Abstract
This PRISMA-ScR driven scoping review aims to evaluate the influence of magnetic field stimulation on dental implant osseointegration. Seven databases were screened adopting ad-hoc strings. All clinical and preclinical studies analyzing the effects of magnetic fields on dental implant osseointegration were included. From 3124 initial items, on the basis of the eligibility criteria, 33 articles, regarding both Pulsed ElectroMagnetic Fields (PEMF) and Static magnetic Fields from permanent Magnets (SFM) were finally included and critically analyzed. In vitro studies showed a positive effect of PEMF, but contrasting effects of SFM on bone cell proliferation, whereas cell adhesion and osteogenic differentiation were induced by both types of stimulation. In vivo studies showed an increased bone-to-implant contact rate in different animal models and clinical studies revealed positive effects on implant stability, under magnetic stimulation. In conclusion, although positive effects of magnetic exposure on osteogenesis activity and osseointegration emerged, this scoping review highlighted the need for further preclinical and clinical studies. More standardized designs, accurate choice of stimulation parameters, adequate methods of evaluation of the outcomes, greater sample size and longer follow-ups are needed to clearly assess the effect of magnetic fields on dental implant osseointegration.
1. Introduction
Implant treatment is a predictable option for the rehabilitation of one or more missing teeth with high long-term dental implant survival rates [1,2]. The osseointegration of dental implants is a fundamental prerequisite for a successful dental implant rehabilitation. The process of osseointegration involves several steps: the formation of a blood coat following implant insertion, the development of mesenchymal tissue, the formation of intramembranous (woven) bone, and, then, of lamellar bone [3,4,5]. A dental implant is considered osseointegrated when a “direct functional and structural connection between living bone and the surface of an implant under load” is reached [6]. Surgical technique, bone quality and quantity, smoking habits, dental implant material/surface and postoperative infections and inflammation are key factors influencing the osseointegration process [7,8,9,10,11]. Different strategies have been proposed to promote and accelerate the osseointegration process, thus extending the clinical indications of dental implants. Among the most relevant, the introduction in the early 90’s of topographic and chemical modifications of dental implant surfaces significantly enhanced their clinical performance with respect to the older unmodified, machined surfaces [12,13,14,15,16]. Following this key step, the application of Electromagnetic and Magnetic Fields (EMFs and MFs) was proposed to further improve tissue healing and regeneration [17,18]. In fact, MFs are known to promote osteogenesis and many preclinical and clinical studies have demonstrated the effects of magnetism on bone healing [19,20,21,22,23,24,25], and the results from orthopedic applications have strongly encouraged the study of their use in dentistry to promote the osseointegration of dental implants [17,26].
EMF/MF exposure in daily life represents a constant feature of modern society, covering many applications from power distribution lines or home appliances to ICT technologies for wireless communications. As early as 1996 the World Health Organization (WHO) established the International EMF Project to assess the environmental and health effects of exposure to static and time varying electric and magnetic fields, cooperating with the International Commission on Non-Ionizing Radiation Protection (ICNIRP) to formulate updated guidelines for limiting exposure to EMFs [27]. Currently, based on the 1979 draft for electromagnetic compatibility standards for medical devices, the Food and Drug Administration (FDA) approve the medical use of magnetic devices, under the constant control of the Center for Devices and Radiological Health (CDRH).
MF sources relevant for medical applications can be either permanent magnets (also known as lodestones) or the stationary flow of unpaired electrons in metal conductors (electric currents), or even the stationary flow of ions present in fluids, e.g., intracellular fluid. When magnets are fixed in space, the generated field does not change with time, and we refer to this case as Static Magnetic Field (SFM) from permanent magnets. Conversely, the more general case of possibly time-varying fields is referred to as EMF, to indicate the coupled nature of electric and magnetic aspects. Finally, when the EMF is generated by sources with a pulsed nature (typically, a sequence of square pulses), we adopt the term Pulsed Electro-Magnetic Field (PEMF), indicating when possible the appropriate details of the field waveform.
So far, only a limited number of studies analyzed the effects of MFs on dental implant osseointegration, mostly involving in vitro and animal models, whereas clinical evidence is still inconsistent. The aim of the present scoping review is to provide a broad perspective on the current knowledge regarding the effects of magnetic stimulations on dental implant osseointegration, to identify evidence and gaps in the literature and to provide indications for future research. The research question was: “what effects can MFs stimulation exerts on dental implant osseointegration?”.
2. Materials and Methods
In effecting this scoping review the PRISMA-ScR (Preferred Reporting Items for Systematic Reviews and Meta-Analyses Extension for Scoping Reviews) [28] model was followed (see Table S1 of Supplementary Material for the detailed PRISMA ScR checklist). First of all, a technical expert panel (TEP) was established, consisting of biochemists, biologists, dentists and engineers. In particular, the TEP was composed of 4 biochemists expert in molecular mechanisms underlying osteoblast precursor growth and osteogenesis induction, 3 engineers expert in low frequency MFs and electronic circuits, 4 dentists expert in implant surfaces, dental implant osseointegration, bone regeneration and oral surgery, and 1 dentist expert in scoping review methodology.
2.1. Databases Selection and Search Strategy
On 23 March 2022 two independent and calibrated reviewers from the TEP scanned the following databases: the National Library of Medicine MEDLINE/PubMed, Scopus, Web of Science, The Cochrane Library, Embase, Clinicaltrials.com and the IEEE Digital Library (the database of publications from the Institute of Electric and Electronic Engineers). For Embase database the following search string was adopted: (“magnetic field” OR “electromagnetic field”) AND (implant OR titanium) AND (dental OR oral OR endosseous OR osseous OR jaw OR bone OR osseointegration OR stability OR osteoblast OR “mesenchymal stromal cell” OR “mesenchymal stem cell”). For all other databases the following search string was adopted: (magnetic field OR electromagnetic field) AND (implant OR titanium) AND (dental OR oral OR endosseous OR osseous OR jaw OR bone OR osseointegration OR stability OR osteoblast OR mesenchymal stromal cell OR mesenchymal stem cell). The major international journals of implantology were also consulted by a hand search; furthermore, reference lists of all selected studies were screened. Only studies in the English language were considered. No other filters were applied. The key points of the search strategy are reported in Table 1.

Table 1.
Search strategy.
2.2. Study Selection
We selected preclinical in vitro and in vivo studies evaluating the effects of MFs on bone cells cultured in contact with Titanium (Ti) surfaces and their effects on Ti implant osseointegration in animal models. We also selected:
- -
- Interventional studies (either randomized or non-randomized controlled clinical trials);
- -
- Observational studies (either analytical or descriptive);
- -
- Case series or Case reports regarding the effects of MFs on osseointegration of dental implants were selected.
Review articles, conference abstracts, editorials and studies regarding the effects of other physical stimulations on osseointegration were disregarded. Disagreements between the two reviewers were resolved via discussion. After title and abstract analysis, a careful evaluation of all full texts for the eligibility criteria (inclusion/exclusion) was performed. Cohen’s kappa coefficient was used to determine inter-rater agreement.
