Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Anti-Biofilm, and Cytotoxic Potential
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Biosynthesis of Silver Nanoparticles (AgNPs)
2.3. Characterization of Aw–AgNPs
2.4. Particle Size and Zeta Potential Analysis
2.5. Evaluation of Anti-Bacterial Activity
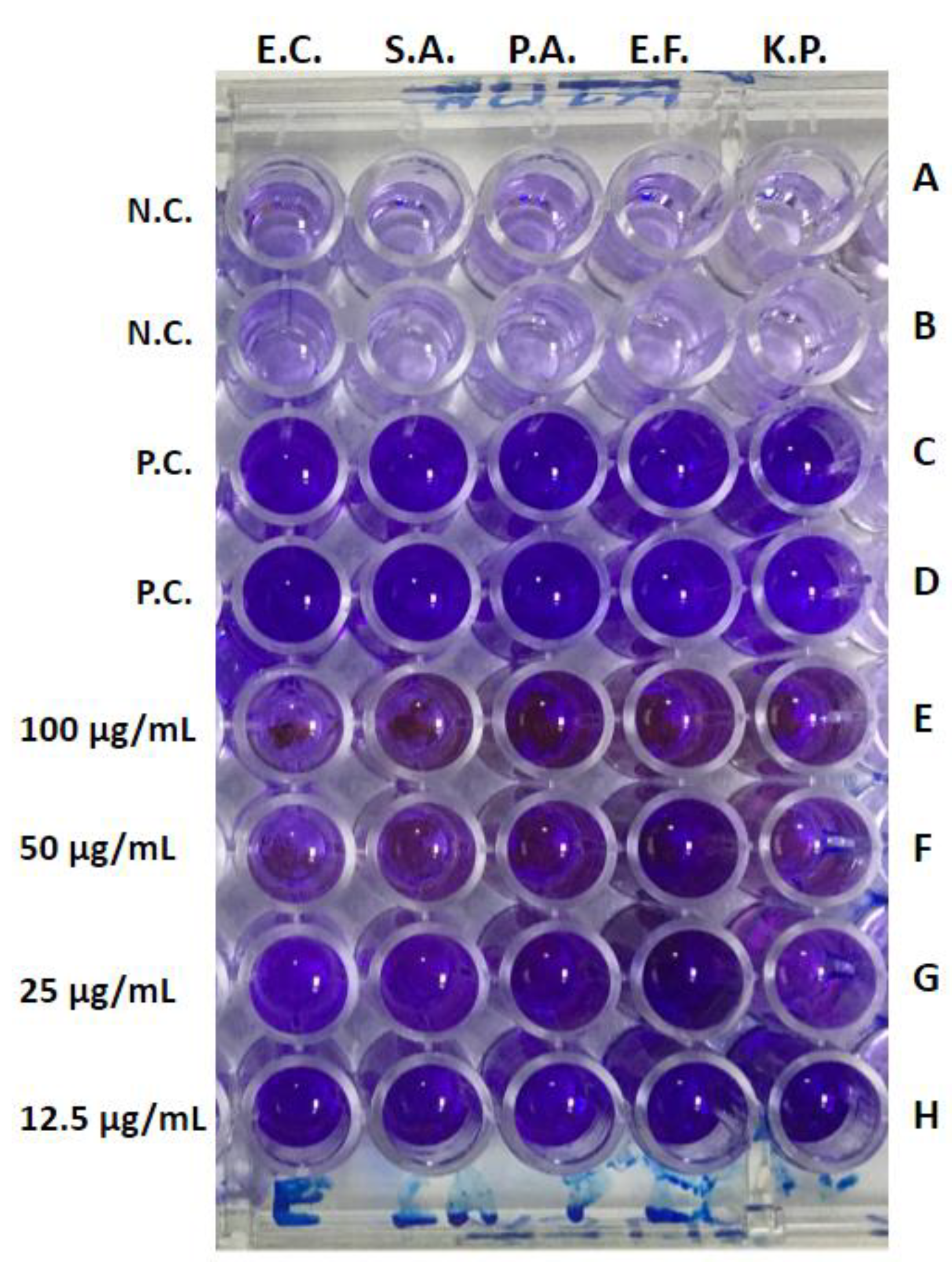
2.6. Detection of Anti-Biofilm Activity
2.7. Anti-Cancer Activity of Aw–AgNPs
2.7.1. Preparation of Cell Culture and Maintenance
2.7.2. Cell Viability Assay
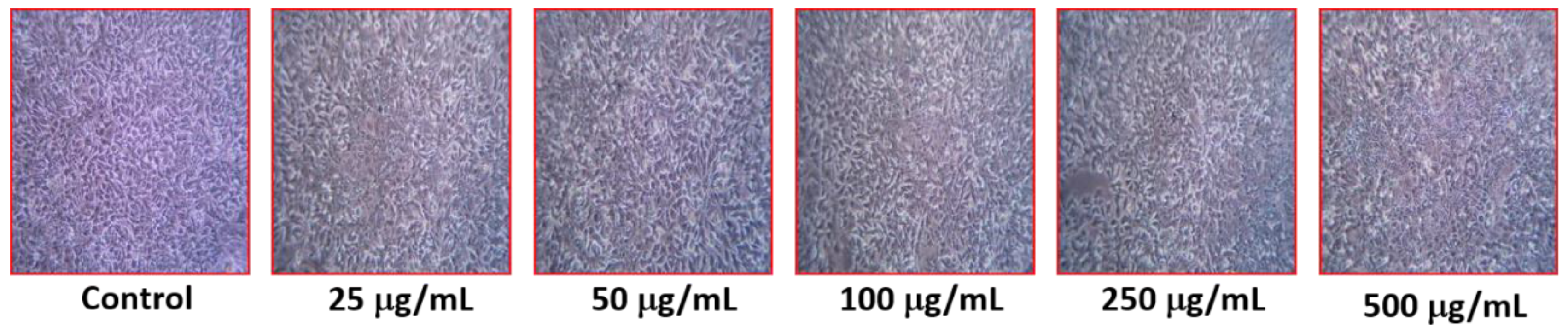
2.7.3. Measurement of Cyto-Morphological Changes in HCC-712
2.8. Statistical Analysis
3. Results and Discussion
3.1. UV–Vis Spectrophotometry
3.2. FTIR Spectroscopy
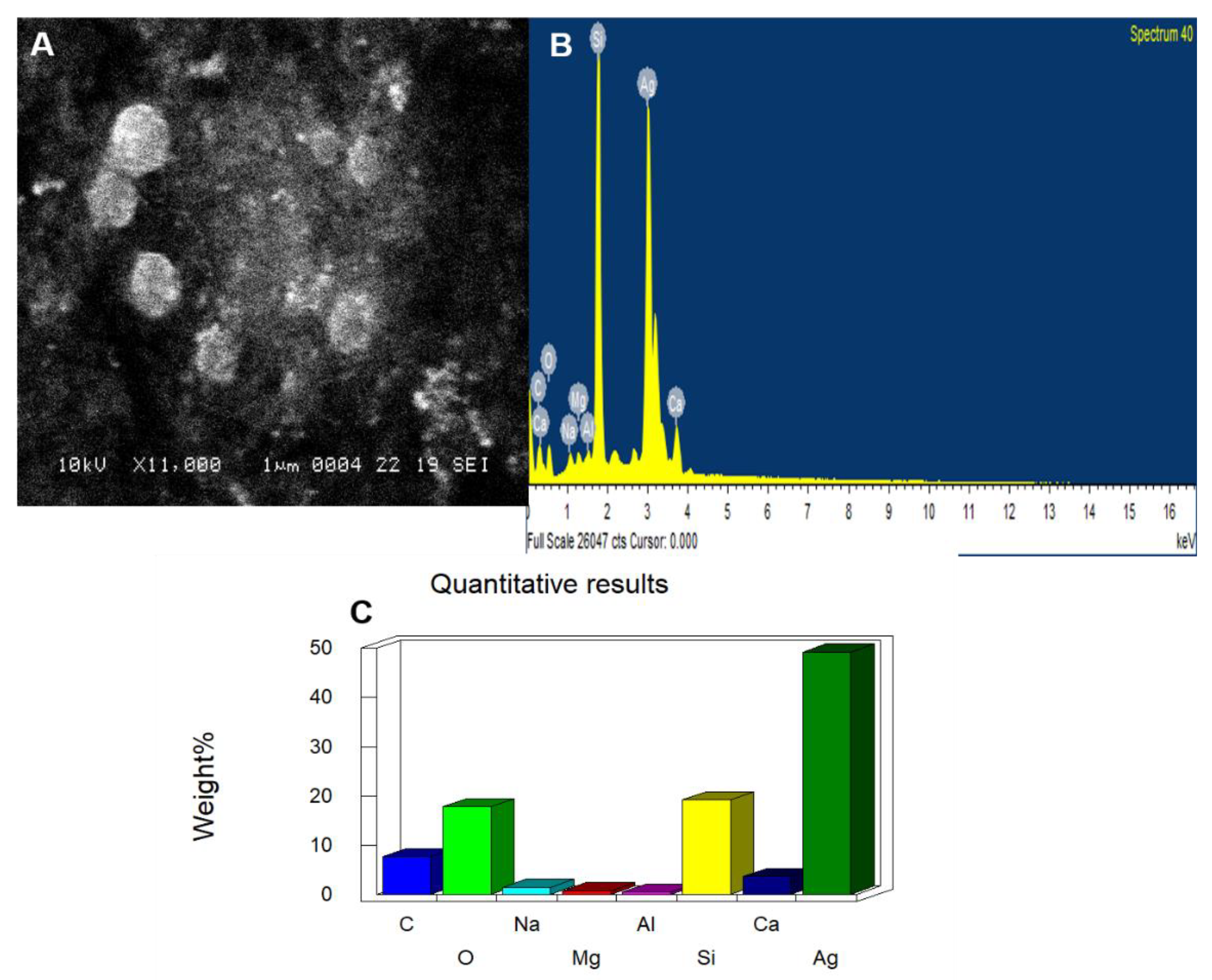
3.3. SEM and EDAX
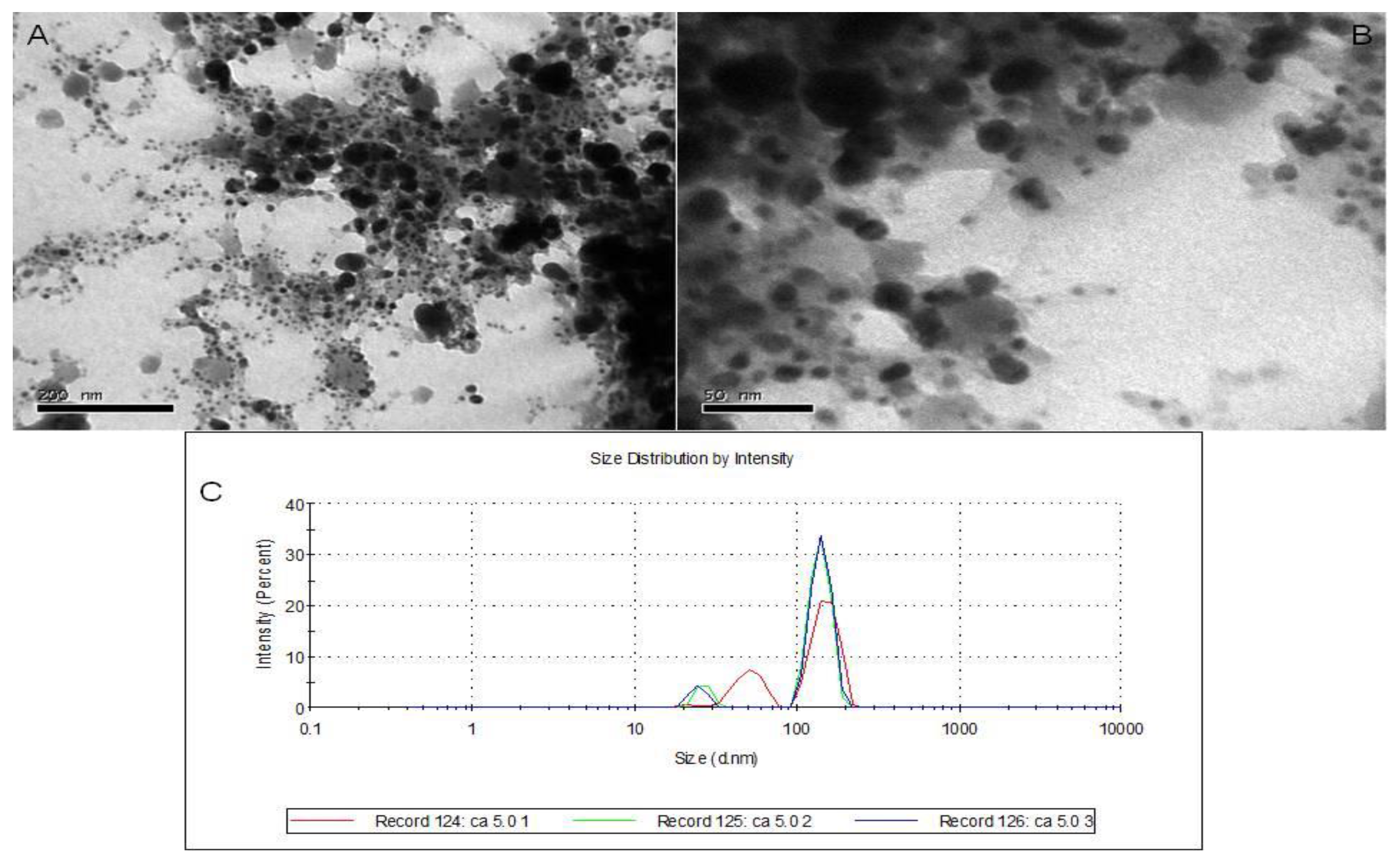
3.4. TEM Analysis and DLS
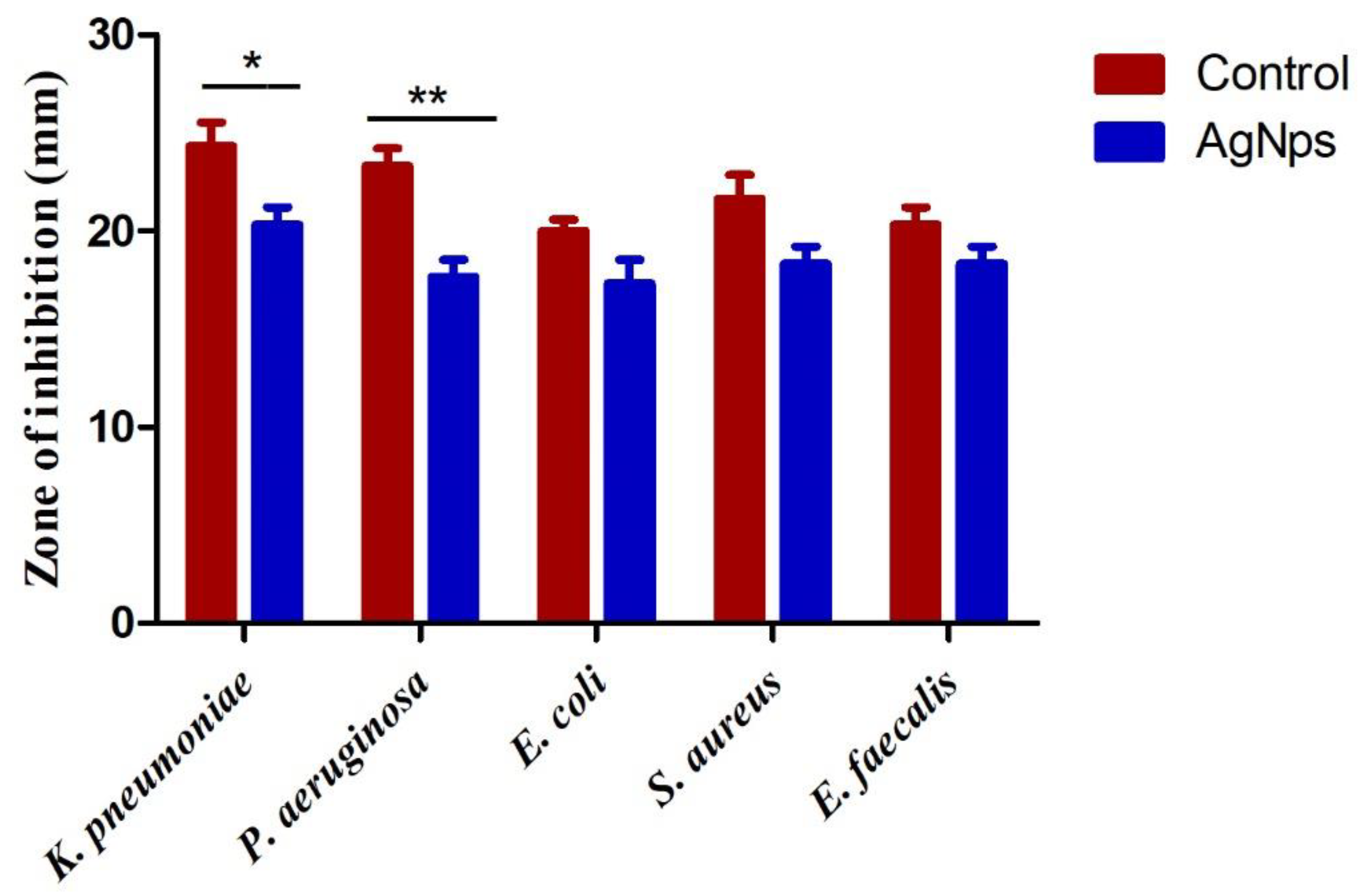
3.5. Assessment of Anti-Bacterial Activity
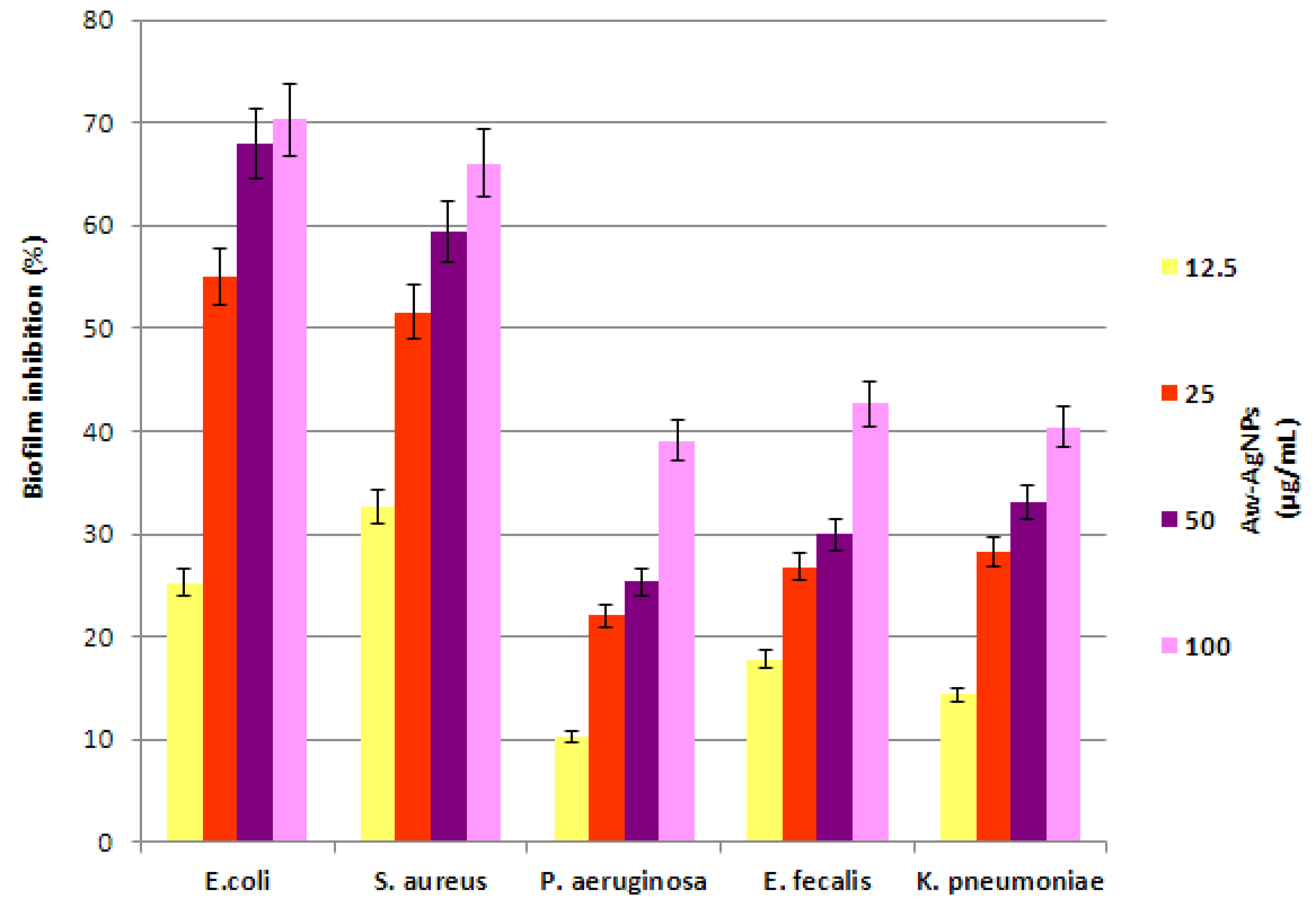
3.6. Anti-Biofilm Activity
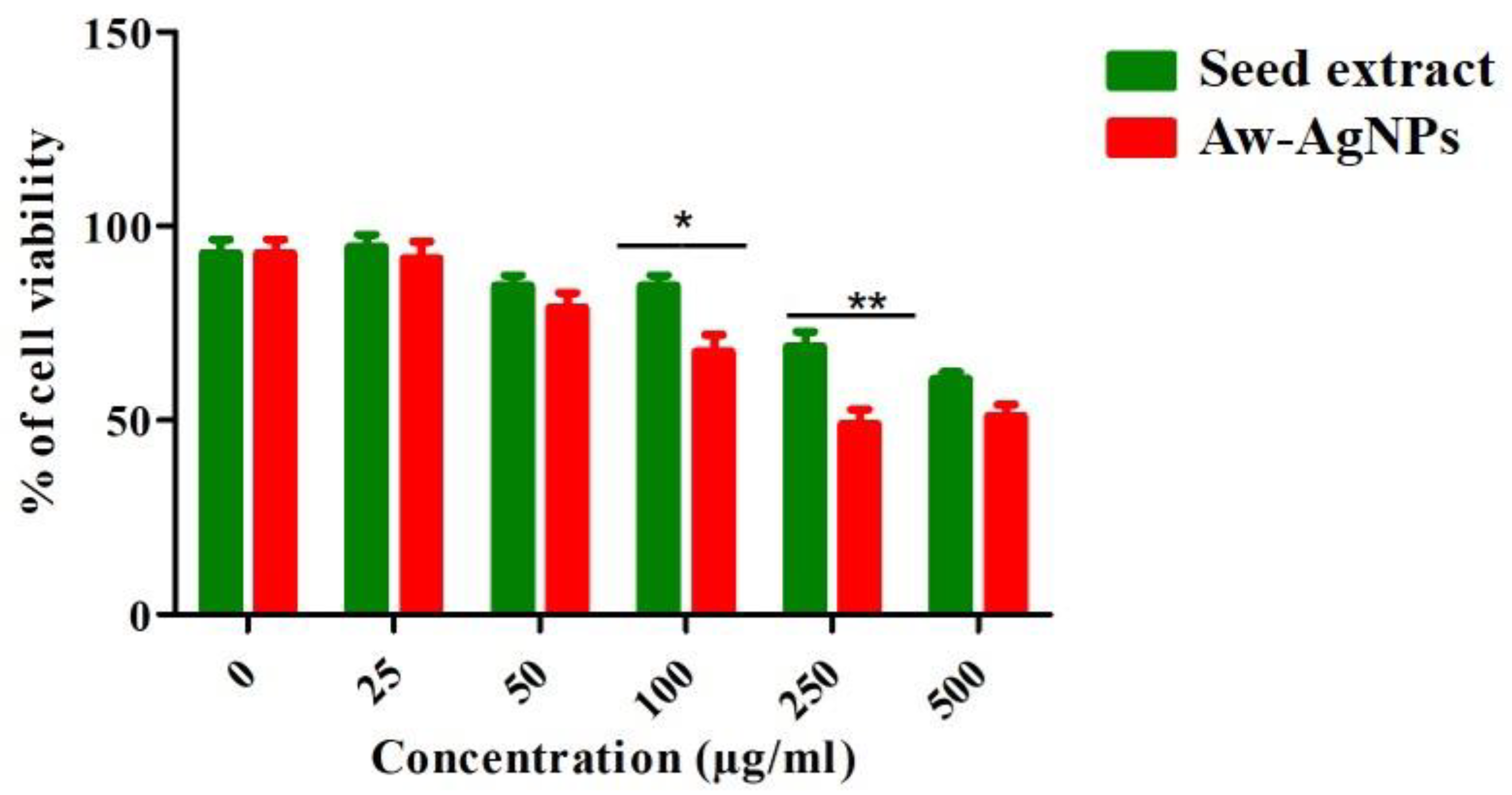
3.7. Effect of Aw–AgNPs against Breast Cancer Cell Line HCC712
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salleh, A.; Naomi, R.; Utami, N.D.; Mohammad, A.W.; Mahmoudi, E.; Mustafa, N.; Fauzi, M.B. The Potential of Silver Nanoparticles for Antiviral and Antibacterial Applications: A Mechanism of Action. Nanomaterials 2020, 10, 1566. