Crystal Structure of Human Lysozyme Complexed with N-Acetyl-α-d-glucosamine
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. X-ray Diffraction Data Collection
2.3. Structure Determination
3. Results and Discussion
3.1. Overall Structure
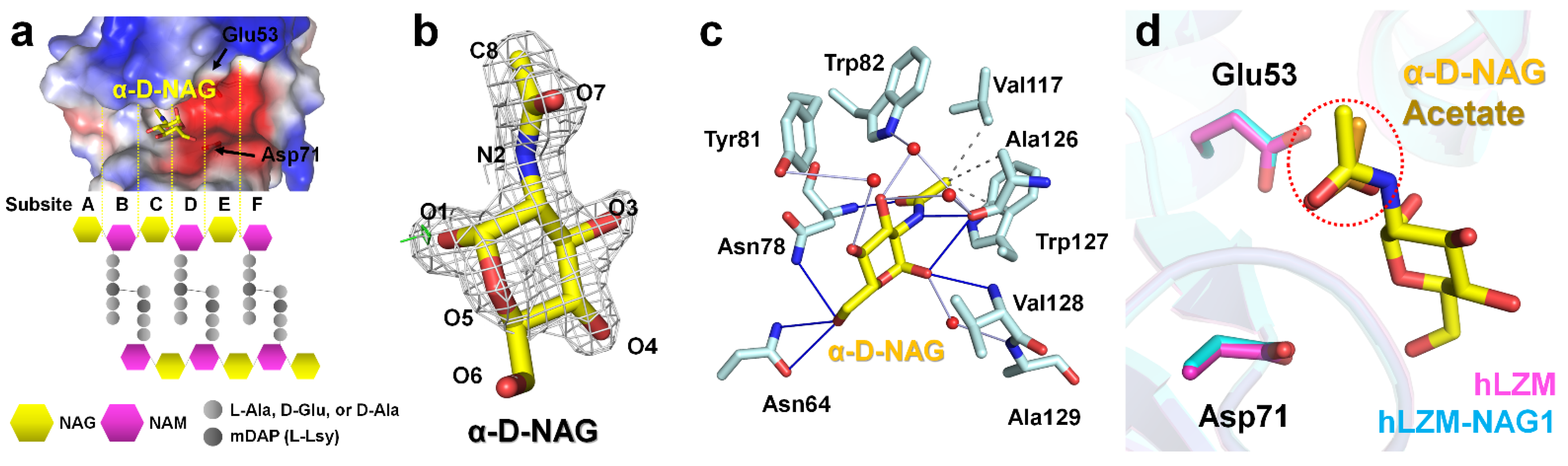
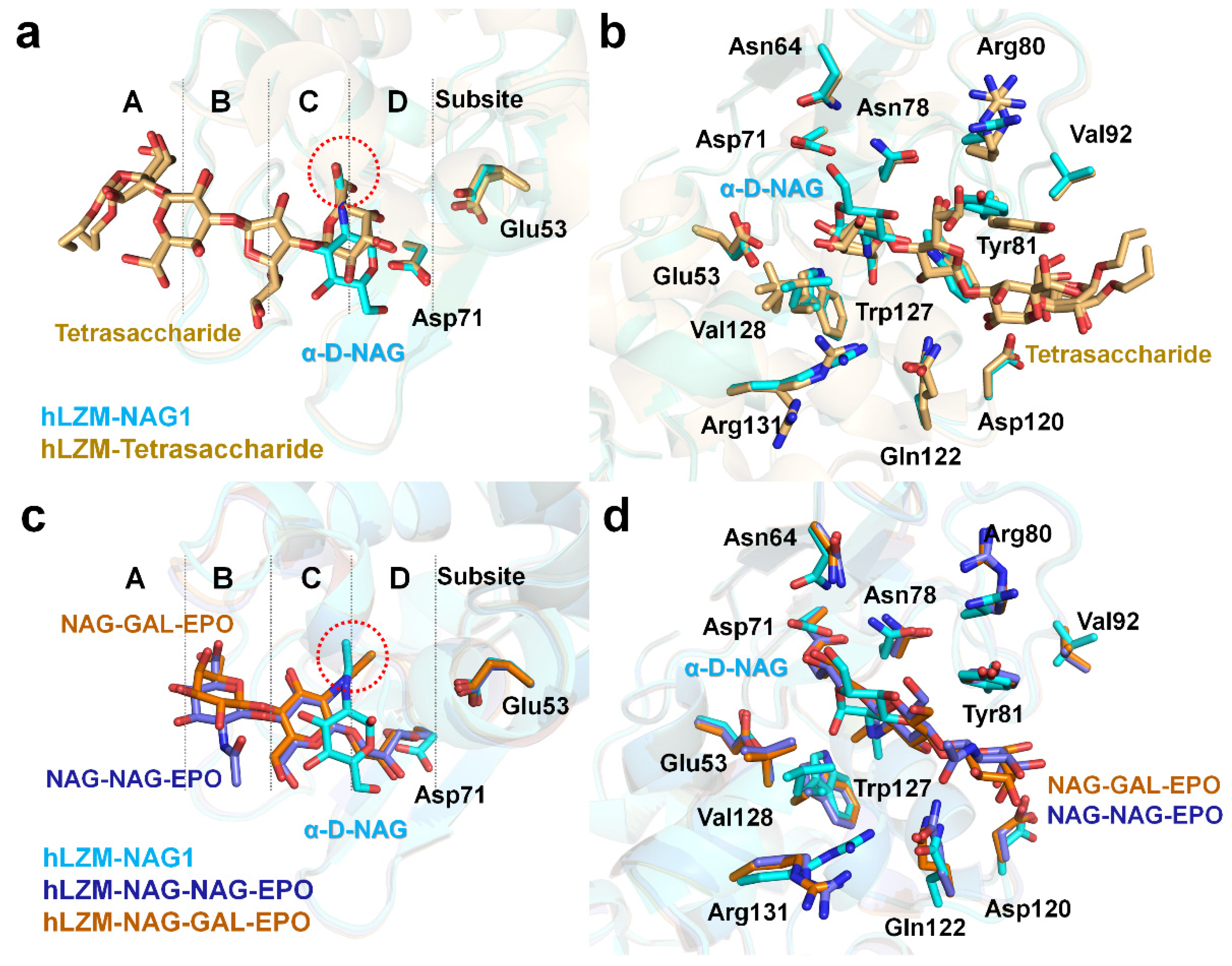
3.2. NAG Binding
3.3. Comparison of Native hLZM and NAG-Bound hLZM
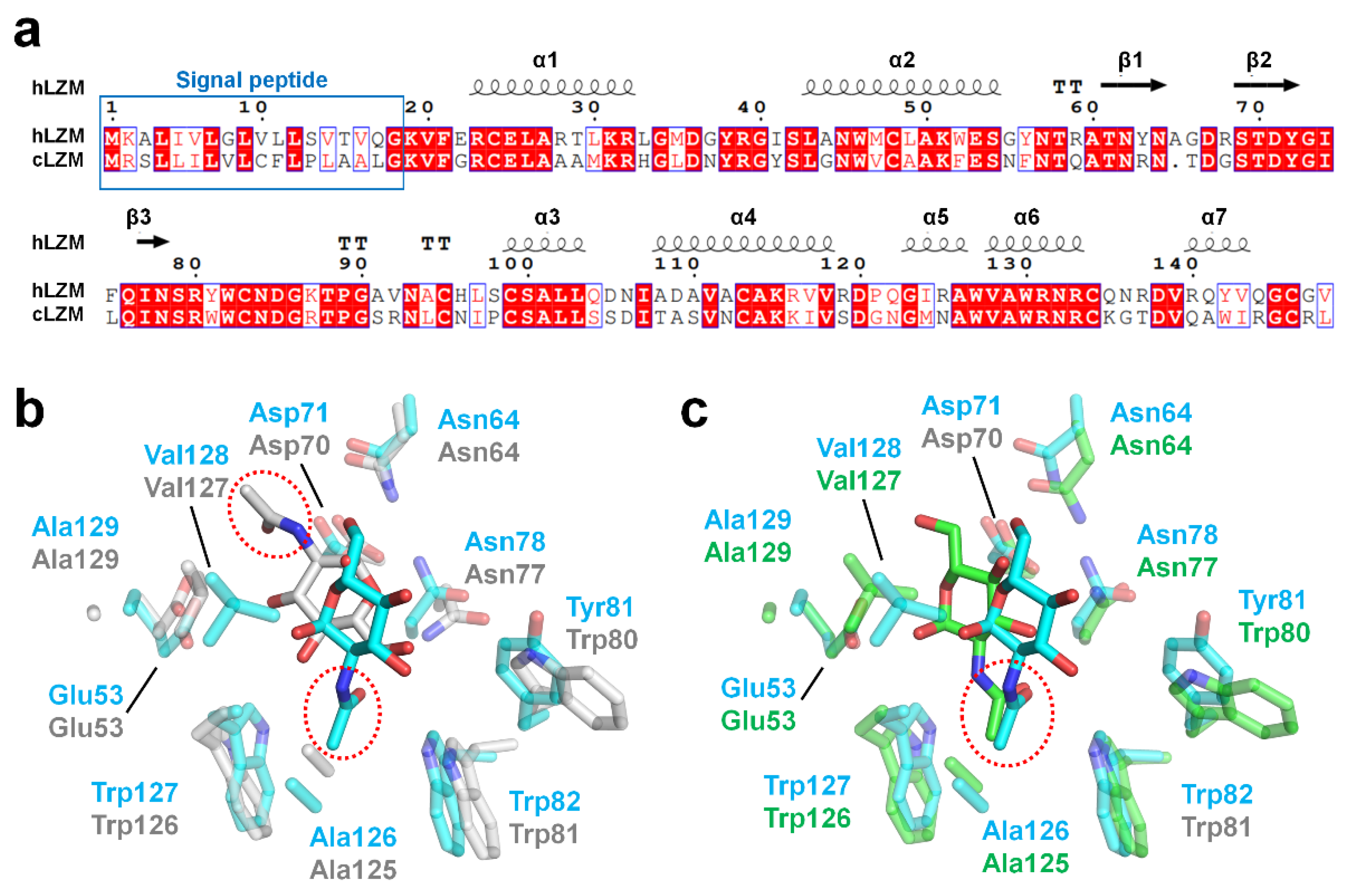
3.4. Structural Comparison of hLZM with Other Lysozyme Structures
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferraboschi, P.; Ciceri, S.; Grisenti, P. Applications of Lysozyme, an Innate Immune Defense Factor, as an Alternative Antibiotic. Antibiotics 2021, 10, 1534. [Google Scholar] [CrossRef]
- Callewaert, L.; Michiels, C.W. Lysozymes in the animal kingdom. J. Biosci. 2010, 35, 127–160. [Google Scholar] [CrossRef]
- Bliska, J.B.; Ragland, S.A.; Criss, A.K. From bacterial killing to immune modulation: Recent insights into the functions of lysozyme. PLoS Pathog. 2017, 13, e1006512. [Google Scholar] [CrossRef]
- Jollès, P.; Jollès, J. What’s new in lysozyme research? Always a model system, today as yesterday. Mol. Cell. Biochem. 1984, 63, 165–189. [Google Scholar] [CrossRef]
- Beintema, J.J.; van Scheltinga, A.C.T. Plant lysozymes. In Lysozymes: Model Enzymes in Biochemistry and Biology; Experientia Supplementum; Springer: Berlin/Heidelberg, Germany, 1996; pp. 75–86. [Google Scholar] [CrossRef]
- Fastrez, J. Phage lysozymes. In Lysozymes: Model Enzymes in Biochemistry and Biology; Experientia Supplementum; Springer: Berlin/Heidelberg, Germany, 1996; pp. 35–64. [Google Scholar] [CrossRef]
