Abstract
Momordica charantia (M. charantia) is rich in flavonoids, which possess a strong antioxidant capacity and may help prevent hair loss. This study aims to develop the microemulsion of M. charantia with antioxidant activity and 5α-reductase (5aR) inhibitory activity. The total phenolic content (TPC), antioxidant activity, and 5aR inhibitory activity of ethanolic and aqueous extracts of the fruit were investigated. The preparation of M. charantia extract-loaded microemulsion (MELM) was optimized and characterized the MELM. The aqueous extract of M. charantia fruit flesh displayed a TPC of 780.75 ± 24.82 mg Gallic acid equivalence/g of extract. ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), and nitric oxide (NO) radical scavenging activities were observed in all the extracts. About 0.461 ± 0.003 mg finasteride equivalence/g of extract of 5aR inhibitory activity was detected in the aqueous extract of the inner tissue of M. charantia fruit. Based on NO radical scavenging and 5aR inhibitory activity, an aqueous extract of the inner tissue (pericarp with seed) of M. charantia fruit was used to prepare the MELM. The MELM was prepared using a different ratio of tween 80 and ethanol as Smix. The results showed that the 1:1 ratio of tween 80: ethanol produced microemulsion of an optimum size, zeta potential, and polydispersity index. The MELM samples were stored at 5, 30, and 40 °C for 12 weeks, and the stability was assessed. The results revealed that the size, zeta potential, and polydispersity index of the formulated MELM remained unchanged during the investigated time. This study primarily reports the 5aR inhibitory activity of M. charantia extract and the development of microemulsion. The prepared MELM could be further developed into cosmetic or pharmacological preparations to manage hair loss.
1. Introduction
Alopecia (hair loss) is a slow transformation of the terminal hair into vellus hair. Many factors can induce alopecia, including pollution, stress, hormones, and drug side effects. There are many types of alopecia; the most common is androgenetic alopecia (AGA), a condition caused by the altered expression of the androgen-related system [1]. The pathology of AGA is associated with the activity of the 5α-reductase (5aR) enzyme in hair follicles and the sebaceous gland. 5aR converts testosterone into dihydrotestosterone (DHT), which is a more potent androgen than testosterone. A high level of DHT can induce many effects, such as acne, hair loss, and enlargement of the prostate [2].
AGA is likely to occur in males and females but is most often found in males. It can be treated with 5aR inhibitors such as finasteride and dutasteride [3]. However, these medicines may cause unacceptable adverse effects such as sexual dysfunction and reduced sexual confidence. The topical minoxidil lotion is another medicine used to treat AGA. It also has side effects such as irritation, itching, rash, and dry skin.
There are two isoforms of 5aR, types 1 and 2 (5aR1 and 5aR2). Several phytochemicals were reported with 5aR inhibitory activity. These include catechins and epicatechin gallate. Flavonoids were reported to inhibit 5aR1 enzymes such as myricetin, quercetin, baicalein, and fisetin. Biochanin A, kaempferol, genistein, and daidzein can also inhibit 5aR activity [4].
Antioxidants such as polyphenols are reported for hair loss prevention and hair growth-promoting activities [5]. Transforming growth factor (TGF)-β1 is a catagen phase inducer released by ROS-inducible and inhibits epithelial cell growth that causing shortening of the human hair cycle [6]. Reactive oxygen species (ROS) are known as radical oxygen, an unstable molecule that can easily react with other molecules in the cell. The reaction of ROS when building up in cells is the cause of damaged DNA, RNA, and proteins, through cell death. ROS also causes hair loss via a reaction with cellular protein in hair. From previous studies, the relationship between ROS and TGF-β1 in epithelial cells has been reported, in which ROS can induce TGF-β. The report showed that antioxidants have the potential to be used as a treatment. M. charantia (known as bitter melon or bitter gourd) belongs to Cucurbitaceae, widely found in Asia, Africa, and the Caribbean [7]. M. charantia is rich in flavonoids that possess a strong antioxidant capacity. Since oxidative stress is related to hair loss, the application of the MELM may prevent the miniaturization of hair. It also contains vitamins, minerals, and fatty acids and has been used in the traditional medicine of Asia and Africa [8]. Bitter melon is a good source of phenolic compounds, including gallic acid, genistic acid, catechin, epicatechin, caffeic acid, chlorogenic acid, p-coumaric acid, and ferulic acid [9]. Bitter melon also has saponins, peptides, and alkaloids. These bioactive compounds potentially impart a wide range of health benefits [10].
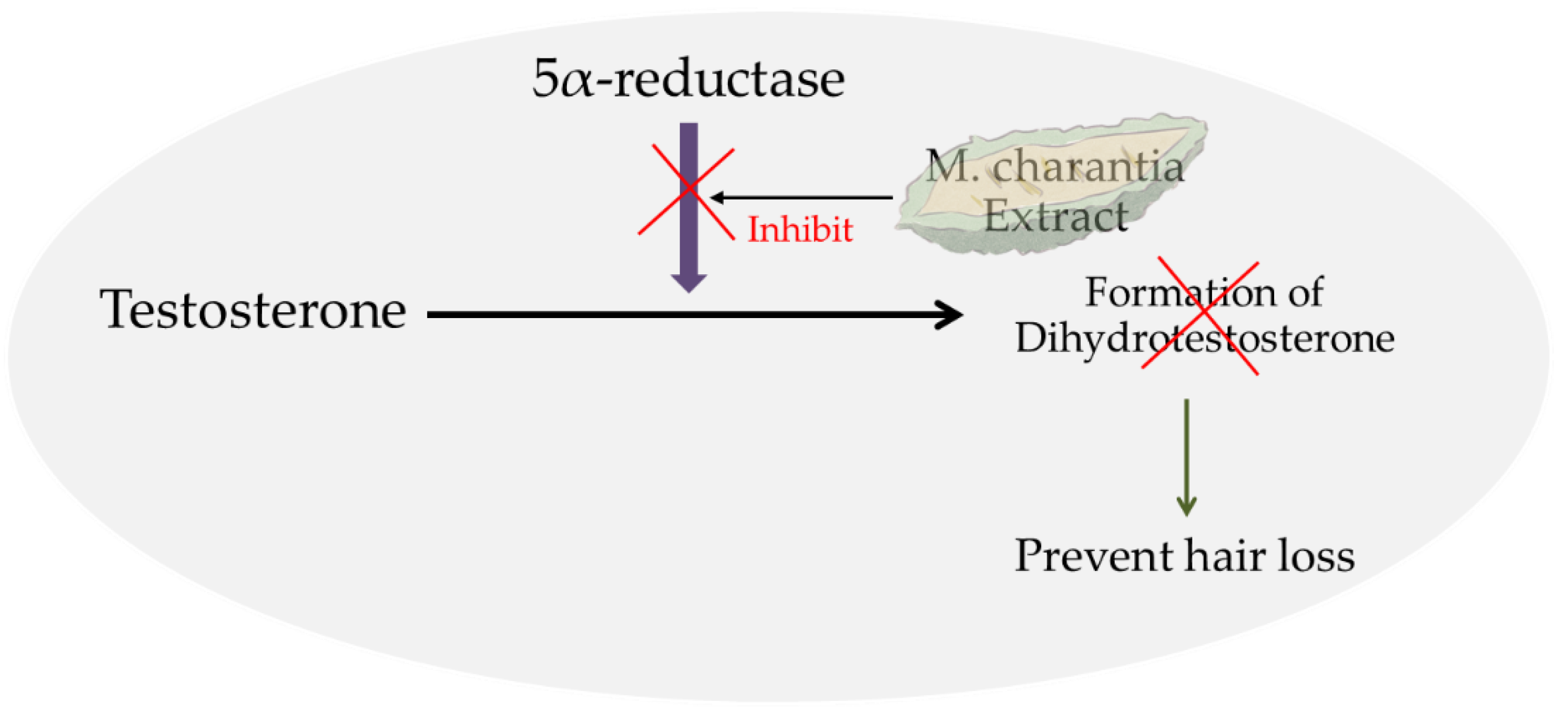
The inhibition of 5aR prevents the conversion of testosterone into DHT, which is a key player involved in AGA, thereby possibly preventing hair loss (Figure 1).
