Trypsin Depolarizes Pacemaker Potentials in Murine Small Intestinal Interstitial Cells of Cajal
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Preparation of ICCs and ICC Clusters
2.3. Patch-Clamp Experiments
2.4. Drugs
2.5. Statistical Analysis
3. Results
3.1. Effects of Trypsin on Pacemaker Potentials in Murine Small Intestinal ICCs
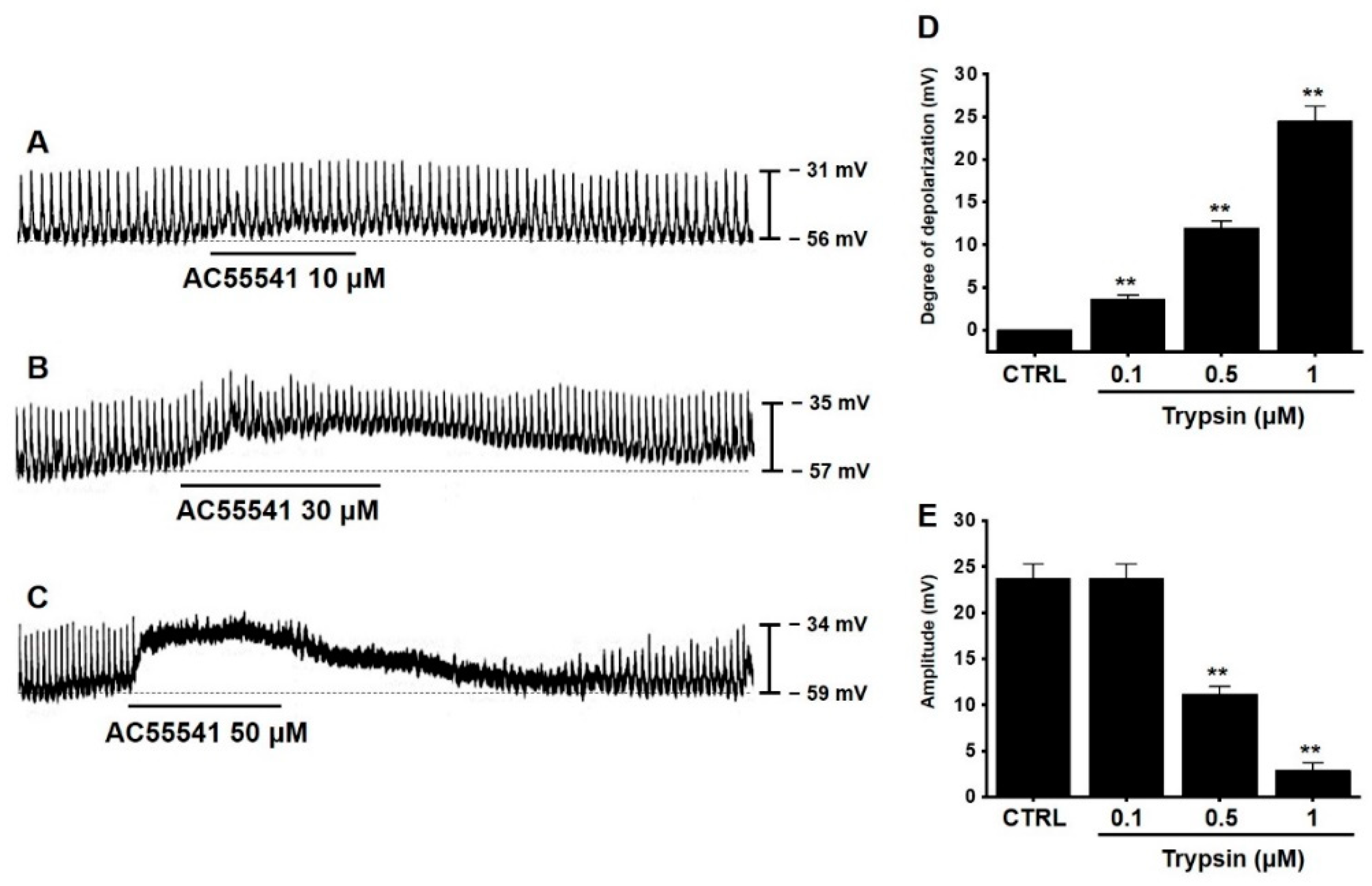
3.2. Effects of AC55541 on Pacemaker Potentials in Murine Small Intestinal ICCs
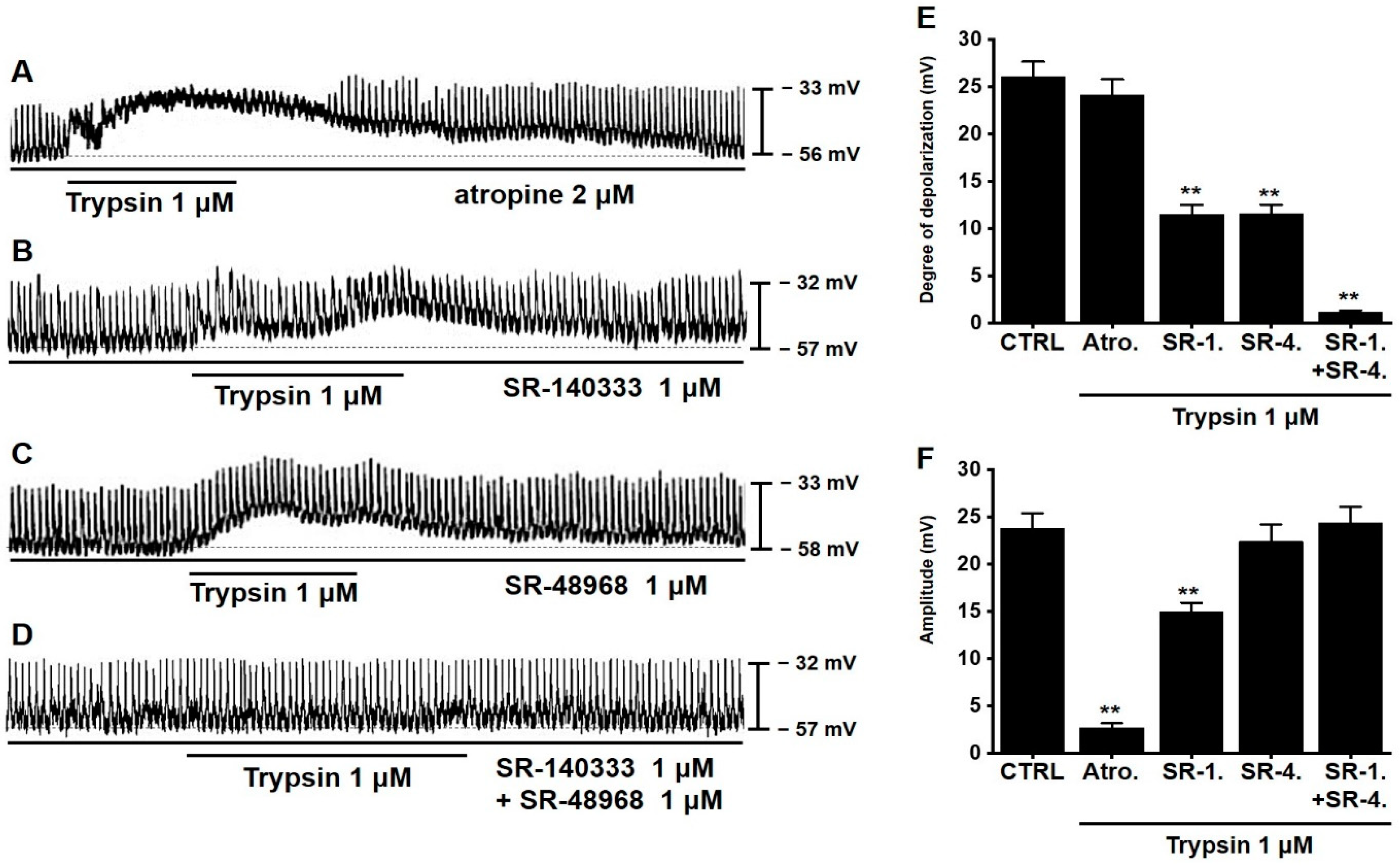