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, A.A.; Umar, K.; Ibrahim, M.N.M. Silver nanoparticles: Various methods of synthesis, size affecting factors and their potential applications—A review. Appl. Nanosci. 2020, 10, 1369–1378. [Google Scholar] [CrossRef]
- Ansari, M.A.; Alzohairy, M.A. One-pot facile green synthesis of silver nanoparticles using seed extract of Phoenix dactylifera and their bactericidal potential against MRSA. Evid.-Based Complement. Altern. Med. 2018, 2, 1860280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, K. Riddle of biofilm resistance. Antimicrob. Agents Chemother. 2001, 45, 999–1007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almatroudi, A.; Khadri, H.; Azam, M.; Rahmani, A.H.; Al Khaleefah, F.K.; Khateef, R.; Ansari, M.A.; Allemailem, K.S. Antibacterial, Antibiofilm and Anticancer Activity of Biologically Synthesized Silver Nanoparticles Using Seed Extract of Nigella sativa. Processes 2020, 8, 388. [Google Scholar] [CrossRef] [Green Version]
- Kora, A.J.; Rastogi, L. Enhancement of antibacterial activity of capped silver nanoparticles in combination with antibiotics, on model gram-negative and gram-positive bacteria. Bioinorg. Chem. Appl. 2013, 8, 871097. [Google Scholar] [CrossRef] [PubMed]