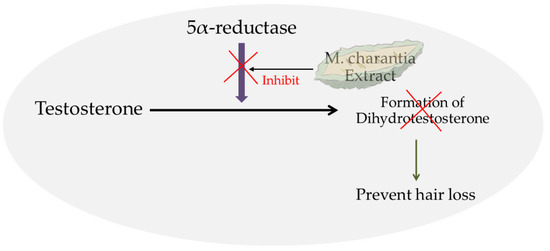
Figure 1.
The schematic representation of the hair-loss prevention properties of M. charantia.
The phenolic compounds can act as 5aR inhibitors, and the overactivity of 5aR is directly related to AGA. This study aimed to evaluate the total phenolic content (TPC), antioxidant activity, and 5aR inhibitory activity of M. charantia extract and formulate M. charantia extract into microemulsion to enhance the delivery of active substances into the hair follicle for hair-loss prevention. Physical properties, stability, and permeation profile of the developed M. charantia extract-loaded microemulsion (MELM) were studied.
2. Materials and Methods
2.1. Chemicals
ABTS (2,2′-azino-bis(3-ethylbenzthiazoline-6-sulphonic acid), DPPH (2,2-diphenyl-1-picrylhydrazyl), trolox (6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid), dithiothreitol, finasteride, gallic acid, N-(1-Naphthyl) ethylenediamine, NADPH, propyl p-hydroxybenzoate, sodium nitroprusside, sucrose, sulfanilamide, and testosterone were purchased from Sigma-Aldrich chemicals. Ethyl alcohol, capric/caprylic triglyceride, polysorbate 20, polysorbate 80, phenoxyethanol, sorbitan laurate, and sorbitan oleate were purchased from Namsiang International Co., Ltd., Bangkok 10250, Thailand. Dibasic sodium phosphate, methyl alcohol, monobasic sodium phosphate, and sodium carbonate were purchased from RCI Labscan, Bangkok 10330, Thailand.
2.2. Animals
Male Sprague–Dawley rats (6 weeks old, 200–220 g) were purchased from Siam Nomura, Bangkok, Thailand. The animals were maintained and fed with a standard laboratory diet and water ad libitum, housed with a 12:12 h light and dark cycle for 7 days before the experiment. The room temperature and humidity were controlled at 23 ± 2 °C and ~60%, respectively. The study protocol was approved by the Ethics Committee of Laboratory Animal Center, Chiang Mai University, Chiang Mai, Thailand (AR07 2564/RT-0007 5-reductase).
2.3. Extraction
M. charantia was purchased from Mueang Mai markets, Chiang Mai. Bitter melons were split into flesh and inner tissue (pericarp with seed). Then, they were cut into small pieces and dried at 45 °C in a hot air oven [11]. The dried samples were grounded into powder and sieved to obtain fine particles. Macerations were prepared at a ratio of 1:10 (powder:solvent), and 20–100% of ethanol or water was served as solvent [12]. The suspensions were stirred two times per day during maceration. After three days, the bitter melon powder was separated from the solvent. The supernatant was concentrated using a rotary evaporator for alcoholic extraction or a freeze dryer for water extraction.
2.4. Determination of Total Phenolic Contents
The samples’ total phenolic content (TPC) was assessed by the Folin–Ciocalteu colorimetric method [13]. Briefly, 0.2 mL of diluted plant extracts were added to 1.0 mL of 0.2 N Folin–Ciocalteu phenol reagent in a test tube and kept for 5 min. After that, 3.0 mL of 7.5% sodium carbonate solution was added. Reactions were kept in the dark for 2 h and then read for UV absorbance at 750 nm. Gallic acid was used as a standard. The TPC of each sample was expressed as mg gallic acid equivalent (GAE) per 1 g extract.
2.5. Determination of DPPH Free Radical Scavenging Activity
DPPH free radical scavenging assay was performed [11]. Briefly, 0.4 mM DPPH solution (750 µL) was mixed with 150 µL of the samples. The mixtures were vortexed and incubated at 25 °C for 30 min in the dark. Then, the absorbance of the mixture was measured at 517 nm, and the DPPH radical scavenging activity was calculated as follows:
DPPH radical scavenging activity (%) = (1 − absorbance of the sample) × 100/absorbance of the control
The results were expressed as mg Trolox equivalent of antioxidant capacity (TEAC)/g of extract.
2.6. Determination of ABTS Free Radical Scavenging Activity
ABTS radical solution was prepared as described previously with slight modifications [11], by mixing 7 mM ABTS with 2.45 mM of potassium persulfate at a ratio of 2:1 and storing it at room temperature in the dark for 6 h. Then, the ABTS solution was diluted to a final absorbance of 0.7 ± 0.01 at 734 nm in 0.1 M potassium phosphate buffer (pH 7.4). For the measurement, 50 µL of the sample and 950 µL of the ABTS solution were mixed and incubated for 15 min at room temperature. After incubation, the solution was measured at 734 nm, and the ABTS radical scavenging activity was calculated as follows:
ABTS radical scavenging activity (%) = (1 − absorbance of the sample) × 100/absorbance of the control
The results were expressed as mg TEAC/g of extract.
2.7. Determination of NO Free Radical Scavenging Activity
Griess reagent was prepared as detailed previously [13]. Briefly, 1% w/v naphthyl ethylenediamine dihydrochloride (dissolved with deionized water) and 1% w/v sulfanilamide (dissolved with phosphoric acid and water) were mixed at a ratio of 1:1. Then, 200 µL of the test sample was mixed with 800 µL of sodium nitroprusside in phosphate buffer pH 7.4 and incubated for 150 min at 37 °C. After incubation, 100 µL of the sample solution was mixed with 100 µL of Griess reagent and incubated at room temperature for 5 min. Then, the absorbance was measured at 540 nm in the dark.
2.8. 5α-Reductase Inhibitory Activity
Rats were sacrificed by injecting Zoletil® 100 (tiletamine HCl and zolazepam HCl with 200 mg/kg body weight) intravenously, and the liver was collected. The liver was rinsed with saline and minced into small pieces in 0.32 M sucrose and 1 mM dithiothreitol. The homogenous solution was centrifuged at 9000× g at 0 °C for 30 min. The supernatant acts as an enzyme source. The inhibition of 5-reductase was determined as previously described [11]. Briefly, the reaction mixture was prepared with 0.2 mL of finasteride or samples, 1.0 mL of phosphate buffer (0.02 M with pH 6.5), 0.3 mL of testosterone (0.5 mg/mL dissolved in methanol), and 0.7 mL of rat microsomal suspension. The reaction was initiated by adding 0.5 mL of NADPH (0.77 mg/mL dissolved in phosphate buffer) and incubated at 37 °C for 30 min. After incubation, the reaction was stopped by adding 5.0 mL of dichloromethane, and 0.5 mL of propyl p-hydroxybenzoate (0.1 mg/mL dissolved in methanol) was added, which acts as an internal standard. Organic phases were collected and evaporated. The leftovers were redissolved in 5.0 mL of methanol. The sample was injected into the HPLC system using Shodex C18 columns (5 µm; 250 × 4.6 mm). Methanol and deionized water were mixed at a ratio of 65:35 and used as the mobile phase. The system was performed with a flow rate of 1.0 mL/min, and an ultraviolet detector at 242 nm was enabled. The results were represented as mg finasteride equivalents per g of extract (mg FE/g of extract) [11].