Three independent reviewers extracted and qualitatively analyzed results and findings from each included study, using an ad-hoc data charting form designed for the present scoping review. The forms report for each study the relevant information about the type of study (in vitro, in vivo, clinical), the details of the stimulation used (SMF, PEMF), the study groups, the duration of the follow-up and the key study findings. Furthermore, the quality of the included clinical studies was assessed using the “Risk Of Bias In Non-randomized Studies of Interventions (ROBINS-I) Tool” [29] and the “Cochrane Risk of Bias Tool” for randomized clinical trials (RoB 2) [30].
3. Results
The bibliographic scan retrieved 4634 suitable items. After removing duplicates, 3124 records underwent the finer selection described above, allowing the exclusion of a further 3083 results. Out of the remaining 41 papers, after a full text reading, 9 were excluded due to the following reasons: (1) they did not focus on dental implant osseointegration (but on other aspects of dental implant therapy, e.g., pain or swelling); (2) they focused on the treatment of bone fractures; (3) physical stimulations different from MFs were used; (4) the description of the magnetic stimulation lacked coherence; (5) bone cells were not cultured on Ti surfaces. Table of the excluded full-texts is provided in Supplementary Material (Table S2).
One adjunctive eligible paper was identified through screening of reference lists of selected studies. Finally, 33 articles published between January 1996 and December 2021 were included in the present scoping review. The k value for the inter-reviewer agreement for potentially pertinent papers was 0.86 (for the selection of titles and abstracts) and 0.92 (for the selection of full-text articles), showing a high level of agreement. The search and selection process flow diagram is presented in Figure 1.
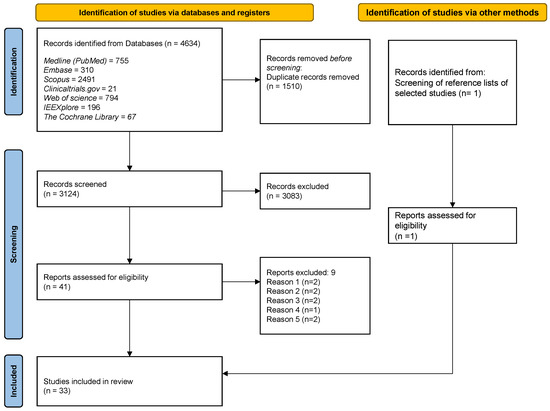
Figure 1.
Flow diagram of the search and selection process.
The results of the articles selection were 9 in vitro studies, 15 in vivo studies, 3 studies presenting both in vitro and in vivo evaluations, 6 clinical studies.
Concerning the stimulation type, we found:
10 studies using SFM, mainly published since 2012: 1 Randomized Controlled Trial (RCT), 1 Controlled Clinical Trial (CCT), 5 in vivo studies (on rabbits and dogs), 1 study presenting both in vivo and in vitro evaluations (on rats) and 2 in vitro studies;
23 studies using PEMF, regularly published since 1996: 2 RCT, 1 retrospective study, 1 CCT, 10 in vivo studies (2 studies in rats and 8 in rabbits), 7 in vitro studies and 2 studies with both in vivo (in rabbits) and in vitro evaluations.
The characteristics and main findings of the in vitro, in vivo and clinical studies are reported in Table 2, Table 3 and Table 4 respectively. Rows are organized for each table according to the stimulation type.

Table 2.
Characteristics and main findings of the in vitro studies included.

Table 3.
Characteristics and main findings of the in vivo studies included.

Table 4.
Characteristics and main findings of the clinical studies included.
3.1. In Vitro Studies
3.1.1. Static Magnetic Fields from Permanent Magnets
Three studies treated the effects of SFM on bone cells cultured on Ti surfaces.
Bambini and colleagues [32] focused on SFM generated by small neodymium-iron-bore (Nd-Fe-B) cover screws as a strategy for non-invasive and local stimulation. They performed in vitro tests, by using MG63 osteoblast-like cell line exposed or not to the magnetic cover screws (with or without Ti implants) for 24, 48 and 72 h. The reported results indicated that SFM application had a negative effect on proliferation; particularly, cells in direct contact with the magnetic cover screw (exposed to the highest magnetic flux density, reportedly 618 mT) showed the lowest proliferation rate compared to cells not exposed to SFM and cells in direct contact with Ti implants at the furthest distance from the magnetic cover screws. Despite its very low thickness, the interposition of the implant surface between cells and magnets reduced the magnetic flux and, therefore, the SFM effect on the proliferation rate. However, the major evidence of a SFM effect was related to the expression of markers of osteoblast differentiation and specifically an upregulation of genes involved in cell differentiation and matrix mineralization processes, such as transforming growth factor-β1 (TGF−β1), COL10A1, Bone Morphogenetic Protein-2 (BMP-2) for an incubation of 72 h, and a downregulation of osteoclastogenesis markers (VCAM-1).
Kim et al. [33] focused first on the interference of various MF intensities (ranging from 0 to 10 mT) with the fibronectin adsorption on the Ti surfaces without observing any significant difference compared to unexposed cells. Then, SMF effects on human osteosarcoma cells (TE-85) grown on the prepared surfaces were evaluated. While differences in the proliferation rate could not be recorded, SFM exposure caused: (i) an enhanced attachment of TE-85 cells on Ti surfaces at 1, 2, 5 and 10 mT and (ii) changes in cell morphology (strand- and sheet-like filopodia) just 2 h after cell seeding, at all applied SFM intensities. The authors hypothesized that SFM has an effect on the 3D structure of fibronectin, which could result in an increase in cell attachment index. In agreement with the reported data [32,33], He et al. [31] found that human bone–derived mesenchymal stem cells (hBMSCs) cultured on 3D-printed porous Ti scaffolds and exposed to increasing intensities of SFMs (50, 100, 150 mT) did not experience differences in viability and proliferation compared to unexposed controls, but showed a higher production and mineralization of ECM and a multipolar and well-spread morphology on Ti surfaces. Furthermore, the SFM exposure led to higher expression (mRNA and protein) of type-I collagen, Alkaline Phosphatase (ALP), Runt-related transcription factor-2 (RUNX2), osteopontin (OPN), and osteocalcin (OCN) at 14 day, emphasizing the SFM-dependent stimulation of osteogenesis. The study also pointed mechanistically (by means of proteomic tools, western blotting and immunofluorescence) to the activation of BMP-SMAD1/5/8 signaling pathway and to SMAD4 protein increase as a key factor in the SMAD-dependent transcription activation of genes involved in osteogenic differentiation.
3.1.2. Pulsed ElectroMagnetic Fields
Nine studies focused on the effects of PEMF on bone-derived cells cultured on Ti surfaces. Atalay et al. [38] tested how PEMF (0.2 mT) influenced the behavior of rat primary osteoblasts on implant surfaces of different chemical compositions at 24, 48 and 72 h exposure. Specifically, discs of commercially pure Ti (cpTi) and Titanium-Zirconium alloy (TiZr) were used. The effect of PEMF exposure in terms of cell viability (biocompatibility), cell proliferation rate, and alkaline phosphatase was clearly stimulative on osteoblasts cultured on the cpTi surface compared to TiZr discs.