- Palanisamy, N.K.; Ferina, N.; Amirulhusni, A.N.; Zain, Z.M.; Hussaini, J.; Ping, L.J.; Durairaj, R. Antibiofilm properties of chemically synthesized silver nanoparticles found against Pseudomonas aeruginosa. J. Nanobiotechnol. 2014, 12, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Christensen, G.; Simpson, W.; Younger, J.J.; Baddour, L.; Barrett, F.; Melton, D.M.; Beachey, E. Adherence of coagulase negative Staphylococci to plastic tissue cultures: A quantitative model for the adherence of staphylococci to medical devices. J. Clin. Microbiol. 1985, 22, 996–1006. [Google Scholar] [CrossRef] [Green Version]
- Ashokkumar, S.; Ravi, S.; Velmurugan, S. Green synthesis of silver nanoparticles from Gloriosa superba L. leaf extract and their catalytic activity. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2013, 115, 388–392. [Google Scholar] [CrossRef]
- Khatami, M.; Shahram Pourseyedi, S. Phoenix dactylifera (date palm) pit aqueous extract mediated novel route for synthesis high stable silver nanoparticles with high antifungal and antibacterial activity. IET Nanobiotechnol. 2015, 9, 184–190. [Google Scholar] [CrossRef]
- Chenthamara, D.; Subramaniam, S.; Ramakrishnan, S.G.; Krishnaswamy, S.; Essa, M.M.; Lin, F.H.; Qoronfleh, M.W. Therapeutic efficacy of nanoparticles and routes of administration. Biomater. Res. 2019, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Michitaka, O.; Naoki, T. Photoreduction of Rhodium (III) Ions in Water with Ultraviolet Light Aiming to Prepare the Dispersions of Ultrafine Particles. Chem. Lett. 1990, 19, 489–492. [Google Scholar] [CrossRef]