2.9. Development of a Microemulsion
To obtain a concentration range of components for the existing microemulsion boundary, pseudoternary phase diagrams were constructed using a water titration method [14]. Various nonionic surfactants (polysorbate 20, sorbitan laurate, polysorbate 80, and sorbitan oleate) were mixed at room temperature with ethanol (cosurfactant) at a ratio of 1:2, 1:1, or 2:1 to obtain a surfactant mixture (Smix). Then, caprylic/capric triglyceride and Smix were mixed at different ratios (0:1, 1:9, 2:8, 3:7, 4:6, 5:5, 6:4, 7:3, 8:2, 9:1, and 1:0), and titrated with the water (Table 1 and Table 2). The volume of water that produces solution turbidity was observed and recorded. The pseudoternary phase diagrams were drawn using STATA software, for which the grey zone is a microemulsion-forming area. A microemulsion consisting of 1 mg/mL aqueous solution of the M. charantia (MELM) was prepared based on the diagrams.

Table 1.
The components of each microemulsion, and their properties.

Table 2.
The ingredients of the formulation.
2.10. The Characterization of MELM
The photon correlation spectroscopy (Nano ZS Zetasizer 90, Worcestershire, UK) method was used to measure the particle size and the zeta potential [15]. The samples were diluted with deionized water at 1:300 (v/v) before measuring, to achieve suitable optical density. The polydispersity index (PDI) and the width of particle size were determined.
The electrophoretic light scattering technique was employed to measure the zeta potential. The scattered light intensity of each formulation was determined at a scattering angle of 173° and performed at 25 °C. All data were evaluated using Zetasizer version 7.02 software.
The viscosity and pH of the samples were measured by Rheometer (Brookfield) and pH meter (Starter3100, OHAUS), respectively, at room temperature, and the values were expressed as average ± standard deviation (SD).
2.11. Stability Study of MELM
The stability of the MELM was assessed at different storage conditions, including 5 ± 3 °C, 30 ± 2 °C, and 40 ± 2 °C, as recommended by the ICH Q1A (R2), the Center for Drug Evaluation and Research, the Food and Drug Administration. The characteristics of the MELM (TPC, zeta potential, PDI, viscosity, particle size, and pH) were determined after three months of storage. The TPC was used as an indicator of the stability of the MELM.
2.12. Determination of Skin Permeability of MELM
The skin permeability of the MELM was conducted as per Nitthikan et al. [16]. The skin permeability of each formulation was measured by the Franz-cell diffusion method (DHC-6TD, USA). The porcine ear skin (purchased from the local market, Chiang Mai) was cleaned with normal saline, and the hairs were removed. The upper skin was carefully cut off and soaked in phosphate buffer (pH 6.5) for 1 h before use. The skin was placed on top of the receiver chamber. Phosphate buffer (pH 5.5) was contained in the receiver chamber as a receiver medium. The amount of 500 µL (94 µg/mL extract) of the MELM samples was placed on the skin, and the receptor media were collected (1 mL) and replaced with the same volume of fresh media after 1, 2, 4, 6, 8, 12, and 24 h intervals. After 24 h, porcine ear skin was ground with a buffer (1 mg of porcine ear skin/1 mL of buffer). The suspensions were centrifuged, and supernatants were collected. Then, the TPC of the supernatants was measured as detailed previously. The total amount of added media was compared with the amount of release at the time point to calculate the % release.
2.13. Statistical Analysis
The values were reported as mean ± SD. All bioactivities were statistically analyzed using one-way analysis of variance (ANOVA) and Duncan’s multiple range test, with a 95% confident interval, using the IBM SPSS Statistics program (Chicago, IL, USA). The Pearson’s correlation coefficient was used to estimate the relationship between two parameters. The pseudoternary phase diagrams were created with the STATA version 15 software (StataCorp LLC, College Station, TX, USA).
3. Results
3.1. Total Phenolic Content of M. charantia Extracts
The TPC of the MELM was varied based on the solvent used for the extraction. The water extraction of flesh displayed 780.75 ± 24.82 mg GAE/g of the extract. The absolute ethanol extraction of flesh displayed 40.20 ± 5.63 mg GAE/g of the extract, whereas 20% ethanol extraction displayed 568.38 ± 88.06 mg GAE/g of the extract. Similarly, the 80% ethanol extract of the inner tissue displayed a TPC of 144.50 ± 6.83 mg GAE/g of extract, whereas water extraction of the inner tissue displayed 61.45 ± 8.22 mg GAE/g of extract (Table 3).

Table 3.
The total phenolic content (TPC), ABTS, DPPH, and nitric oxide radical scavenging activities of M. charantia extracts.
3.2. Antioxidant Activities of MELM
The flesh extracted with deionized water gave the highest DPPH radical scavenging activity of 153.36 ± 11.86 mg TEAC/g extract, whereas 40% ethanol extract of flesh displayed only 102.72 ± 7.04 mg TEAC/g extract of radical scavenging activity. On the other hand, the inner tissue extracted with absolute ethanol displayed the highest activity of 136.05 ± 8.19 mg TEAC/g extract, whereas 40% ethanol extract exhibited the lowest activity (78.34 ± 22.80 mg TEAC/g extract) (Table 3).
Flesh extracted with 60% ethanol and inner tissue extracted with 60% ethanol displayed the highest ABTS radical inhibition activity of 241.79 ± 2.71 and 244.39 ± 12.81 mg TEAC/g extract, respectively. Water extract of flesh and inner tissue displayed less ABTS radical inhibition activity of 76.31 ± 3.73 and 77.88 ± 3.15 mg TEAC/g extract, respectively (Table 1).
Flesh extracted with 20% ethanol and the water extract of the inner tissue exhibited the highest NO radical inhibition activity of 4235.20 ± 215.25, and 6201.75 ± 157.04 mg TEAC/g extract, respectively (Table 3).
3.3. 5α-Reductase Inhibitory Activity
The standard curve for 5aR inhibitory activity was prepared. The 5aR inhibitory activity of finasteride was 0.27 µM (IC50). According to the standard curve, the 5aR inhibitory activity of extracts was calculated. All the extracts displayed inhibition in the range from 0.349 ± 0.007 to 0.469 ± 0.001 mg finasteride equivalent (FE)/g extract. The flesh extracted with 40% ethanol and the water extract of the inner tissue displayed a maximum of 0.469 ± 0.001 and 0.461 ± 0.003 mg FE/g extract of the 5aR inhibition, respectively. At the same time, less inhibitory activity was observed in the flesh extracted with 20% ethanol (Table 4).

Table 4.
The 5a-reductase inhibitory activity of M. charantia extracts.
