Recent Advances in Intrinsically Fluorescent Polydopamine Materials
Abstract
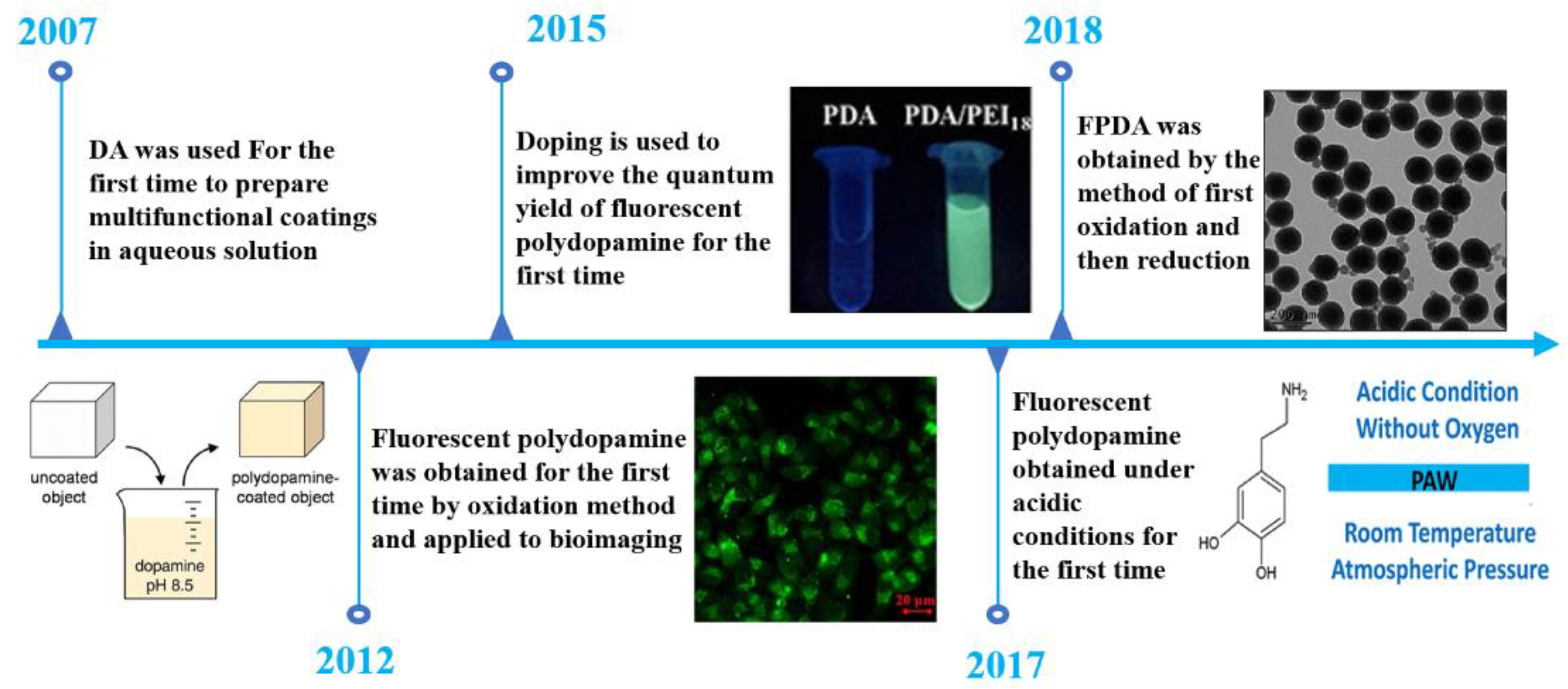
:1. Introduction
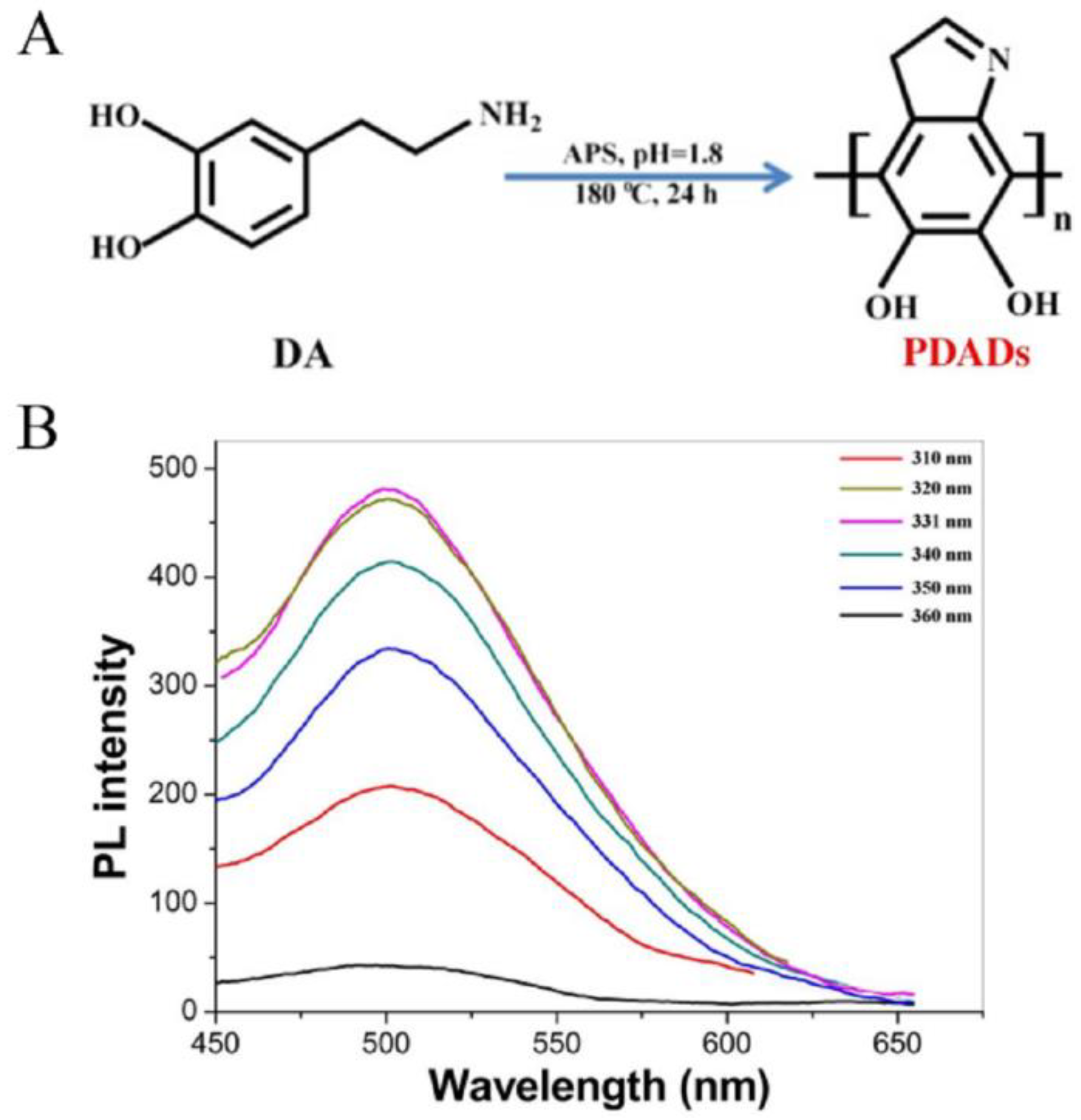
2. Preparation Principle
2.1. Redshift Phenomenon
2.2. Size Distribution
3. Application
3.1. Detection of Metal Ions
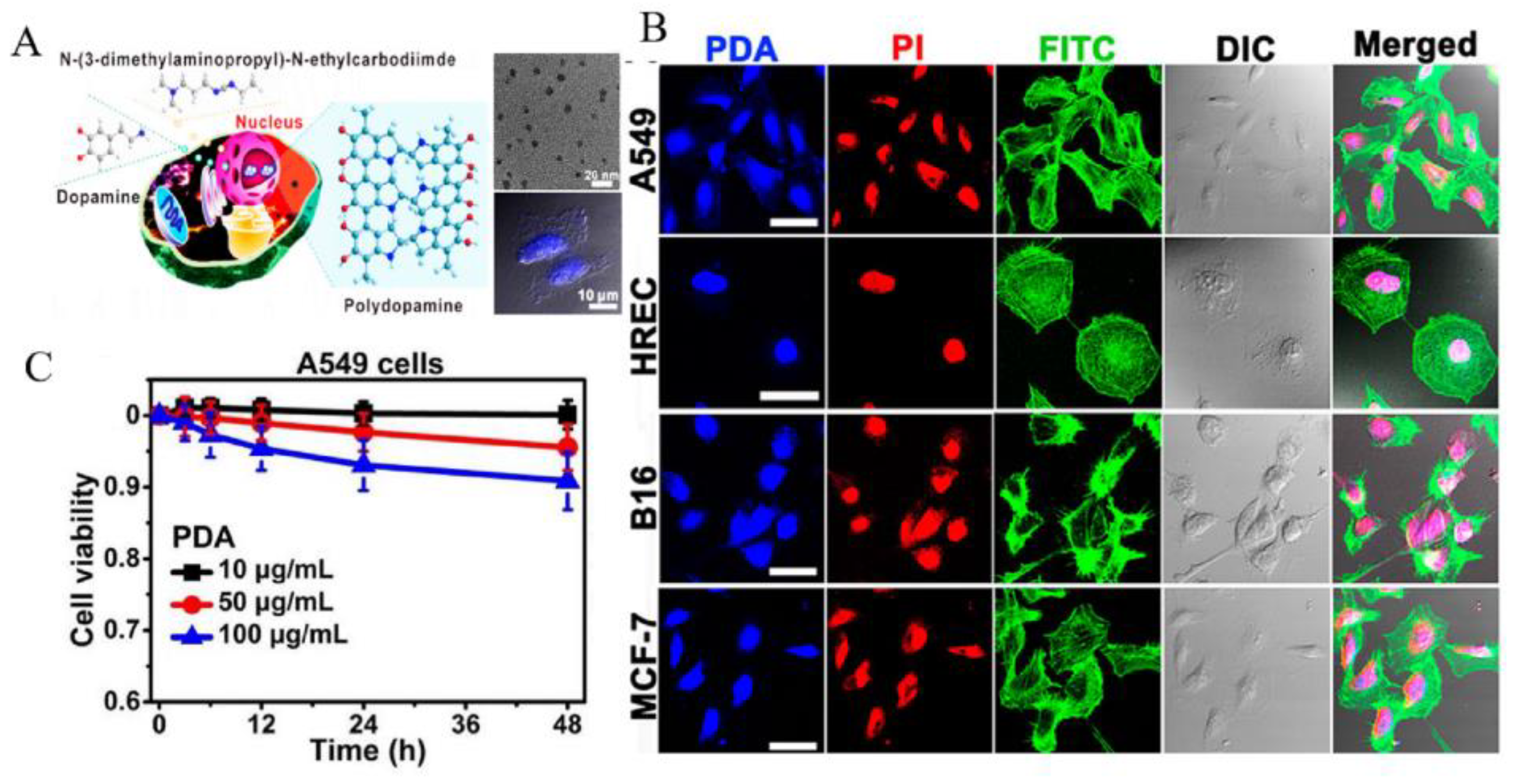
3.2. Bioimaging
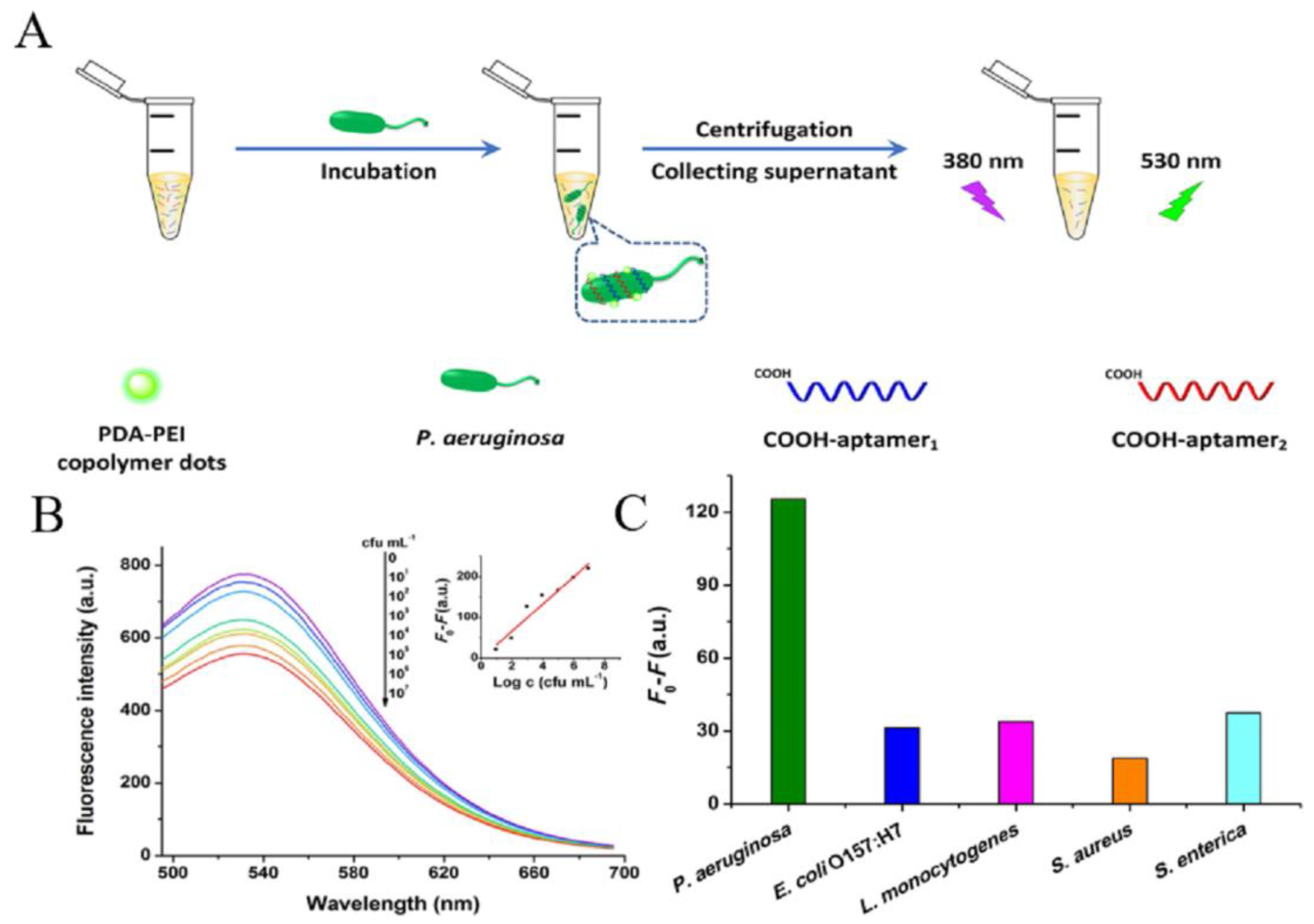
3.3. Detection of Bacteria
3.4. Detection of Biomolecules
3.5. Other Applications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chinnathambi, S.; Chen, S.; Ganesan, S.; Hanagata, N. Silicon quantum dots for biological applications. Adv. Healthc. Mater. 2014, 3, 10–29. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Xiang, X.; Liu, Y.; Zhou, G.; Ji, X.; He, Z. Dual-color determination of protein via terminal protection of small-molecule-linked DNA and the enzymolysis of exonuclease III. Biosens. Bioelectron. 2014, 58, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.; Huh, M.S.; Ryu, J.H.; Lee, D.-E.; Sun, I.-C.; Choi, K.; Kim, K.; Kwon, I.C. Nanoprobes for biomedical imaging in living systems. Nano Today 2011, 6, 204–220. [Google Scholar] [CrossRef]
- Pacheco-Linan, P.J.; Bravo, I.; Nueda, M.L.; Albaladejo, J.; Garzon-Ruiz, A. Functionalized CdSe/ZnS Quantum Dots for Intracellular pH Measurements by Fluorescence Lifetime Imaging Microscopy. ACS Sens. 2020, 5, 2106–2117. [Google Scholar] [CrossRef]
- Kim, E.J.; Kim, E.B.; Lee, S.W.; Cheon, S.A.; Kim, H.J.; Lee, J.; Lee, M.K.; Ko, S.; Park, T.J. An easy and sensitive sandwich assay for detection of Mycobacterium tuberculosis Ag85B antigen using quantum dots and gold nanorods. Biosens. Bioelectron. 2017, 87, 150–156. [Google Scholar] [CrossRef]
- Ji, X.; Palui, G.; Avellini, T.; Na, H.B.; Yi, C.; Knappenberger, K.L., Jr.; Mattoussi, H. On the pH-dependent quenching of quantum dot photoluminescence by redox active dopamine. J. Am. Chem. Soc. 2012, 134, 6006–6017. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Ma, Y.; Ge, H.; Luo, J.; Peng, B.; Deng, Z. Design and Synthesis of Self-Healable Superhydrophobic Coatings for Oil/Water Separation. Langmuir 2020, 36, 15309–15318. [Google Scholar] [CrossRef]
- Liu, Y.; Fan, Q.; Huo, Y.; Liu, C.; Li, B.; Li, Y. Construction of a Mesoporous Polydopamine@GO/Cellulose Nanofibril Composite Hydrogel with an Encapsulation Structure for Controllable Drug Release and Toxicity Shielding. ACS Appl. Mater. Interfaces 2020, 12, 57410–57420. [Google Scholar] [CrossRef]
- Cao, X.; Zhang, K.; Yan, W.; Xia, Z.; He, S.; Xu, X.; Ye, Y.; Wei, Z.; Liu, S. Calcium ion assisted fluorescence determination of microRNA-167 using carbon dots-labeled probe DNA and polydopamine-coated Fe3O4 nanoparticles. Mikrochim. Acta 2020, 187, 212. [Google Scholar] [CrossRef]
- Jia, M.; Bi, Z.; Shi, C.; Zhao, N.; Guo, X. Polydopamine Coated Lithium Lanthanum Titanate in Bilayer Membrane Electrolytes for Solid Lithium Batteries. ACS Appl. Mater. Interfaces 2020, 12, 46231–46238. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Hong, W.; Zhang, H.; Zhang, T.T.; Chen, Z.; Yuan, S.; Peng, P.; Xiao, M.; Xu, L. Photothermally triggered cytosolic drug delivery of glucose functionalized polydopamine nanoparticles in response to tumor microenvironment for the GLUT1-targeting chemo-phototherapy. J. Control Release 2020, 317, 232–245. [Google Scholar] [CrossRef]
- Della Vecchia, N.F.; Avolio, R.; Alfè, M.; Errico, M.E.; Napolitano, A.; d’Ischia, M. Building-Block Diversity in Polydopamine Underpins a Multifunctional Eumelanin-Type Platform Tunable Through a Quinone Control Point. Adv. Funct. Mater. 2013, 23, 1331–1340. [Google Scholar] [CrossRef]
