3D Virtual Modeling for Morphological Characterization of Pituitary Tumors: Preliminary Results on Its Predictive Role in Tumor Resection Rate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design
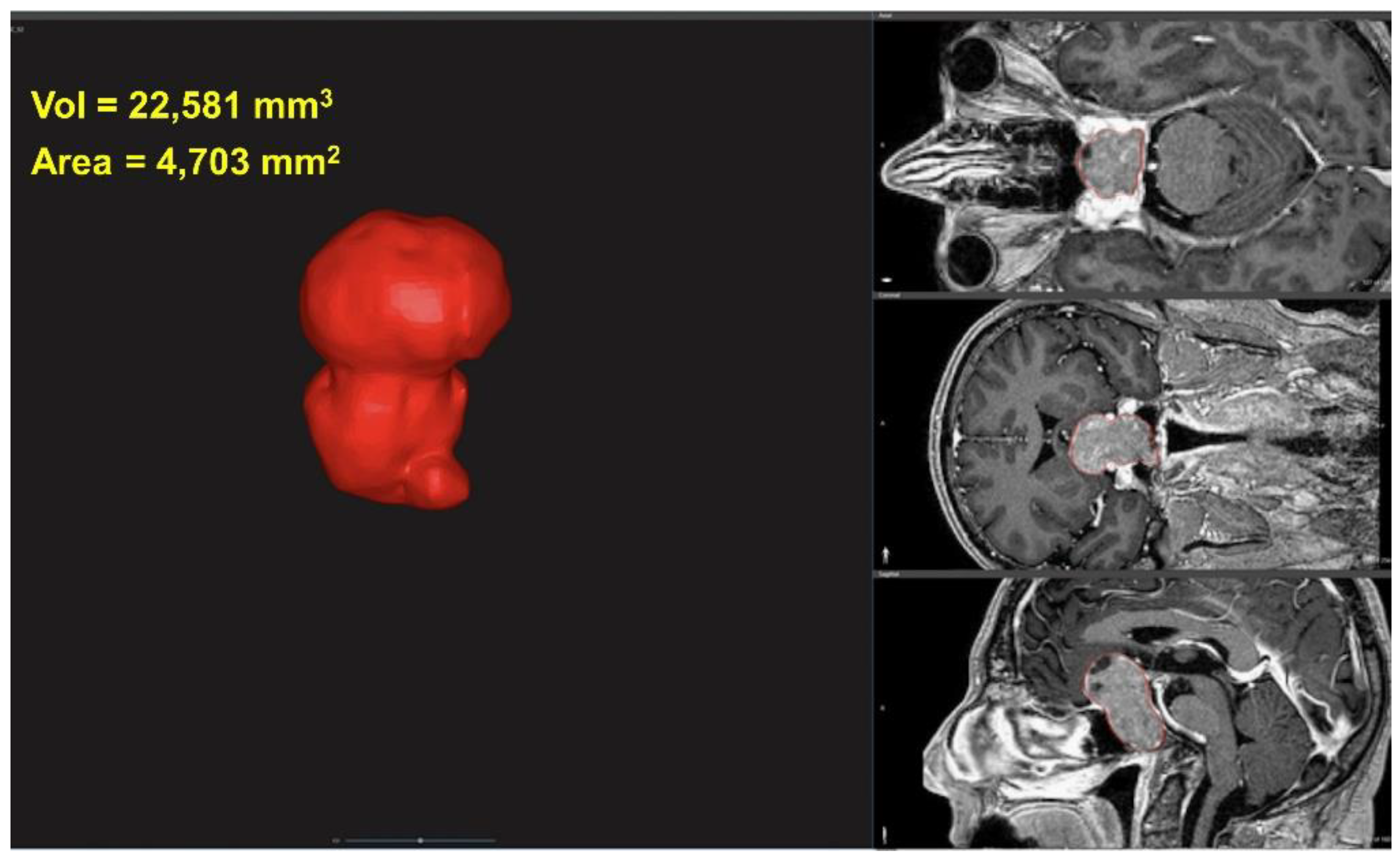
2.2. 3D Characterization of Tumor
2.3. Tumor Volume and Area
2.4. Tumor Sphericity
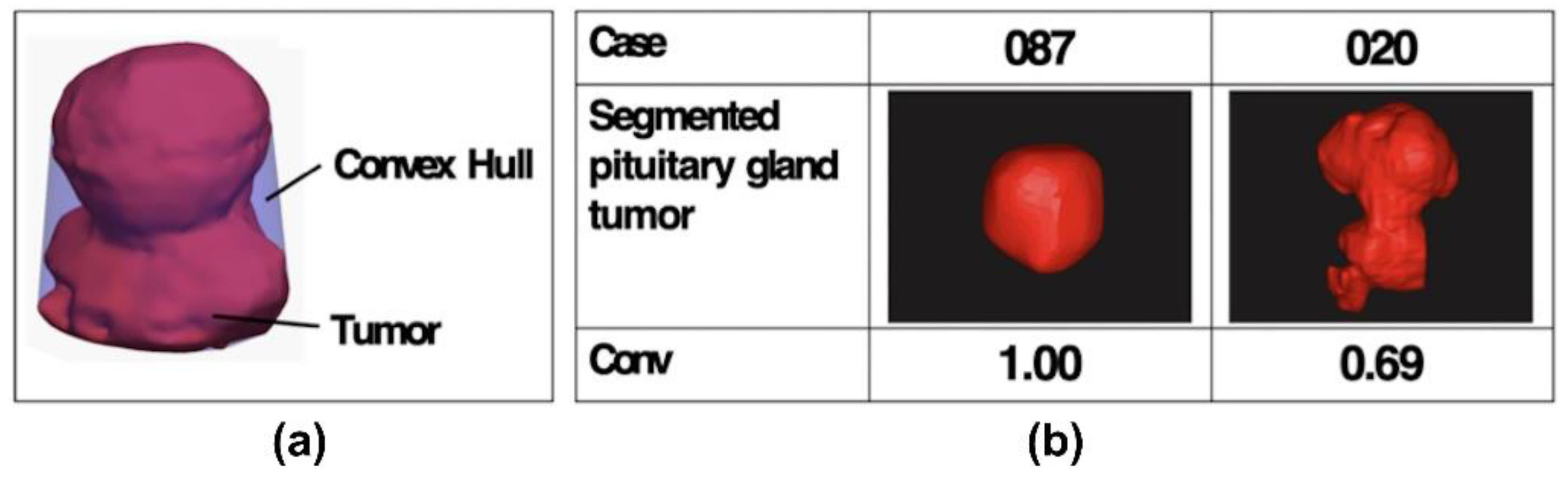
2.5. Tumor Convexity
2.6. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Buchfelder, M.; Schlaffer, S.; Zhao, Y. The optimal surgical techniques for pituitary tumors. Best Pract. Res. Clin. Endocrinol. Metab. 2019, 33, 101299. [Google Scholar] [CrossRef] [PubMed]
- Laws, E.R.; Thapar, K. Pituitary surgery. Endocrinol. Metab. Clin. N. Am. 1999, 28, 119–131. [Google Scholar] [CrossRef]
- Frank, G.; Pasquini, E.; Farneti, G.; Mazzatenta, D.; Sciarretta, V.; Grasso, V.; Fustini, M.F. The Endoscopic versus the Traditional Approach in Pituitary Surgery. Neuroendocrinology 2006, 83, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Liu, W.; Cao, P.; Zheng, Y.; Bu, Z.; Zhou, T. Endoscopic Versus Microscopic Transsphenoidal Surgery in the Treatment of Pituitary Adenoma: A Systematic Review and Meta-Analysis. World Neurosurg. 2017, 101, 236–246. [Google Scholar] [CrossRef]
- Marcus, H.J.; Vercauteren, T.; Ourselin, S.; Dorward, N.L. Intraoperative Ultrasound in Patients Undergoing Transsphenoidal Surgery for Pituitary Adenoma: Systematic Review. World Neurosurg. 2017, 106, 680–685. [Google Scholar] [CrossRef] [Green Version]
- Pennacchietti, V.; Garzaro, M.; Grottoli, S.; Pacca, P.; Garbossa, D.; Ducati, A.; Zenga, F. Three-Dimensional Endoscopic Endonasal Approach and Outcomes in Sellar Lesions: A Single-Center Experience of 104 Cases. World Neurosurg. 2016, 89, 121–125. [Google Scholar] [CrossRef]
- Vasudevan, K.; Saad, H.; Oyesiku, N.M. The Role of Three-Dimensional Endoscopy in Pituitary Adenoma Surgery. Neurosurg. Clin. N. Am. 2019, 30, 421–432. [Google Scholar] [CrossRef]
- Asioli, S.; Righi, A.; Iommi, M.; Baldovini, C.; Ambrosi, F.; Guaraldi, F.; Zoli, M.; Mazzatenta, D.; Faustini-Fustini, M.; Rucci, P.; et al. Validation of a clinicopathological score for the prediction of post-surgical evolution of pituitary adenoma: Retrospective analysis on 566 patients from a tertiary care centre. Eur. J. Endocrinol. 2019, 180, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Lopes, M.B.S. The 2017 World Health Organization classification of tumors of the pituitary gland: A summary. Acta Neuropathol. 2017, 134, 521–535. [Google Scholar] [CrossRef]
