Study of Elevation Forces and Resilience of the Schneiderian Membrane Using a New Balloon Device in Maxillary Sinus Elevations on Pig Head Cadavers
Abstract
1. Introduction
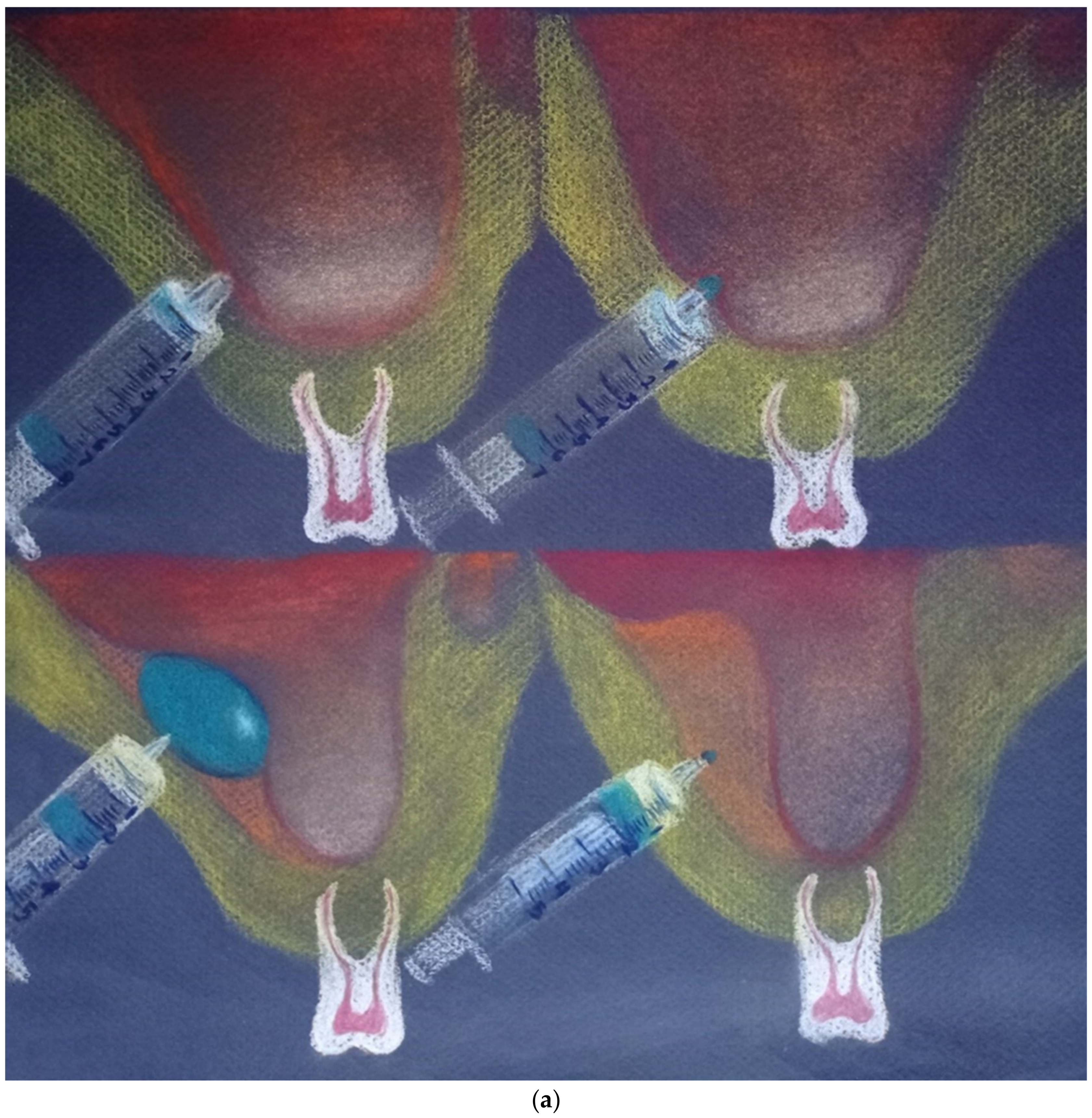
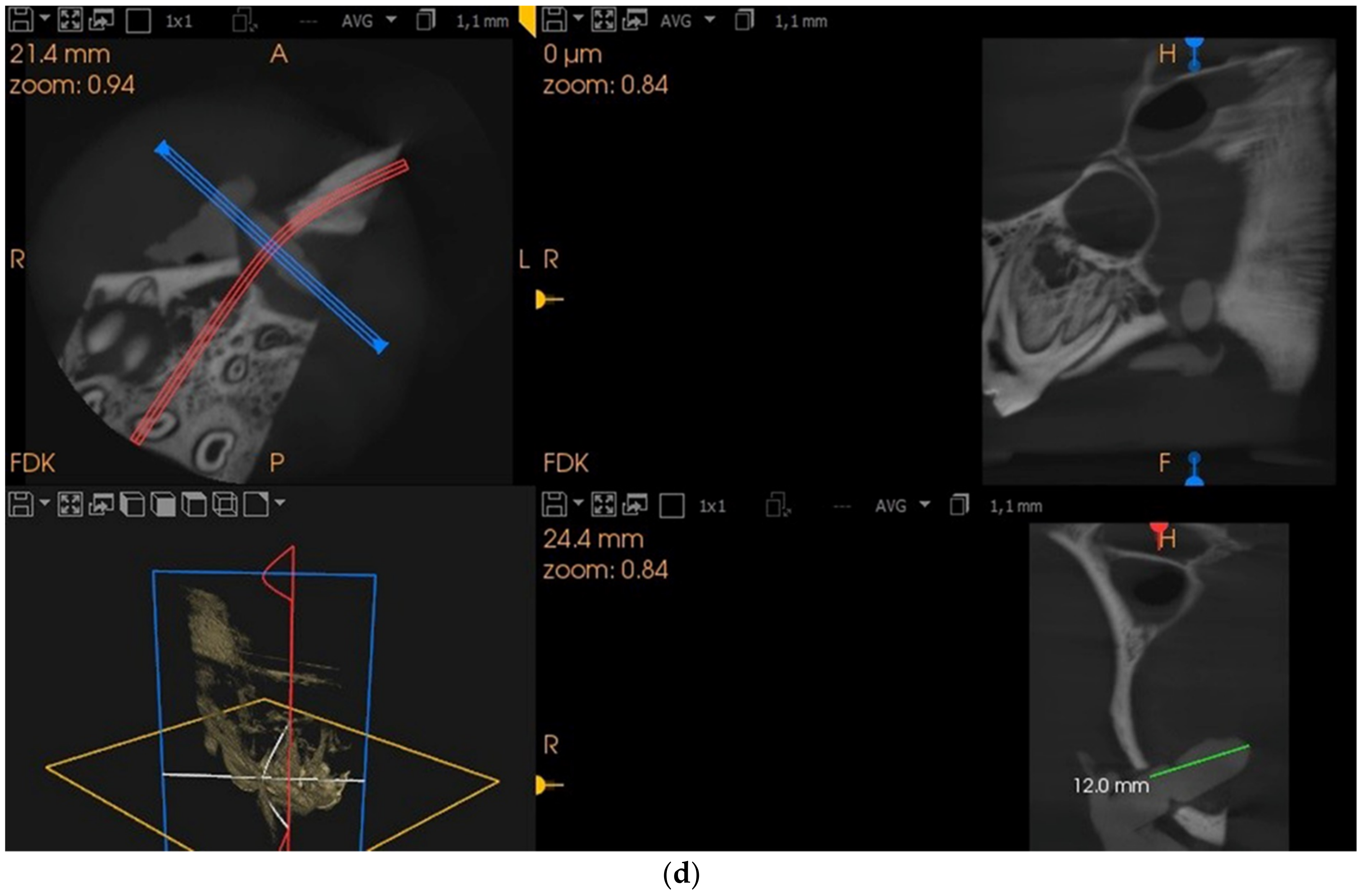
2. Materials and Methods
2.1. Measurement of Balloon Pressure and Resistance
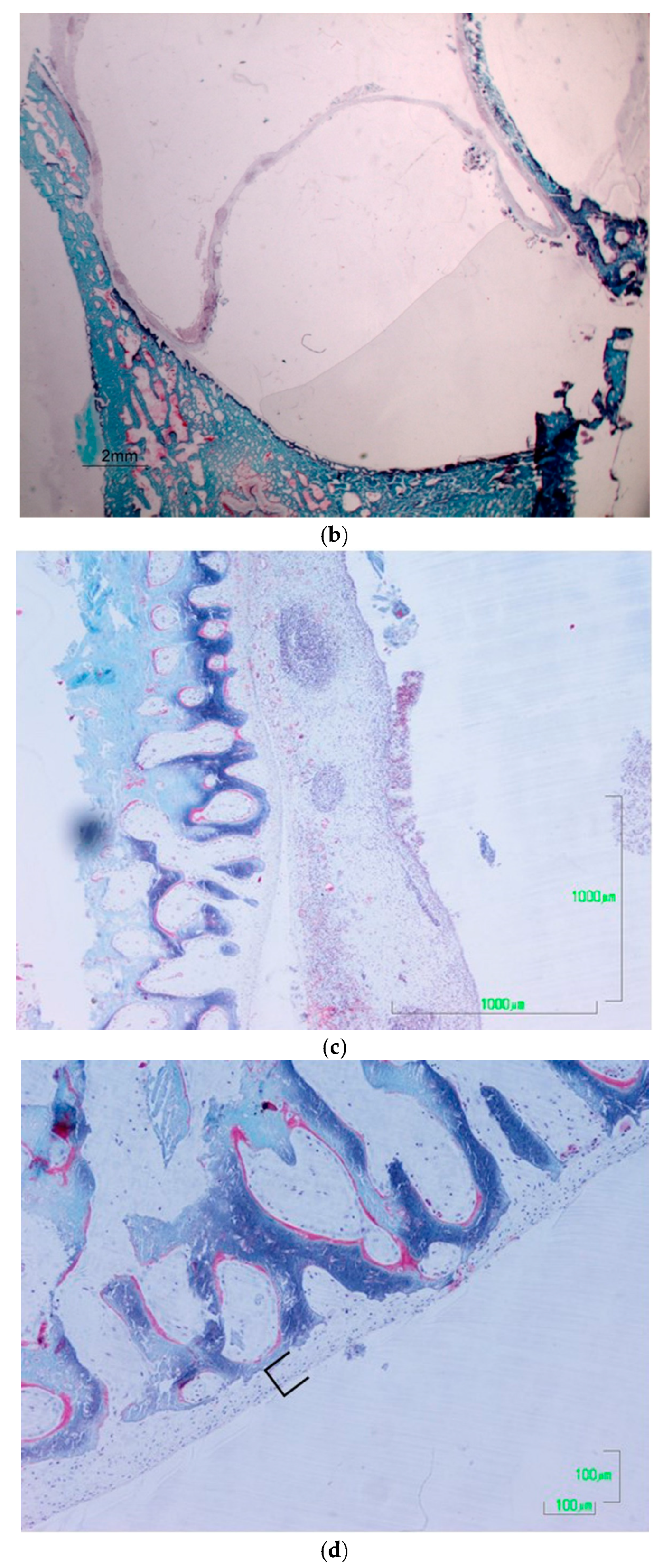
2.2. Histological Processing: Non-Decalcified Bone Samples Embedded in Plastic
3. Results
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jung, R.E.; Fenner, N.; Hämmerle, C.H.; Zitzmann, N.U. Long-term outcome of implants placed with guided bone regeneration (GBR) using resorbable and non-resorbable membranes after 12–14 years. Clin. Oral Implants Res. 2013, 24, 1065–1073. [Google Scholar] [CrossRef] [PubMed]
- Sagheb, K.; Schiegnitz, E.; Moergel, M.; Walter, C.; Al-Nawas, B.; Wagner, W. Clinical outcome of alveolar ridge augmentation with individualized CAD-CAM-produced titanium mesh. Int. J. Implant Dent. 2017, 3, 36. [Google Scholar] [CrossRef] [PubMed]
- Zita Gomes, R.; Paraud Freixas, A.; Han, C.H.; Bechara, S.; Tawil, I. Alveolar Ridge Reconstruction with Titanium Meshes and Simultaneous Implant Placement: A Retrospective, Multicenter Clinical Study. BioMed Res. Int. 2016, 2016, 5126838. [Google Scholar] [CrossRef]
- Pommer, B.; Frantal, S.; Willer, J.; Posch, M.; Watzek, G.; Tepper, G. Impact of dental implant length on early failure rates: A meta-analysis of observational studies. J. Clin. Periodontol. 2011, 38, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Winkler, S.; Morris, H.F.; Ochi, S. Implant Survival to 36 Months as Related to Length and Diameter. Ann. Periodontol. 2000, 5, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Mangano, F.G.; Macchi, A.; Luongo, G.; Mangano, C. Custom-Made Synthetic Scaffolds for Bone Reconstruction: A Retrospective, Multicenter Clinical Study on 15 Patients. BioMed Res. Int. 2016, 2016, 5862586. [Google Scholar] [CrossRef] [PubMed]