- Meredith, P.; Riesz, J. Radiative Relaxation Quantum Yields for Synthetic Eumelanin ¶. Photochem. Photobiol. 2004, 79, 211–216. [Google Scholar] [CrossRef]
- Kayatz, P.; Thumann, G.; Luther, T.T.; Jordan, J.F.; Bartz-Schmidt, K.U.; Esser, P.J.; Schraermeyer, U. Oxidation causes melanin fluorescence. Investig. Ophthalmol. Vis. Sci. 2001, 42, 241–246. [Google Scholar]
- Nighswander-Rempel, S.P.; Riesz, J.; Gilmore, J.; Meredith, P. A quantum yield map for synthetic eumelanin. J. Chem. Phys. 2005, 123, 194901. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meredith, P.; Sarna, T. The physical and chemical properties of eumelanin. Pigment. Cell Res. 2006, 19, 572–594. [Google Scholar] [CrossRef]
- Ai, K.; Liu, Y.; Ruan, C.; Lu, L.; Lu, G.M. Sp2 C-dominant N-doped carbon sub-micrometer spheres with a tunable size: A versatile platform for highly efficient oxygen-reduction catalysts. Adv. Mater. 2013, 25, 998–1003. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Miller, D.J.; Freeman, B.D.; Paul, D.R.; Bielawski, C.W. Elucidating the structure of poly(dopamine). Langmuir 2012, 28, 6428–6435. [Google Scholar] [CrossRef]
- Pan, C.; Sugiyasu, K.; Wakayama, Y.; Sato, A.; Takeuchi, M. Thermoplastic fluorescent conjugated polymers: Benefits of preventing pi-pi stacking. Angew. Chem. Int. Ed. 2013, 52, 10775–10779. [Google Scholar] [CrossRef]
- Stark, K.B.; Gallas, J.M.; Zajac, G.W.; Golab, J.T.; Gidanian, S.; McIntire, T.; Farmer, P.J. Effect of stacking and redox state on optical absorption spectra of melanins -- comparison of theoretical and experimental results. J. Phys. Chem. B 2005, 109, 1970–1977. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wang, S.; Xu, L.; Feng, L.; Ji, Y.; Tao, L.; Li, S.; Wei, Y. Biocompatible polydopamine fluorescent organic nanoparticles: Facile preparation and cell imaging. Nanoscale 2012, 4, 5581–5584. [Google Scholar] [CrossRef] [PubMed]
- Xue, Q.; Cao, X.; Zhang, C.; Xian, Y. Polydopamine nanodots are viable probes for fluorometric determination of the activity of alkaline phosphatase via the in situ regulation of a redox reaction triggered by the enzyme. Mikrochim. Acta 2018, 185, 231. [Google Scholar] [CrossRef]
- Kong, X.J.; Wu, S.; Chen, T.T.; Yu, R.Q.; Chu, X. MnO2-induced synthesis of fluorescent polydopamine nanoparticles for reduced glutathione sensing in human whole blood. Nanoscale 2016, 8, 15604–15610. [Google Scholar] [CrossRef] [PubMed]
- Corani, A.; Huijser, A.; Iadonisi, A.; Pezzella, A.; Sundstrom, V.; d’Ischia, M. Bottom-up approach to eumelanin photoprotection: Emission dynamics in parallel sets of water-soluble 5,6-dihydroxyindole-based model systems. J. Phys. Chem. B 2012, 116, 13151–13158. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, F.; Yoshimura, Y.; Nakazawa, H.; Ariga, T. Free radical scavenging activity of grape seed extract and antioxidants by electron spin resonance spectrometry in an H2O2/NaOH/DMSO system. J. Agric. Food Chem. 1999, 47, 2544–2548. [Google Scholar] [CrossRef]
- Yoshimura, Y.; Inomata, T.; Nakazawa, H.; Kubo, H.; Yamaguchi, F.; Ariga, T. Evaluation of free radical scavenging activities of antioxidants with an H2O2/NaOH/DMSO system by electron spin resonance. J. Agric. Food Chem. 1999, 47, 4653–4656. [Google Scholar] [CrossRef]
- Lin, J.H.; Yu, C.J.; Yang, Y.C.; Tseng, W.L. Formation of fluorescent polydopamine dots from hydroxyl radical-induced degradation of polydopamine nanoparticles. PCCP 2015, 17, 15124–15130. [Google Scholar] [CrossRef]
- Quignard, S.; d’Ischia, M.; Chen, Y.; Fattaccioli, J. Ultraviolet-Induced Fluorescence of Polydopamine-Coated Emulsion Droplets. ChemPlusChem 2014, 79, 1254–1257. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Cui, J.; Wang, Y.; Postma, A.; Hao, J.; Hosta-Rigau, L.; Caruso, F. Monodisperse Polymer Capsules: Tailoring Size, Shell Thickness, and Hydrophobic Cargo Loading via Emulsion Templating. Adv. Funct. Mater. 2010, 20, 1625–1631. [Google Scholar] [CrossRef]