Wang et al. [37] analyzed the response of rat osteoblasts cultured on surfaces with different topographies: polished flat surfaces, sand-blasted with large grit and acid etched surfaces (micro-topographic modification) and anodized nanotubular-structured surfaces (nano-topographic modificcation). The authors reported that PEMF stimulation led to higher osteoblast adhesion and proliferation and augmentation of ECM mineralization on all the tested Ti surfaces, with nano-topography showing the better PEMF-dependent increment with respect to the control group of unexposed cells. Importantly, no differences in rate proliferation were observed between the different surfaces without PEMF stimulation.
Fassina et al. analyzed the effects on osteoblast-like cells in four consecutive studies [39,40,41,42], after the application of PEMF (2 mT, 75 Hz). In two of these studies, human osteosarcoma cells (SAOS-2) were cultured on Ti devices with a complex texture, Ti sintered grids or Ti fiber-mesh sheets, showing an increased proliferation rate and the concomitant production of ECM components such as decorin, osteopontin, and type-I collagen after PEMF stimulation [40,41]. The authors obtained similar results by culturing the same cell type on Ti plasma spray surfaces: once again, a higher proliferation rate and production of an autologous ECM were observed with PEMF exposure [39,42].
Bloise et al. [35] reported similar effects on ECM deposition using human bone marrow mesenchymal stem cell (hBM-MSCs), cultured on a TiO2 surface of unspecified topography. In particualr, the authors showed that the same PEMF intensity used by Fassina’s group (2 mT; 75 Hz) led to a higher expression of Runx-2, Bone SialoProtein (BSP), Osterix, OSC, BMP-2 and a higher production of ALP, type-I collagen, type-III collagen and FN. Furthermore, as complementing data, they analized the ion flux, showing that the exposure was also able to enhance cellular Ca2+ currents, especially in the initial phase of the osteogenic process, with a higher intracellular Ca2+ concentration, and the externalization of type-I collagen and collagen network formation.
Finally, two publications, analyzing PEMF effects both in vivo and in vitro, can be described partially in this section for their in vitro results [34,36]. Two different research groups corroborated the positive effect of pulsed stimulation on cell growth independently on the used models (BM-MSC from osteoporotic rabbits [34] and osteoblast-like MC3T3-E1 cells [36]) and at very different exposure times and peak intensities of PEMF (4–7 days/1 mT and 6–12 weeks/2 mT, respectively).
3.2. In Vivo Studies
3.2.1. Static Magnetic Fields from Permanent Magnets
Six studies investigated the effect of SFM on implant osseointegration in vivo: one in rats, four in rabbits, and one in dogs. In addition to in vitro results, He et al. [31] also reported a positive effect of moderate SMF on histologically evaluated osteogenesis and osteointegration of the 3D-printed Ti scaffolds implanted in mandibular rats.
Kim et al. [46] demonstrated that Ti implants receiving SFM from a magnetic cover screw (Neodymium magnet generating a magnetism of 15 mT) showed a higher mean percentage of bone-to-implant contact (BIC) than the control groups at all investigated time-points (1, 4 and 8 weeks) and improved peri-implant bone formation in rabbits. Furthermore, by using microarrays the authors found an upregulation of 293 gene transcripts of the 20,000 analyzed, many of which have been described as participating in bone formation, angiogenesis and ECM-deposition processes. Moreover, the SFM-induced transcripts also included genes related to osteoclasts and bone resorption, highlighting very puzzling and sometimes contrasting effects of SFM in the formation and maintenance of bone around dental implants.
Further studies confirmed the BIC increase after SFM exposure at 15 and 30 days [45] and at 12 weeks [44], respectively. In the study by Bambini et al. [45], dental implants inserted in the tibia of New Zealand rabbits after SFM stimulation showed a higher BIC both in the earlier and in the later osseointegration period, supporting the ability of SFM to reduce the bone healing period. Naito et al. used similar implants placed into rabbit femurs and confirmed a faster osseointegration by measuring BIC after 12 weeks of healing. In fact BIC values were significantly higher in the test group compared to the controls (32.4 ± 16.6% and 17.1 ± 4.5%, respectively) [44].
Some benefits in the use of static magnetism were reported by Leesungbok et al. [47] when studying the osseointegration of commercial sandblasted/large-grit/acid-etched–treated Ti implants inserted in rabbit tibia with or without a neodymium magnet on the cover screw. Indeed, the SFM was shown to increase BIC in implants just after 3 weeks, although at longer times (6 weeks) BIC values remained almost unchanged, both in the test and control groups.
The only in vivo study evaluating the effects of SFM in the jawbone was performed by Li et al. [43] on dogs. The authors designed a Ti implant with sand-blasted surfaces and with a magnet inside (mTi) able to generate a moderate SFM (0.3–9.4 mT middle position and 0.2–1.4 mT upper-lower position within 5 mm from the implant), avoiding the use of a fixed external magnetic source. The effect of a filling of superparamagnetic hydroxyapatite (HYH-Fe) added directly around the implant was also evaluated. Thus, Ti + hydroxyapatite (HA), mTi + HA and mTi + superparamagnetic hydroxyapatite (HYH-Fe) implants were inserted into the jawbone of two dogs and the formation of new bone was evaluated both by histological analysis and by sequential fluorescent labeling at 8 and 12 weeks. This preliminary study reported increased trabecular bone formation around mTi implants with HYH-Fe compared to the other tested combinations.
3.2.2. Pulsed ElectroMagnetic Fields
Two studies focused on the effects of PEMF on implant osseointegration in rats.
Grana et al. [51] found that rats receiving PEMF twice a day (in sessions of 30 min) had increased ossification and BIC percentages after 20 days. Recently, Nunes’s group [48] showed that PEMF exposure positively affected bone parameters such as volume percentage, trabecular thickness and bone mineral density (BMD) and also removal torque and BIC of implants placed in rats. However, different times of exposure per day led to different results at various follow-ups.
Eight studies evaluated the effects of PEMF on implant osseointegration in rabbits.
Barak et al. [50] demonstrated that implants with a PEMF-emitting healing cap showed a 48% and 42% greater BIC (after 2 and 4 weeks, respectively) compared to implants without exposure. More specifically, the authors also reported that BIC was not statistically affected by PEMF in the apical region, suggesting a putative relationship between the area of bone regeneration and the distance from the PEMF emitter.