- De Avila, E.D.; Scarso Filho, J.; de Oliveira Ramalho, L.T.; Gabrielli, M.F.R.; Pereira Filho, V.A. Alveolar ridge augmentation with the perforated and nonperforated bone grafts. J. Periodontal Implant Sci. 2014, 44, 33–38. [Google Scholar] [CrossRef]
- Gultekin, B.A.; Bedeloglu, E.; Kose, T.E.; Mijiritsky, E. Comparison of Bone Resorption Rates after Intraoral Block Bone and Guided Bone Regeneration Augmentation for the Reconstruction of Horizontally Deficient Maxillary Alveolar Ridges. BioMed Res. Int. 2016, 2016, 4987437. [Google Scholar] [CrossRef]
- Yang, J.-W.; Park, H.-J.; Yoo, K.-H.; Chung, K.; Jung, S.; Oh, H.-K.; Kim, H.-S.; Kook, M.-S. A comparison study between periosteum and resorbable collagen membrane on iliac block bone graft resorption in the rabbit calvarium. Head Face Med. 2014, 10, 15. [Google Scholar] [CrossRef][Green Version]
- Sakkas, A.; Ioannis, K.; Winter, K.; Schramm, A.; Wilde, F. Clinical results of autologous bone augmentation harvested from the mandibular ramus prior to implant placement. An analysis of 104 cases. GMS Interdiscip. Plast. Reconstr. Surg. DGPW 2016, 5, Doc21. [Google Scholar]
- Cucchi, A.; Vignudelli, E.; Napolitano, A.; Marchetti, C.; Corinaldesi, G. Evaluation of complication rates and vertical bone gain after guided bone regeneration with non-resorbable membranes versus titanium meshes and resorbable membranes. A randomized clinical trial. Clin. Implant Dent. Relat. Res. 2017, 19, 821–832. [Google Scholar] [CrossRef] [PubMed]
- Dal Polo, M.R.; Poli, P.P.; Rancitelli, D.; Beretta, M.; Maiorana, C. Alveolar ridge reconstruction with titanium meshes: A systematic review of the literature. Med. Oral Patol. Oral Y Cir. Bucal 2014, 19, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Jegham, H.; Masmoudi, R.; Ouertani, H.; Blouza, I.; Turki, S.; Khattech, M. Ridge augmentation with titanium mesh: A case report. J. Stomatol. Oral Maxillofac. Surg. 2017, 118, 181–186. [Google Scholar] [CrossRef] [PubMed]
