Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. In Vivo Study Design
2.2. Analysis of Plasma Fatty Acids
2.3. Analysis of Adipose Tissue Fatty Acids
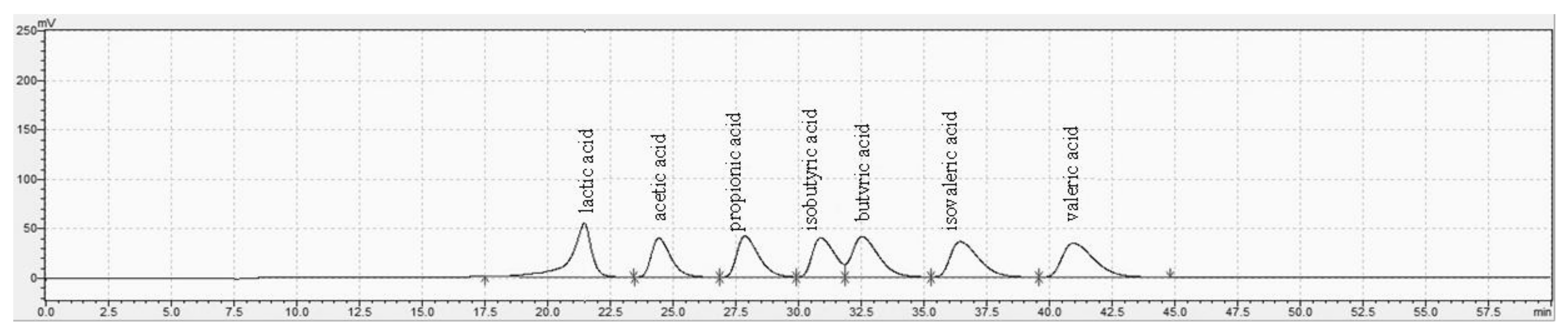
2.4. Analysis of Fecal SCFAs and Lactic Acid
2.5. Statistical Analysis
3. Results
3.1. Plasma Fatty Acid Profiles
3.2. Adipose Tissue Fatty Acid Profiles
3.3. Fecal SCFAs and Lactic Acid
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Diabetes. Available online: https://www.who.int/news-room/fact-sheets/detail/diabetes (accessed on 20 January 2022).
- Facts & Figures. Available online: https://www.idf.org/aboutdiabetes/what-is-diabetes/facts-figures.html (accessed on 20 January 2022).
- Mobasseri, M.; Shirmohammadi, M.; Amiri, T.; Vahed, N.; Hosseini Fard, H.; Ghojazadeh, M. Prevalence and incidence of Type 1 diabetes in the world: A systematic review and meta-analysis. Health Promot. Perspect. 2020, 10, 98–115. [Google Scholar] [CrossRef] [PubMed]
- DiMeglio, L.A.; Evans-Molina, C.; Oram, R.A. Type 1 diabetes. Lancet 2018, 391, 2449–2462. [Google Scholar] [CrossRef]
- Sciurti, M.; Fornaroli, F.; Gaiani, F.; Bonaguri, C.; Leandro, G.; Di Mario, F.; De’ Angelis, G.L. Genetic susceptibilty and celiac disease: What role do HLA haplotypes play? Acta Biomed. 2018, 89, 17–21. [Google Scholar] [PubMed]
- Mejía-León, M.E.; de la Barca, A.M.C. Diet, Microbiota and immune system in Type 1 diabetes development and evolution. Nutrients 2015, 7, 9171–9184. [Google Scholar] [CrossRef] [Green Version]
- Pircalabioru, G.G.; Corcionivoschi, N.; Gundogdu, O.; Chifiriuc, M.-C.; Marutescu, L.G.; Ispas, B.; Savu, O. Dysbiosis in the development of Type I diabetes and associated complications: From mechanisms to targeted gut microbes manipulation therapies. Int. J. Mol. Sci. 2021, 22, 2763. [Google Scholar] [CrossRef]
- Hamdy, O.; Barakatun-Nisak, M.-Y. Nutrition in diabetes. Endocrinol. Metab. Clin. North Am. 2016, 45, 799–817. [Google Scholar] [CrossRef]
- Hernández-Alonso, P.; Bulló, M.; Salas-Salvadó, J. Pistachios for health: What do we know about this multifaceted nut? Nutr. Today 2016, 51, 133–138. [Google Scholar] [CrossRef] [Green Version]
- Sales-Campos, H.; de Souza, P.R.; Peghini, B.C.; da Silva, J.S.; Cardoso, C.R. An overview of the modulatory effects of oleic acid in health and disease. Mini Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar]
- Granado-Casas, M.; Mauricio, D. Oleic acid in the diet and what it does: Implications for diabetes and its complications. In Bioactive Food as Dietary Interventions for Diabetes, 2nd ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 211–229. [Google Scholar]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.S.; Karathanos, V.T. Nutritional evaluation and health promoting activities of nuts and seeds cultivated in Greece. Int. J. Food Sci. Nutr. 2013, 64, 757–767. [Google Scholar] [CrossRef]
- Mustad, V.A.; Etherton, T.D.; Cooper, A.D.; Mastro, A.M.; Pearson, T.A.; Jonnalagadda, S.S.; Kris-Etherton, P.M. Reducing saturated fat intake is associated with increased levels of LDL receptors on mononuclear cells in healthy men and women. J. Lipid Res. 1997, 38, 459–468. [Google Scholar] [CrossRef]
- Berglund, L.; Lefevre, M.; Ginsberg, H.N.; Kris-Etherton, P.M.; Elmer, P.J.; Stewart, P.W.; Ershow, A.; Pearson, T.A.; Dennis, B.H.; Roheim, P.S.; et al. Comparison of monounsaturated fat with carbohydrates as a replacement for saturated fat in subjects with a high metabolic risk profile: Studies in the fasting and postprandial states. Am. J. Clin. Nutr. 2007, 86, 1611–1620. [Google Scholar] [CrossRef]