3.4. Development of Microemulsion
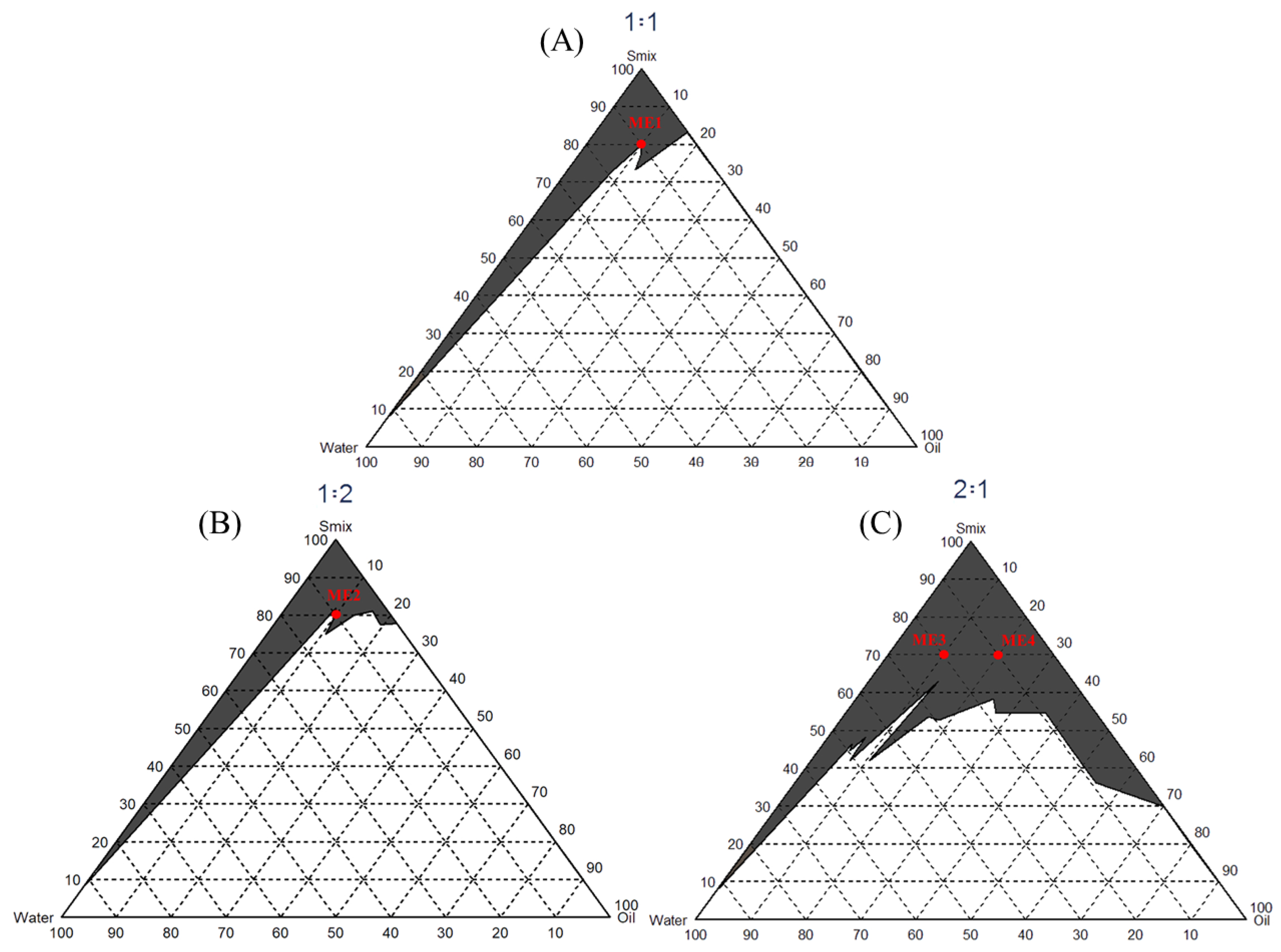
The microemulsion is composed of water, oil, and surfactant, with droplet sizes ranging from 10 to 100 nm, translucent or transparent. It is a thermodynamically stable system and can be formed spontaneously with no need for energy or heating. Oil and Smix are mixed at different ratios to observe the amount of water that causes the solution turbidity. Hydrophile–Lipophile Balance (HLB) is a numerical system used to define the relationship between nonionic surfactants’ water-soluble and oil-soluble parts. This study selected caprylic/capric triglyceride as oil (HLB = 13.8). Smix was prepared in the ratio of 2:1, 1:1, and 1:2. A pseudoternary phase diagram (Figure 2) was performed to find a region of microemulsion forming area. Different ratios of surfactant and ethanol were mixed with caprylic/capric triglyceride and titrated by water. The parts A, B, and C were mixed to form the microemulsion (Table 2).
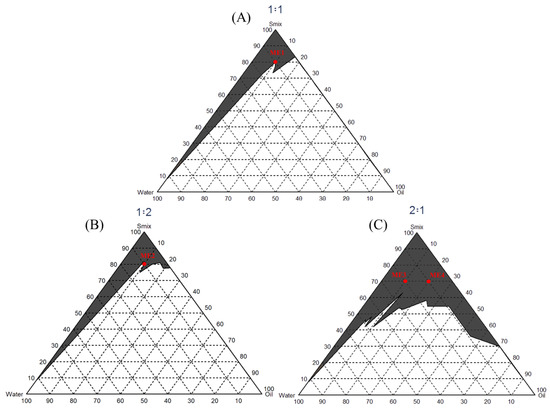
Figure 2.
The pseudoternary phase diagram of the microemulsion formation with different tween 80 and ethanol ratios. The black area indicates the microemulsion formation. Red dots indicate the selected formula, which produces the microemulsion with less surfactant. (A) a 1:1 ratio of tween 80: ethanol, (B) a 1:2 ratio of tween 80: ethanol, and (C) a 2:1 ratio of tween 80: ethanol.