- Raverot, G.; Dantony, E.; Beauvy, J.; Vasiljevic, A.; Mikolasek, S.; Borson-Chazot, F.; Jouanneau, E.; Roy, P.; Trouillas, J. Risk of Recurrence in Pituitary Neuroendocrine Tumors: A Prospective Study Using a Five-Tiered Classification. J. Clin. Endocrinol. Metab. 2017, 102, 3368–3374. [Google Scholar] [CrossRef]
- Trouillas, J.; Roy, P.; Sturm, N.; Dantony, E.; Cortet-Rudelli, C.; Viennet, G.; Bonneville, J.-F.; Assaker, R.; Auger, C.; Brue, T.; et al. A new prognostic clinicopathological classification of pituitary adenomas: A multicentric case–control study of 410 patients with 8 years post-operative follow-up. Acta Neuropathol. 2013, 126, 123–135. [Google Scholar] [CrossRef] [PubMed]
- Luomaranta, T.; Raappana, A.; Saarela, V.; Liinamaa, M.J. Factors Affecting the Visual Outcome of Pituitary Adenoma Patients Treated with Endoscopic Transsphenoidal Surgery. World Neurosurg. 2017, 105, 422–431. [Google Scholar] [CrossRef] [PubMed]
- Póczoš, P.; Kremláček, J.; Česák, T.; Macháčková, M.; Jirásková, N. The Use of Optical Coherence Tomography in Chiasmal Compression. Czech Slovak Ophthalmol. 2019, 75, 120–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.H.; Lee, J.H.; Lee, J.H.; Hong, A.R.; Kim, Y.J.; Kim, Y.H. Endoscopic Transsphenoidal Surgery Outcomes in 331 Nonfunctioning Pituitary Adenoma Cases After a Single Surgeon Learning Curve. World Neurosurg. 2018, 109, e409–e416. [Google Scholar] [CrossRef] [PubMed]
- Knosp, E.; Steiner, E.; Kitz, K.; Matula, C. Pituitary adenomas with invasion of the cavernous sinus space: A magnetic resonance imaging classification compared with surgical findings. Neurosurgery 1993, 33, 610–618. [Google Scholar] [CrossRef]
- Micko, A.; Oberndorfer, J.; Weninger, W.J.; Vila, G.; Höftberger, R.; Wolfsberger, S.; Knosp, E. Challenging Knosp high-grade pituitary adenomas. J. Neurosurg. 2019, 132, 1739–1746. [Google Scholar] [CrossRef]
- Micko, A.; Woehrer, A.; Wolfsberger, S.; Knosp, E. Invasion of the cavernous sinus space in pituitary adenomas: Endoscopic verification and its correlation with an MRI-based classification. J. Neurosurg. 2015, 122, 803–811. [Google Scholar] [CrossRef]