The dependence of the BIC on the intensity of the PEMF and on the time-interval of exposure was investigated by Matsumoto et al. [55]. Specifically, rabbits with implants treated with different intensities of PEMF (0.2 mT–0.3 mT–0.8 mT) showed increased BIC compared to animals with unexposed implants. We note that results seemed not to show dependence on PEMF intensity, treatment schedule and duration (measurements taken up to four weeks). Conversely, at longer exposure time (8 week treatment), Ozen et al. [53] demonstrated that there was a higher number of osteoblasts and new trabecular bone formation around implants exposed to PEMF compared to implants without PEMF exposure. The influence of exposure cycle, in terms of hours/day, was also tested: Ijiri et al. [56] found that rabbits receiving PEMF stimulation (10 or 5 h/day) showed greater bone formation around porous Ti implants compared to unexposed controls and that 10 h/day exposure led to a greater bone formation compared to 5 h/day exposure. Similarly, Jing et al. [36] found that implants placed in rabbits receiving PEMF (2 mT; 75 Hz) increased new trabecular bone formation compared to controls without PEMF. PEMF also stimulated the expression of osteogenic markers, such as RUNX2, BMP2 and OCN. In contrast to the reported observations, the histological evaluation by Buzzà et al. [54] showed no difference in terms of peri-implant bone and removal torque between implants with or without PEMF stimulation, although details of important variables such as duration and intensity of the electromagnetic stimulation were not provided.
Studies on rabbits affected by systemic pathologies are also available. Diabetic rabbits receiving PEMF stimulation showed better results in terms of peri-implant bone formation and bone histomorphometry parameters compared to the diabetic control group [21]. In a different study [49], the same authors found that in glucocorticoid-induced osteoporotic rabbits PEMF exposure significantly affected the peri-implant bone formation around porous Ti implants, leading to a histomorphometry of the PEMF-exposed glucocorticoid treated animals similar to that of the controls (healthy rabbits). The authors also evaluated the effects of PEMF on osteoblast and osteocyte functionality by assessing circulating levels of osteoblast-related factors such as osterix (OSX), OCN, RUNX-2 and bone-resorbing cytokines (i.e., serum TRAcP5b and CTX-1) showing a PEMF-dependent increase in their level [49]. In addition, Ye et al. [34] found that PEMF stimulation (1 mT 2 h/day) led to increased bone formation, measured by microcomputed tomography and histomorphometry, on the porous surface of Ti implants placed in the femurs of osteoporotic rabbits after 6 and 12 weeks. Conversely, Akca et al. [52] found that peri-implant bone volume of osteoporotic rabbits receiving PEMF stimulation 4 h/day was not improved by the treatment and was similar to the peri-implant bone of osteoporotic animals not receiving stimulation.
3.3. Clinical Studies
3.3.1. Static Magnetic Fields from Permanent Magnets
Only two studies have clinically evaluated the effects of SFM on dental implant osseointegration, reaching similar results with respect to its advantageous effects. Gujjalpudi et al. [57] reported a significant increase in stability, measured by implant stability quotient (ISQ), for implants receiving SFM (50–245 mT intensities) from circular isotrophic Neodymium-Iron-Boron magnets placed in the denture vs. unexposed implants at 1 (73. 25 ± 4.53 test, 68.45 ± 4.46 control), 2 (76.05 ± 4.26 test, 72.05 control) and 3 months (78.95 ± 3.50 test, 74.45 ± 3.83 control) after positioning.
The second clinical study showed that immediately placed maxillary implants had signifantly higher ISQ values measured by resonance frequency analysis (RFA) when exposed to SFM compared to unexposed implants only after the first month of healing (55.0 ± 1.2 test, 51.3 ± 4.9 control). Furthermore, the control group showed also more bone loss in the second month (0.30 ± 0.10 test, 0.39 ± 0.16, control), while at 3 months both groups had similar bone levels [58].
3.3.2. Pulsed ElectroMagnetic Fields
Four clinical studies focused on the effects of PEMF on implant osseointegration by using emitting healing caps and other PEMF emitting devices. Two studies are RCTs, one is a CCT and one is a retrospective study. The first RTC study [62], pioneered the evaluation of PEMF advantages in clinical settings, showing that implants exposed to PEMF (2 h/day for 12 days with a frequency of 2 Hz applied in the first hour, 4 Hz in the second hour) had a higher radiodensity immediately postoperatively and until 1, 3, 6, and 12 months compared to unexposed ones. Also, a significantly lower bone loss was measured up to 1 year follow-up, but no statistical differences were observed regarding the stability of the implants, measured by RFA, probably due to the small size of the analyzed groups. In 2020, Nayak et al. [60] in their RCT found that implants receiving a healing abutment able to provide a PEMF stimulation (0.05–0.5 mT), showed an increased stability, measured by RFA, and less bone loss compared to implants not receiving PEMF up to 6 months of follow-up. An increased implant stability due to PEMF exposure was also demonstrated in the retrospective CCT by Barak et al. [61], in which the authors also analyzed some differences regarding the maxillary and mandibular location of tested implants. However, Bud et al. [59] did not find statistically significant differences in terms of bone radiodensity measured by CBCT at 60 days between implants receiving PEMF from healing caps and implants with conventional healing caps.
4. Discussion
To the best of our knowledge, this is the first PRISMA-driven scoping review which analyzes the effects of MFs on the dental implant osseointegration. Considering that clinical studies are still limited in number, are pioneering and are not supported by a solid and unequivocal preclinical assessment, a scoping review approach was chosen [63,64] in order to identify and analyse the available evidence, but also knowledge gaps, and to provide indications for future research.
It must be emphasised that an assessment of applied protocols indicates wide intensity and frequency ranges for the MFs used, with some studies not even reporting any MF characteristics. This makes any accurate results comparison extremely difficult. In addition, the cellular models vary considerably in the studies with both cell-lines and primary cultures used to test the in vitro effects of MFs. Particularly relevant are the investigations performed on primary hbMSC. These multipotent cells can differentiate into osteoblasts and therefore represent a particularly suitable system for evaluating the interference of chemical and physical agents on osteogenesis [65,66,67,68].
Regarding the analysis of the effect of the MFs on the implant surface adhesion, both PEMF and SFM were effective in promoting the adhesion on Ti surfaces. Some differences emerge between the SFM and PEMF effects on the proliferation rate of exposed cells: in general, all the results from PEMF application show an increased proliferation rate compared to unexposed controls, regardless of the cell type used. Conversely, the application of an SFM determined no significant differences or even a reduction of the proliferation rate [32].
With regard to implant materials, some studies highlight the influence of the topographic and chemical properties of the implant surface on PEMF-induced cell response. In particular, nano-rough surface topographies proved to be the most effective in inducing early cell adhesion, early cell proliferation and osteogenic differentiation under PEMF stimulation [37], Furthermore, cell viability, proliferation, and early differentiation was significantly more pronounced on osteoblasts cultured on the cpTi surface compared to TiZr discs [38].
Finally, both PEMF and SFM appear to increase the osteoblast function in terms of upregulation of genes related to osteogenic differentiation, with a concomitant deposition of mineralized ECM [34,35,37,40,41,42,49].
The mechanisms through which MFs stimulate osteogenesis have been the object of several studies (reviewed in Galli et al., 2019 [69] and Zhang et al., 2020 [70]). A direct effect seems to be exerted on the cell membrane, involving sensor structures (i.e., primary cilia) and ion, particularly calcium, flux. While PEMF treatment directly applies electric currents, SFMs can generate a biological-derived EMF with a cascade of intracellular signaling pathways. Activation of, among others, Ca- Calmodulin, PKA, MAPK, WnT and BMP-SMADs pathways have been reported as responsible for MF-dependent induction of osteogenesis markers, although they are extremely dependent on the physical parameters of the applied MF and on the cellular context, i.e., cell type, developmental stage and tissue environment [70]. From the analysis of the studies selected for this review, it appears that PEMF might improve bone anabolism through canonical Wnt/β-catenin signaling [21], while activation of BMP-SMADs signaling pathway appears to be involved in SMF-induced osteogenesis [31].
