Use of Fe (II) and H2O2 along with Heating for the Estimation of the Browning Susceptibility of White Wine
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Wine Samples
2.2. Chemicals and Reagents
2.3. Equipment
2.4. Experimental Procedure
2.5. Methods of Analysis
2.5.1. Determination of Browning Index
2.5.2. Determination of Sulfur Dioxide
2.5.3. Visible and Ultraviolet Spectra
2.5.4. Determination of Hydroxycinnamic Acids
2.5.5. Determination of Flavanols
2.5.6. Determination of Total Phenolic Content
2.5.7. Determination of Total Free Sulfhydryls
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of Fe (II) + H2O2 on the Browning Index of Roditis–Malagouzia and Debina White Wines during Storage at 18 °C or 45 °C
3.2. Effect of Fe (II) + H2O2 on the Levels of Antioxidants of Roditis–Malagouzia White Wine during Storage at 18 °C or 45 °C
3.2.1. Total Sulphur Dioxide
3.2.2. Total Phenolics
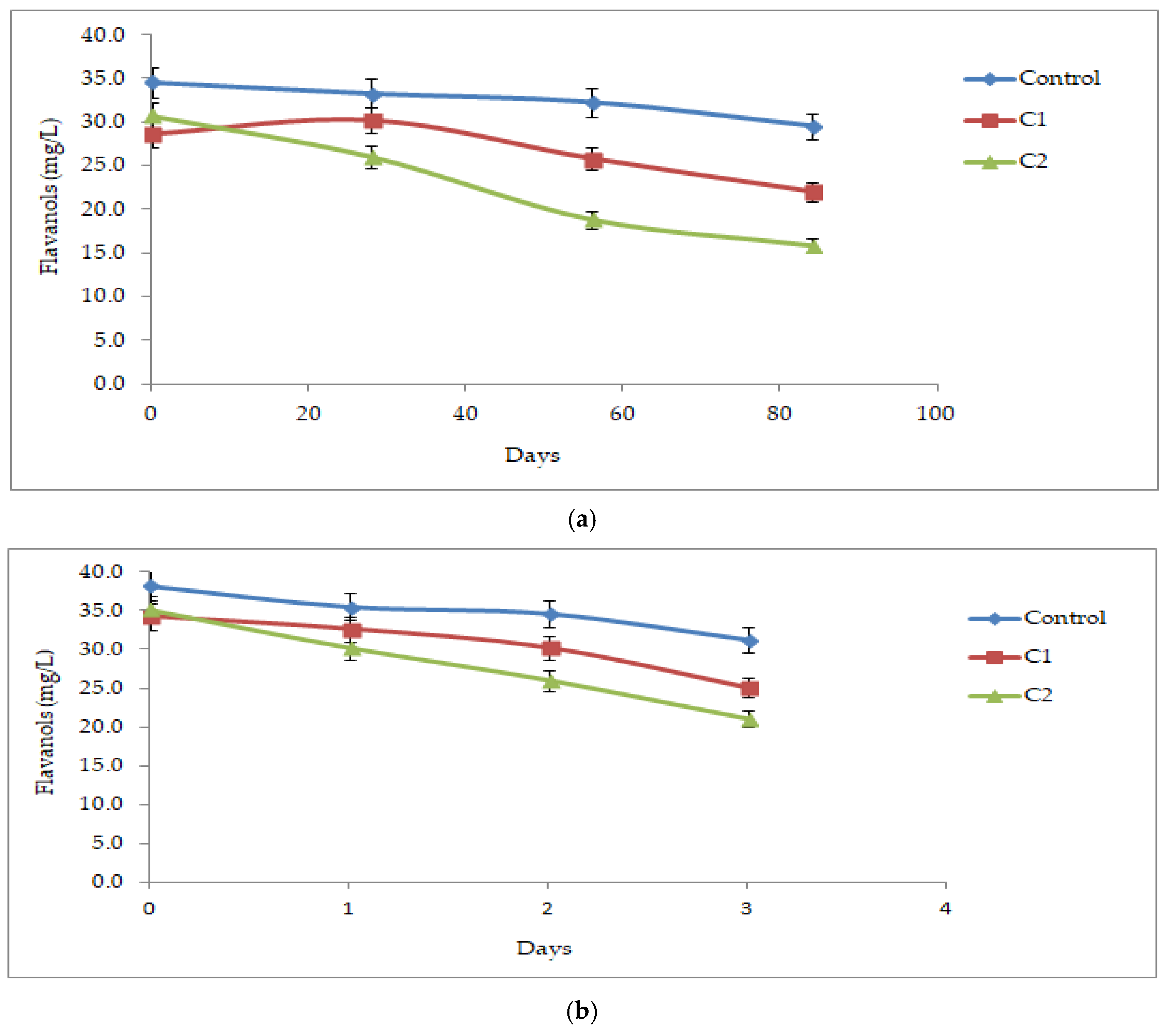
3.2.3. Flavanols
3.2.4. Hydroxycinnamates
3.2.5. Total Free Sulfhydryls
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singleton, V.L. Oxygen with phenols and related reactions in musts, wines, and model systems: Observations and practical implications. Am. J. Enol. Vitic. 1987, 38, 69–77. [Google Scholar]
- Fernãndez-Zurbano, P.; Ferreira, V.; Peña, C.; Escudero, A.; Serrano, F.; Cacho, J. Prediction of oxidative browning in white wines as a function of their chemical composition. J. Agric. Food Chem. 1995, 43, 2813–2817. [Google Scholar] [CrossRef]
- Vaimakis, V.; Roussis, I.G. Must oxygenation together with glutathione addition in the oxidation of white wine. Food Chem. 1996, 57, 419–422. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Papadopoulou, D. Inhibition of the decline of volatile esters and terpenols during oxidative storage of Muscat-white and Xinomavro-red wine by caffeic acid and N-acetyl-cysteine. Food Chem. 2005, 93, 485–492. [Google Scholar] [CrossRef]
- Danilewicz, J.C. Review of reaction mechanisms of oxygen and proposed intermediate reduction products in wine: Central role of iron and copper. Am. J. Enol. Vitic. 2003, 54, 73–85. [Google Scholar]
- Danilewicz, J.C. Interaction of sulfur dioxide, polyphenols, and oxygen in a wine-model system: Central role of iron and copper. Am. J. Enol. Vitic. 2007, 58, 53–60. [Google Scholar]
- Danilewicz, J.C. Reactions involving iron in mediating catechol oxidation in model wine. Am. J. Enol. Vitic. 2013, 64, 316–324. [Google Scholar] [CrossRef]