- Ros, E. Dietary cis-monounsaturated fatty acids and metabolic control in Type 2 diabetes. Am. J. Clin. Nutr. 2003, 78, 617S–625S. [Google Scholar] [CrossRef] [Green Version]
- Manco, M.; Calvani, M.; Mingrone, G. Effects of dietary fatty acids on insulin sensitivity and secretion. Diabetes Obes. Metab. 2004, 6, 402–413. [Google Scholar] [CrossRef]
- Wu, H.-J.; Wu, E. The role of gut microbiota in immune homeostasis and autoimmunity. Gut Microbes 2012, 3, 4–14. [Google Scholar] [CrossRef] [Green Version]
- Rosés, C.; Cuevas-Sierra, A.; Quintana, S.; Riezu-Boj, J.I.; Martínez, J.A.; Milagro, F.I.; Barceló, A. Gut microbiota bacterial species associated with mediterranean diet-related food groups in a northern spanish population. Nutrients 2021, 13, 636. [Google Scholar] [CrossRef]
- Bezirtzoglou, E.; Stavropoulou, E.; Kantartzi, K.; Tsigalou, C.; Voidarou, C.; Mitropoulou, G.; Prapa, I.; Santarmaki, V.; Kompoura, V.; Yanni, A.E.; et al. Maintaining digestive health in diabetes: The role of the gut microbiome and the challenge of functional foods. Microorganisms 2021, 9, 516. [Google Scholar] [CrossRef]
- Yanni, A.E.; Mitropoulou, G.; Prapa, I.; Agrogiannis, G.; Kostomitsopoulos, N.; Bezirtzoglou, E.; Kourkoutas, Y.; Karathanos, V.T. Functional modulation of gut microbiota in diabetic rats following dietary intervention with pistachio nuts (Pistacia vera L.). Metabol Open 2020, 7, 100040. [Google Scholar] [CrossRef]
- Terzo, S.; Mulè, F.; Caldara, G.F.; Baldassano, S.; Puleio, R.; Vitale, M.; Cassata, G.; Ferrantelli, V.; Amato, A. Pistachio consumption alleviates inflammation and improves gut microbiota composition in mice fed a high-fat diet. Int. J. Mol. Sci. 2020, 21, 365. [Google Scholar] [CrossRef] [Green Version]
- Paun, A.; Danska, J.S. Modulation of Type 1 and Type 2 diabetes risk by the intestinal microbiome: Role of gut microbiome in diabetes. Pediatr. Diabetes 2016, 17, 469–477. [Google Scholar] [CrossRef]
- Shao, L.; Feng, B.; Zhang, Y.; Zhou, H.; Ji, W.; Min, W. The role of adipose-derived inflammatory cytokines in Type 1 diabetes. Adipocyte 2016, 5, 270–274. [Google Scholar] [CrossRef] [Green Version]
- Zipris, D. Visceral adipose tissue: A new target organ in virus-induced Type 1 diabetes. Front. Immunol. 2021, 12, 702506. [Google Scholar] [CrossRef]
- Risérus, U. Fatty acids and insulin sensitivity. Curr. Opin. Clin. Nutr. Metab. Care 2008, 11, 100–105. [Google Scholar] [CrossRef]
- Priyadarshini, M.; Lednovich, K.; Xu, K.; Gough, S.; Wicksteed, B.; Layden, B.T. FFAR from the gut microbiome crowd: SCFA receptors in T1D pathology. Metabolites 2021, 11, 302. [Google Scholar] [CrossRef]
- Fernández-Millán, E.; Guillén, C. Multi-organ crosstalk with endocrine pancreas: A focus on how gut microbiota shapes pancreatic Beta-cells. Biomolecules 2022, 12, 104. [Google Scholar] [CrossRef] [PubMed]
- Garonzi, C.; Forsander, G.; Maffeis, C. Impact of fat intake on blood glucose control and cardiovascular risk factors in children and adolescents with Type 1 diabetes. Nutrients 2021, 13, 2625. [Google Scholar] [CrossRef] [PubMed]
- Furman, B.L. Streptozotocin-induced diabetic models in mice and rats. Curr. Protoc. 2021, 1, e78. [Google Scholar] [CrossRef] [PubMed]