- Poli, P.P.; Beretta, M.; Cicciù, M.; Maiorana, C. Alveolar Ridge Augmentation with Titanium Mesh. A Retrospective Clinical Study. Open Dent. J. 2014, 8, 148–158. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Ahn, K.-J.; Yun, P.-Y. A retrospective study on the prognosis of single implant placed at the sinus bone graft site. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 118, 181–186. [Google Scholar] [CrossRef]
- Chirilă, L.; Rotaru, C.; Filipov, I.; Săndulescu, M. Management of acute maxillary sinusitis after sinus bone grafting procedures with simultaneous dental implants placement—A retrospective study. BMC Infect. Dis. 2016, 16, 94. [Google Scholar] [CrossRef]
- Kayabasoglu, G.; Nacar, A.; Altundag, A.; Cayonu, M.; Muhtarogullari, M.; Cingi, C. A retrospective analysis of the relationship between rhinosinusitis and sinus lift dental implantation. Head Face Med. 2014, 10, 53. [Google Scholar] [CrossRef]
- Kim, Y.-K.; Hwang, J.-Y.; Yun, P.-Y. Relationship Between Prognosis of Dental Implants and Maxillary Sinusitis Associated with the Sinus Elevation Procedure. Int. J. Oral Maxillofac. Implants 2013, 28, 178–183. [Google Scholar] [CrossRef]
- Park, J.S.; Kim, B.C.; Choi, B.; Lee, J. ORAL SURGERY Facial skin fistula as a postoperative complication related to maxillary sinus grafting: A case report. Quintessence Int. 2015, 46, 145–149. [Google Scholar]
- Nooh, N. Effect of schneiderian membrane perforation on posterior maxillary implant survival. J. Int. Oral Health 2013, 5, 28–34. [Google Scholar]