- Waterhouse, A.L.; Laurie, V.F. Oxidation of wine phenolics: A critical evaluation and hypotheses. Am. J. Enol. Vitic. 2006, 57, 306–313. [Google Scholar]
- Nguyen, T.H.; Waterhouse, A.L. Redox cycling of iron: Effects of chemical composition on reaction rates with phenols and oxygen in model wine. Am. J. Enol. Vitic. 2021, 72, 209–216. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Waterhouse, A.L. Acid complexation of iron controls the fate of hydrogen peroxide in model wine. Food Chem. 2022, 377, 131910. [Google Scholar] [CrossRef]
- Li, H.; Guo, A.; Wang, H. Mechanisms of oxidative browning of wine. Food Chem. 2008, 108, 1–13. [Google Scholar] [CrossRef]
- Oliveira, C.M.; Ferreira, A.C.S.; De Freitas, V.; Silva, A.M.S. Oxidation mechanisms occurring in wines. Food Res. Int. 2011, 44, 1115–1126. [Google Scholar] [CrossRef]
- Elias, R.J.; Waterhouse, A.L. Controlling the Fenton reaction in wine. J. Agric. Food Chem. 2010, 58, 1699–1707. [Google Scholar] [CrossRef] [PubMed]
- Elias, R.J.; Andersen, M.L.; Skibsted, L.H.; Waterhouse, A.L. Identification of free radical intermediates in oxidized wine using electron paramagnetic resonance spin trapping. J. Agric. Food Chem. 2009, 57, 4359–4365. [Google Scholar] [CrossRef]
- Danilewicz, J.C.; Seccombe, J.T.; Whelan, J. Mechanism of interaction of polyphenols, oxygen, and sulfur dioxide in model wine and wine. Am. J. Enol. Vitic. 2008, 59, 128–136. [Google Scholar]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of SO2 on the formation and evolution of volatile compounds in wines. Food Control 2007, 18, 1501–1506. [Google Scholar] [CrossRef]
- Roussis, I.G.; Sergianitis, S. Protection of some aroma volatiles in a model wine medium by sulphur dioxide and mixtures of glutathione with caffeic acid or gallic acid. Flavour Fragr. J. 2008, 23, 35–39. [Google Scholar] [CrossRef]
- Roussis, I.G.; Patrianakou, M.; Drossiadis, A. Protection of aroma volatiles in a red wine with low sulphur dioxide by a mixture of glutathione, caffeic acid and gallic acid. S. Afr. J. Enol. Vitic. 2013, 34, 262–265. [Google Scholar] [CrossRef]
- Roussis, I.G.; Papadopoulou, D.; Sakarellos-Daitsiotis, M. Protective effect of thiols on wine aroma volatiles. Open Food Sci. J. 2009, 3, 98–102. [Google Scholar] [CrossRef]
- Galuska, S.; Makris, D.P. The effect of chlorogenic acid, catechin and SO2 on browning development in white wine model solutions. J. Inst. Brew. 2013, 119, 309–313. [Google Scholar] [CrossRef]
- Cheynier, V.; Rigaud, J.; Souquet, J.M.; Barillere, J.M.; Moutounet, M. Effect of pomace contact and hyperoxidation on the phenolic composition and quality of Grenache and Chardonnay wines. Am. J. Enol. Vitic. 1989, 40, 36–42. [Google Scholar]