- Serra, C.; Burkhardt, J.-K.; Esposito, G.; Bozinov, O.; Pangalu, A.; Valavanis, A.; Holzmann, D.; Schmid, C.; Regli, L. Pituitary surgery and volumetric assessment of extent of resection: A paradigm shift in the use of intraoperative magnetic resonance imaging. Neurosurg. Focus 2016, 40, E17. [Google Scholar] [CrossRef] [Green Version]
- Zoli, M.; Milanese, L.; Bonfatti, R.; Sturiale, C.; Pasquini, E.; Frank, G.; Mazzatenta, D. Cavernous sinus invasion by pituitary ad-enomas: Role of endoscopic endonasal surgery. J. Neurosurg. Sci. 2016, 60, 485–494. [Google Scholar]
- Lopez-Garcia, R.; Abarca-Olivas, J.; Monjas-Cánovas, I.; Alfonso, A.P.; Moreno-López, P.; Gras-Albert, J. Endonasal endoscopic surgery in pituitary adenomas: Surgical results in a series of 86 consecutive patients. Neurocirugia 2018, 29, 161–169. [Google Scholar] [CrossRef]
- Mastorakos, P.; Mehta, G.U.; Chatrath, A.; Moosa, S.; Lopes, M.-B.; Payne, S.C.; Jane, J.A. Tumor to Cerebellar Peduncle T2-Weighted Imaging Intensity Ratio Fails to Predict Pituitary Adenoma Consistency. J. Neurol. Surg. Part B Skull Base 2018, 80, 252–257. [Google Scholar] [CrossRef] [PubMed]
- Pappy, A.L.; Savinkina, A.; Bicknese, C.; Neill, S.; Oyesiku, N.M.; Ioachimescu, A.G. Predictive modeling for pituitary adenomas: Single center experience in 501 consecutive patients. Pituitary 2019, 22, 520–531. [Google Scholar] [CrossRef] [PubMed]
- Serra, C.; Staartjes, V.E.; Maldaner, N.; Muscas, G.; Akeret, K.; Holzmann, D.; Soyka, M.B.; Schmid, C.; Regli, L. Predicting extent of resection in transsphenoidal surgery for pituitary adenoma. Acta Neurochir. 2018, 160, 2255–2262. [Google Scholar] [CrossRef] [PubMed]
- Do, H.; Kshettry, V.R.; Siu, A.; Belinsky, I.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Extent of Resection, Visual, and Endocrinologic Outcomes for Endoscopic Endonasal Surgery for Recurrent Pituitary Adenomas. World Neurosurg. 2017, 102, 35–41. [Google Scholar] [CrossRef]
- Rana, M.; Essig, H.; Eckardt, A.M.; Tavassol, F.; Ruecker, M.; Schramm, A.; Gellrich, N.-C. Advances and Innovations in Computer-Assisted Head and Neck Oncologic Surgery. J. Craniofacial Surg. 2012, 23, 272–278. [Google Scholar] [CrossRef]
- Pellegrino, G.; Ferri, A.; Cercenelli, L.; Marcelli, E.; Marchetti, C.; Tarsitano, A.; Ciocca, L. 3D planning of ear prosthesis and navigated flapless surgery for craniofacial implants: A pilot study. J. Stomatol. Oral Maxillofac. Surg. 2021, 122, 391–396. [Google Scholar] [CrossRef]