3.3. Involvement of Neurokinin Receptors in Trypsin-Induced Pacemaker Potential Depolarization in Murine Small Intestinal ICCs
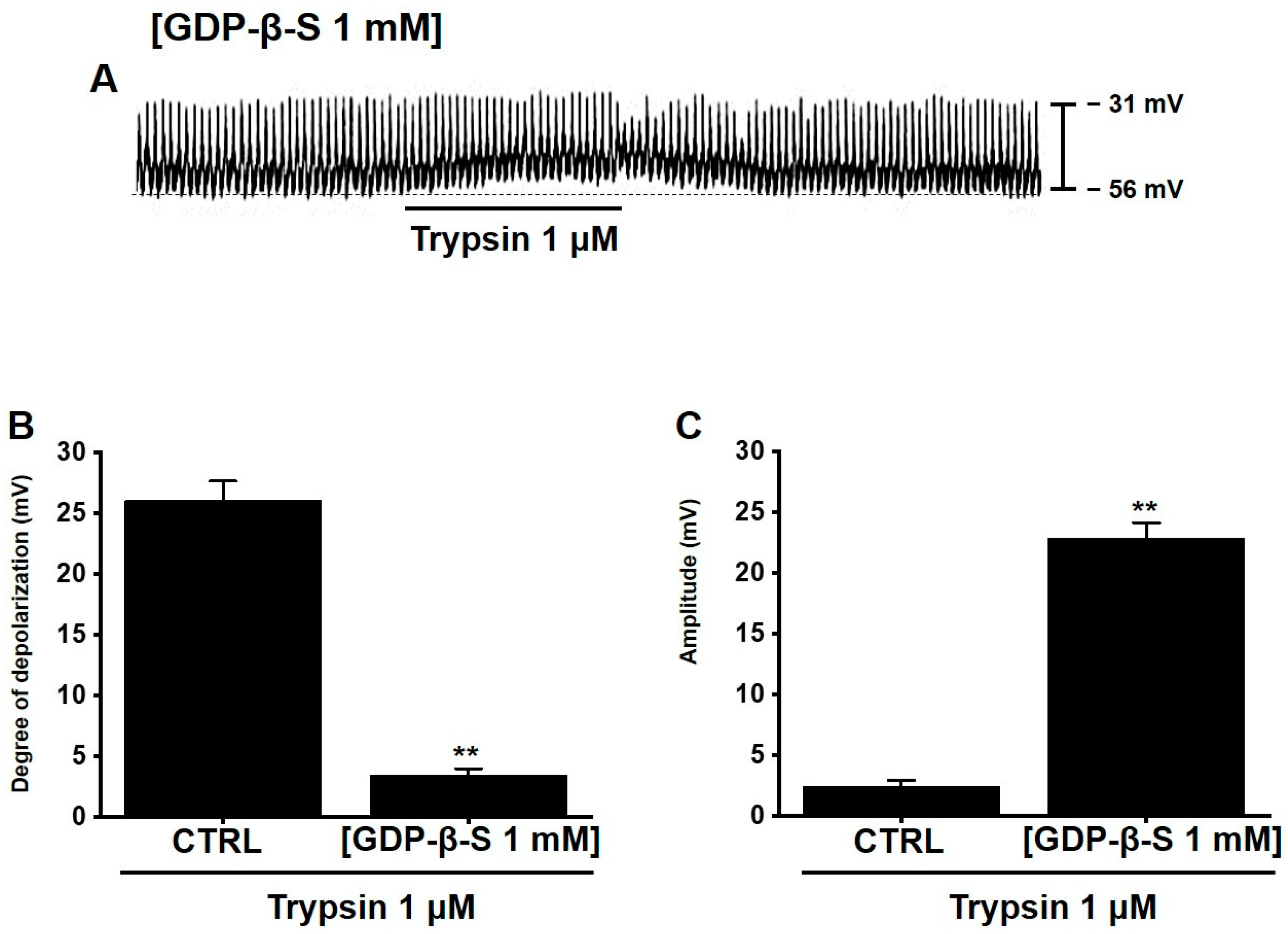
3.4. Involvement of G Proteins in Trypsin-Induced Pacemaker