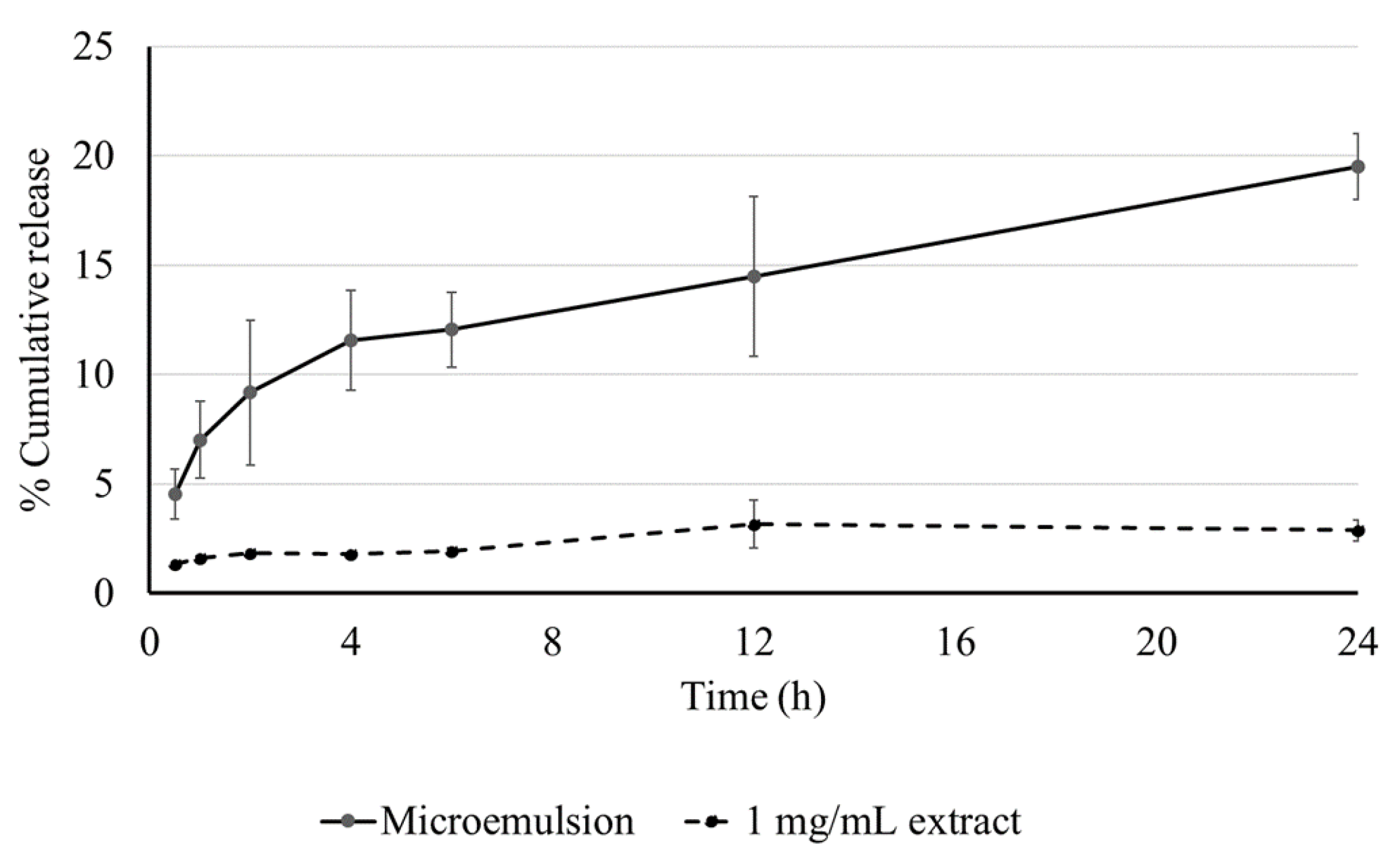
3.5. Skin Permeability of MELM
In vitro Franz cell diffusion study was used to determine the skin permeability of the MELM. The TPC content of reservoir solutions was collected at different periods. The accumulation of extract solution in the skin was only 2.88 ± 0.47%, while the MELM displayed 19.52 ± 1.52% released at 24 h (Figure 3).
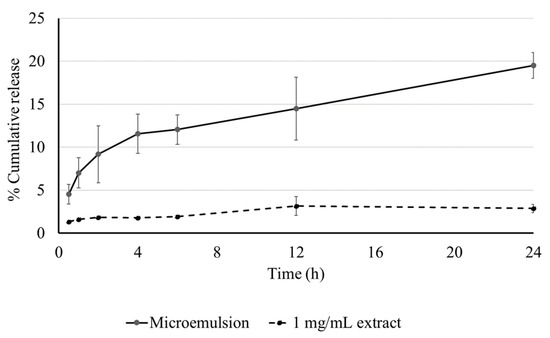
Figure 3.
The release profile of the microemulsion and extract at various time intervals. The data are represented as % cumulative release.
3.6. Stability of Microemulsion in Accelerated and Normal Conditions
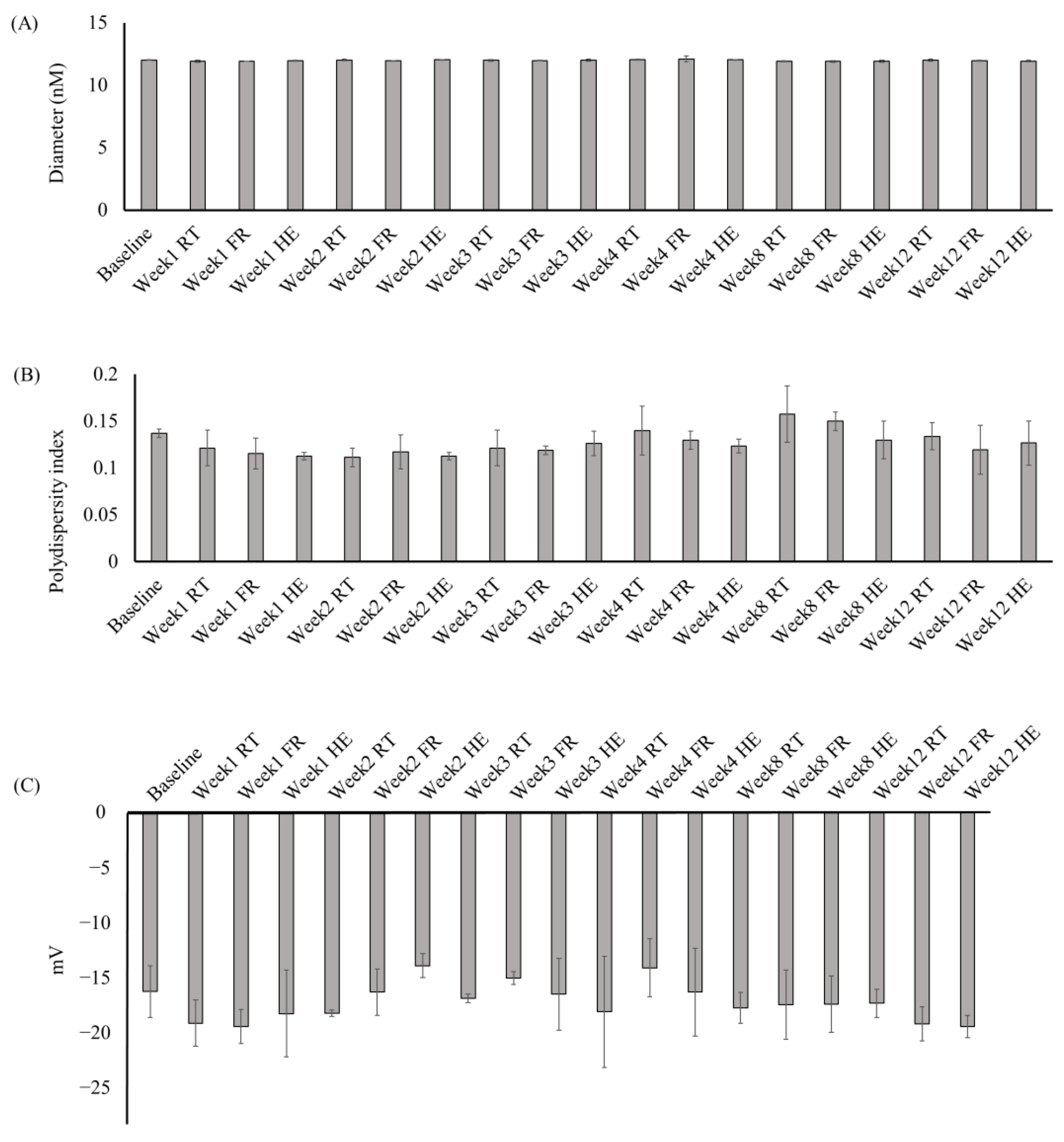
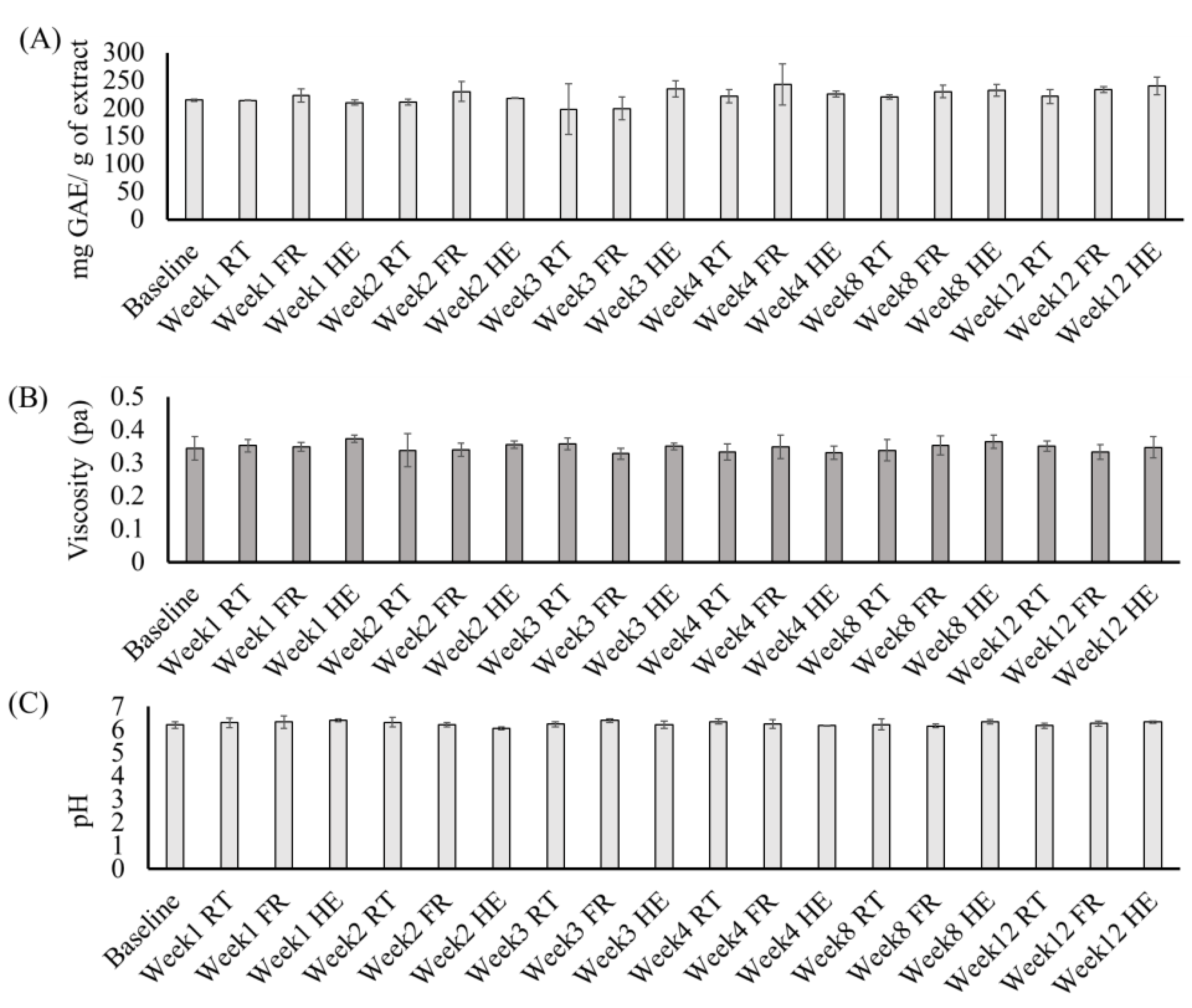
The stability of the MELM was determined after 12 weeks of storage under different conditions. The diameter (11.82 to 12.08 nm), PDI (0.099 to 0.19), zeta potential (−22.3 to −11.3 mV) (Figure 4), TPC (11.7–22.83 mg GAE/g extract), viscosity (0.31 to 0.40 pa), and pH (6.08 to 6.5) (Figure 5) of the MELM samples were not changed significantly (p = 0.05) at any of the storage conditions, compared with the baseline.
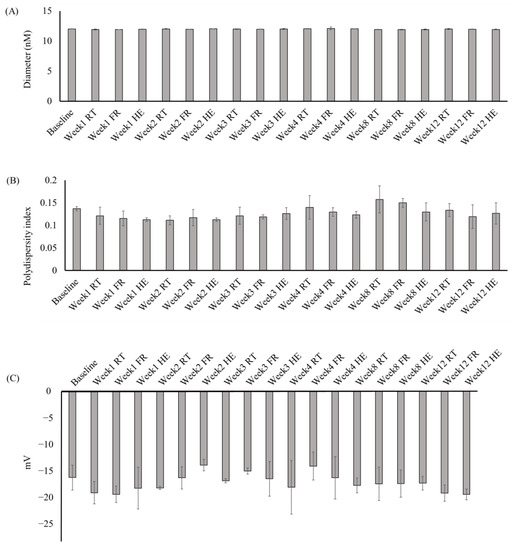
Figure 4.
The changes in the diameter (A), polydispersity index (B), and zeta potential (C) of the MELM were stored under different conditions for 12 weeks.
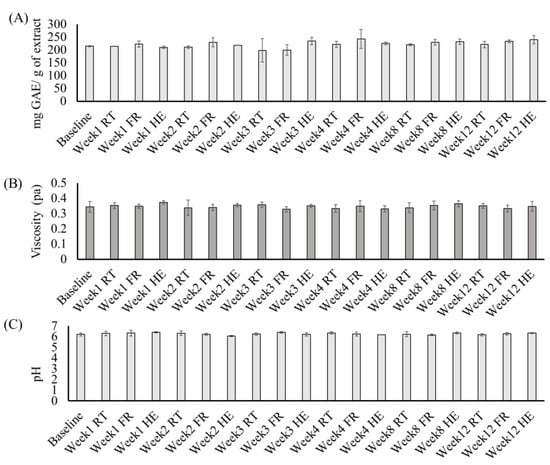
Figure 5.
The changes in the total phenolic content (A), viscosity (B), and pH (C) of the MELM were stored under different conditions for 12 weeks.
4. Discussion