- Checcucci, E.; Amparore, D.; Fiori, C.; Manfredi, M.; Ivano, M.; Di Dio, M.; Niculescu, G.; Piramide, F.; Cattaneo, G.; Piazzolla, P.; et al. 3D imaging applications for robotic urologic surgery: An ESUT YAUWP review. World J. Urol. 2019, 38, 869–881. [Google Scholar] [CrossRef]
- Bianchi, L.; Barbaresi, U.; Cercenelli, L.; Bortolani, B.; Gaudiano, C.; Chessa, F.; Angiolini, A.; Lodi, S.; Porreca, A.; Bianchi, F.M.; et al. The Impact of 3D Digital Reconstruction on the Surgical Planning of Partial Nephrectomy: A Case-control Study. Still Time for a Novel Surgical Trend? Clin. Genitourin. Cancer 2020, 18, e669–e678. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Bortolani, B.; Cercenelli, L.; Gaudiano, C.; Carpani, G.; Rustici, A.; Droghetti, M.; Mottaran, A.; Boschi, S.; et al. Interpreting nephrometry scores with three-dimensional virtual modelling for better planning of robotic partial nephrectomy and predicting complications. Urol. Oncol. Semin. Orig. Investig. 2021, 39, 836.e1–836.e9. [Google Scholar] [CrossRef]
- Panesar, S.S.; Magnetta, M.; Mukherjee, D.; Abhinav, K.; Branstetter, B.F.; Gardner, P.A.; Iv, M.; Fernandez-Miranda, J.C. Patient-specific 3-dimensionally printed models for neurosurgical planning and education. Neurosurg. Focus 2019, 47, E12. [Google Scholar] [CrossRef] [Green Version]
- Tarsitano, A.; Ricotta, F.; Cercenelli, L.; Bortolani, B.; Battaglia, S.; Lucchi, E.; Marchetti, C.; Marcelli, E. Pretreatment tumor volume and tumor sphericity as prognostic factors in patients with oral cavity squamous cell carcinoma. J. Cranio-Maxillofac. Surg. 2019, 47, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Casanueva, F.F.; Barkan, A.L.; Buchfelder, M.; Klibanski, A.; Laws, E.R.; Loeffler, J.S.; Melmed, S.; Mortini, P.; Wass, J.; Giustina, A. Criteria for the definition of Pituitary Tumor Centers of Excellence (PTCOE): A Pituitary Society Statement. Pituitary 2017, 20, 489–498. [Google Scholar] [CrossRef] [PubMed]
- Faustini-Fustini, M.; Pasquini, E.; Zoli, M.; Mazzatenta, D.; Frank, G. Pituitary Centers of Excellence. Neurosurgery 2013, 73, E557. [Google Scholar] [CrossRef] [PubMed]
- Barazi, S.A.; Pasquini, E.; D’Urso, P.I.; Zoli, M.; Mazzatenta, D.; Sciarretta, V.; Frank, G. Extended endoscopic transplanum–transtuberculum approach for pituitary adenomas. Br. J. Neurosurg. 2012, 27, 374–382. [Google Scholar] [CrossRef] [PubMed]