- El Hosry, L.; Auezova, L.; Sakr, A.; Hajj-Moussa, E. Browning susceptibility of white wine and antioxidant effect of glutathione. Int. J. Food Sci. Technol. 2009, 44, 2459–2463. [Google Scholar] [CrossRef]
- Singleton, V.L.; Kramlinga, T.E. Browning of white wines and an accelerated test for browning capacity. Am. J. Enol. Vitic. 1976, 27, 157–160. [Google Scholar]
- Mayén, M.; Barón, R.; Mérida, J.; Medina, M. Changes in phenolic compounds during accelerated browning in white wines from cv. Pedro Ximenez and cv. Baladi grapes. Food Chem. 1997, 58, 89–95. [Google Scholar] [CrossRef]
- Muller-Spath, H. Der POM-test. Deutsche Weinbau 1992, 23, 1099–1100. [Google Scholar]
- Fia, G.; Gori, C.; Bucalossi, G.; Borghini, F.; Zanoni, B. A Naturally occurring antioxidant complex from unripe grapes: The case of Sangiovese (v. Vitis vinifera). Antioxidants 2018, 7, 27. [Google Scholar] [CrossRef] [Green Version]
- Palma, M.; Barroso, C.G. Application of a new analytical method to determine the susceptibility of wine to browning. Eur. Food Res. Technol. 2002, 214, 441–443. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. Compendium of International Methods of Wine and Must Analysis, 2020 ed.; International Organisation of Vine and Wine: Paris, France, 2021; Volume 1, ISBN 978-2-85038-053-2. [Google Scholar]
- Mladenoska, I.; Petkova, V.; Kadifkova Panovska, T. Pre-fermentative treatment of a model wine with aim to serve as functional food with decreased alcohol content. Maced. Pharm. Bull. 2017, 63, 47–53. [Google Scholar] [CrossRef]
- Roussis, I.G.; Lambropoulos, I.; Tzimas, P.; Gkoulioti, A.; Marinos, V.; Tsoupeis, D.; Boutaris, I. Antioxidant activities of some Greek wines and wine phenolic extracts. J. Food Comp. Anal. 2008, 21, 614–621. [Google Scholar] [CrossRef]
- Kontogeorgos, N.; Roussis, I.G. Total free sulphydryls of several white and red wines. S. Afr. J. Enol. Vitic. 2014, 35, 125–127. [Google Scholar]
- Patrianakou, M.; Roussis, I.G. Decrease of Wine Volatile Aroma Esters by Oxidation. S. Afr. J. Enol. Vitic. 2013, 34, 241–245. [Google Scholar] [CrossRef]
- Kallithraka, S.; Salacha, M.I.; Tzourou, I. Changes in phenolic composition and antioxidant activity of white wine during bottle storage: Accelerated browning test versus bottle storage. Food Chem. 2009, 113, 500–505. [Google Scholar] [CrossRef]