- Zou, W.S.; Zhao, Q.C.; Zhang, J.; Chen, X.M.; Wang, X.F.; Zhao, L.; Chen, S.H.; Wang, Y.Q. Enhanced photoresponsive polyethyleneimine/citric acid co-carbonized dots for facile and selective sensing and intracellular imaging of cobalt ions at physiologic pH. Anal. Chim. Acta 2017, 970, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Wang, W.; Wu, H.; Fang, F.; Wang, N.; Zhang, X.; Xu, S. Polyethyleneimine modified fluorescent carbon dots and their application in cell labeling. Colloids Surf. B Biointerfaces 2012, 100, 209–214. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Ji, J.; Zhang, X.; Zhang, X.; Yang, B.; Deng, F.; Li, Z.; Wang, K.; Yang, Y.; Wei, Y. Self-polymerization of dopamine and polyethyleneimine: Novel fluorescent organic nanoprobes for biological imaging applications. J. Mater. Chem. B 2015, 3, 3476–3482. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Z.; Jia, L. Room temperature preparation of water-soluble polydopamine-polyethyleneimine copolymer dots for selective detection of copper ions. Talanta 2019, 197, 584–591. [Google Scholar] [CrossRef]
- Zhong, Z.; Gao, R.; Chen, Q.; Jia, L. Dual-aptamers labeled polydopamine-polyethyleneimine copolymer dots assisted engineering a fluorescence biosensor for sensitive detection of Pseudomonas aeruginosa in food samples. Spectrochim. Acta Pt. A Mol. Biomol. Spectrosc. 2020, 224, 117417. [Google Scholar] [CrossRef]
- Wang, Z.; Xu, C.; Lu, Y.; Wei, G.; Ye, G.; Sun, T.; Chen, J. Microplasma electrochemistry controlled rapid preparation of fluorescent polydopamine nanoparticles and their application in uranium detection. Chem. Eng. J. 2018, 344, 480–486. [Google Scholar] [CrossRef]
- Liu, B.; Han, X.; Liu, J. Iron oxide nanozyme catalyzed synthesis of fluorescent polydopamine for light-up Zn2+ detection. Nanoscale 2016, 8, 13620–13626. [Google Scholar] [CrossRef]
- Xiong, H.; Xu, J.; Yuan, C.; Wang, X.; Wen, W.; Zhang, X.; Wang, S. Oxidation-controlled synthesis of fluorescent polydopamine for the detection of metal ions. Microchem. J. 2019, 147, 176–182. [Google Scholar] [CrossRef]
- Wang, X.; Jin, B.; Lin, X. In-situ FTIR spectroelectrochemical study of dopamine at a glassy carbon electrode in a neutral solution. Anal. Sci. 2002, 18, 931–933. [Google Scholar] [CrossRef] [Green Version]
- Dalsin, J.L.; Messersmith, P.B. Bioinspired antifouling polymers. Mater. Today 2005, 8, 38–46. [Google Scholar] [CrossRef]
- van der Leeden, M.C. Are conformational changes, induced by osmotic pressure variations, the underlying mechanism of controlling the adhesive activity of mussel adhesive proteins? Langmuir 2005, 21, 11373–11379. [Google Scholar] [CrossRef] [PubMed]
- Wei, Q.; Zhang, F.; Li, J.; Li, B.; Zhao, C. Oxidant-induced dopamine polymerization for multifunctional coatings. Polym. Chem. 2010, 1, 1430–1433. [Google Scholar] [CrossRef]
- Łuczak, T. Preparation and characterization of the dopamine film electrochemically deposited on a gold template and its applications for dopamine sensing in aqueous solution. Electrochim. Acta 2008, 53, 5725–5731. [Google Scholar] [CrossRef]
- Hong, S.; Na, Y.S.; Choi, S.; Song, I.T.; Kim, W.Y.; Lee, H. Non-Covalent Self-Assembly and Covalent Polymerization Co-Contribute to Polydopamine Formation. Adv. Funct. Mater. 2012, 22, 4711–4717. [Google Scholar] [CrossRef]
- d’Ischia, M.; Napolitano, A.; Ball, V.; Chen, C.T.; Buehler, M.J. Polydopamine and eumelanin: From structure-property relationships to a unified tailoring strategy. Acc. Chem. Res. 2014, 47, 3541–3550. [Google Scholar] [CrossRef]