- Uversky, V.N.; Wohlkönig, A.; Huet, J.; Looze, Y.; Wintjens, R. Structural Relationships in the Lysozyme Superfamily: Significant Evidence for Glycoside Hydrolase Signature Motifs. PLoS ONE 2010, 5, e15388. [Google Scholar] [CrossRef] [Green Version]
- Ellison, R.T.; Giehl, T.J. Killing of gram-negative bacteria by lactoferrin and lysozyme. J. Clin. Investig. 1991, 88, 1080–1091. [Google Scholar] [CrossRef]
- Oliver, W.T.; Wells, J.E. Lysozyme as an alternative to growth promoting antibiotics in swine production. J. Anim. Sci. Biotechnol. 2015, 6, 35. [Google Scholar] [CrossRef] [Green Version]
- Tenovuo, J. Clinical applications of antimicrobial host proteins lactoperoxidase, lysozyme and lactoferrin in xerostomia: Efficacy and safety. Oral Dis. 2002, 8, 23–29. [Google Scholar] [CrossRef]
- Masschalck, B.; Michiels, C.W. Antimicrobial Properties of Lysozyme in Relation to Foodborne Vegetative Bacteria. Crit. Rev. Microbiol. 2008, 29, 191–214. [Google Scholar] [CrossRef]
- Lu, D.; Liu, S.; Ding, F.; Wang, H.; Li, J.; Li, L.; Dai, Y.; Li, N. Large-scale production of functional human lysozyme from marker-free transgenic cloned cows. Sci. Rep. 2016, 6, 22947. [Google Scholar] [CrossRef] [Green Version]
- Travis, S.M.; Conway, B.-A.D.; Zabner, J.; Smith, J.J.; Anderson, N.N.; Singh, P.K.; Peter Greenberg, E.; Welsh, M.J. Activity of Abundant Antimicrobials of the Human Airway. Am. J. Respir. Cell Mol. Biol. 1999, 20, 872–879. [Google Scholar] [CrossRef]
- Siwicki, A.K.; Klein, P.; Morand, M.; Kiczka, W.; Studnicka, M. Immunostimulatory effects of dimerized lysozyme (KLP-602) on the nonspecific defense mechanisms and protection against furunculosis in salmonids. Vet. Immunol. Immunopathol. 1998, 61, 369–378. [Google Scholar] [CrossRef]