- Khademalrasool, M.; Farbod, M. A simple and high yield solvothermal synthesis of uniform silver nanowires with controllable diameters. J. Nanostruct. 2015, 5, 415–422. [Google Scholar]
- Mizukoshi, Y.; Okisu, K.; Maeda, Y.; Yamamoto, T.A.; Oshima, R.; Nagata, Y. Sonochemical preparation of bimetallic nanoparticles of gold/palladium in aqueous solution. J. Am. Phys. Chem. B 1997, 101, 7033–7037. [Google Scholar] [CrossRef]
- Darvishi, S.; Borghei, S.M.; Hashemizadeh, S.A. Structural, optical and electrical properties of silver nanoparticles deposited by spin coating method. J. Nanostruct. 2012, 2, 501–504. [Google Scholar]
- Khatami, M.; Alijani, H.; Nejad, M.; Varma, R. Core@ shell nanoparticles: Greener synthesis using natural plant products. Appl. Sci. 2018, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Phongtongpasuk, S.; Poadang, S.; Yongvanich, N. Environmental-friendly method for synthesis of silver nanoparticles from dragon fruit peel extract and their antibacterial activities. Energy Procedia 2016, 89, 239–247. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, U.C.; Mittal, A.K.; Chisti, Y. Research review paper: Synthesis of metallic nanoparticles using plant extract. Biotechnol. Adv. 2013, 31, 346–356. [Google Scholar]