- Zhang, C.; Ou, Y.; Lei, W.X.; Wan, L.S.; Ji, J.; Xu, Z.K. CuSO4/H2O2-Induced Rapid Deposition of Polydopamine Coatings with High Uniformity and Enhanced Stability. Angew. Chem. Int. Ed. Engl. 2016, 55, 3054–3057. [Google Scholar] [CrossRef]
- Bai, Y.; Zhang, B.; Chen, L.; Lin, Z.; Zhang, X.; Ge, D.; Shi, W.; Sun, Y. Facile One-Pot Synthesis of Polydopamine Carbon Dots for Photothermal Therapy. Nanoscale Res. Lett. 2018, 13, 287. [Google Scholar] [CrossRef]
- Yildirim, A.; Bayindir, M. Turn-on fluorescent dopamine sensing based on in situ formation of visible light emitting polydopamine nanoparticles. Anal. Chem. 2014, 86, 5508–5512. [Google Scholar] [CrossRef]
- Ci, Q.; Liu, J.; Qin, X.; Han, L.; Li, H.; Yu, H.; Lim, K.L.; Zhang, C.W.; Li, L.; Huang, W. Polydopamine Dots-Based Fluorescent Nanoswitch Assay for Reversible Recognition of Glutamic Acid and Al3+ in Human Serum and Living Cell. ACS Appl. Mater. Interfaces 2018, 10, 35760–35769. [Google Scholar] [CrossRef]
- Xiong, S.; Wang, Y.; Yu, J.; Chen, L.; Zhu, J.; Hu, Z. Polydopamine particles for next-generation multifunctional biocomposites. J. Mater. Chem. A 2014, 2, 7578–7587. [Google Scholar] [CrossRef]
- Sikora, F.J.; Mcbride, M.B. Aluminum complexation by catechol as determined by ultraviolet spectrophotometry. Environ. Sci. Technol. 1989, 23, 349–356. [Google Scholar] [CrossRef]
- Ponzio, F.; Barthès, J.; Bour, J.; Michel, M.; Bertani, P.; Hemmerlé, J.; d’Ischia, M.; Ball, V. Oxidant Control of Polydopamine Surface Chemistry in Acids: A Mechanism-Based Entry to Superhydrophilic-Superoleophobic Coatings. Chem. Mater. 2016, 28, 4697–4705. [Google Scholar] [CrossRef]
- Zhao, S.; Song, X.; Bu, X.; Zhu, C.; Wang, G.; Liao, F.; Yang, S.; Wang, M. Polydopamine dots as an ultrasensitive fluorescent probe switch for Cr(VI) in vitro. J. Appl. Polym. Sci. 2017, 134, 44784. [Google Scholar] [CrossRef]
- Chen, T.P.; Liu, T.; Su, T.L.; Liang, J. Self-Polymerization of Dopamine in Acidic Environments without Oxygen. Langmuir 2017, 33, 5863–5871. [Google Scholar] [CrossRef]
- Du, X.; Li, L.; Li, J.; Yang, C.; Frenkel, N.; Welle, A.; Heissler, S.; Nefedov, A.; Grunze, M.; Levkin, P.A. UV-triggered dopamine polymerization: Control of polymerization, surface coating, and photopatterning. Adv. Mater. 2014, 26, 8029–8033. [Google Scholar] [CrossRef]
- Xiong, B.; Chen, Y.; Shu, Y.; Shen, B.; Chan, H.N.; Chen, Y.; Zhou, J.; Wu, H. Highly emissive and biocompatible dopamine-derived oligomers as fluorescent probes for chemical detection and targeted bioimaging. Chem. Commun. 2014, 50, 13578–13580. [Google Scholar] [CrossRef]
- Bettinger, C.J.; Bruggeman, J.P.; Misra, A.; Borenstein, J.T.; Langer, R. Biocompatibility of biodegradable semiconducting melanin films for nerve tissue engineering. Biomaterials 2009, 30, 3050–3057. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Cao, J.; Li, H.; Li, J.; Jin, Q.; Ren, K.; Ji, J. Mussel-inspired polydopamine: A biocompatible and ultrastable coating for nanoparticles in vivo. ACS Nano 2013, 7, 9384–9395. [Google Scholar] [CrossRef]
- Priyam, A.; Nagar, P.; Sharma, A.K.; Kumar, P. Mussel-inspired polydopamine-polyethylenimine conjugated nanoparticles as efficient gene delivery vectors for mammalian cells. Colloids Surf. B Biointerfaces 2018, 161, 403–412. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Enrique, J.; Marcelo, G.; Martin Del Valle, E.M. Size Matters in the Cytotoxicity of Polydopamine Nanoparticles in Different Types of Tumors. Cancers 2019, 11, 1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Ma, L.; Liu, D.; Wang, Z. Cytotoxicity of Gold Nanoparticles. Chin. J. Appl. Chem. 2016, 33, 1009–1016. [Google Scholar]