- Tsoukas, M.A.; Ko, B.-J.; Witte, T.R.; Dincer, F.; Hardman, W.E.; Mantzoros, C.S. Dietary Walnut suppression of colorectal cancer in mice: Mediation by MiRNA patterns and fatty acid incorporation. J. Nutr. Biochem. 2015, 26, 776–783. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Song, R.; Nguyen, C.; Zerlin, A.; Karp, H.; Naowamondhol, K.; Thames, G.; Gao, K.; Li, L.; Tseng, C.-H.; et al. Pistachio nuts reduce triglycerides and body weight by comparison to refined carbohydrate snack in obese subjects on a 12-week weight loss program. J. Am. Coll. Nutr. 2010, 29, 198–203. [Google Scholar] [CrossRef]
- Ukhanova, M.; Wang, X.; Baer, D.J.; Novotny, J.A.; Fredborg, M.; Mai, V. Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross-over human feeding study. Br. J. Nutr. 2014, 111, 2146–2152. [Google Scholar] [CrossRef]
- Lepage, G.; Roy, C.C. Direct transesterification of all classes of lipids in a one-step reaction. J. Lipid Res. 1986, 27, 114–120. [Google Scholar] [CrossRef]
- Mamalakis, G.; Kafatos, A.; Manios, Y.; Kalogeropoulos, N.; Andrikopoulos, N. Adipose fat quality vs. quantity: Relationships with children’s serum lipid levels. Prev. Med. 2001, 33, 525–535. [Google Scholar] [CrossRef]
- Chaia, A.P.; Zárate, G. Dairy propionibacteria from milk or cheese diets remain viable and enhance propionic acid production in the mouse cecum. Lait 2005, 85, 85–98. [Google Scholar] [CrossRef] [Green Version]
- Nikolaou, A.; Galanis, A.; Kanellaki, M.; Tassou, C.; Akrida-Demertzi, K.; Kourkoutas, Y. Assessment of free and immobilized kefir culture in simultaneous alcoholic and malolactic cider fermentations. LWT Food Sci.Technol. 2017, 76, 67–78. [Google Scholar] [CrossRef]
- Huda-Faujan, N.; Abdulamir, A.S.; Fatimah, A.B.; Anas, O.M.; Shuhaimi, M.; Yazid, A.M.; Loong, Y.Y. The impact of the level of the intestinal short chain fatty acids in inflammatory bowel disease patients versus healthy subjects. Open Biochem. J. 2010, 4, 53–58. [Google Scholar] [CrossRef]
- Needell, J.C.; Brown, M.N.; Zipris, D. Involvement of adipose tissue inflammation and dysfunction in virus-induced Type 1 diabetes. J. Endocrinol. 2018, 238, 61–75. [Google Scholar] [CrossRef] [Green Version]
- Gastaldelli, A.; Gaggini, M.; DeFronzo, R.A. Role of adipose tissue insulin resistance in the natural history of Type 2 diabetes: Results from the San Antonio Metabolism Study. Diabetes 2017, 66, 815–822. [Google Scholar] [CrossRef] [Green Version]
- Chait, A.; den Hartigh, L.J. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Markovic-Jovanovic, S. Nutritional management in Type 1 diabetes. In Mellitus. In Type 1 Diabetes; IntechOpen: London, UK, 2013. [Google Scholar]
- Franz, M.J.; Bantle, J.P.; Beebe, C.A.; Brunzell, J.D.; Chiasson, J.-L.; Garg, A.; Holzmeister, L.A.; Hoogwerf, B.; Mayer-Davis, E.; Mooradian, A.D.; et al. Evidence-based nutrition principles and recommendations for the treatment and prevention of diabetes and related complications. Diabetes Care 2003, 26, S51–S61. [Google Scholar] [CrossRef] [Green Version]
- Johns, I.; Frost, G.; Dornhorst, A. Increasing the proportion of plasma MUFA, as a result of dietary intervention, is associated with a modest improvement in insulin sensitivity. J. Nutr. Sci. 2019, 9, e6. [Google Scholar] [CrossRef] [Green Version]
- Ryan, M.; McInerney, D.; Owens, D.; Collins, P.; Johnson, A.; Tomkin, G.H. Diabetes and the mediterranean diet: A beneficial effect of oleic acid on insulin sensitivity, adipocyte glucose transport and endothelium-dependent vasoreactivity. QJM 2000, 93, 85–91. [Google Scholar] [CrossRef] [Green Version]
- Martín-Peláez, S.; Fito, M.; Castaner, O. Mediterranean diet effects on Type 2 diabetes prevention, disease progression, and related mechanisms. A review. Nutrients 2020, 12, 2236. [Google Scholar] [CrossRef]
- Introduction. Diabetes Care 2016, 39, S1–S2.