The extraction methods, solvents used, and plant parts are the influencing factors in gaining bioactive phytocompounds. The fruits of M. charantia are rich in vitamins, minerals, protein, and lipids. Its seed is a good source of polyunsaturated fatty acids, lipids, and linolenic acid [17,18]. M. charantia is rich in phenolic compounds that prevent oxidative damage. The fruit, pericarp, and seeds of M. charantia contain various phenolic compounds and phytosterols [19]. The main bioactive compounds of bitter melon are gallic acid, genistic acid, catechin, epicatechin, caffeic acid, chlorogenic acid, p-coumaric acid, ferulic acid, saponins, polypeptide, fatty acids, and alkaloids [8].
The TPC of the aqueous extract of M. charantia fruit flesh and inner tissue displayed 780.75 ± 24.82 and 61.45 ± 8.22 mg GAE/g of extract, respectively, which indicates flesh has higher TPC than inner tissue. The solvent with decreased ethanol displayed high TPC in flesh samples. At the same time, this phenomenon was not observed in the inner tissue samples. The blending of ethanol and water in different ratios creates many levels of solvent polarities. The polarities of the solvent affect the composition and types of compounds in the extracts, so the bioactivity of the extract is varied. Accordingly, finding an optimum solvent is critical to extracting the bioactive compounds, especially phenolics, from M. charantia (Table 1). Individual antioxidants may act via multiple mechanisms, and therefore, no single antioxidation assay would accurately reflect the antioxidation activity of the sample.