According to the pro-osteogenetic effects observed in vitro, animal studies evaluating the effects of SFM on osseointegration reported higher BIC values for implants receiving SFM compared to controls [44,45,46]. However, more precise kinetic studies should be performed in order to characterize such effects in the different phases of osteogenesis, since no univocal results about have yet been provided [47]. The majority of animal investigations that focused on PEMF showed a higher BIC in animals that received PEMF stimulation compared to the control groups [48,50,51,55] with high variability depending on factors such as the times of exposure, intensity, emitter distance, as well as the animal model employed, which makes any accurate results comparison extremely difficult.
The effects of PEMF were also investigated on implants placed in animals affected by systemic pathologies such as osteoporosis and diabetes. Uncontrolled diabetes [71] and also impairments of systemic bone metabolism may be risk factors for osseointegration and its maintenance over time [72]. Interestingly, the majority of the analysed studies [21,34,49], demonstrated that PEMF treatment is able to reverse or reduce the negative influence of such systemic pathologies on bone tissue and therefore on the osseointegration process.
Clinical studies still appear to be very limited in number and do not show univocal results.
Successful osseointegration is a prerequisite for functional dental implants and primary implant stability is a prerequisite for successful osseointegration. Primary implant stability is a mechanical phenomenon related to local bone quality and quantity, the type of implant and the placement technique used. Secondary implant stability depends on the bone formation and remodeling at the implant/tissue interface and in the surrounding bone during the osseointegration process [1].
The majority of clinical investigations regarding PEMF application showed positive effects on implant osseointegration, with an increased implant stability, a higher perimplant radiodensity and a significantly lower bone loss compared to controls [60,61,62]. Bud et al. [59] did not report statistically significant differences in radiodensity around implants (considered as an approximation of actual bone contact with implants) receiving PEMF stimulation or not.
Regarding SFM stimulation, the still limited available clinical studies showed a statistically significant higher stability of implants exposed to SFM compared to controls in the early phase of osseointegration (first month) [57,58], whereas no effect has been described at longer time intervals (2–3 months) [58].
The transition from primary to secondary stability is one of the crutial phase during implant osseointegration [73]. The chance to enhance and accelerate implant stability during such a delicate transition phase by means of MFs, makes the abovementioned results particularly interesting and deservable of more in-depth investigations.
Concerning the aspects of handling of possible MF devices for clinical use, it must be considered that SFM, differently from PEMF, does not depend on external electrical supplies, thereby avoiding the risk for heat or electric hazards to tissues [74]. Thus, the application of SFM to dentistry might be of benefit.
Other review articles from Qi et al. [26] and Lew et al. [17] focused on the application of magnetic fields in implant dentistry, highlighting, similar to that which emerged from the present review, some positive effects exerted by SFM and PEMF on dental implant osseointegration. Qi et al., however, dealt exclusively with PEMF stimulation. Differently from the previous reviews, the present scoping review has been conducted following the PRISMA protocol, leading us to the individuation and the inclusion of a higher number of articles with the aim of providing a wider perspective on the subject, from the preclinical evidence to the clinical one.
4.1. Limitations of Available Scientific Research
The major limitations of all the available pre-clinical studies on MFs are related to their extemely high heterogeneity in terms of implant surface and composition, intensity and duration of MF stimulations, experimental conditions and cell types or animal models used, as well as of the parameters and outcomes analyzed (proliferation rate, cell adhesion, ECM deposition, ECM mineralization, cell morphology, markers of differentiation). Also, clinical studies, for whom relevance is per se limited by the low number of participants, and their moderate risk of bias, adopt very different and unstandardized stimulation protocols, and different experimemtal clinical conditions (edentulous site location, implant characteristics, follow-up, outcomes). All these aspects significantly impair a reliable inter-study comparison and analysis.
4.2. Indications for Future Research
Further in vitro investigations are needed, aiming, in particular, to unravel the biochemical keys of MF effects on intracellular pathways, cell morphology and cytoskeleton (actin filament, vimentin intermediate filaments, and microtubules) remodelling, in order to clarify how MFs interact with bone cells.
Future preclinical studies should also aim to evaluate how MFs influence osseointegration in relation to factors such as implant surface, or bone-affecting systemic conditions.
Additional controlled clinical trials with well-defined protocols are required: such studies should adopt a standardized and more accurate control method for implant stability, in order to better define the influence of MFs on dental implant osseointegration, in particular during the transition from primary to secondary stability, and their possible clinical applications.
All the future studies should also accurately evaluate how MF parameters such as intensity, amplitude, frequency, can variate the effects of the stimulation. Statements regarding the rationale behind the choice of stimulation parameters would represent an added value in any study. Alternatively, a comparative assessment of the stimulation amplitudes and frequencies should be pursued as a routine tool.
5. Conclusions
The high heterogeneity in methodological approaches and related results of in vivo and in vitro studies makes a translation to clinical settings extremely difficult. From in vitro studies, a positive effect of PEMF on bone cells proliferation emerged, and both PEMF and SFM showed a pro-osteogenic effect, also with an improved adhesion to Ti surfaces.
Also, in vivo studies showed an overall positive effect of magnetic stimulation on the osseointegration of Ti implants in terms of increased bone-to-implant contact rate.
As regards available clinical studies, the majority of them show an early increase in the levels of implant stability under MF stimulation, allowing us to speculate a positive influence of MFs on the transition from primary to secondary stability. However, more well-designed in vitro, in vivo and clinical studies perfomed according to the aforementioned indications for future research, are needed in order to better understand the influence of MFs on dental implant osseointegration and to evaluate their possible clinical application.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/app12094496/s1, Table S1: PRISMA ScR checklist; Table S2: Excluded studies and reasons for exclusion.