- Van Staveren, W.A.; Deurenberg, P.; Katan, M.B.; Burema, J.; de Groot, L.C.; Hoffmans, M.D. Validity of the fatty acid composition of subcutaneous fat tissue microbiopsies as an estimate of the long-term average fatty acid composition of the diet of separate individuals. Am. J. Epidemiol. 1986, 123, 455–463. [Google Scholar]
- Cantwell, M.M. Assessment of individual fatty acid intake. Proc. Nutr. Soc. 2000, 59, 187–191. [Google Scholar]
- Ahn, M.Y.; Seo, Y.J.; Ji, S.D.; Han, J.W.; Hwang, J.S.; Yun, E.Y. Fatty acid composition of adipose tissues in obese mice and SD rats fed with Isaria sinclairii powder. Toxicol. Res. 2010, 26, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Montanaro, M.A.; Bernasconi, A.M.; González, M.S.; Rimoldi, O.J.; Brenner, R.R. Effects of fenofibrate and insulin on the biosynthesis of unsaturated fatty acids in streptozotocin diabetic rats. Prostaglandins Leukot. Essent. Fat. Acids 2005, 73, 369–378. [Google Scholar] [CrossRef]
- Malaisse, W.J.; Zhang, Y.; Louchami, K.; Sener, A.; Portois, L.; Carpentier, Y.A. Brain phospholipid and triglyceride fatty acid content and pattern in Type 1 and Type 2 diabetic rats. Neurosci. Lett. 2006, 409, 75–79. [Google Scholar] [CrossRef]
- Carta, G.; Murru, E.; Banni, S.; Manca, C. Palmitic acid: Physiological Role, metabolism and nutritional implications. Front. Physiol. 2017, 8, 902. [Google Scholar] [CrossRef] [Green Version]
- Nogoy, K.M.C.; Kim, H.J.; Lee, Y.; Zhang, Y.; Yu, J.; Lee, D.H.; Li, X.Z.; Smith, S.B.; Seong, H.A.; Choi, S.H. High dietary oleic acid in olive oil-supplemented diet enhanced Omega-3 fatty acid in blood plasma of rats. Food Sci. Nutr. 2020, 8, 3617–3625. [Google Scholar] [CrossRef]
- Bozzetto, L.; Alderisio, A.; Clemente, G.; Giorgini, M.; Barone, F.; Griffo, E.; Costabile, G.; Vetrani, C.; Cipriano, P.; Giacco, A.; et al. Gastrointestinal effects of extra-virgin olive oil associated with lower postprandial glycemia in Type 1 diabetes. Clin. Nutr. 2019, 38, 2645–2651. [Google Scholar] [PubMed]
- Griffin, M.D.; Sanders, T.A.B.; Davies, I.G.; Morgan, L.M.; Millward, D.J.; Lewis, F.; Slaughter, S.; Cooper, J.A.; Miller, G.J.; Griffin, B.A. Effects of altering the ratio of dietary n-6 to n-3 fatty acids on insulin sensitivity, lipoprotein size, and postprandial lipemia in men and postmenopausal women aged 45-70 y: The OPTILIP Study. Am. J. Clin. Nutr. 2006, 84, 1290–1298. [Google Scholar] [PubMed] [Green Version]