- Badiali, G.; Marcelli, E.; Bortolani, B.; Marchetti, C.; Cercenelli, L. An average three-dimensional virtual human skull for a template-assisted maxillofacial surgery. Int. J. Artif. Organs 2019, 42, 566–574. [Google Scholar] [CrossRef]
- Battaglia, S.; Ricotta, F.; Maiolo, V.; Savastio, G.; Contedini, F.; Cipriani, R.; Bortolani, B.; Cercenelli, L.; Marcelli, E.; Marchetti, C.; et al. Computer-assisted surgery for reconstruction of complex mandibular defects using osteomyocutaneous microvascular fibular free flaps: Use of a skin paddle-outlining guide for soft-tissue reconstruction. A technical report. J. Cranio-Maxillofac. Surg. 2018, 47, 293–299. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Barbaresi, U.; Angiolini, A.; Pultrone, C.V.; Manferrari, F.; Bortolani, B.; Cercenelli, L.; Borghesi, M.; Chessa, F.; et al. 3D Reconstruction and physical renal model to improve percutaneous punture during PNL. Int. Braz. J. Urol. 2019, 45, 1281–1282. [Google Scholar] [CrossRef]
- Ricotta, F.; Cercenelli, L.; Battaglia, S.; Bortolani, B.; Savastio, G.; Marcelli, E.; Marchetti, C.; Tarsitano, A. Navigation-guided resection of maxillary tumors: Can a new volumetric virtual planning method improve outcomes in terms of control of resection margins? J. Cranio-Maxillofac. Surg. 2018, 46, 2240–2247. [Google Scholar] [CrossRef]
- Schiavina, R.; Bianchi, L.; Borghesi, M.; Chessa, F.; Cercenelli, L.; Marcelli, E.; Brunocilla, A. Three-dimensional digital reconstruction of renal model to guide preoperative planning of robot-assisted partial nephrectomy. Int. J. Urol. 2019, 26, 931–932. [Google Scholar] [CrossRef]
- Wadell, H. Volume, Shape, and Roundness of Quartz Particles. J. Geol. 1935, 43, 250–280. [Google Scholar] [CrossRef]
- Dhar, S.; Tremmel, M.; Mocco, J.; Kim, M.; Yamamoto, J.; Siddiqui, A.H.; Hopkins, L.N.; Meng, H. Morphology parameters for intracranial aneurysm rupture risk assessment. Neurosurgery 2008, 63, 185–197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dallapiazza, R.F.; Grober, Y.; Starke, R.M.; Laws, E.R.; Jane, J.A. Long-term Results of Endonasal Endoscopic Transsphenoidal Resection of Nonfunctioning Pituitary Macroadenomas. Neurosurgery 2014, 76, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Dehdashti, A.R.; Ganna, A.; Karabatsou, K.; Gentili, F. Pure endoscopic endonasal approach for pituitary adenomas: Early surgical results in 200 patients and comparison with previous micro- surgical series. Neurosurgery 2008, 62, 1006–1017. [Google Scholar] [CrossRef] [PubMed]