- Kumar, V.; Yadav, S.K. Plant-mediated synthesis of silver and gold nanoparticles and their applications. Chem. Technol. Biotechnol. 2009, 84, 151–157. [Google Scholar] [CrossRef]
- Malik, P.; Shankar, R.; Malik, V.; Sharma, N.; Mukherjee, T.K. Green chemistry based benign routes for nanoparticle synthesis. Nanoparticle 2014, 2014, 302429. [Google Scholar] [CrossRef] [Green Version]
- Al-Alawi, R.A.; Al-Mashiqri, J.H.; Al-Nadabi, J.S.M.; Al-Shihi, B.I.; Baqi, Y. Date Palm Tree (Phoenix dactylifera L.): Natural Products and Therapeutic Options. Front. Plant Sci. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, F.; Khan, T.J.; Kalamegam, G.; Pushparaj, P.N.; Chaudhary, A.; Abuzenadah, A.; Kumosani, T.; Barbour, E.; Al-Qahtani, M. Anti-cancer effects of Ajwa dates (Phoenix dactylifera L.) in diethylnitrosamine induced hepatocellular carcinoma in Wistar rats. BMC Complement. Altern. Med. 2017, 17, 418. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Mohieldein, A. In Vivo Evaluation of Anti Diabetic, Hypolipidemic, Antioxidative Activities of Saudi Date Seed Extract on Streptozotocin Induced Diabetic Rats. J. Clin. Diagn. Res. 2016, 10, FF06–FF12. [Google Scholar] [CrossRef] [PubMed]