- Giacco, R.; Cuomo, V.; Vessby, B.; Uusitupa, M.; Hermansen, K.; Meyer, B.J.; Riccardi, G.; Rivellese, A.A.; KANWU Study Group. Fish oil, insulin sensitivity, insulin secretion and glucose tolerance in healthy people: Is there any effect of fish oil supplementation in relation to the type of background diet and habitual dietary intake of n-6 and n-3 fatty acids? Nutr. Metab. Cardiovasc. Dis. 2007, 17, 572–580. [Google Scholar] [CrossRef] [PubMed]
- Björntorp, P. “Portal” adipose tissue as a generator of risk factors for cardiovascular disease and diabetes. Arteriosclerosis 1990, 10, 493–496. [Google Scholar] [CrossRef] [Green Version]
- Houghton, D.; Hardy, T.; Stewart, C.; Errington, L.; Day, C.P.; Trenell, M.I.; Avery, L. Systematic review assessing the effectiveness of dietary intervention on gut microbiota in adults with Type 2 diabetes. Diabetologia 2018, 61, 1700–1711. [Google Scholar] [CrossRef] [Green Version]
- Nishitsuji, K.; Xiao, J.; Nagatomo, R.; Umemoto, H.; Morimoto, Y.; Akatsu, H.; Inoue, K.; Tsuneyama, K. Analysis of the gut microbiome and plasma short-chain fatty acid profiles in a spontaneous mouse model of metabolic syndrome. Sci. Rep. 2017, 7, 15876. [Google Scholar] [CrossRef]
- Mariño, E.; Richards, J.L.; McLeod, K.H.; Stanley, D.; Yap, Y.A.; Knight, J.; McKenzie, C.; Kranich, J.; Oliveira, A.C.; Rossello, F.J.; et al. Erratum: Gut microbial metabolites limit the frequency of autoimmune T cells and protect against Type 1 diabetes. Nat. Immunol. 2017, 18, 951. [Google Scholar]
- Hove, H.; Mortensen, P.B. Influence of Intestinal Inflammation (IBD) and small and large bowel length on fecal short-chain fatty acids and lactate. Dig. Dis. Sci. 1995, 40, 1372–1380. [Google Scholar]
- Wang, S.P.; Rubio, L.A.; Duncan, S.H.; Donachie, G.E.; Holtrop, G.; Lo, G.; Farquharson, F.M.; Wagner, J.; Parkhill, J.; Louis, P.; et al. Pivotal roles for PH, lactate, and lactate-utilizing bacteria in the stability of a human colonic microbial ecosystem. Msystems 2020, 5, e00645-20. [Google Scholar] [CrossRef]
- Hatti-Kaul, R.; Chen, L.; Dishisha, T.; Enshasy, H.E. Lactic acid bacteria: From starter cultures to producers of chemicals. FEMS Microbiol. Lett. 2018, 365, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Perini, M.P.; Rentas, M.F.; Pedreira, R.; Amaral, A.R.; Zafalon, R.V.A.; Rodrigues, R.B.A.; Henríquez, L.B.F.; Zanini, L.; Vendramini, T.H.A.; Balieiro, J.C.C.; et al. Duration of prebiotic intake is a key-factor for diet-induced modulation of immunity and fecal fermentation products in dogs. Microorganisms 2020, 8, 1916. [Google Scholar]
