum with signal attribution is shown in
Figure 3. The DD mol% in Gal was around 30% for both PLA-Gal
1–5KDa and PLA-Gal
5–10KDa, respectively.
3.4. Molecular Characteristics of PEG and PLA Derivatives
All the synthetized PEG and PLA derivatives were characterized by mean of GPC analysis. The molecular weight distributions were derived by the raw RI detector response vs. elution volume distribution, through a calibration curve consisting of polystyrene standards with known molecular weights. The number-average molecular weight (Mn), weight-average molecular weight (Mw), and the dispersity index (Ð = Mw/Mn) were obtained. In the case of the MeO-PEG-PBAPin and MeO-PEG-PBA, the lack of multimodal distribution as well as the PDI close to 1, proves that the functionalization steps did not affect the integrity of the polymeric chains (please refer to
Figures S5 and S6 in Supplementary Materials).
Table 1 summarizes the molecular characteristics of PEG and PLA derivatives.
3.5. Boronate Ester Formation
The development of a system like the one here proposed requires knowledge of the binding affinity between boronic acid and diol moieties. To this scope, an analytical method based on UV-Vis spectroscopy was used. Alizarin Red S (ARS) was chosen as reporter model diol to study the MeO-PEG
2000-PBA complexation according with the scheme reported below (
Scheme 3).
ARS shows a drastic shift of the spectral band at around 500 nm in response to the formation of a covalent reversible boronic ester. Since MeO-PEG-PBA lack a significant overlapping absorption in the 400–600 nm spectral region, the only absorbing species are free ARS and the ARS-boronic ester. The amount of complex formed ([AB]) can be expressed as a function of the constant of the equilibrium between ARS and MeO-PEG2000-PBA (Kars) and of the initial concentrations of the reactants, Cars and CPEG-PBA.
The assay was conducted at two different pH: 7.4 and 10.0. Note that the absorption of ARS and ARS-boronic ester at pH 7.4 are almost overlapped (511 and 457 nm, respectively), and the increase in the complex appears as a shift of the absorption band (
Figure 4a) instead of a balancing of two bands around an isosbestic point. Differently, at pH 10, the complex absorption maximum is shifted to 413 nm, thus revealing, upon the change of free ARS/ARS-boronic ester ratio, an isosbestic point at 451 nm (
Figure 4c).
The constant for the formation of MeO-PEG
2000-PBGal-PLA in principle can be obtained by displacement of ARS from the ARS/MeO-PEG
2000-PBA complex. Because of the insolubility of the hydrophobic block in the aqueous analysis medium, it was not possible to determine it directly. Thus, the study was conducted using the water-soluble D-galactose, the moiety that would interact in the case of MeO-PEG
2000-PBGal-PLA formation. Even if it is not accurately the same, the measures give a thermodynamic order of magnitude of the binding event. Exploration of the binding at neutral or basic pH can give hints about the best conditions to be applied for the two blocks coupling and the formation of MeO-PEG
2000-PBGal-PLA nanoparticles. Therefore, the assay was conducted at two different pH: 7.4 and 10.0. The addition of increasing concentration of Gal to an ARS/MeO-PEG
2000-PBA complex 1:1 solution reverted the trend observed before, giving a bathochromic shift of the band around 500 nm in the case of the experiment conducted at pH 7.4. At pH 10, the addition of Gal provoked an intensity increase in the band of the free ARS and a simultaneous decrease in the intensity of the band with a max at 413 nm of the ARS/MeO-PEG
2000-PBA complex (
Figure 4d). Results of the binding experiments of MeO-PEG
2000-PBA with ARS and Gal are summarized in
Table 2.
The study reveals that the PBA moiety installed on PEG is able to interact with Gal and the overall affinities of the two different diols for MeO-PEG
2000-PBA, ARS and Gal, are in agreement with what was previously reported [
8,
14]. Moreover, the binding affinity was found to increase with increasing pH of the medium. In effect, the K value for Gal at pH 10 was considerably high (378 M
−1), being around 90 times the K at pH 7.4 (378 M
−1).
It is evident that the best condition to form the desired assembly is an aqueous environment at pH 10. A first attempt was explored for the formation of nano-assemblies under these conditions by nanoprecipitation. 1H NMR on the obtained samples revealed an extensive degradation of the polyester.
3.6. Critical Issues of the Approach and Possible Solutions: Transforming the Idea into Working Nanoparticles
Boronic acid responsive materials are fascinating and promising in many subfields of biomedical sciences such as biosensing and drug delivery. Accordingly with the method of preparation of those materials, different architectures and supramolecular constructs can be obtained, from crosslinked hydrogels to core-shell nanoparticles. Our approach, to the best of our knowledge, is the first example of a ligand presentation strategy combined and synchronized with the hydrophilic shell loss, which would solve the double dilemma discussed above. If from one side, the preliminary results discussed in this communication encourage us to further explore the approach, from the other side many critical issues emerged, and a contingency plan need to be developed to put at work this new idea. Three main problems have been identified: (1) limited efficiency of block functionalization; (2) oxidative susceptibility of B-C bond; (3) PBA/Gal ester formation between the two block and nanoparticles formation.