- Dhandapani, S.; Singh, H.; Negm, H.M.; Cohen, S.; Anand, V.K.; Schwartz, T.H. Cavernous Sinus Invasion in Pituitary Adenomas: Systematic Review and Pooled Data Meta-Analysis of Radiologic Criteria and Comparison of Endoscopic and Microscopic Surgery. World Neurosurg. 2016, 96, 36–46. [Google Scholar] [CrossRef]
- Nishioka, H.; Hara, T.; Nagata, Y.; Fukuhara, N.; Yamaguchi-Okada, M.; Yamada, S. Inherent Tumor Characteristics That Limit Effective and Safe Resection of Giant Nonfunctioning Pituitary Adenomas. World Neurosurg. 2017, 106, 645–652. [Google Scholar] [CrossRef]
- Juraschka, K.; Khan, O.H.; Godoy, B.L.; Monsalves, E.; Kilian, A.; Krischek, B.; Ghare, A.; Vescan, A.; Gentili, F.; Zadeh, G. Endoscopic endonasal transsphenoidal approach to large and giant pituitary adenomas: Institutional experience and predictors of extent of resection. J. Neurosurg. 2014, 121, 75–83. [Google Scholar] [CrossRef]
- Sylvester, P.T.; Evans, J.A.; Zipfel, G.J.; Chole, R.A.; Uppaluri, R.; Haughey, B.H.; Getz, A.E.; Silverstein, J.; Rich, K.M.; Kim, A.H.; et al. Combined high-field intraoperative magnetic resonance imaging and endoscopy increase extent of resection and progression-free survival for pituitary adenomas. Pituitary 2015, 18, 72–85. [Google Scholar] [CrossRef] [Green Version]
- Hofstetter, C.P.; Nanaszko, M.J.; Mubita, L.L.; Tsiouris, J.; Anand, V.K.; Schwartz, T.H. Volumetric classification of pituitary macroadenomas predicts outcome and morbidity following endoscopic endonasal transsphenoidal surgery. Pituitary 2011, 15, 450–463. [Google Scholar] [CrossRef]
- Little, A.S.; Chicoine, M.R.; Kelly, D.F.; Sarris, C.E.; Mooney, M.A.; White, W.L.; Gardner, P.A.; Fernandez-Miranda, J.C.; Barkhoudarian, G.; Chandler, J.P.; et al. Evaluation of Surgical Resection Goal and Its Relationship to Extent of Resection and Patient Outcomes in a Multicenter Prospective Study of Patients With Surgically Treated, Nonfunctioning Pituitary Adenomas: A Case Series. Oper. Neurosurg. 2019, 18, 26–33. [Google Scholar] [CrossRef]