Author Contributions
Conceptualization L.G., A.B., G.C. and D.B.; methodology L.G., A.B., G.C., D.B., M.A. and A.F.; study selection P.A.V. and E.S.; data extraction P.A.V. and E.S.; data analysis M.A., A.F., G.C., D.B., L.G. and A.B.; writing—original draft preparation. G.C., D.B., M.A., A.F., N.C., F.D.R., A.P., E.S., P.A.V., L.Z., L.G. and A.B.; writing—review and editing G.C., D.B., M.A., A.F., N.C., F.D.R., A.P., L.Z., L.G. and A.B.; supervision L.G., A.B., G.C. and D.B.; All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Zarb, G.; Worthington, P.; Eriksson, A. The long-term efficacy of currently used dental implants: A review and proposed criteria of success. Int. J. Oral Maxillofac. Implants 1986, 1, 11–25. [Google Scholar] [PubMed]
- Overmann, A.L.; Aparicio, C.; Richards, J.T.; Mutreja, I.; Fischer, N.G.; Wade, S.M.; Potter, B.K.; Davis, T.A.; Bechtold, J.E.; Forsberg, J.A.; et al. Orthopaedic osseointegration: Implantology and future directions. J. Orthop. Res. 2020, 38, 1445–1454. [Google Scholar] [CrossRef] [PubMed]
- Grzeskowiak, R.M.; Schumacher, J.; Dhar, M.S.; Harper, D.P.; Mulon, P.Y.; Anderson, D.E. Bone and cartilage interfaces with orthopedic implants: A literature review. Front. Surg. 2020, 7, 601244. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Albrektsson, T.; Brånemark, P.I.; Hansson, H.A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta Orthop. 1981, 52, 155–170. [Google Scholar] [CrossRef]
- Goiato, M.C.; Dos Santos, D.M.; Santiago, J.F.; Moreno, A.; Pellizzer, E.P. Longevity of dental implants in type IV bone: A systematic review. Int. J. Oral Maxillofac. Surg. 2014, 43, 1108–1116. [Google Scholar] [CrossRef]
- Esposito, M.; Hirsch, J.M.; Lekholm, U.; Thomsen, P. Biological factors contributing three major determinants for late implant failures in the Brånemark system. Eur. J. Oral Sci. 1998, 106, 527–551. [Google Scholar] [CrossRef]
- Huynh-Ba, G.; Friedberg, J.R.; Vogiatzi, D.; Ioannidou, E. Implant failure predictors in the posterior maxilla: A retrospective study of 273 consecutive implants. J. Periodontol. 2008, 79, 2256–2261. [Google Scholar] [CrossRef]
- Sverzut, A.T.; Stabile, G.A.V.; de Moraes, M.; Mazzonetto, R.; Moreira, R.W.F. The influence of tobacco on early dental implant failure. J. Oral Maxillofac. Surg. 2008, 66, 1004–1009. [Google Scholar] [CrossRef]
- Urban, T.; Kostopoulos, L.; Wenzel, A. Immediate implant placement in molar regions: Risk factors for early failure. Clin. Oral Implants Res. 2012, 23, 220–227. [Google Scholar] [CrossRef] [PubMed]
- Cooper, L.F. Endosseous implants. J. Am. Dent. Assoc. 2001, 132, 1452. [Google Scholar]
- Lupi, S.M.; Torchia, M.; Rizzo, S. Biochemical modification of titanium oral implants: Evidence from in vivo studies. Materials 2021, 14, 2798. [Google Scholar] [CrossRef] [PubMed]
- Yeo, I.S.L. Modifications of dental implant surfaces at the micro- and nano-level for enhanced osseointegration. Materials 2019, 13, 89. [Google Scholar] [CrossRef]
- Wennerberg, A.; Albrektsson, T. On implant surfaces: A review of current knowledge and opinions. Int. J. Oral Maxillofac. Implants 2010, 25, 63–74. [Google Scholar]
- Annunziata, M.; Guida, L. The effect of titanium surface modifications on dental implant osseointegration. Front. Oral Biol. 2015, 17, 62–77. [Google Scholar]
- Lew, W.Z.; Feng, S.W.; Lee, S.Y.; Huang, H.M. The review of bioeffects of static magnetic fields on the oral tissue-derived cells and its application in regenerative medicine. Cells 2021, 10, 2662. [Google Scholar] [CrossRef]
- Costantini, E.; Sinjari, B.; D’Angelo, C.; Murmura, G.; Reale, M.; Caputi, S. Human gingival fibroblasts exposed to extremely low-frequency electromagnetic fields: In vitro model of wound-healing improvement. Int. J. Mol. Sci. 2019, 20, 2108. [Google Scholar] [CrossRef]
- Androjna, C.; Fort, B.; Zborowski, M.; Midura, R.J. Pulsed electromagnetic field treatment enhances healing callus biomechanical properties in an animal model of osteoporotic fracture. Bioelectromagnetics 2014, 35, 396–405. [Google Scholar] [CrossRef]
- Liu, Y.; Hao, L.; Jiang, L.; Li, H. Therapeutic effect of pulsed electromagnetic field on bone wound healing in rats. Electromagn. Biol. Med. 2021, 40, 26–32. [Google Scholar] [CrossRef]
- Cai, J.; Li, W.; Sun, T.; Li, X.; Luo, E.; Jing, D. Pulsed electromagnetic fields preserve bone architecture and mechanical properties and stimulate porous implant osseointegration by promoting bone anabolism in type 1 diabetic rabbits. Osteoporos. Int. 2018, 29, 1177–1191. [Google Scholar] [CrossRef] [PubMed]
- Grace, K.L.R.; Revell, W.J.; Brookes, M. The effects of pulsed electromagnetism on fresh fracture healing: Osteochondral repair in the rat femoral groove. Orthopedics 1998, 21, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Glazer, P.A.; Heilmann, M.R.; Lotz, J.C.; Bradford, D.S. Use of electromagnetic fields in a spinal fusion: A rabbit model. Spine 1997, 22, 2351–2356. [Google Scholar] [CrossRef] [PubMed]
- Takano-Yamamoto, T.; Kawakami, M.; Sakuda, M. Effect of a pulsing electromagnetic field on demineralized bone-matrix-induced bone formation in a bony defect in the premaxilla of rats. J. Dent. Res. 1992, 71, 1920–1925. [Google Scholar] [CrossRef]
- Bruce, G.K.; Howlett, C.R.; Huckstep, R.L. Effect of a static magnetic field on fracture healing in a rabbit radius. Preliminary results. Clin. Orthop. Relat. Res. 1987, 222, 300–306. [Google Scholar] [CrossRef]
- Qi, Y.; Zhang, S.; Zhang, M.; Zhou, Z.; Zhang, X.; Li, W.; Cai, H.; Zhao, B.C.; Lee, E.S.; Jiang, H.B. Effects of physical stimulation in the field of oral health. Scanning 2021, 2021, 5517567. [Google Scholar] [CrossRef]
- International Commission on Non-Ionizing Radiation Protection (ICNIRP). Guidelines for limiting exposure to electromagnetic fields (100 kHz to 300 GHz). Health Phys. 2020, 118, 483–524. [Google Scholar] [CrossRef]
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. PRISMA extension for scoping reviews (PRISMA-ScR): Checklist and explanation. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef]