- Chen, P.; Wang, H.; He, M.; Chen, B.; Yang, B.; Hu, B. Size-dependent cytotoxicity study of ZnO nanoparticles in HepG2 cells. Ecotoxicol. Environ. Saf. 2019, 171, 337–346. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Neuss, S.; Leifert, A.; Fischler, M.; Wen, F.; Simon, U.; Schmid, G.; Brandau, W.; Jahnen-Dechent, W. Size-Dependent Cytotoxicity of Gold Nanoparticles. Small 2007, 3, 1941–1949. [Google Scholar] [CrossRef]
- Mai, T.T.; Hamai, A.; Hienzsch, A.; Caneque, T.; Muller, S.; Wicinski, J.; Cabaud, O.; Leroy, C.; David, A.; Acevedo, V.; et al. Salinomycin kills cancer stem cells by sequestering iron in lysosomes. Nat. Chem. 2017, 9, 1025–1033. [Google Scholar] [CrossRef] [Green Version]
- Jo, D.H.; Kim, J.H.; Lee, T.G.; Kim, J.H. Size, surface charge, and shape determine therapeutic effects of nanoparticles on brain and retinal diseases. Nanomedicine 2015, 11, 1603–1611. [Google Scholar] [CrossRef]
- Gaumet, M.; Vargas, A.; Gurny, R.; Delie, F. Nanoparticles for drug delivery: The need for precision in reporting particle size parameters. Eur. J. Pharm. Biopharm. 2008, 69, 1–9. [Google Scholar] [CrossRef]
- Cindric, M.; Matysik, F.M. Hyphenation of Electrochemistry with Mass Spectrometry for Bioanalytical Studies. In Advances in Chemical Bioanalysis; Springer: Cham, Switzerland, 2013; pp. 237–259. [Google Scholar]
- Yin, C.; Cai, J.; Gao, L.; Yin, J.; Zhou, J. Highly efficient degradation of 4-nitrophenol over the catalyst of Mn2O3/AC by microwave catalytic oxidation degradation method. J. Hazard. Mater. 2016, 305, 15–20. [Google Scholar] [CrossRef]
- Rahman, M.M.; Byanju, B.; Grewell, D.; Lamsal, B.P. High-power sonication of soy proteins: Hydroxyl radicals and their effects on protein structure. Ultrason. Sonochem. 2020, 64, 105019. [Google Scholar] [CrossRef]
- Chen, X.; Yan, Y.; Mullner, M.; van Koeverden, M.P.; Noi, K.F.; Zhu, W.; Caruso, F. Engineering fluorescent poly(dopamine) capsules. Langmuir 2014, 30, 2921–2925. [Google Scholar] [CrossRef]
- Hider, R.C.; Howlin, B.; Miller, J.R.; Mohd-Nor, A.R.; Silver, J. Model compounds for microbial iron-transport compounds. Part IV. Further solution chemistry and Mssbauer studies on iron(II) and iron(III) catechol complexes. Inorg. Chim. Acta 1983, 80, 51–56. [Google Scholar] [CrossRef]
- Schweigert, N.; Zehnder, A.J.; Eggen, R.I. Chemical properties of catechols and their molecular modes of toxic action in cells, from microorganisms to mammals. Environ. Microbiol. 2001, 3, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Mo, S.; Liu, S.G.; Liao, L.L.; Li, N.B.; Luo, H.Q. Synthesis of fluorescent polydopamine nanoparticles by Michael addition reaction as an analysis platform to detect iron ions and pyrophosphate efficiently and construction of an IMPLICATION logic gate. Sens. Actuators B Chem. 2018, 255, 754–762. [Google Scholar] [CrossRef]
- Yin, H.; Zhang, K.; Wang, L.; Zhou, K.; Zeng, J.; Gao, D.; Xia, Z.; Fu, Q. Redox modulation of polydopamine surface chemistry: A facile strategy to enhance the intrinsic fluorescence of polydopamine nanoparticles for sensitive and selective detection of Fe3. Nanoscale 2018, 10, 18064–18073. [Google Scholar] [CrossRef]
- Huang, X.; Schubert, A.B.; Chrisman, J.D.; Zacharia, N.S. Formation and Tunable Disassembly of Polyelectrolyte–Cu2+ Layer-by-Layer Complex Film. Langmuir 2013, 29, 12959–12968. [Google Scholar] [CrossRef]
- Qi, P.; Zhang, D.; Wan, Y. Morphology-tunable polydopamine nanoparticles and their application in Fe3+ detection. Talanta 2017, 170, 173–179. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Xue, S.F.; Chen, Z.H.; Han, X.Y.; Shi, G.; Zhang, M. Bioinspired Copolymers Based Nose/Tongue-Mimic Chemosensor for Label-Free Fluorescent Pattern Discrimination of Metal Ions in Biofluids. Anal. Chem. 2018, 90, 8248–8253. [Google Scholar] [CrossRef]