- Nam, K.Y.; Kim, J.B. Treatment of dental implant-related maxillary sinusitis with functional endoscopic sinus surgery in combinationmwith an intra-oral approach. J. Korean Assoc. Oral Maxillofac. Surg. 2014, 40, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Alkan, A.; Çelebi, N.; Baş, B. Acute Maxillary Sinusitis Associated with Internal Sinus Lifting: Report of a Case. Eur. J. Dent. 2008, 02, 69–72. [Google Scholar] [CrossRef]
- Emmerich, D.; Att, W.; Stappert, C. Sinus floor elevation using os- teotomes: A systematic review and meta-analysis. J. Periodontol. 2005, 76, 1237–1251. [Google Scholar] [CrossRef]
- Le Gall, M.G. Localized sinus elevation and osteocompression with single-stage tapered dental implants: Technical note. Int. J. Oral Maxillofac. Implants 2004, 19, 431–437. [Google Scholar] [PubMed]
- Ho, S.C.; Wallace, S.S.; Froum, S.J.; Tarnow, D. Influence of anatomy on Schneiderian membrane perforations during sinus elevation surgery: Three-dimensional analysis. Pract. Proced. Aesthetic Dent. 2001, 13, 160–163. [Google Scholar]
- Shlomi, B.; Horowitz, I.; Kahn, A.; Dobriyan, A.; Chaushu, G. The effect of sinus membrane perforation and repair with Lambone on the outcome of maxillary sinus floor augmentation: A radiographic assessment. Int. J. Oral Maxillofac. Implants 2004, 19, 559–562. [Google Scholar]
- Berengo, M.; Sivolella, S.; Majzoub, Z.; Cordioli, G. Endoscopic evaluation of the bone-added osteotome sinus floor elevation procedure. Int. J. Oral Maxillofac. Surg. 2004, 33, 189–194. [Google Scholar] [CrossRef]
- Reiser, G.M.; Rabinovitz, Z.; Bruno, J.; Damoulis, P.D.; Griffin, T.J. Evaluation of maxillary sinus membrane response following elevation with the crestal osteotome technique in human cadavers. Int. J. Oral Maxillofac. Implants 2002, 16, 833–840. [Google Scholar]
- Nkenke, E.; Schlegel, A.; Schultze-Mosgau, S.; Neukam, F.W.; Wiltfang, J. The endoscopically controlled osteotome sinus floor elevation: A preliminary prospective study. Int. J. Oral Maxillofac. Implants 2002, 17, 557–566. [Google Scholar]