- Zhang, R.; Wu, L.; Eckert, T.; Burg-Roderfeld, M.; Rojas-Macias, M.A.; Lütteke, T.; Krylov, V.B.; Argunov, D.A.; Datta, A.; Markart, P.; et al. Lysozyme’s lectin-like characteristics facilitates its immune defense function. Q. Rev. Biophys. 2017, 50, e9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osserman, E.F.; Klockars, M.; Halper, J.; Fischel, R.E. Effects of Lysozyme on Normal and Transformed Mammalian Cells. Nature 1973, 243, 331–335. [Google Scholar] [CrossRef]
- Lee-Huang, S.; Maiorov, V.; Huang, P.L.; Ng, A.; Lee, H.C.; Chang, Y.-T.; Kallenbach, N.; Huang, P.L.; Chen, H.-C. Structural and Functional Modeling of Human Lysozyme Reveals a Unique Nonapeptide, HL9, with Anti-HIV Activity. Biochemistry 2005, 44, 4648–4655. [Google Scholar] [CrossRef]
- Song, H.; Inaka, K.; Maenaka, K.; Matsushima, M. Structural Changes of Active Site Cleft and Different Saccharide Binding Modes in Human Lysozyme Co-crystallized with Hexa-N-acetyl-chitohexaose at pH 4.0. J. Mol. Biol. 1994, 244, 522–540. [Google Scholar] [CrossRef]
- Nam, K.H.; Cho, Y. Stable sample delivery in a viscous medium via a polyimide-based single-channel microfluidic chip for serial crystallography. J. Appl. Crystallogr. 2021, 54, 1081–1087. [Google Scholar] [CrossRef]
- Otwinowski, Z.; Minor, W. Processing of X-ray diffraction data collected in oscillation mode. Methods Enzymol. 1997, 276, 307–326. [Google Scholar] [CrossRef]
- Vagin, A.; Teplyakov, A. Molecular replacement with MOLREP. Acta Crystallogr. D Biol. Crystallogr. 2010, 66, 22–25. [Google Scholar] [CrossRef]
- Nam, K.H. Beef tallow injection matrix for serial crystallography. Sci. Rep. 2022, 12, 694. [Google Scholar] [CrossRef]