The ethanol/water extract of M. charantia exhibits stronger antioxidant activities than other solvent extracts [20]. The alcoholic and water extracts were reported for the DPPH scavenging activity and inhibited the peroxidase and lipid peroxidation [21]. Deng et al. reported that the aqueous extract M. charantia reduced the serum level NO, aspartate transaminase, alanine aminotransferase, and inducible nitric oxide synthase [22]. The stems, pulp, and fruits of M. charantia are reported for the ABTS scavenging activity, the hydroxyl radical scavenging activity, reducing power, and the metal-chelating activity [23,24]. M. charantia contains various biologically active chemicals such as cucurbitane-type triterpenoids, saponin, kuguacins F-S, vicine, and charantin, which may be responsible for free radical scavenging activity, α-amylase inhibitory activity, and NO suppression. However, further studies regarding the purification of active compounds and the evaluation of their biological activities are necessary [25].
The result of the present study also indicates the superior free radical scavenging activities of the ethanolic and water extract of M. charantia fruit (Table 3). A strong NO scavenging activity was noticed in both the aqueous and ethanolic extract of flesh and the inner tissue of M. charantia fruit (Table 3), which displayed the anti-inflammatory properties of the plant. NO plays a role in the pathogenesis of inflammation [26]. M. charantia extract displayed high NO scavenging activity (Table 3), and the water extract obtained from an inner tissue displayed the highest NO scavenging activity (6201.75 ± 157.04 mg TEAC/g of the extract).
Caffeine is a well-studied phytochemical for its role in hair-loss prevention. The antioxidant activity of caffeine helps maintain a healthy scalp, which stimulates scalp hair growth [27]. In vitro tests confirmed that caffeine could modulate IGF-1, keratinocyte growth factor, and TGF-β2 the same as minoxidil [28]. The polyphenols are versatile bioactive phytocompounds and have antioxidant, anti-inflammation, etc., activities. Epigallocatechin-3-gallate (EGCG) is reported as the cure for autoimmune hair loss; EGCG could inhibit Janus kinase-2 expression, thereby blocking the IFN-γ pathway, reducing the immune privilege of the hair follicle [29]. As per our knowledge, there is a gap in the 5aR activity and the possible use of M. charantia-based treatment to manage hair loss.
The 5aR inhibitory activity of the ethanol and water extracts of M. charantia was observed. A maximum of 0.469 ± 0.001 mg FE/g of the extract was noticed in 40% ethanolic extract of flesh (Table 4), followed by an inner tissue extracted with water showing 0.461 ± 0.001 mg FE/g of the extract. NO scavenging and 5aR inhibition properties are important factors related to hair-loss prevention; thus, the water extract of inner tissue was selected for further studies.
The microemulsion was chosen for loading the extract. It is a promising delivery system that significantly enhances the topical delivery of many substances. It has a smaller particle size, which can penetrate the hair follicles more effectively and deeply than non-particulate substances. In addition, surfactant systems in the microemulsion also enhance the topical delivery of the substances [30]. In the present study, a small amount of surfactant was used to prepare the small size particles, to reduce the irritation, which is the possible adverse effect of the microemulsion. The small droplet size is the key that facilitates increased permeability. So, Smix with a ratio of 1:1 was selected to formulate and load the extract. The results showed that the MELM has higher permeability compared to extract solution (Figure 3). Natural compounds such as terpenes and their derivatives, and saponin, can act as percutaneous absorption enhancers [31,32]. The inner tissue of M. charantia contains saponins and some terpenes. Terpenes are non-polar compounds, whereas saponin is polar. Since we used water extract for the study, the extract may have contained saponin, affecting the in vitro permeability. Further experiments are needed to confirm the above statement. The extract was used as a control in the penetration study to remove the bias from the effect of the natural percutaneous absorption enhancer.
The stability of the MELM has been assessed by storing them at various temperatures for 12 weeks. The changes in the particle size, PDI, zeta potential, TPC, viscosity, and pH were determined at constant intervals. No significant changes were detected in any of the assessed parameters, which indicated that the prepared MELM was stable for 12 weeks at 5–40 °C (Figure 4 and Figure 5). The production cost of the MELM was less than 10 USD per kg of the microemulsion. Thus, the prepared MELM could be a valuable candidate for use in cosmetic or pharmacological formulations to confer the beneficial effects of M. charantia. Further studies are required to transfer the findings into cosmetological applications. Nonetheless, the production cost may vary based on the availability of the raw materials, human resources, and mechanical costs.
We primarily reported the 5aR activity of the ethanolic and aqueous extract of M. charantia. Further investigations are required to extract M. charantia bioactive compounds using different solvent systems and clinical trials in human volunteers to formulate the optimum MELM for cosmetic applications.
5. Conclusions
A stable microemulsion of the M. charantia extract was successfully established. Further in vivo investigation is needed to establish the human skin permeation and skin irritation properties of the developed microemulsion, which could aid in the development of topical hair-loss prevention drugs or cosmetic products.
Author Contributions
Conceptualization, C.C., S.S. and N.D.; methodology, C.C., S.S., N.D. and W.R.; software, C.C.; validation, C.C., N.D. and S.S.; formal analysis, S.S. and P.T.; investigation, P.T. and N.D.; resources, C.C., S.P. and W.R.; data curation, P.T. and S.S.; writing—original draft preparation, P.T., B.S.S., C.C., N.D. and W.R.; writing—review and editing, P.T., B.S.S., C.C., N.D. and W.R.; visualization, P.T.; supervision, C.C., S.S. and N.D.; Project administration, C.C. and S.P.; funding acquisition, C.C. and S.P. All authors have read and agreed to the published version of the manuscript.
Funding
Patarapan Trakoolthong was funded by the Research and Researchers for Industries Program (RRI) under the Thailand Research Fund (TRF) (grant number MSD60I0124). This research was supported by Chiang Mai University, Chiang Mai, Thailand.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Laboratory Animal Center, Chiang Mai University, Chiang Mai, Thailand (AR07 2564/RT-0007 5-reductase).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available within the article.