- Sterne, J.A.; Hernán, M.A.; Reeves, B.C.; Savović, J.; Berkman, N.D.; Viswanathan, M.; Henry, D.; Altman, D.G.; Ansari, M.T.; Boutron, I.; et al. ROBINS-I: A tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016, 355, i4919. [Google Scholar] [CrossRef]
- Sterne, J.A.C.; Savović, J.; Page, M.J.; Elbers, R.G.; Blencowe, N.S.; Boutron, I.; Cates, C.J.; Cheng, H.Y.; Corbett, M.S.; Eldridge, S.M.; et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 2019, 366, I4898. [Google Scholar] [CrossRef]
- He, Y.; Yu, L.; Liu, J.; Li, Y.; Wu, Y.; Huang, Z.; Wu, D.; Wang, H.; Wu, Z.; Qiu, G. Enhanced osteogenic differentiation of human bone-derived mesenchymal stem cells in 3-dimensional printed porous titanium scaffolds by static magnetic field through up-regulating Smad4. FASEB J. 2019, 33, 6069–6081. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Memè, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of supercharged cover screw as static magnetic field generator for bone healing, 1st part: In vitro enhancement of osteoblast-like cell differentiation. J. Biol. Regul. Homeost. Agents 2017, 31, 215–220. [Google Scholar] [PubMed]
- Kim, H.J.; Chang, I.T.; Heo, S.J.; Koak, J.Y.; Kim, S.K.; Jang, J.H. Effect of magnetic field on the fibronectin adsorption, cell attachment and proliferation on titanium surface. Clin. Oral Implants Res. 2005, 16, 557–562. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Liu, W.; Yan, L.; Cheng, S.; Li, X.; Qiao, S. 3D-printed Ti6Al4V scaffolds combined with pulse electromagnetic fields enhance osseointegration in osteoporosis. Mol. Med. Rep. 2021, 23, 410. [Google Scholar] [CrossRef]
- Bloise, N.; Petecchia, L.; Ceccarelli, G.; Fassina, L.; Usai, C.; Bertoglio, F.; Balli, M.; Vassalli, M.; De Angelis, M.G.C.; Gavazzo, P.; et al. The effect of pulsed electromagnetic field exposure on osteoinduction of human mesenchymal stem cells cultured on nano-TiO2 surfaces. PLoS ONE 2018, 13, e0199046. [Google Scholar] [CrossRef]
- Jing, D.; Zhai, M.; Tong, S.; Xu, F.; Cai, J.; Shen, G.; Wu, Y.; Li, X.; Xie, K.; Liu, J.; et al. Pulsed electromagnetic fields promote osteogenesis and osseointegration of porous titanium implants in bone defect repair through a Wnt/β-catenin signaling-associated mechanism. Sci. Rep. 2016, 6, 32045. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; An, Y.; Li, F.; Li, D.; Jing, D.; Guo, T.; Luo, E.; Ma, C. The effects of pulsed electromagnetic field on the functions of osteoblasts on implant surfaces with different topographies. Acta Biomater. 2014, 10, 975–985. [Google Scholar] [CrossRef] [PubMed]
- Atalay, B.; Aybar, B.; Ergüven, M.; Emes, Y.; Bultan, Ö.; Akça, K.; Yalçin, S.; Baysal, U.; Işsever, H.; Çehreli, M.C.; et al. The effects of pulsed electromagnetic field (PEMF) on osteoblast-like cells cultured on titanium and titanium-zirconium surfaces. J. Craniofac. Surg. 2013, 24, 2127–2134. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Sbarra, M.S.; Visai, L.; De Angelis, M.G.C.; Mazzini, G.; Benazzo, F.; Magenes, G. Ultrasonic and electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto a titanium plasma-spray surface. Tissue Eng. Part C Methods 2009, 15, 233–242. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Magenes, G. Electromagnetically enhanced coating of a sintered titanium grid with human SAOS-2 osteoblasts and extracellular matrix. In Proceedings of the 2008 30th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Vancouver, BC, Canada, 20–25 August 2008; pp. 3582–3585. [Google Scholar]
- Fassina, L.; Saino, E.; Visai, L.; Silvani, G.; De Angelis, M.G.C.; Mazzini, G.; Benazzo, F.; Magenes, G. Electromagnetic enhancement of a culture of human SAOS-2 osteoblasts seeded onto titanium fiber-mesh scaffolds. J. Biomed. Mater. Res. Part A 2008, 87, 750–759. [Google Scholar] [CrossRef]
- Fassina, L.; Saino, E.; Visai, L.; Magenes, G. Physically enhanced coating of a titanium plasma-spray surface with human SAOS-2 osteoblasts and extracellular matrix. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; pp. 6415–6418. [Google Scholar]
- Li, X.; Wu, J.; Li, D.; Zou, Q.; Man, Y.; Zou, L.; Li, W. Pro-osteogenesis and in vivo tracking investigation of a dental implantation system comprising novel mTi implant and HYH-Fe particles. Bioact. Mater. 2021, 6, 2658–2666. [Google Scholar] [CrossRef] [PubMed]
- Naito, Y.; Yamada, S.; Jinno, Y.; Arai, K.; Galli, S.; Ichikawa, T.; Jimbo, R. Bone-forming effect of a static magnetic field in rabbit femurs. Int. J. Periodontics Restor. Dent. 2019, 39, 259–264. [Google Scholar] [CrossRef] [PubMed]
- Bambini, F.; Santarelli, A.; Putignano, A.; Procaccini, M.; Orsini, G.; Diiorio, D.; Meme, L.; Sartini, D.; Emanuelli, M.; Lo Muzio, L. Use of supercharged cover screw as static magnetic field generator for bone healing, 2nd part: In vivo enhancement of bone regeneration in rabbits. J. Biol. Regul. Homeost. Agents 2017, 31, 481–485. [Google Scholar] [PubMed]
- Kim, E.C.; Leesungbok, R.; Lee, S.W.; Hong, J.Y.; Ko, E.J.; Ahn, S.J. Effects of static magnetic fields on bone regeneration of implants in the rabbit: Micro-CT, histologic, microarray, and real-time PCR analyses. Clin. Oral Implants Res. 2017, 28, 396–405. [Google Scholar] [CrossRef] [PubMed]
- Leesungbok, R.; Ahn, S.J.; Lee, S.W.; Park, G.H.; Kang, J.S.; Choi, J.J. The effects of a static magnetic field on bone formation around a sandblasted, large-grit, acid-etched-treated titanium implant. J. Oral Implantol. 2013, 39, 248–255. [Google Scholar] [CrossRef]
- Nunes, C.M.M.; Ferreira, C.L.; Bernardo, D.V.; Lopes, C.C.R.; Collino, L.; da Silva Lima, V.C.; de Camargo Reis Mello, D.; de Vasconcellos, L.M.R.; Jardini, M.A.N. Evaluation of pulsed electromagnetic field protocols in implant osseointegration: In vivo and in vitro study. Clin. Oral Investig. 2021, 25, 2925–2937. [Google Scholar] [CrossRef]
- Cai, J.; Shao, X.; Yang, Q.; Yang, Y.; Yan, Z.; Luo, E.; Feng, X.; Jing, D. Pulsed electromagnetic fields modify the adverse effects of glucocorticoids on bone architecture, bone strength and porous implant osseointegration by rescuing bone-anabolic actions. Bone 2020, 133, 115266. [Google Scholar] [CrossRef]