- Emsley, P.; Cowtan, K. Coot: Model-building tools for molecular graphics. Acta Crystallogr. D Biol. Crystallogr. 2004, 60, 2126–2132. [Google Scholar] [CrossRef] [Green Version]
- Liebschner, D.; Afonine, P.V.; Baker, M.L.; Bunkoczi, G.; Chen, V.B.; Croll, T.I.; Hintze, B.; Hung, L.W.; Jain, S.; McCoy, A.J.; et al. Macromolecular structure determination using X-rays, neutrons and electrons: Recent developments in Phenix. Acta Crystallogr. D Struct. Biol. 2019, 75, 861–877. [Google Scholar] [CrossRef] [Green Version]
- Williams, C.J.; Headd, J.J.; Moriarty, N.W.; Prisant, M.G.; Videau, L.L.; Deis, L.N.; Verma, V.; Keedy, D.A.; Hintze, B.J.; Chen, V.B.; et al. MolProbity: More and better reference data for improved all-atom structure validation. Protein Sci. 2018, 27, 293–315. [Google Scholar] [CrossRef]
- Salentin, S.; Schreiber, S.; Haupt, V.J.; Adasme, M.F.; Schroeder, M. PLIP: Fully automated protein–ligand interaction profiler. Nucleic Acids Res. 2015, 43, W443–W447. [Google Scholar] [CrossRef]
- Sievers, F.; Wilm, A.; Dineen, D.; Gibson, T.J.; Karplus, K.; Li, W.; Lopez, R.; McWilliam, H.; Remmert, M.; Soding, J.; et al. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011, 7, 539. [Google Scholar] [CrossRef]
- Gouet, P.; Courcelle, E.; Stuart, D.I.; Metoz, F. ESPript: Analysis of multiple sequence alignments in PostScript. Bioinformatics 1999, 15, 305–308. [Google Scholar] [CrossRef] [Green Version]
- Von Dreele, R.B. Binding of N-acetylglucosamine to chicken egg lysozyme: A powder diffraction study. Acta Crystallogr. D Biol. 2001, 57, 1836–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Butryn, A.; Simon, P.S.; Aller, P.; Hinchliffe, P.; Massad, R.N.; Leen, G.; Tooke, C.L.; Bogacz, I.; Kim, I.-S.; Bhowmick, A.; et al. An on-demand, drop-on-drop method for studying enzyme catalysis by serial crystallography. Nat. Commun. 2021, 12, 4461. [Google Scholar] [CrossRef]