- Elshazly, K.; Kshettry, V.R.; Farrell, C.J.; Nyquist, G.; Rosen, M.; Evans, J.J. Clinical Outcomes After Endoscopic Endonasal Resection of Giant Pituitary Adenomas. World Neurosurg. 2018, 114, e447–e456. [Google Scholar] [CrossRef]
- Bianchi, L.; Schiavina, R.; Bortolani, B.; Cercenelli, L.; Gaudiano, C.; Mottaran, A.; Droghetti, M.; Chessa, F.; Boschi, S.; Molinaroli, E.; et al. Novel Volumetric and Morphological Parameters Derived from Three-dimensional Virtual Modeling to Improve Comprehension of Tumor’s Anatomy in Patients with Renal Cancer. Eur. Urol. Focus 2021. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Porpiglia, F.; Amparore, D.; Checcucci, E.; Manfredi, M.; Stura, I.; Migliaretti, G.; Autorino, R.; Ficarra, V.; Fiori, C. Three-dimensional virtual imaging of renal tumours: A new tool to improve the accuracy of nephrometry scores. Br. J. Urol. 2019, 124, 945–954. [Google Scholar] [CrossRef] [PubMed]

| No. of Patients | % | ||
|---|---|---|---|
| Previous Treatment | none | 72 | 96% |
| surgery | 3 | 4% | |
| radiotherapy | 0 | 0% | |
| Tumor | GH-secreting tumors | 6 | 8% |
| PRL-secreting tumors | 9 | 12% | |
| ACTH-secreting tumors | 2 | 2.7% | |
| Non-functioning tumors | 58 | 77.3% | |
| Endocrine Functions | none | 36 | 48% |
| anterior hypopituitarism | 26 | 34.7% | |
| Diabetes Insipidus (DI) | 1 | 1.3% | |
| Acromegaly | 6 | 8% | |
| Cushing disease | 2 | 2.7% | |
| HyperPRL (>200 ng/mL) | 9 | 12% | |
| Visual Symptoms | no | 35 | 46.7% |
| present | 40 | 53.3% | |
| Ophthalmoplegia | no | 69 | 92% |
| present | 6 | 8% |
| No. of Patients | % | ||
|---|---|---|---|
| Hardy | 1 | 6 | 8% |
| 2 | 43 | 57.4% | |
| 3 | 16 | 21.3% | |
| 4 | 10 | 13.3% | |
| Wilson | A | 33 | 44% |
| B | 30 | 40% | |
| C | 3 | 4% | |
| D | 1 | 1.3% | |
| E | 8 | 10.7% | |
| Knosp | 0 | 26 | 34.7% |
| 1 | 10 | 13.3% | |
| 2 | 18 | 24% | |
| 3 | 16 | 21.3% | |
| 4 | 5 | 6.7% | |
| Zurich Score | 1 | 5 | 6.7% |
| 2 | 59 | 78.6% | |
| 3 | 6 | 8% | |
| 4 | 5 | 6.7% |
| Parameter | Mean ± SD | Complete Tumor Removal | Incomplete Tumor Removal |
|---|---|---|---|
| Vol | 9117 ± 8423 mm3 | 8400 ± 7792 mm3 | 11,365 ± 10,086 mm3 |
| Conv | 0.88 ± 0.08 | 0.91 ± 0.06 | 0.80 ± 0.10 |
| Knosp grade 0 | 26 | 24 | 2 |
| Knosp grade 1 | 10 | 8 | 2 |
| Knosp grade 2 | 18 | 14 | 4 |
| Knosp grade 3 | 16 | 11 | 5 |
| Knosp grade 4 | 5 | 0 | 5 |
| Parameter | z | p | 95% Conf. Interval | Odds Ratio | ||