- Barak, S.; Neuman, M.; Iezzi, G.; Piattelli, A.; Perrotti, V.; Gabet, Y. A new device for improving dental implants anchorage: A histological and micro-computed tomography study in the rabbit. Clin. Oral Implants Res. 2016, 27, 935–942. [Google Scholar] [CrossRef]
- Grana, D.R.; Marcos, H.J.A.; Kokubu, G.A. Pulsed electromagnetic fields as adjuvant therapy in bone healing and peri-implant bone formation: An experimental study in rats. Acta Odontol. Latinoam. 2008, 21, 77–83. [Google Scholar]
- Akca, K.; Sarac, E.; Baysal, U.; Fanuscu, M.; Chang, T.L.; Cehreli, M. Micro-morphologic changes around biophysically-stimulated titanium implants in ovariectomized rats. Head Face Med. 2007, 3, 28. [Google Scholar] [CrossRef][Green Version]
- Özen, J.; Atay, A.; Orucß, S.; Dalkiz, M.; Beydemir, B.; Develi, S. Evaluation of pulsed electromagnetic fields on bone healing after implant placement in the rabbit mandibular model. Turk. J. Med. Sci. 2004, 34, 91–95. [Google Scholar]
- Buzzá, E.P.; Shibli, J.A.; Barbeiro, R.H.; de Albergaria Barbosa, J.R. Effects of electromagnetic field on bone healing around commercially pure titanium surface: Histologic and mechanical study in rabbits. Implant Dent. 2003, 12, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, H.; Ochi, M.; Abiko, Y.; Hirose, Y.; Kaku, T.; Sakaguchi, K. Pulsed electromagnetic fields promote bone formation around dental implants inserted into the femur of rabbits. Clin. Oral Implants Res. 2000, 11, 354–360. [Google Scholar] [CrossRef]
- Ijiri, K.; Matsunaga, S.; Fukuyama, K.; Maeda, S.; Sakou, T.; Kitano, M.; Senba, I. The effect of pulsing electromagnetic field on bone ingrowth into a porous coated implant. Anticancer Res. 1996, 16, 2853–2856. [Google Scholar] [PubMed]
- Gujjalapudi, M.; Anam, C.; Mamidi, P.; Chiluka, R.; Gautamkumar, A.; Bibinagar, R. Effect of magnetic field on bone healing around endosseous implants—An in-vivo study. J. Clin. Diagn. Res. 2016, 10, ZF01–ZF04. [Google Scholar] [CrossRef] [PubMed]
- Siadat, H.; Bassir, S.H.; Alikhasi, M.; Shayesteh, Y.S.; Khojasteh, A.; Monzavi, A. Effect of static magnetic fields on the osseointegration of immediately placed implants: A randomized controlled clinical trial. Implant Dent. 2012, 21, 491–495. [Google Scholar] [CrossRef] [PubMed]
- Bud, E.S.; Bud, A.; Păcurar, M.; Vlasa, A.; Lazăr, A.P.; Lazăr, L. Clinical studies regarding electromagnetic stimulation in proximity of dental implants on patients with/without orthodontic treatment. J. Clin. Med. 2020, 9, 3983. [Google Scholar] [CrossRef]
- Nayak, B.P.; Dolkart, O.; Satwalekar, P.; Kumar, Y.P.; Chandrasekar, A.; Fromovich, O.; Yakobson, E.; Barak, S.; Dayube, U.; Shibli, J.A. Effect of the pulsed electromagnetic field (PEMF) on dental implants stability: A randomized controlled clinical trial. Materials 2020, 13, 1667. [Google Scholar] [CrossRef]
- Barak, S.; Matalon, S.; Dolkart, O.; Zavan, B.; Mortellaro, C.; Piattelli, A. Miniaturized electromagnetic device abutment improves stability of the dental implants. J. Craniofac. Surg. 2019, 30, 1055–1057. [Google Scholar] [CrossRef]
- EI Fadly, M.A.; Selim, H.A.; Katamish, M.A.; Metwally, S.A. Evaluation of the effect of pulsed electromagnetic fields on osseointegration of immediate dental implants. Egypt. J. Oral Maxillofac. Surg. 2014, 5, 84–91. [Google Scholar] [CrossRef]
- Munn, Z.; Peters, M.D.J.; Stern, C.; Tufanaru, C.; McArthur, A.; Aromataris, E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med. Res. Methodol. 2018, 18, 143. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, R.; Hall, B.J.; Doyle, J.; Waters, E. “Scoping the scope” of a cochrane review. J. Public Health 2011, 33, 147–150. [Google Scholar] [CrossRef] [PubMed]
- Bruder, S.P.; Fink, D.J.; Caplan, A.I. Mesenchymal stem cells in bone development, bone repair, and skeletal regeneration therapy. J. Cell. Biochem. 1994, 56, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Vater, C.; Kasten, P.; Stiehler, M. Culture media for the differentiation of mesenchymal stromal cells. Acta Biomater. 2011, 7, 463–477. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Speranza, M.C.; Scianguetta, S.; Tramontano, A.; Bencivenga, D.; Stampone, E.; Negri, A.; Nobili, B.; Locatelli, F.; et al. Iron overload enhances human mesenchymal stromal cell growth and hampers matrix calcification. Biochim. Biophys. Acta 2016, 1860, 1211–1223. [Google Scholar] [CrossRef]
- Borriello, A.; Caldarelli, I.; Bencivenga, D.; Stampone, E.; Perrotta, S.; Oliva, A.; Della Ragione, F. Tyrosine kinase inhibitors and mesenchymal stromal cells: Effects on self-renewal, commitment and functions. Oncotarget 2017, 8, 5540–5565. [Google Scholar] [CrossRef]
- Galli, C.; Pedrazzi, G.; Guizzardi, S. The cellular effects of pulsed electromagnetic fields on osteoblasts: A review. Bioelectromagnetics 2019, 40, 211–233. [Google Scholar] [CrossRef]
- Zhang, B.; Xie, Y.; Ni, Z.; Chen, L. Effects and mechanisms of exogenous electromagnetic field on bone cells: A review. Bioelectromagnetics 2020, 41, 263–278. [Google Scholar] [CrossRef]
- Naujokat, H.; Kunzendorf, B.; Wiltfang, J. Dental implants and diabetes mellitus—A systematic review. Int. J. Implant Dent. 2016, 2, 5. [Google Scholar] [CrossRef]
- De Medeiros, F.C.F.L.; Kudo, G.A.H.; Leme, B.G.; Saraiva, P.P.; Verri, F.R.; Honório, H.M.; Pellizzer, E.P.; Santiago Junior, J.F. Dental implants in patients with osteoporosis: A systematic review with meta-analysis. Int. J. Oral Maxillofac. Surg. 2018, 47, 480–491. [Google Scholar] [CrossRef]
- Raghavendra, S.; Wood, M.C.; Taylor, T.D. Early wound healing around endosseous implants: A review of the literature. Int. J. Oral Maxillofac. Implants 2005, 20, 425–431. [Google Scholar] [PubMed]
- Gaetani, R.; Ledda, M.; Barile, L.; Chimenti, I.; De Carlo, F.; Forte, E.; Ionta, V.; Giuliani, L.; D’Emilia, E.; Frati, G.; et al. Differentiation of human adult cardiac stem cells exposed to extremely low-frequency electromagnetic fields. Cardiovasc. Res. 2009, 82, 411–420. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).