- Sirry, S.M.; Samah Ali, S.; Abdelaziz, A.; Mohamed, A. Biosynthesis of silver nanoparticles using the extracts of palm date seeds (Phoenix dactylifera L.) and its application in increasing the anti-inflammatory effect of piroxicam drug. Adv. Nat. Sci. Nanosci. Nanotechnol. 2020, 11, 035017. [Google Scholar] [CrossRef]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, Fifteenth Informational Supplement, CLSI Document; 2006 M100-S16, vol 26-3; M7-A7, vol 26-2; M2-A9, vol 26-1; CLSI: Wayne, PA, USA, 2006. [Google Scholar]
- Jadhav, S.; Shah, R.; Bhave, M.; Palombo, E.A. Inhibitory activity of yarrow essential oil on listeria planktonic cells and biofilms. Food Control 2013, 29, 125–130. [Google Scholar] [CrossRef]
- Das, G.; Patra, J.K.; Debnath, T.; Ansari, A.; Shin, H.-S. Investigation of antioxidant, antibacterial, antidiabetic, and cytotoxicity potential of silver nanoparticles synthesized using the outer peel extract of Ananas comosus (L.). PLoS ONE 2019, 14, e0220950. [Google Scholar] [CrossRef] [Green Version]
- Erdogan, O.; Abbak, M.; Demirbolat, G.M.; Birtekocak, F.; Aksel, M.; Pasa, S.; Ozge, C. Green synthesis of silver nanoparticles via Cynara scolymus leaf extracts: The characterization, anticancer potential with photodynamic therapy in MCF7 cells. PLoS ONE 2019, 14, e0216496. [Google Scholar] [CrossRef] [Green Version]
- Hamouda, R.A.; Hussein, M.H.; Abo-Elmagd, R.A.; Bawazir, S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019, 9, 13071. [Google Scholar] [CrossRef]