|---|---|---|---|---|---|---|
| Sex | −1.43 | 0.15 | 0.31 | 1.72 | ||
| Pre-treat | 1.07 | 0.29 | 0.15 | 582.45 | ||
| Hardy grade | −0.74 | 0.46 | 0.19 | 2.09 | ||
| Knosp | 2 | 0.54 | 0.59 | 0.45 | 743.09 | 18.2 |
| 3 | 1.06 | 0.29 | 0.20 | 205.21 | 6.5 | |
| 4 | 1.94 | 0.05 | 1.6 | 1582.61 | 50.5 | |
| Tumor | PRL | 0.53 | 0.59 | 0.05 | 158.28 | |
| ACTH | −0.09 | 0.93 | 0.01 | 177.43 | ||
| NF | −1.52 | 0.13 | 0.01 | 2.06 | ||
| ICD | −0.47 | 0.54 | 0.68 | 1.27 | 0.9 | |
| Vol | 2.44 | 0.01 | 1.01 | 1.03 | 1.02 | |
| Conv | −2.35 | 0.02 | 0.69 | 0.97 | 0.8 | |
| Parameter | z | p | 95% Conf. Interval | Odds Ratio | |
|---|---|---|---|---|---|
| Conv | −3.87 | 0.00 | 0.73 | 0.90 | 0.81 |
| Vol | 1.61 | 0.11 | 1.00 | 1.01 | 1.01 |
| Parameter | z | p | 95% Conf. Interval | Odds Ratio | |
| Conv | −3.02 | 0.03 | 0.73 | 0.93 | 0.83 |
| Knosp 2 | 1.61 | 0.073 | 0.80 | 136.7 | 10.48 |
| Knosp 3 | 1.81 | 0.108 | 0.66 | 65.31 | 6.56 |
| Knosp 4 | 2.62 | 0.070 | 0.86 | 54.33 | 6.82 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cercenelli, L.; Zoli, M.; Bortolani, B.; Curti, N.; Gori, D.; Rustici, A.; Mazzatenta, D.; Marcelli, E. 3D Virtual Modeling for Morphological Characterization of Pituitary Tumors: Preliminary Results on Its Predictive Role in Tumor Resection Rate. Appl. Sci. 2022, 12, 4275. https://doi.org/10.3390/app12094275
Cercenelli L, Zoli M, Bortolani B, Curti N, Gori D, Rustici A, Mazzatenta D, Marcelli E. 3D Virtual Modeling for Morphological Characterization of Pituitary Tumors: Preliminary Results on Its Predictive Role in Tumor Resection Rate. Applied Sciences. 2022; 12(9):4275. https://doi.org/10.3390/app12094275
Chicago/Turabian StyleCercenelli, Laura, Matteo Zoli, Barbara Bortolani, Nico Curti, Davide Gori, Arianna Rustici, Diego Mazzatenta, and Emanuela Marcelli. 2022. "3D Virtual Modeling for Morphological Characterization of Pituitary Tumors: Preliminary Results on Its Predictive Role in Tumor Resection Rate" Applied Sciences 12, no. 9: 4275. https://doi.org/10.3390/app12094275
APA StyleCercenelli, L., Zoli, M., Bortolani, B., Curti, N., Gori, D., Rustici, A., Mazzatenta, D., & Marcelli, E. (2022). 3D Virtual Modeling for Morphological Characterization of Pituitary Tumors: Preliminary Results on Its Predictive Role in Tumor Resection Rate. Applied Sciences, 12(9), 4275. https://doi.org/10.3390/app12094275