- Vernia, P.; Caprilli, R.; Latella, G.; Barbetti, F.; Magliocca, F.M.; Cittadini, M. Fecal lactate and ulcerative colitis. Gastroen-Terology 1988, 95, 1564–1568. [Google Scholar] [CrossRef]
- Song, W.-B.; Lv, Y.-H.; Zhang, Z.-S.; Li, Y.-N.; Xiao, L.-P.; Yu, X.-P.; Wang, Y.-Y.; Ji, H.-L.; Ma, L. Soluble intercellular adhesion molecule-1, D-lactate and diamine oxidase in patients with inflammatory bowel disease. World J. Gastroenterol. 2009, 15, 3916–3919. [Google Scholar] [CrossRef] [PubMed]
- Duzgun, A.P.; Bugdayci, G.; Sayin, B.; Ozmen, M.M.; Ozer, M.V.; Coskun, F. Serum D-lactate: A useful diagnostic marker for acute appendicitis. Hepatogastroenterology 2007, 54, 1483–1486. [Google Scholar] [PubMed]
- Belenguer, A.; Duncan, S.H.; Holtrop, G.; Anderson, S.E.; Lobley, G.E.; Flint, H.J. Impact of PH on lactate formation and utilization by human fecal microbial communities. Appl. Environ. Microbiol. 2007, 73, 6526–6533. [Google Scholar] [CrossRef] [Green Version]
- Windey, K.; François, I.; Broekaert, W.; De Preter, V.; Delcour, J.A.; Louat, T.; Herman, J.; Verbeke, K. High dose of prebiotics reduces fecal water cytotoxicity in healthy subjects. Mol. Nutr. Food Res. 2014, 58, 2206–2218. [Google Scholar] [CrossRef]
- Neis, E.P.J.G.; Dejong, C.H.C.; Rensen, S.S. The role of microbial amino acid metabolism in host metabolism. Nutrients 2015, 7, 2930–2946. [Google Scholar] [CrossRef] [Green Version]
- Oliphant, K.; Allen-Vercoe, E. Macronutrient metabolism by the human gut microbiome: Major fermentation by-products and their impact on host health. Microbiome 2019, 7, 91. [Google Scholar] [CrossRef]
- Heimann, E.; Nyman, M.; Pålbrink, A.-K.; Lindkvist-Petersson, K.; Degerman, E. Branched short-chain fatty acids modulate glucose and lipid metabolism in primary adipocytes. Adipocyte 2016, 5, 359–368. [Google Scholar] [CrossRef]
- Nagal, R.; Wang, S.; Solberg Woods, L.C.; Seshie, O.; Chung, S.T.; Shively, C.A.; Register, T.C.; Craft, S.; McClain, D.A.; Yadav, H. Comparative microbiome signatures and short-chain fatty acids in mouse, rat, non-human primate, and human feces. Front. Microbiol. 2018, 9, 2897. [Google Scholar] [CrossRef] [Green Version]
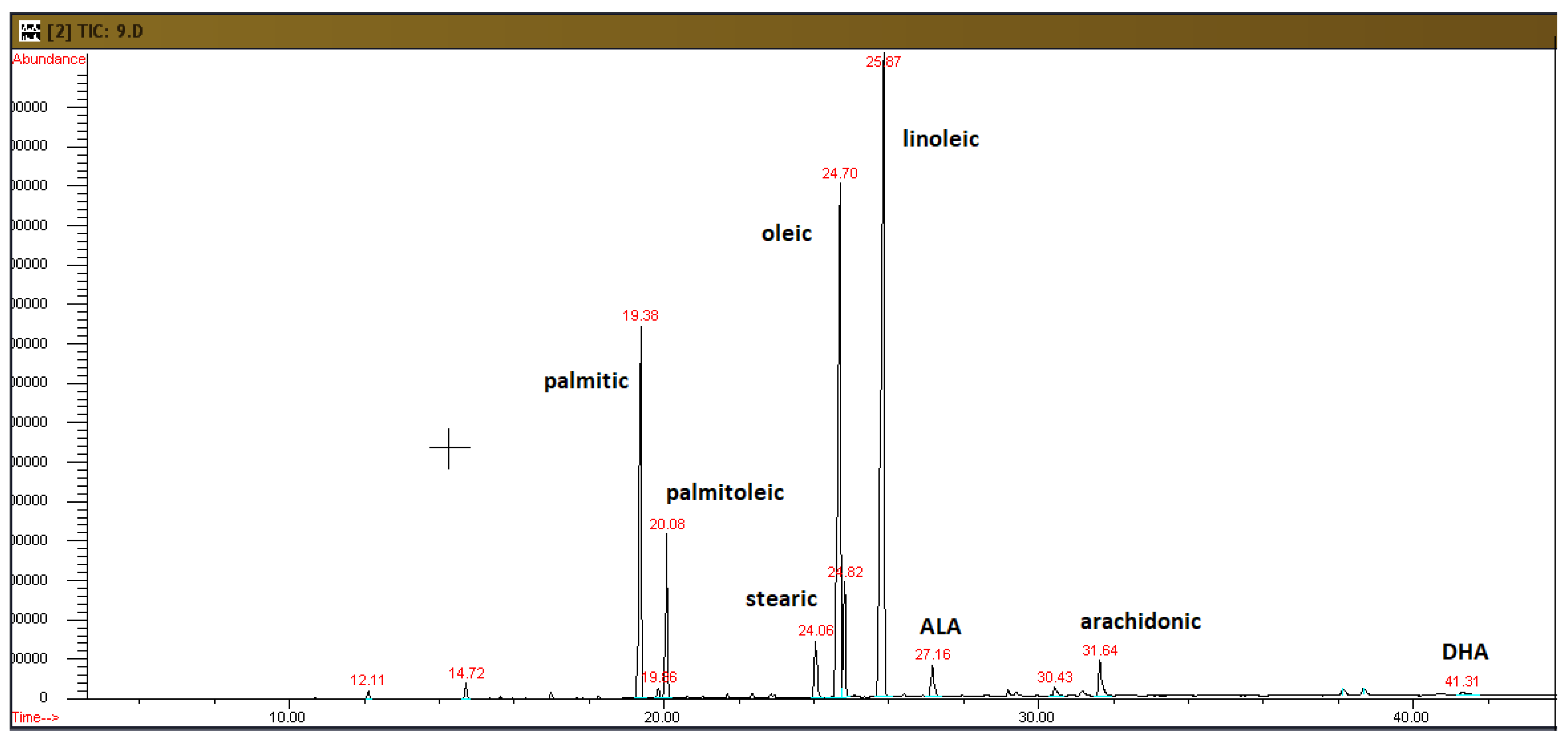
| Fatty Acids (%) | CD | PD | DCD | DPD |
|---|---|---|---|---|
| 16:0 | 17.26 ± 0.36 | 17.64 ± 0.51 | 16.92 ± 1.84 | 18.04 ± 0.53 |