- Lin, Z.Y.; Qu, Z.B.; Chen, Z.H.; Han, X.Y.; Deng, L.X.; Luo, Q.; Jin, Z.; Shi, G.; Zhang, M. The Marriage of Protein and Lanthanide: Unveiling a Time-Resolved Fluorescence Sensor Array Regulated by pH toward High-Throughput Assay of Metal Ions in Biofluids. Anal. Chem. 2019, 91, 11170–11177. [Google Scholar] [CrossRef]
- Pepperkok, R.; Ellenberg, J. High-throughput fluorescence microscopy for systems biology. Nat. Rev. Mol. Cell Biol. 2006, 7, 690–696. [Google Scholar] [CrossRef]
- Song, X.; Hu, J.; Zeng, H. Two-dimensional semiconductors: Recent progress and future perspectives. J. Mater. Chem. C 2013, 1, 2952–2969. [Google Scholar] [CrossRef]
- Liu, Y.; Zhou, J.; Wang, L.; Hu, X.; Liu, X.; Liu, M.; Cao, Z.; Shangguan, D.; Tan, W. A Cyanine Dye to Probe Mitophagy: Simultaneous Detection of Mitochondria and Autolysosomes in Live Cells. J. Am. Chem. Soc. 2016, 138, 12368–12374. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Sahu, S.; Yau, Y.H.; Wang, X.; Shochat, S.G.; Nielsen, P.H.; Dueholm, M.S.; Otzen, D.E.; Lee, J.; Delos Santos, M.M.; et al. Detection of Pathogenic Biofilms with Bacterial Amyloid Targeting Fluorescent Probe, CDy11. J. Am. Chem. Soc. 2016, 138, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Alamudi, S.H.; Satapathy, R.; Kim, J.; Su, D.; Ren, H.; Das, R.; Hu, L.; Alvarado-Martinez, E.; Lee, J.Y.; Hoppmann, C.; et al. Development of background-free tame fluorescent probes for intracellular live cell imaging. Nat. Commun. 2016, 7, 11964. [Google Scholar] [CrossRef]
- Wang, L.; Frei, M.S.; Salim, A.; Johnsson, K. Small-Molecule Fluorescent Probes for Live-Cell Super-Resolution Microscopy. J. Am. Chem. Soc. 2019, 141, 2770–2781. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ding, G.; Duan, Y.; Zhu, Y.; Zhu, G.; Wang, M.; Li, X.; Zhang, Y.; Qin, X.; Hung, C.H. A novel triphenylamine-based bis-Schiff bases fluorophores with AIE-Activity as the hydrazine fluorescence turn-off probes and cell imaging in live cells. Talanta 2020, 217, 121029. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Ni, Z.; Li, P.; Li, Y.; Pang, X.; Xie, R.; Zhou, Z.; Li, H.; Zhang, Y. A near-infrared fluorescent probe for monitoring and imaging of beta-galactosidase in living cells. Talanta 2020, 219, 121307. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Xu, D.; Liu, M.; Fu, L.; Wan, Q.; Mao, L.; Dai, Y.; Wen, Y.; Zhang, X.; Wei, Y. Room temperature preparation of fluorescent starch nanoparticles from starch-dopamine conjugates and their biological applications. Mater. Sci. Eng. C-Mater. Biol. Appl. 2018, 82, 204–209. [Google Scholar] [CrossRef]
- Zhao, Y.; Chang, C.; Gai, P.; Han, L.; Li, F.; Li, B. One-step synthesis of fluorescent organic nanoparticles: The application to label-free ratiometric fluorescent pH sensor. Sens. Actuators B Chem. 2018, 273, 1479–1486. [Google Scholar] [CrossRef]
- Ho, C.C.; Ding, S.J. The pH-controlled nanoparticles size of polydopamine for anti-cancer drug delivery. J. Mater. Sci. Mater. Med. 2013, 24, 2381–2390. [Google Scholar] [CrossRef]
- Ma, B.; Liu, F.; Zhang, S.; Duan, J.; Kong, Y.; Li, Z.; Tang, D.; Wang, W.; Ge, S.; Tang, W.; et al. Two-photon fluorescent polydopamine nanodots for CAR-T cell function verification and tumor cell/tissue detection. J. Mater. Chem. B 2018, 6, 6459–6467. [Google Scholar] [CrossRef]