- Ardekian, L.; Oved-Peleg, E.; Mactei, E.E.; Peled, M. The clinical signifi-cance of sinus membrane perforation during augmentation of the maxillary sinus. J. Oral Maxillofac. Surg. 2006, 64, 277–282. [Google Scholar] [CrossRef]
- Stelzle, F.; Rohde, M. Elevation forces and resilience of the sinus membrane during sinus floor elevation: Preliminary measurements using a balloon method on ex vivo pig heads. Int. J. Oral Maxillofac. Implants 2014, 29, 550–557. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fenner, M.; Vairaktaris, E.; Fischer, K.; Schlegel, K.A.; Neukam, F.W.; Nkenke, E. Influence of residual alveolar bone height on osseointegration of implants in the maxilla: A pilot study. Clin. Oral Implants Res. 2009, 20, 555–559. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, K.A.; Zimmermann, R.; Thorwarth, M.; Neukam, F.-W.; Klongnoi, B.; Nkenke, E.; Felszeghy, E. Sinus floor elevation using autogenous bone or bone substitute combined with platelet-rich plasma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007, 104, e15–e25. [Google Scholar] [CrossRef] [PubMed]
- Mueller, C.K.; Solcher, P.; Peisker, A.; Mtsariashvilli, M.; Schlegel, K.A.; Hildebrand, G.; Rost, J.; Liefeith, K.; Chen, J.; Schultze-Mosgau, S. Analysis of the influence of the macro- and microstructure of dental zirconium implants on osseointegration: A minipig study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2013, 116, e1–e8. [Google Scholar] [CrossRef] [PubMed]
- Stelzle, F.; Benner, K.-U. An animal model for sinus floor elevation with great elevation heights. Macroscopic, microscopic, radiological and micro-CT analysis: Ex vivo. Clin. Oral Implants Res. 2010, 21, 1370–1378. [Google Scholar] [CrossRef] [PubMed]
- Aimetti, M.; Massei, G.; Morra, M.; Cardesi, E.; Romano, F. Correlation between gingival phenotype and Schneiderian membrane thickness. Int. J. Oral Maxillofac. Implants 2009, 23, 1128–1132. [Google Scholar]