| Data Collection | hLZM | hLZM-NAG1 | hLZM-NAG2 |
| Wavelength (Å) | 0.979 | 0.979 | 0.979 |
| Space group | P212121 | P212121 | P212121 |
| Unit cell dimension (Å) | |||
| a | 33.035 | 32.796 | 32.980 |
| b | 56.062 | 55.758 | 56.049 |
| c | 61.063 | 60.859 | 60.939 |
| Resolution range (Å) a | 50.0–1.30(1.32–1.30) | 50.0–1.55 (1.58–1.55) | 50.0–1.60 (1.63–1.60) |
| Unique reflections | 28,633 (1358) | 16,579 (797) | 15,319 (688) |
| Completeness (%) | 99.5 (94.8) | 99.6 (98.2) | 99.3 (90.3) |
| Redundancy | 6.4 (4.4) | 9.5 (5.0) | 6.0 (4.6) |
| Mean I/σ(I) | 40.42 (4.53) | 23.20 (3.37) | 27.59 (3.23) |
| Rmerge | 0.095 (0.428) | 0.293 (1.637) | 0.108 (0.524) |
| CC1/2 | 0.989 (0.863) | 0.981 (0.502) | 0.993 (0.832) |
| CC* | 0.997 (0.963) | 0.995 (0.818) | 0.998 (0.953) |
| Refinement | |||
| Resolution range (Å) | 50.0–1.30 | 50.0–1.55 | 50.0–1.60 |
| (1.33–1.30) | (1.60–1.55) | (1.65–1.60) | |
| Rwork | 0.1513 (0.2055) | 0.1686 (0.2236) | 0.1683 (0.2034) |
| Rfree | 0.1710 (0.2056) | 0.1910 (0.2664) | 0.1844 (0.2572) |
| RMS deviations | |||
| Bonds (Å) | 0.022 | 0.009 | 0.006 |
| Angles (degree) | 1.639 | 1.146 | 0.902 |
| Average B factor (Å2) | |||
| Protein | 12.30 | 15.19 | 17.95 |
| Ligand | 26.48 | 32.57 | |
| Water | 24.01 | 25.01 | 27.55 |
| Ramachandran plot | |||
| Most favored (%) | 97.66 | 96.09 | 96.88 |
| Allowed (%) | 2.34 | 3.91 | 3.12 |
| Hydrophobic Interactions | |||||
|---|---|---|---|---|---|