- Awasthi, A.K.; Gupta, S.; Thakur, J.; Gupta, S.; Pal, S.; Bajaj, A.; Srivastava, A. Polydopamine-on-liposomes: Stable nanoformulations, uniform coatings and superior antifouling performance. Nanoscale 2020, 12, 5021–5030. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Gao, X.; Huang, X.; Ge, H.; Xie, M.; Qian, J.; Song, J.; Li, Y.; Zhu, X.; Zhang, C. Polydopamine-coated nucleic acid nanogel for siRNA-mediated low-temperature photothermal therapy. Biomaterials 2020, 245, 119976. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Wang, X.; Wang, P.; Ruan, S.; Xie, A.; Shen, Y.; Zhu, M. 4-in-1 phototheranostics: PDA@CoPA-LA nanocomposite for photothermal imaging/photothermal/in-situ O2 generation/photodynamic combination therapy. Chem. Eng. J. 2020, 387, 124113. [Google Scholar] [CrossRef]
- Hoelz, A.; Debler, E.W.; Blobel, G. The structure of the nuclear pore complex. Annu. Rev. Biochem. 2011, 80, 613–643. [Google Scholar] [CrossRef] [Green Version]
- Gundersen, G.G.; Worman, H.J. Nuclear positioning. Cell 2013, 152, 1376–1389. [Google Scholar] [CrossRef] [Green Version]
- Grimm, J.B.; English, B.P.; Chen, J.; Slaughter, J.P.; Zhang, Z.; Revyakin, A.; Patel, R.; Macklin, J.J.; Normanno, D.; Singer, R.H.; et al. A general method to improve fluorophores for live-cell and single-molecule microscopy. Nat. Methods 2015, 12, 244–250. [Google Scholar] [CrossRef]
- Ding, P.; Wang, H.; Song, B.; Ji, X.; Su, Y.; He, Y. In Situ Live-Cell Nucleus Fluorescence Labeling with Bioinspired Fluorescent Probes. Anal. Chem. 2017, 89, 7861–7868. [Google Scholar] [CrossRef]
- Hameed, S.; Xie, L.; Ying, Y. Conventional and emerging detection techniques for pathogenic bacteria in food science: A review. Trends Food Sci. Technol. 2018, 81, 61–73. [Google Scholar] [CrossRef]
- Fan, X.L.; Li, H.Y.; Ye, W.Y.; Zhao, M.Q.; Huang, D.N.; Fang, Y.; Zhou, B.Q.; Ren, K.F.; Ji, J.; Fu, G.S. Magainin-modified polydopamine nanoparticles for photothermal killing of bacteria at low temperature. Colloids Surf. B Biointerfaces 2019, 183, 110423. [Google Scholar] [CrossRef]
- Ye, Y.; Zheng, L.; Wu, T.; Ding, X.; Chen, F.; Yuan, Y.; Fan, G.C.; Shen, Y. Size-Dependent Modulation of Polydopamine Nanospheres on Smart Nanoprobes for Detection of Pathogenic Bacteria at Single-Cell Level and Imaging-Guided Photothermal Bactericidal Activity. ACS Appl. Mater. Interfaces 2020, 12, 35626–35637. [Google Scholar] [CrossRef]
- Chien, C.T.; Li, S.S.; Lai, W.J.; Yeh, Y.C.; Chen, H.A.; Chen, I.S.; Chen, L.C.; Chen, K.H.; Nemoto, T.; Isoda, S.; et al. Tunable photoluminescence from graphene oxide. Angew. Chem. Int. Ed. Engl. 2012, 51, 6662–6666. [Google Scholar] [CrossRef] [PubMed]
- Mazrad Zihnil, A.I.; In, I.; Park, S.Y. Reusable Fe3O4 and WO3 immobilized onto montmorillonite as a photo-reactive antimicrobial agent. RSC Adv. 2016, 6, 54486–54494. [Google Scholar] [CrossRef]
- Islamy Mazrad, Z.A.; In, I.; Lee, K.D.; Park, S.Y. Rapid fluorometric bacteria detection assay and photothermal effect by fluorescent polymer of coated surfaces and aqueous state. Biosens. Bioelectron. 2017, 89 Pt 2, 1026–1033. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Nam, Y. Polydopamine-doped conductive polymer microelectrodes for neural recording and stimulation. J. Neurosci. Methods 2019, 326, 108369. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Bian, F.; Cai, L.; Wang, T.; Kong, T.; Zhao, Y. Bioinspired photonic barcodes for multiplexed target cycling and hybridization chain reaction. Biosens. Bioelectron. 2019, 143, 111629. [Google Scholar] [CrossRef]
- Jiang, H.; Xia, Q.; Liu, D.; Ling, K. Calcium-cation-doped polydopamine-modified 2D black phosphorus nanosheets as a robust platform for sensitive and specific biomolecule sensing. Anal. Chim. Acta 2020, 1121, 1–10. [Google Scholar] [CrossRef]