| 16:1 | 0.30 ± 0.02 | 0.35 ± 0.05 | N/D | N/D |
| 17:0 | 0.30 ± 0.01 | 0.31 ± 0.03 | N/D | N/D |
| 18:0 | 11.27 ± 0.65 | 11.94 ± 0.55 | 11.32 ± 1.29 | 13.83 ± 0.33 |
| 18:1ω9 | 10.72 ± 0.61 | 10.90 ± 1.37 | 9.77± 1.43 | 14.43 a ± 0.80 |
| 18:2ω6 | 28.46 ± 0.95 | 21.50 ± 0.55 | 26.71 ± 3.78 | 22.36 ± 0.95 |
| 18:3ω6 | 1.06 ± 0.06 | N/D | 1.20 ± 0.13 | N/D |
| 20:4ω6 | 23.26 ± 1.14 | 24.08 ± 1.77 | 18.47 ± 3.19 | 23.33 ± 2.06 |
| 22:6ω3 | 1.66 ± 0.06 | 1.87 ± 0.12 | 1.81 ± 0.28 | 1.99 ± 0.18 |
| SFA | 28.83 ± 0.80 | 29.90 ± 0.96 | 28.24 ± 2.96 | 31.87 ± 0.85 |
| MUFA | 11.02 ± 0.63 | 11.25 ± 1.33 | 9.77 ± 1.43 | 14.43 a ± 0.80 |
| PUFA | 54.43 ± 1.16 | 47.44 ± 1.72 | 48.19 ± 4.28 | 47.68 ± 1.38 |
| ω6 | 52.77 ± 1.11 | 45.57 ± 1.67 | 46.38 ± 4.06 | 45.69 ± 1.47 |
| ω3 | 1.66 ± 0.06 | 1.87 ± 0.12 | 1.81 ± 0.28 | 1.99 ± 0.18 |
| ω6/ω3 | 31.93 ± 0.70 | 24.37 ± 1.32 | 28.66 ± 3.83 | 24.01 ± 2.38 |
| Fatty Acids (%) | CD | PD | DCD | DPD |
|---|---|---|---|---|
| 12:0 | 0.03 ± 0.04 | 0.04 ± 0.01 | 0.02 ± 0.00 | 0.03 ± 0.00 |
| 14:0 | 0.39 ± 0.01 | 0.40 ± 0.03 | 0.34 ± 0.03 | 0.35 ± 0.02 |
| 15:0 | 0.24 ± 0.01 | 0.23 ± 0.01 | 0.20 ± 0.01 | 0.20 ± 0.01 |
| 15:1 | 0.05 ± 0.00 | 0.01 ± 0.00 | 0.002 ± 0.00 | 0.05 ± 0.01 |
| 16:0 | 17.91 ± 0.24 | 17.61 ± 0.31 | 15.16 b,c ± 0.78 | 14.92 b,c ± 0.56 |
| 16:1 | 1.26 ± 0.05 | 1.42 ± 0.20 | 0.90 ± 0.29 | 0.70 ± 0.10 |
| 17:0 | 0.22 ± 0.01 | 0.21 ± 0.01 | 0.23 ± 0.01 | 0.22 ± 0.01 |
| 17:1 | 0.09 ± 0.01 | 0.08 ± 0.01 | 0.06 ± 0.01 | 0.06 ± 0.01 |
| 18:0 | 2.67 ± 0.04 | 2.56 ± 0.07 | 3.06 ± 0.18 | 3.07 ± 0.16 |
| 18:1ω9 | 25.53 ± 0.10 | 30.22 b ± 0.80 | 26.80 c ± 0.31 | 31.59 a,b ± 0.67 |
| 18:1ω7 | 2.87 ± 0.05 | 3.01 ± 0.07 | 3.11 ± 0.20 | 3.22 ± 0.08 |
| 18:2ω6 | 43.35 ± 0.31 | 39.33 b ± 0.25 | 44.80 c ± 0.70 | 40.81 a,b ± 0.55 |
| 18:3ω6 | 0.11 ± 0.01 | 0.12 ± 0.01 | 0.10 ± 0.01 | 0.11 ± 0.03 |
| 18:3ω3 (ALA) | 1.92 ± 0.04 | 1.74 ± 0.09 | 1.26 b ± 0.18 | 1.07 b,c ± 0.08 |
| 20:0 | 0.10 ± 0.01 | 0.10 ± 0.00 | 0.16 ± 0.03 | 0.16 ± 0.01 |
| 20:1ω9 | 0.23 ± 0.00 | 0.24 ± 0.00 | 0.34 ± 0.05 | 0.34 ± 0.03 |
| 20:2ω6 | 0.32 ± 0.01 | 0.30 ± 0.03 | 0.38 ± 0.04 | 0.35 ± 0.05 |
| 20:3ω6 | 0.17 ± 0.02 | 0.15 ± 0.01 | 0.23± 0.02 | 0.21± 0.03 |
| 20:4ω6 | 0.73 ± 0.05 | 0.64 ± 0.08 | 0.59 ± 0.08 | 0.50 ±0.13 |
| 24:0 | 0.12 ± 0.01 | 0.12 ± 0.01 | 0.20 ± 0.04 | 0.19 ±0.05 |
| 22:6ω3 (DHA) | 0.11 ± 0.01 | 0.11 ± 0.03 | 0.15± 0.04 | 0.15 ± 0.06 |
| SFA | 21.68 ± 0.24 | 21.26 ± 0.35 | 19.37 b,c ± 0.62 | 19.14 b,c ± 0.38 |