- Stelzle, F.; Benner, K.-U. Evaluation of Different Methods of Indirect Sinus Floor Elevation for Elevation Heights of 10 mm: An Experimental Ex Vivo Study. Clin. Implant Dent. Relat. Res. 2011, 13, 124–133. [Google Scholar] [CrossRef]
- Bornstein, M.M.; Wasmer, J.; Sendi, P.; Janner, S.F.; Buser, D.; Von Arx, T. Characteristics and dimensions of the Schneiderian membrane and apical bone in maxillary molars referred for apical surgery: A comparative radiographic analysis using limited cone beam computed tomography. J. Endod. 2012, 38, 51–57. [Google Scholar] [CrossRef]
- Janner, S.F.M.; Caversaccio, M.D.; Dubach, P.; Sendi, P.; Buser, D.; Bornstein, M.M. Characteristics and dimensions of the Schneiderian membrane: A radiographic analysis using cone beam computed tomography in patients referred for dental implant surgery in the posterior maxilla. Clin. Oral Implants Res. 2011, 22, 1446–1453. [Google Scholar] [CrossRef]





| Sinus | Sinus Wall Thickness (mm) | Elevation Height Measured with CBCT (mm) | Membrane Perforated during Aperture | Membrane Perforated during Elevation | Elevation of All Layers. Histological Analysis |
|---|---|---|---|---|---|
| 1 | 1 | 10.6 | No | No | Yes |
| 2 | 1.1 | 11 | No | No | No |
| 3 | 1 | 10.1 | No | No | Yes |
| 4 | 0.9 | 10.7 | No | No | Yes |
| 5 | 1.8 | 10.1 | No | No | Yes |
| 6 | 0.9 | 12.5 | No | No | No |
| 7 | 2.2 | 9.7 | No | No | Yes |
| 8 | 2.4 | 8 | No | No | Yes |
| 9 | 1.2 | 13.3 | No | No | Yes |
| 10 | 1.8 | 9.8 | No | No | No |
| 11 | 1.1 | 10.1 | No | No | No |
| 12 | 1 | 11.8 | No | No | Yes |
| 13 | 1.4 | 10.2 | No | No | Yes |
| 14 | 1.7 | 12 | No | No | Yes |
| 15 | 2.3 | 9.6 | No | No | Yes |
| Sinus Floor Thickness (mm) | Elevation Height Measured with CBCT (mm) | |||
|---|---|---|---|---|
| Men | 1.45 | 10.63 | ||
| SD | 0.53 | 1.32 | ||
| Median | 1.20 | 10.20 | ||
| Minimum value | 0.90 | 8.00 | ||