- Yang, M.; Zhou, H.; Zhang, Y.; Hu, Z.; Niu, N.; Yu, C. Controlled synthesis of polydopamine: A new strategy for highly sensitive fluorescence turn-on detection of acetylcholinesterase activity. Mikrochim Acta 2018, 185, 132. [Google Scholar] [CrossRef]
- Xiong, Y.; Liu, Q.; Yin, X. Synthesis of alpha-glucosidase-immobilized nanoparticles and their application in screening for alpha-glucosidase inhibitors. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 2016, 1022, 75–80. [Google Scholar] [CrossRef]
- Li, G.; Kong, W.; Zhao, M.; Lu, S.; Gong, P.; Chen, G.; Xia, L.; Wang, H.; You, J.; Wu, Y. A fluorescence resonance energy transfer (FRET) based “Turn-On” nanofluorescence sensor using a nitrogen-doped carbon dot-hexagonal cobalt oxyhydroxide nanosheet architecture and application to alpha-glucosidase inhibitor screening. Biosens. Bioelectron. 2016, 79, 728–735. [Google Scholar] [CrossRef]
- Chen, H.; Zhang, J.; Wu, H.; Koh, K.; Yin, Y. Sensitive colorimetric assays for alpha-glucosidase activity and inhibitor screening based on unmodified gold nanoparticles. Anal. Chim. Acta 2015, 875, 92–98. [Google Scholar] [CrossRef]
- Wei, X.; Zhang, Z.; Wang, Z. A simple dopamine detection method based on fluorescence analysis and dopamine polymerization. Microchem. J. 2019, 145, 55–58. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, Z.; Yang, X.; Li, Z.L.; Sun, L.; Ma, J.; Jiang, H. The determination of alpha-glucosidase activity through a nano fluorescent sensor of F-PDA-CoOOH. Anal. Chim. Acta 2019, 1080, 170–177. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Sun, T.; Grattan, K.T.V. A Turn-On Fluorescence-Based Fibre Optic Sensor for the Detection of Mercury. Sensors 2019, 19, 2142. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Li, Z.; Liu, D.; Chen, M.; Wang, S.C.; Chan, Y.T.; Wang, P. Tetraphenylethylene(TPE)-Containing Metal-Organic Nanobelt and Its Turn-on Fluorescence for Sulfide (S2−). Inorg. Chem. 2020, 59, 6640–6645. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Zhao, Y.; Jia, P.; Wang, Q.; Liu, Y.; Bu, T.; Zhang, M.; Bai, F.; Wang, L. Dual-Emission Zr-MOF-Based Composite Material as a Fluorescence Turn-On Sensor for the Ultrasensitive Detection of Al3. Inorg. Chem. 2020, 59, 18205–18213. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-Y.; Li, L.; Yu, R.-Q.; Chen, T.-T.; Chu, X. CoOOH-induced synthesis of fluorescent polydopamine nanoparticles for the detection of ascorbic acid. Anal. Methods 2017, 9, 5518–5524. [Google Scholar] [CrossRef]
- Yang, Q.; Wang, X.; Peng, H.; Arabi, M.; Li, J.; Xiong, H.; Choo, J.; Chen, L. Ratiometric fluorescence and colorimetry dual-mode assay based on manganese dioxide nanosheets for visual detection of alkaline phosphatase activity. Sens. Actuators B Chem. 2020, 302, 127176. [Google Scholar] [CrossRef]
- Chang, L.; Chen, F.; Zhang, X.; Kuang, T.; Li, M.; Hu, J.; Shi, J.; Lee, L.J.; Cheng, H.; Li, Y. Synthetic Melanin E-Ink. ACS Appl. Mater. Interfaces 2017, 9, 16553–16560. [Google Scholar] [CrossRef]
- Jastrzebska, M.M.; Isotalo, H.; Paloheimo, J.; Stubb, H. Electrical conductivity of synthetic DOPA-melanin polymer for different hydration states and temperatures. J. Biomater. Sci. Polym. Ed. 1996, 7, 577–586. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Su, H.; Zhao, F. Recent Advances in Intrinsically Fluorescent Polydopamine Materials. Appl. Sci. 2022, 12, 4560. https://doi.org/10.3390/app12094560
Su H, Zhao F. Recent Advances in Intrinsically Fluorescent Polydopamine Materials. Applied Sciences. 2022; 12(9):4560. https://doi.org/10.3390/app12094560
Chicago/Turabian StyleSu, Hang, and Fei Zhao. 2022. "Recent Advances in Intrinsically Fluorescent Polydopamine Materials" Applied Sciences 12, no. 9: 4560. https://doi.org/10.3390/app12094560