| MUFA | 30.05 ± 0.13 | 35.03 b ± 0.58 | 31.26 c ± 0.47 | 35.95 a,b ± 0.61 |
| PUFA | 46.73 ± 0.21 | 42.39 b ± 0.30 | 47.51 c ± 0.62 | 43.20 a,b ± 0.71 |
| ω6 | 44.69 ± 0.24 | 40.54 b ± 0.24 | 46.10 c ± 0.64 | 41.98 a,b ± 0.68 |
| ω3 | 2.04 ± 0.05 | 1.85 ± 0.12 | 1.40 b ± 0.10 | 1.22 b,c ± 0.09 |
| ω6/ω3 | 22.01 ± 0.60 | 22.32 ± 1.20 | 36.20 b,c ± 5.49 | 35.23 ± 2.71 |
| CD | PD | DCD | DPD | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End | Baseline | End | Baseline | End | Baseline | End | |
| lactic acid | 0.798 ± 0.063 | 0.921 ± 0.216 | 0.760 ± 0.056 | 1.345 c ± 0.625 | 1.429 ± 0.314 | 0.819 c ± 0.109 | 1.508 ± 0.490 | 0.711 c ± 0.073 |
| acetic acid | 5.224 ± 0.785 | 7.274 ± 2.169 | 5.303 ± 0.671 | 6.617 ± 0.720 | 5.744 ± 2.963 | 9.171 ± 2.410 | 6.424 ± 2.764 | 5.845 ± 1.827 |
| propionic acid | 0.911 ± 0.150 | 0.960 ± 0.145 | 0.965 ± 0.073 | 1.078 ± 0.241 | 0.916 ± 0.438 | 1.225 ± 0.284 | 1.019 ± 0.407 | 0.766 ± 0.124 |
| isobutyric acid | 0.199 ± 0.012 | 0.213 ± 0.038 | 0.206 ± 0.012 | 0.249 ± 0.046 | 0.173 ± 0.026 | 0.215 ± 0.039 | 0.164 ± 0.020 | 0.180 ± 0.024 |
| butyric acid | 0.937 ± 0.149 | 0.845 ± 0.159 | 0.834 ± 0.175 | 0.936 ± 0.312 | 0.606 ± 0.196 | 0.764 ± 0.213 | 0.653 ± 0.223 | 0.580 ± 0.198 |
| isovaleric acid | 0.040 ± 0.009 | 0.074 ± 0.016 | 0.056 ± 0.010 | 0.034 b ± 0.012 | 0.030 ± 0.016 | 0.068 ± 0.048 | 0.033 ± 0.013 | 0.026 a ±0.014 |
| valeric acid | 0.109 ± 0.033 | 0.191 ± 0.031 | 0.135 ± 0.034 | 0.134 ± 0.039 | 0.120 ± 0.041 | 0.191 ± 0.067 | 0.183 ± 0.054 | 0.182 ± 0.064 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Prapa, I.; Yanni, A.E.; Nikolaou, A.; Kostomitsopoulos, N.; Kalogeropoulos, N.; Bezirtzoglou, E.; Karathanos, V.T.; Kourkoutas, Y. Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat. Appl. Sci. 2022, 12, 4606. https://doi.org/10.3390/app12094606
Prapa I, Yanni AE, Nikolaou A, Kostomitsopoulos N, Kalogeropoulos N, Bezirtzoglou E, Karathanos VT, Kourkoutas Y. Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat. Applied Sciences. 2022; 12(9):4606. https://doi.org/10.3390/app12094606
Chicago/Turabian StylePrapa, Ioanna, Amalia E. Yanni, Anastasios Nikolaou, Nikolaos Kostomitsopoulos, Nick Kalogeropoulos, Eugenia Bezirtzoglou, Vaios T. Karathanos, and Yiannis Kourkoutas. 2022. "Dietary Pistachio (Pistacia vera L.) Beneficially Alters Fatty Acid Profiles in Streptozotocin-Induced Diabetic Rat" Applied Sciences 12, no. 9: 4606. https://doi.org/10.3390/app12094606