| hLZM Residue [Atom] | Distance (Å) | α-D-NAG Atom | |||
| Val117 [CG1] | 3.60 | C8 | |||
| Ala126 [CB] | 3.97 | C8 | |||
| Trp127 [CD2] | 3.70 | C8 | |||
| Hydrogen Bonds | |||||
| hLZM Residue [Atom] | Distance (Å) H-A 1 | Distance (Å) D-A 2 | Donor Angle (°) | α-D-NAG Atom | |
| Asn64 [ND2] | 2.06 | 2.92 | 145.86 | O6 | |
| Asn64 [OD1] | 2.17 | 3.16 | 177.97 | O6 | |
| Asn78 [ND2] | 2.31 | 3.27 | 164.54 | O6 | |
| Asn78 [N] | 2.90 | 3.57 | 127.25 | O7 | |
| Ala126 [O] | 3.24 | 3.94 | 130.39 | O1 | |
| Ala126 [O] | 3.60 | 3.92 | 101.36 | N2 | |
| Val128 [N] | 3.79 | 4.08 | 100.14 | O1 | |
| Water Bridges | |||||
| hLZM Residue [Atom] | Distance (Å) A-W 3 | Distance (Å) D-W 4 | Donor Angle (°) | Water Angle (°) | α-D-NAG Atom |
| Tyr81 [OH] | 4.00 | 3.48 | 127.96 | 112.25 | O4 |
| Trp82 [NE1] | 3.02 | 2.72 | 144.92 | 87.42 | O3 |
| Ala126 [O] | 2.79 | 3.71 | 102.23 | 107.74 | O3 |
| Ala126 [O] | 4.02 | 2.72 | 144.92 | 114.21 | O3 |
| Ala129 [N] | 3.01 | 3.23 | 158.53 | 77.42 | O1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, K.H. Crystal Structure of Human Lysozyme Complexed with N-Acetyl-α-d-glucosamine. Appl. Sci. 2022, 12, 4363. https://doi.org/10.3390/app12094363
Nam KH. Crystal Structure of Human Lysozyme Complexed with N-Acetyl-α-d-glucosamine. Applied Sciences. 2022; 12(9):4363. https://doi.org/10.3390/app12094363
Chicago/Turabian StyleNam, Ki Hyun. 2022. "Crystal Structure of Human Lysozyme Complexed with N-Acetyl-α-d-glucosamine" Applied Sciences 12, no. 9: 4363. https://doi.org/10.3390/app12094363
APA StyleNam, K. H. (2022). Crystal Structure of Human Lysozyme Complexed with N-Acetyl-α-d-glucosamine. Applied Sciences, 12(9), 4363. https://doi.org/10.3390/app12094363