Post-polymerization modification reactions have been used to distally modify the PEG chain with a PBA moiety. The choice of PBA bearing reactant appears to be crucial, since underrated or under reported oxidative susceptibility can affect the stability of the obtained products, leading to poor degree of functionalization. Limited (≤50%) efficiency of block functionalization means the existence of a mixture of raw and functionalized blocks difficult to separate for the similar chemico-physical characteristics and solubility. This could be a problem during particles formation, leading to non-uniform, high polydisperse system.
Combinatorial study on the PBA bearing moiety oxidative susceptibility and the affinity with Gal, would be auspicial to find out a structural compromise toward a high efficiency of functionalization and a satisfying binding constant. It has recently found how the installation of a pendant carboxyl group from the phenyl ring can increase the oxidative stability and utility in biological contexts [
15].
A second problem arise from finding out the optimized condition for the PBA ester formation between the PBA bearing hydrophilic block and the core forming galactosylated polymer. Several factor, such as the choice of a good base, the presence of dissolved gasses as well as finding a good solvent for both blocks, have to be adjusted and may represent obstacles that need to be addressed case by case. In the case here reported, it is evident that the major difficulty consists in operate the conjugation between blocks in a basic environment (which have been found to be optimal for boronic ester formation between the PBA and Gal) without compromising the integrity of the polyester chain.
4. Conclusions
Overcoming the “PEG dilemma”, prolonging nanosystem blood circulation, and enhancing their tumor accumulation appears an urgent need in nanomedicine and personalized medicine. Preventing cellular uptake and endosomal escape processes is particularly important for drug targeting hepatocytes.
Although active targeting can improve the uptake in a specific cell/tissue, it is not always a solution for the dilemma, but it requires the ligand to be appropriately presented. The best scenario would be the one in which PEG detachment and ligand presentation are concerted and do not depend on different physical phenomena characterized each by their own kinetic and pH sensibility. The strategy proposed in this research is the development of a liver targeted drug delivery system where the block condensation, the stimulo-responsiveness for hydrophilic shell loss, and the active targeting ligand presentation coexist in a unique element. This is a pH-reversible boronic ester formed between a Gal and a PBA residues at the core–shell interface. During the research, we successfully settled a synthetic pathway leading to MeO-PEG2000-PBA and PLA-Gal of two different Mws in order to explore a broader applicability. Overall, the study confirms the presence of a functional free boronic acid end-cap on the PEG derivative and shows the ability of MeO-PEG-PBA to form a pH adaptive boronic ester with Gal, efficiently targeting hepatocytes, being recognized by the ASGPR receptor since asialoglycoprotein is hyper-expressed on hepatocytes’ membrane. It shows also that the formation of the two blocks should occur in a basic environment to maximize the yield of the hydrophilic corona installation. The obtained preliminary results encourage us to move towards a future perspective of exploring the best conditions, which enable the preservation of the integrity of the polyester and the use of a suitable base, in aqueous or organic medium. Light should be also shed on the kinetic stability of the construct: being the bond at the interface between the hydrophobic core and the hydrophilic shell, it is likely that this would influence the dissociation of the blocks, the rate at which the PEG dissociates at pH 7.4 from the nanoparticle surface. Finally, the work represents the proof of concept for our approach suggesting an alternative way of thinking about active targeting and the ligand presentation strategy.




 Gal/MeO-PEG2000-PBA + ARS. The binding constant between Gal and MeO-PEG2000-PBA is calculated as the product of Kars and Kexc. In the insert, the molar extinction coefficients (ɛ) for free and complexed ARS are reported at pH 7.4 and 10. Detailed information on the analytical methods used for the calculation of binding constants is reported in Supplementary material.
Gal/MeO-PEG2000-PBA + ARS. The binding constant between Gal and MeO-PEG2000-PBA is calculated as the product of Kars and Kexc. In the insert, the molar extinction coefficients (ɛ) for free and complexed ARS are reported at pH 7.4 and 10. Detailed information on the analytical methods used for the calculation of binding constants is reported in Supplementary material.
 Gal/MeO-PEG2000-PBA + ARS. The binding constant between Gal and MeO-PEG2000-PBA is calculated as the product of Kars and Kexc. In the insert, the molar extinction coefficients (ɛ) for free and complexed ARS are reported at pH 7.4 and 10. Detailed information on the analytical methods used for the calculation of binding constants is reported in Supplementary material.
Gal/MeO-PEG2000-PBA + ARS. The binding constant between Gal and MeO-PEG2000-PBA is calculated as the product of Kars and Kexc. In the insert, the molar extinction coefficients (ɛ) for free and complexed ARS are reported at pH 7.4 and 10. Detailed information on the analytical methods used for the calculation of binding constants is reported in Supplementary material.