- Narayanaswamy, K.; Athimoolam, R.; Ayyavoo, J. Green Synthesis of Silver Nanoparticles Using Leaf Extracts of Clitoria ternatea and Solanum nigrum and Study of Its Antibacterial Effect against Common Nosocomial Pathogens. J. Nanosci. 2015, 2015, 928204. [Google Scholar] [CrossRef] [Green Version]
- Salem, S.H. Biosynthesis of Silver Nanoparticles from Date (Phoenix dactylifera) Seeds Extract and Evaluation of Antibacterial Activity against Pathogenic Bacteria. Orient. J. Chem. 2020, 36, 1189–1193. [Google Scholar]
- Al-Tamimi, A.; Alfarhan, A.; Rajagopal, R. Antimicrobial and anti-biofilm activities of polyphenols extracted from different Saudi Arabian date cultivars against human pathogens. J. Infect. Public Health 2021, 14, 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Kalishwaralal, K.; BarathManiKanth, S.; Pandian, S.R.; Deepak, V.; Gurunathan, S. Silver nanoparticles impede the biofilm formation by Pseudomonas aeruginosa and Staphylococcus epidermidis. Colloids Surf. B Biointerfaces 2010, 79, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Shahbaz, K.; Asif, J.A.; Liszen, T.; Nurul, A.A.; Alam, M.K. Cytotoxic and Antioxidant Effects of Phoenix dactylifera L. (Ajwa Date Extract) on Oral Squamous Cell Carcinoma Cell Line. BioMed Res. Int. 2022, 2022, 5792830. [Google Scholar] [CrossRef] [PubMed]

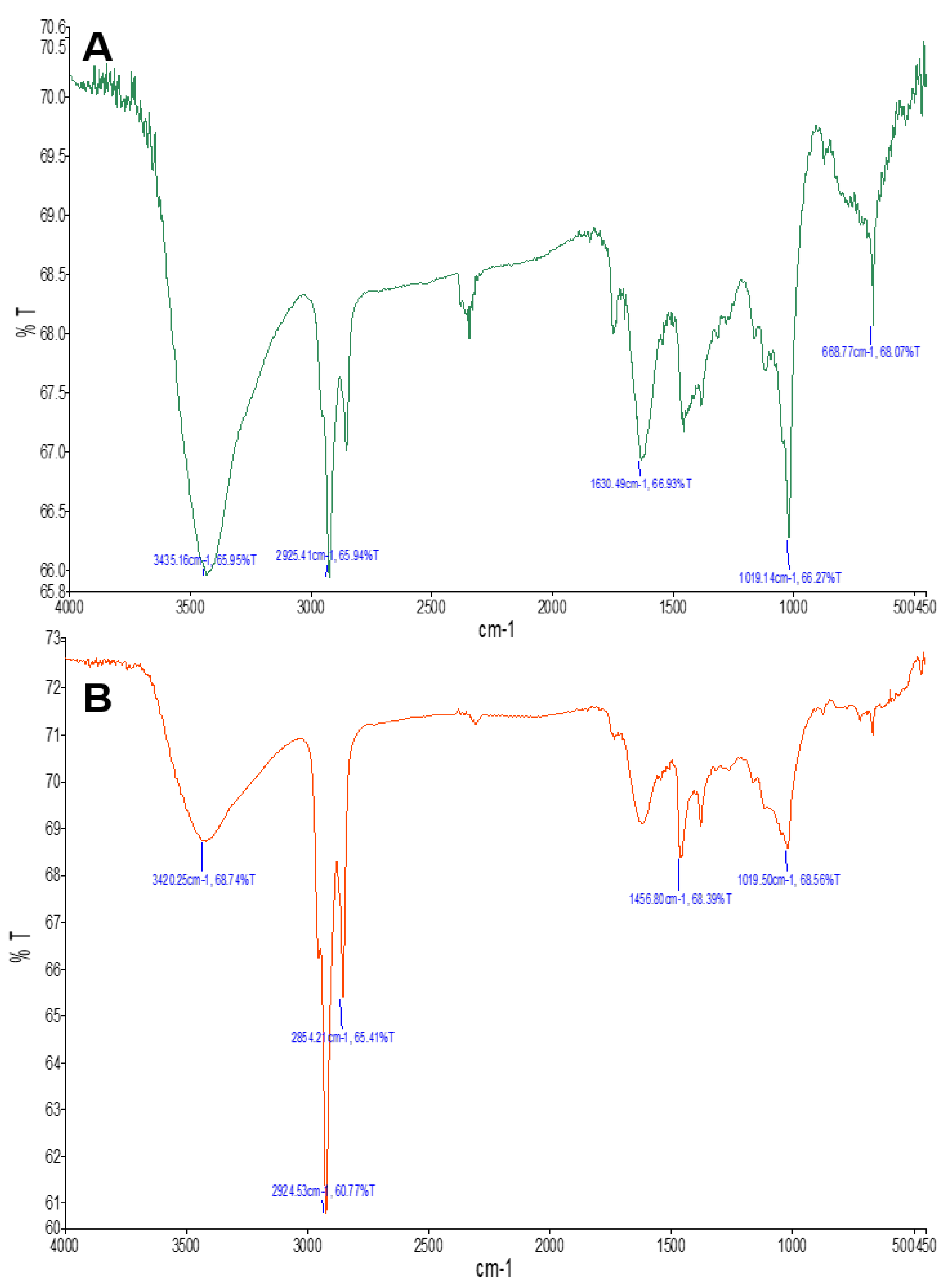