| Maximum value | 2.40 | 13.30 | ||
| Groups | Thickness < 1.45 mm | Thickness > 1.45 mm | Height < 10.63 mm | Height > 10.63 mm |
| Mean | 1.06 | 2.03 | 9.80 | 11.88 |
| SD | 0.15 | 0.30 | 0.73 | 0.95 |
| Median | 1.00 | 2.00 | 10.10 | 11.90 |
| Minimum value | 0.90 | 1.70 | 8.00 | 10.70 |
| Maximum value | 1.40 | 2.40 | 10.60 | 13.30 |
| p-value T-student | 0.000002 | 0.0014 | ||
| Global Sample | |||||||
|---|---|---|---|---|---|---|---|
| Variables | n | % | p-Value | ||||
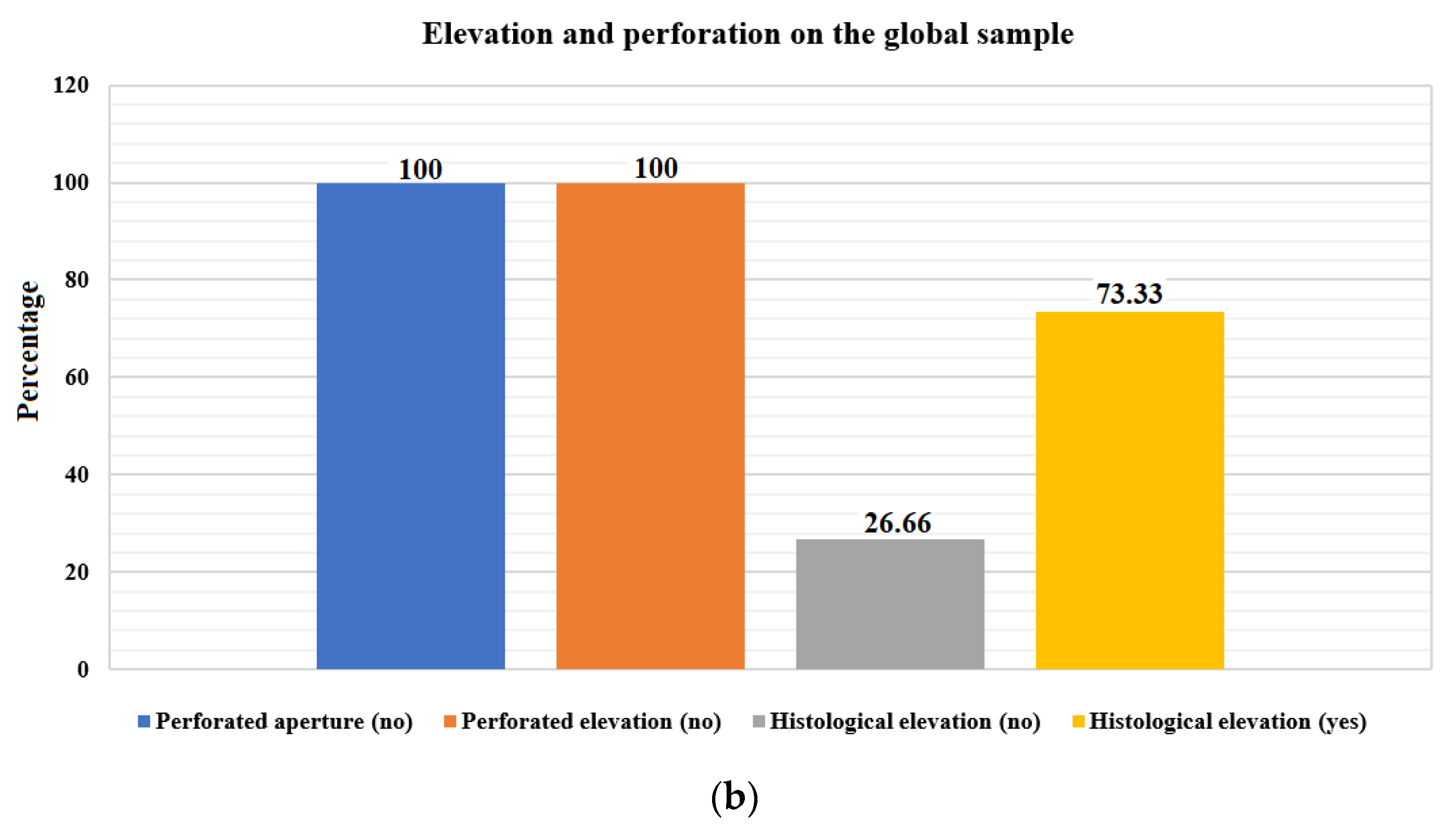
| Membrane perforated during aperture (no) | 15 | 100.00 | 1.000 | ||||
| Membrane perforated during elevation (no) | 15 | 100.00 | |||||
| Elevation of all layers. Histological analysis (no) | 4 | 26.66 | 0.0268 | ||||
| Elevation of all layers. Histological analysis (yes) | 11 | 73.33 | |||||
| Subgroups | Height < 10.63 mm | Height > 10.63 mm | Total | Fisher’s Exact Test | |||
| Variables | n | % | n | % | % | n | p-Value |
| Membrane perforated during aperture (no) | 9 | 60.00 | 6 | 40.00 | 15 | 100.00 | 0.4661 |
| Membrane perforated during elevation (no) | 9 | 60.00 | 6 | 40.00 | 15 | 100.00 | 0.4661 |
| Elevation of all layers. Histological analysis (no) | 2 | 13.33 | 13 | 86.67 | 15 | 100.00 | 0.0001 |
| Elevation of all layers. Histological analysis (yes) | 7 | 46.67 | 8 | 53.33 | 15 | 100.00 | 1.0000 |
| Max | Septum | Pressure on Membrane | |
|---|---|---|---|
| SINUS 1 | 598.8 | NO | 280.7 |
| SINUS 2 | 620.2 | NO | 302.1 |
| SINUS 3 | 680.2 | SI | 362.1 |
| SINUS 4 | 650.7 | NO | 332.6 |
| SINUS 5 | 601.9 | NO | 283.8 |
| SINUS 6 | 630.8 | NO | 312.7 |
| SINUS 7 | 654.9 | SI | 335.8 |
| SINUS 8 | 635.4 | NO | 317.3 |
| SINUS 9 | 726.2 | SI | 408.1 |
| SISUS 10 | 659.7 | NO | 341.6 |
| SINUS11 | 704.4 | NO | 386.3 |
| SINUS 12 | 658.2 | SI | 340.1 |
| SINUS 13 | 706.8 | NO | 388.7 |
| SINUS 14 | 698.6 | NO | 380.5 |
| SINUS 15 | 712.6 | SI | 394.5 |