- Aleixandre-Tudo, J.L.; Weightman, C.; Panzeri, V.; Nieuwoudt, H.H.; du Toit, W.J. Effect of skin contact before and during alcoholic fermentation on the chemical and sensory profile of South African Chenin blanc white wines. S. Afr. J. Enol. Vitic. 2015, 36, 366–377. [Google Scholar] [CrossRef]
- Galuska, S.; Makris, D.P.; Kefalas, P. Browning development in wine-like liquid model matrices: Dependence on phenolic, Fe (III) and SO2 concentrations. J. Food Process. Eng. 2010, 33, 934–945. [Google Scholar] [CrossRef]
- Recamales, Á.F.; Sayago, A.; González-Miret, M.L.; Hernanz, D. The effect of time and storage conditions on the phenolic composition and colour of white wine. Food Res. Int. 2006, 39, 220–229. [Google Scholar] [CrossRef]
- Simpson, R.F. Factors affecting oxidative browning of white wine. Vitis 1982, 21, 233–239. [Google Scholar]
- Fernãndez-Zurbano, P.; Ferreira, V.; Escudero, A.; Cacho, J. Role of hydroxycinnamic acids and flavanols in the oxidation and browning of white wines. J. Agric. Food Chem. 1998, 46, 4937–4944. [Google Scholar] [CrossRef]
- Gislason, N.E.; Currie, B.L.; Waterhouse, A.L. Novel antioxidant reactions of cinnamates in wine. J. Agric. Food Chem. 2011, 59, 6221–6226. [Google Scholar] [CrossRef]
- Kritzinger, E.C.; Bauer, F.F.; du Toit, W.J. Role of glutathione in winemaking: A review. J. Agric. Food Chem. 2013, 61, 269–277. [Google Scholar] [CrossRef] [Green Version]
- Sarakbi, A.; Kauffmann, J.-M. A new chemical criteria for white wine: The glutathione equivalent capacity. Food Chem. 2014, 153, 321–326. [Google Scholar] [CrossRef]
- Ugliano, M.; Kwiatkowski, M.; Vidal, S.; Capone, D.; Siebert, T.; Dieval, J.-B.; Aagaard, O.; Waters, E.J. Evolution of 3-mercaptohexanol, hydrogen sulfide, and methyl mercaptan during bottle storage of Sauvignon blanc wines. Effect of glutathione, copper, oxygen exposure, and closure-derived oxygen. J. Agric. Food Chem. 2011, 59, 2564–2572. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Voltea, S.; Karabagias, I.K.; Roussis, I.G. Use of Fe (II) and H2O2 along with Heating for the Estimation of the Browning Susceptibility of White Wine. Appl. Sci. 2022, 12, 4422. https://doi.org/10.3390/app12094422
Voltea S, Karabagias IK, Roussis IG. Use of Fe (II) and H2O2 along with Heating for the Estimation of the Browning Susceptibility of White Wine. Applied Sciences. 2022; 12(9):4422. https://doi.org/10.3390/app12094422
Chicago/Turabian StyleVoltea, Sofia, Ioannis K. Karabagias, and Ioannis G. Roussis. 2022. "Use of Fe (II) and H2O2 along with Heating for the Estimation of the Browning Susceptibility of White Wine" Applied Sciences 12, no. 9: 4422. https://doi.org/10.3390/app12094422