Acknowledgments
The authors gratefully acknowledge Chiang Mai University, Thailand, for their support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Boisvert, W.A.; Yu, M.; Choi, Y.; Jeong, G.H.; Zhang, Y.L.; Cho, S.; Choi, C.; Lee, S.; Lee, B.H. Hair growth-promoting effect of Geranium sibiricum extract in human dermal papilla cells and C57BL/6 mice. BMC Complement. Altern. Med. 2017, 17, 109. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kure, K.; Isago, T.; Hirayama, T. Changes in the sebaceous gland in patients with male pattern hair loss (androgenic alopecia). J. Cosmet. Dermatol. 2015, 14, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Yin, T.; Qiao, Z.; Li, Y.; Li, D.; Jiang, M.; An, C.; Wang, F.; Zuo, M.; Hu, K.; Li, Q. Comparisons of the efficacy and safety of finasteride and dutasteride for benign prostatic hyperplasia: A network meta-analysis. Am. J. Ther. 2017, 24, 517–523. [Google Scholar] [CrossRef] [PubMed]
- Hiipaka, R.A.; Zhang, H.Z.; Dai, W.; Dai, Q.; Liao, S. Structure-activity relationships for inhibition of human 5a-reductase by polyphenols. Biochem. Pharmacol. 2002, 63, 1165–1176. [Google Scholar] [CrossRef]
- Daniels, G.; Akram, S.; Westgate, G.E.; Tamburic, S. Can plant-derived phytochemicals provide symptom relief for hair loss? A critical review. Int. J. Cosmet. Sci. 2019, 41, 332–345. [Google Scholar] [CrossRef]
- Shin, H.; Yoo, H.G.; Inui, S.; Itami, S.; Kim, I.G.; Cho, A.R.; Lee, D.H.; Park, W.S.; Kwon, O.; Cho, K.H.; et al. Induction of transforming growth factor-beta 1 by androgen is mediated by reactive oxygen species in hair follicle dermal papilla cells. BMB Rep. 2013, 46, 460–464. [Google Scholar] [CrossRef]
- Braca, A.; D’Arrigo, M.; Germanò, M.P.; Siciliano, T. Chemical composition and antimicrobial activity of Momordica charantia seed essential oil. Fitoterapia 2008, 79, 123–125. [Google Scholar] [CrossRef]
- Kumar, K.P.S.; Bhowmik, D. Traditional medicinal uses and therapeutic benefits of Momordica charantia Linn. Int. J. Pharm. Sci. Rev. Res. 2010, 4, 23–28. [Google Scholar]
- Sing, P.T.; Tuyen, C.K.; Sophie, E.P.; Paul, D.R. Bitter melon (Momordica charantia L.) bioactive composition and health benefits: A review. Food Rev. Int. 2016, 32, 181–202. [Google Scholar]
- Keseru, A.; Andronie, L.; Ioana, P.; Rotaru, A.; Danut, M.; Coroian, A.; Răducu, C. Characterization of Momordica charantia Using FT-IR Spectroscopy. Bull. UASVM Hortic. 2016, 73, 245–246. [Google Scholar]
- Kumar, N.; Chaiyasut, C. Health promotion potential of vegetables cultivated in northern Thailand: A preliminary screening of tannin and flavonoid contents, 5α-reductase inhibition, astringent activity, and antioxidant activities. J. Evid. Based Complement. Altern. Med. 2017, 22, 573–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, Y.; Yin Chia, Y.; Lee, C.H.; Sheng Sow, H.; Yap, W.-S. Effectiveness of Maceration periods with different extraction solvents on in-vitro antimicrobial activity from fruit of Momordica charantia L. JAPS 2016, 4, 16–23. [Google Scholar] [CrossRef]
- Pengkumsri, N.; Chaiyasut, C.; Saenjum, C.; Sirilun, S.; Peerajan, S.; Suwannalert, P.; Sirisattha, S.; Sivamaruthi, B.S. Physicochemical and antioxidative properties of black, brown and red rice varieties of northern Thailand. Food Sci. Technol. 2015, 35, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Chaiyana, W.; Leelapornpisid, P.; Phongpradist, R.; Kiattisin, K. Enhancement of antioxidant and skin moisturizing effects of olive oil by incorporation into microemulsions. Nanomater. Nanotechnol. 2016, 6, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Anantaworasakul, P.; Chaiyana, W.; Michniak-Kohn, B.B.; Rungseevijitprapa, W.; Ampasavate, C. Enhanced Transdermal Delivery of Concentrated Capsaicin from Chili Extract-Loaded Lipid Nanoparticles with Reduced Skin Irritation. Pharmaceutics 2020, 12, 463. [Google Scholar] [CrossRef]
- Nitthikan, N.; Leelapornpisid, P.; Natakankitkul, S.; Chaiyana, W.; Mueller, M.; Viernstein, H.; Kiattisin, K. Improvement of Stability and transdermal delivery of bioactive compounds in green Robusta coffee beans extract loaded nanostructured lipid carriers. J. Nanotechnol. 2018, 2018, 7865024. [Google Scholar] [CrossRef] [Green Version]
- Gupta, M.; Sharma, S.; Gautam, A.K.; Bhadauria, R. Momordica charantia Linn, (karela): Nature’s silent healer. Int. J. Pharm. Sci. Rev. Res. 2011, 11, 32–37. [Google Scholar]
- Yoshime, L.T.; de Melo, I.L.P.; Sattler, J.A.G.; de Carvalho, E.B.T.; Mancini-Filho, J. Bitter gourd (Momordica charantia L.) seed oil as a naturally rich source of bioactive compounds for nutraceutical purposes. Nutrire 2016, 41, 12. [Google Scholar] [CrossRef] [Green Version]
- Jia, S.; Shen, M.; Zhang, F.; Xie, J. Recent Advances in Momordica charantia: Functional Components and Biological Activities. Int. J. Mol. Sci. 2017, 18, 2555. [Google Scholar] [CrossRef] [Green Version]
- Padmashree, A.; Sharma, G.K.; Semwal, A.D. Studies on the antioxygenic activity of bitter gourd (Momordica charantia) and its fractions using various in vitro models. J. Sci. Food Agric. 2011, 91, 776–782. [Google Scholar] [CrossRef]
- Wu, S.J.; Ng, L.T. Antioxidant and free radical scavenging activities of wild bitter melon (Momordica charantia Linn. var. abbreviata Ser.) in Taiwan. LWT-Food Sci. Technol. 2008, 41, 323–330. [Google Scholar] [CrossRef]
- Deng, Y.; Tang, Q.; Zhang, Y.; Zhang, R.; Wei, Z.; Tang, X.; Zhang, M. Protective effect of Momordica charantia water extract against liver injury in restraint-stressed mice and the underlying mechanism. Food Nutr. Res. 2017, 61, 1348864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Yen, M.H.; Tsang, S.F.; Gan, K.H.; Hsu, H.Y.; Lin, C.N. Antioxidant triterpenoids from the stems of Momordica charantia. Food Chem. 2010, 118, 751–756. [Google Scholar] [CrossRef]
- Fan, T.; Hu, J.; Fu, L.; Zhang, L. Optimization of enzymolysis-ultrasonic assisted extraction of polysaccharides from Momordica charantia L. by response surface methodology. Carbohydr. Polym. 2015, 115, 701–706. [Google Scholar] [CrossRef]
- Pham, T.M.H.; Ngo, D.H.; Ngo, D.N.; Vo, T.S. Investigation of biological activities of wild bitter melon (Momordica charantia Linn. Var. Abbreviata Ser.). Biomolecules 2019, 9, 211. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Herman, A.; Herman, A.P. Caffeine’s mechanisms of action and its cosmetic use. Skin Pharmacol. Physiol. 2013, 26, 8–14. [Google Scholar] [CrossRef]
- Fischer, T.W.; Herczeg-Lisztes, E.; Funk, W.; Zillikens, D.; Bíró, T.; Paus, R. Differential effects of caffeine on hair shaft elongation, matrix and outer root sheath keratinocyte proliferation, and transforming growth factor-beta2/insulin-like growth factor-1-mediated regulation of the hair cycle in male and female human hair follicles in vitro. Br. J. Dermatol. 2014, 171, 1031–1043. [Google Scholar]
- Hamed, F.N.; McDonagh, A.J.G.; Almaghrabi, S.; Bakri, Y.; Messenger, A.G.; Tazi-Ahnini, R. Epigallocatechin-3 gallate inhibits STAT-1/JAK2/IRF-1/HLA-DR/HLAB and reduces CD8 MKG2D lymphocytes of alopecia areata patients. Int. J. Environ. Res. Public Health 2018, 15, 2882. [Google Scholar] [CrossRef] [Green Version]
- Tampucci, S.; Paganini, V.; Burgalassi, S.; Chetoni, P.; Monti, D. Nanostructured drug delivery systems for targeting 5-α-reductase inhibitors to the hair follicle. Pharmaceutics 2022, 14, 286. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef] [PubMed]
- Patil, U.K.; Saraogi, R. Natural products as potential drug permeation enhancer in transdermal drug delivery system. Arch. Dermatol. Res. 2014, 306, 419–426. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).