| Maximum Pressure mmHg | Pressure on Membrane | |||
|---|---|---|---|---|
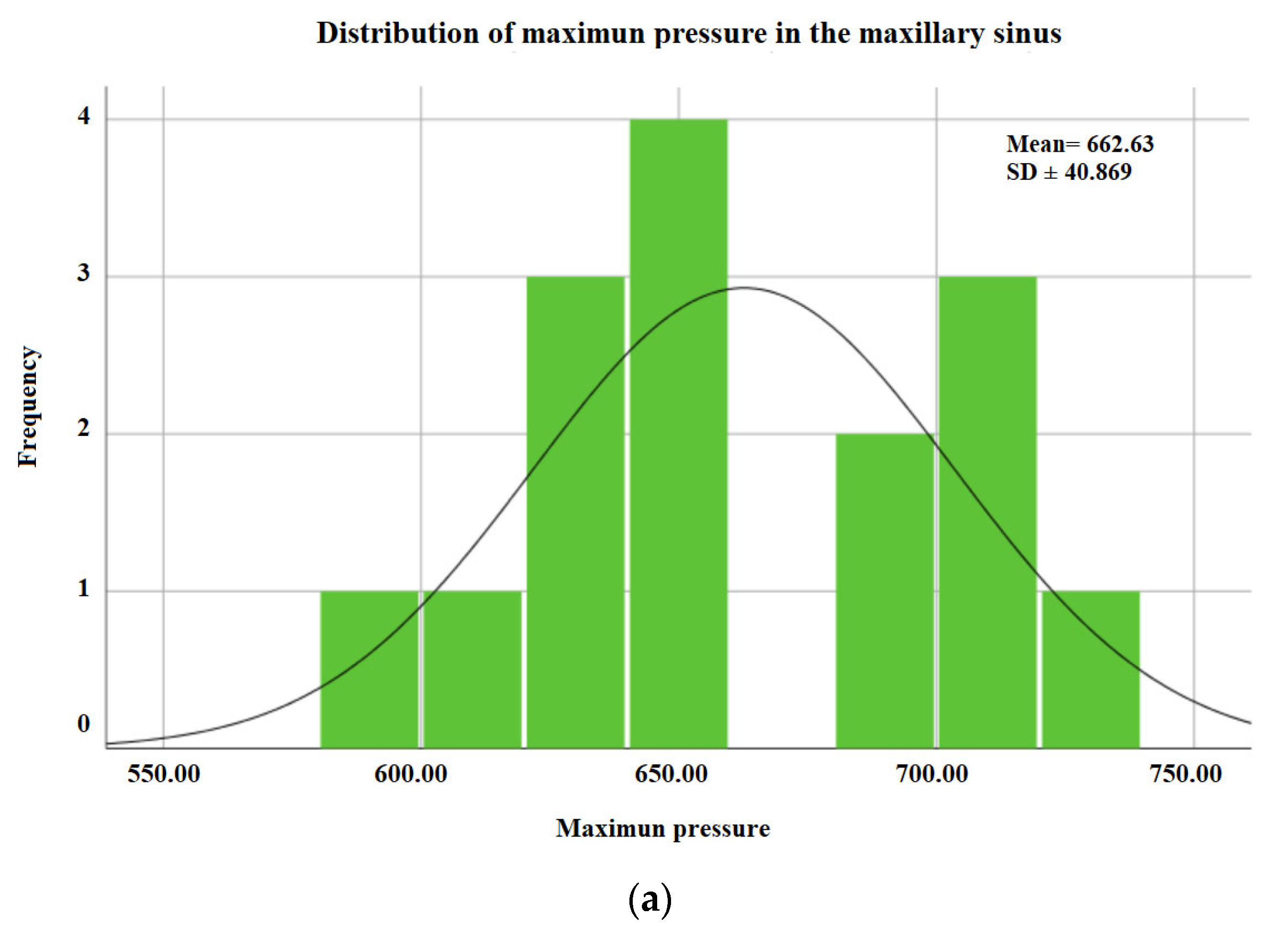
| Mean | 662.62 | 344.46 | ||
| SD | 40.86 | 40.88 | ||
| Median | 658.20 | 340.10 | ||
| Minimum value | 598.80 | 280.70 | ||
| Maximum value | 726.20 | 408.10 | ||
| Groups | Without septum | With septum | Without septum | With septum |
| Media | 650.73 | 686.42 | 332.63 | 368.12 |
| SD | 50.88 | 32.00 | 40.88 | 32.24 |
| Median | 643.050 | 680.20 | 324.95 | 362.10 |
| Minimum value | 598.80 | 654.90 | 280.70 | 335.80 |
| Maximum value | 706.80 | 726.20 | 388.70 | 408.10 |
| p-value | 0.093 | 0.096 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernández Castellano, E.R.; Marquez Sanchez, M.T.; Flores Fraile, J. Study of Elevation Forces and Resilience of the Schneiderian Membrane Using a New Balloon Device in Maxillary Sinus Elevations on Pig Head Cadavers. Appl. Sci. 2022, 12, 4406. https://doi.org/10.3390/app12094406
Fernández Castellano ER, Marquez Sanchez MT, Flores Fraile J. Study of Elevation Forces and Resilience of the Schneiderian Membrane Using a New Balloon Device in Maxillary Sinus Elevations on Pig Head Cadavers. Applied Sciences. 2022; 12(9):4406. https://doi.org/10.3390/app12094406
Chicago/Turabian StyleFernández Castellano, Erick Rafael, Magaly Teresa Marquez Sanchez, and Javier Flores Fraile. 2022. "Study of Elevation Forces and Resilience of the Schneiderian Membrane Using a New Balloon Device in Maxillary Sinus Elevations on Pig Head Cadavers" Applied Sciences 12, no. 9: 4406. https://doi.org/10.3390/app12094406
APA StyleFernández Castellano, E. R., Marquez Sanchez, M. T., & Flores Fraile, J. (2022). Study of Elevation Forces and Resilience of the Schneiderian Membrane Using a New Balloon Device in Maxillary Sinus Elevations on Pig Head Cadavers. Applied Sciences, 12(9), 4406. https://doi.org/10.3390/app12094406