| Aw–AgNPs Wave Number (cm−1) | Probable Functional Group | Compound Class |
|---|---|---|
| 3420 | N-H Stretch | Amines |
| 2854 | C-H Stretch | Alkanes |
| 2985 | C-H Stretch | Alkanes |
| 1030 | C-O stretch | Ester |
| 1019 | C-4-OH stretch | Typical for glucose residue of disaccharides |
| 668 | C=C stretch | Alkene |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allemailem, K.S.; Khadri, H.; Azam, M.; Khan, M.A.; Rahmani, A.H.; Alrumaihi, F.; Khateef, R.; Ansari, M.A.; Alatawi, E.A.; Alsugoor, M.H.; et al. Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Anti-Biofilm, and Cytotoxic Potential. Appl. Sci. 2022, 12, 4537. https://doi.org/10.3390/app12094537
Allemailem KS, Khadri H, Azam M, Khan MA, Rahmani AH, Alrumaihi F, Khateef R, Ansari MA, Alatawi EA, Alsugoor MH, et al. Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Anti-Biofilm, and Cytotoxic Potential. Applied Sciences. 2022; 12(9):4537. https://doi.org/10.3390/app12094537
Chicago/Turabian StyleAllemailem, Khaled S., Habeeb Khadri, Mohd Azam, Masood Alam Khan, Arshad Husain Rahmani, Faris Alrumaihi, Riazunnisa Khateef, Mohammad Azam Ansari, Eid A. Alatawi, Mahdi H. Alsugoor, and et al. 2022. "Ajwa-Dates (Phoenix dactylifera)-Mediated Synthesis of Silver Nanoparticles and Their Anti-Bacterial, Anti-Biofilm, and Cytotoxic Potential" Applied Sciences 12, no. 9: 4537. https://doi.org/10.3390/app12094537