Potential Depolarization in Murine Small Intestinal ICCs
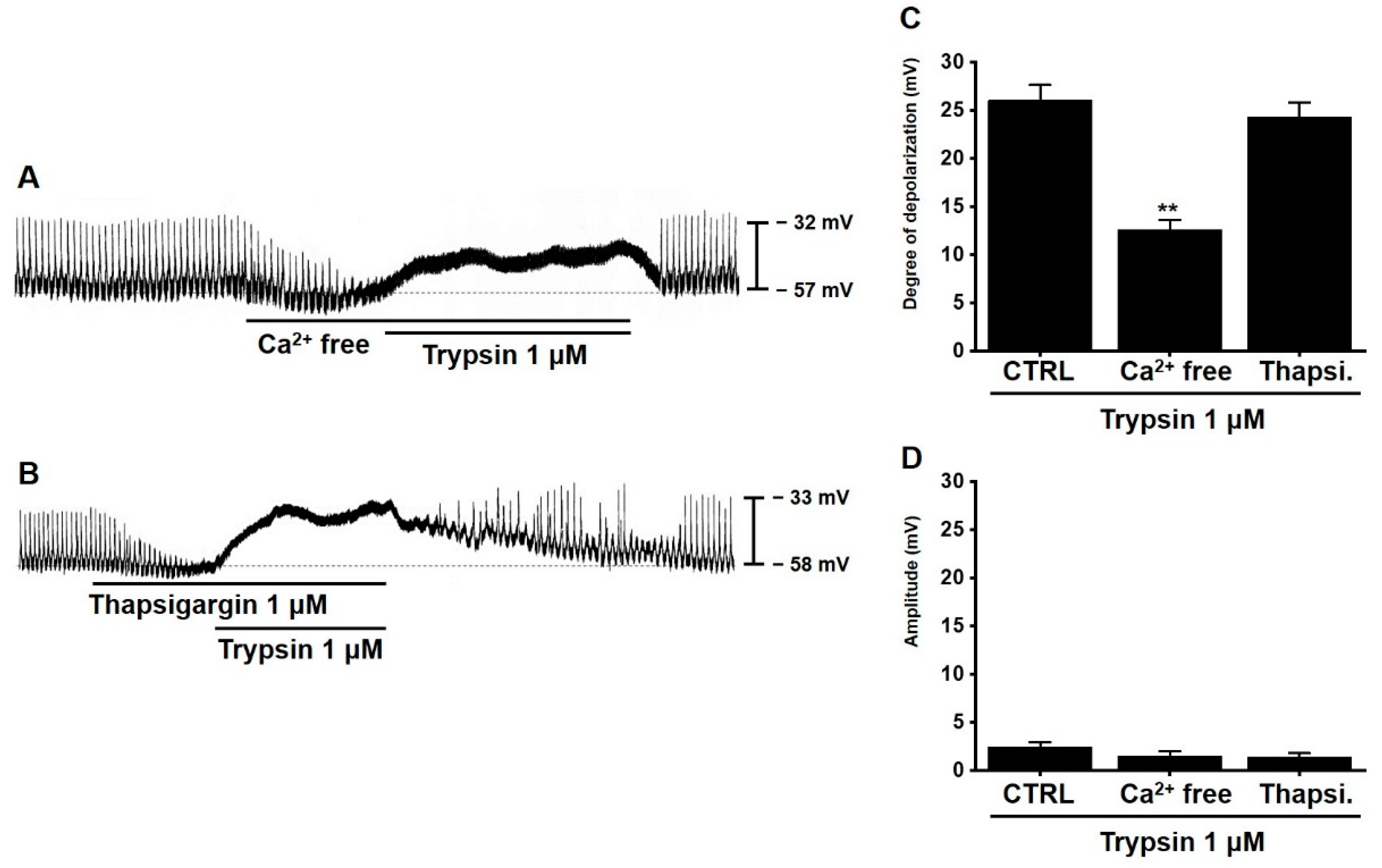
3.5. Involvement of Extracellular and Intracellular Calcium Ions in Trypsin-Induced Pacemaker Potential Depolarization in Murine Small Intestinal ICCs
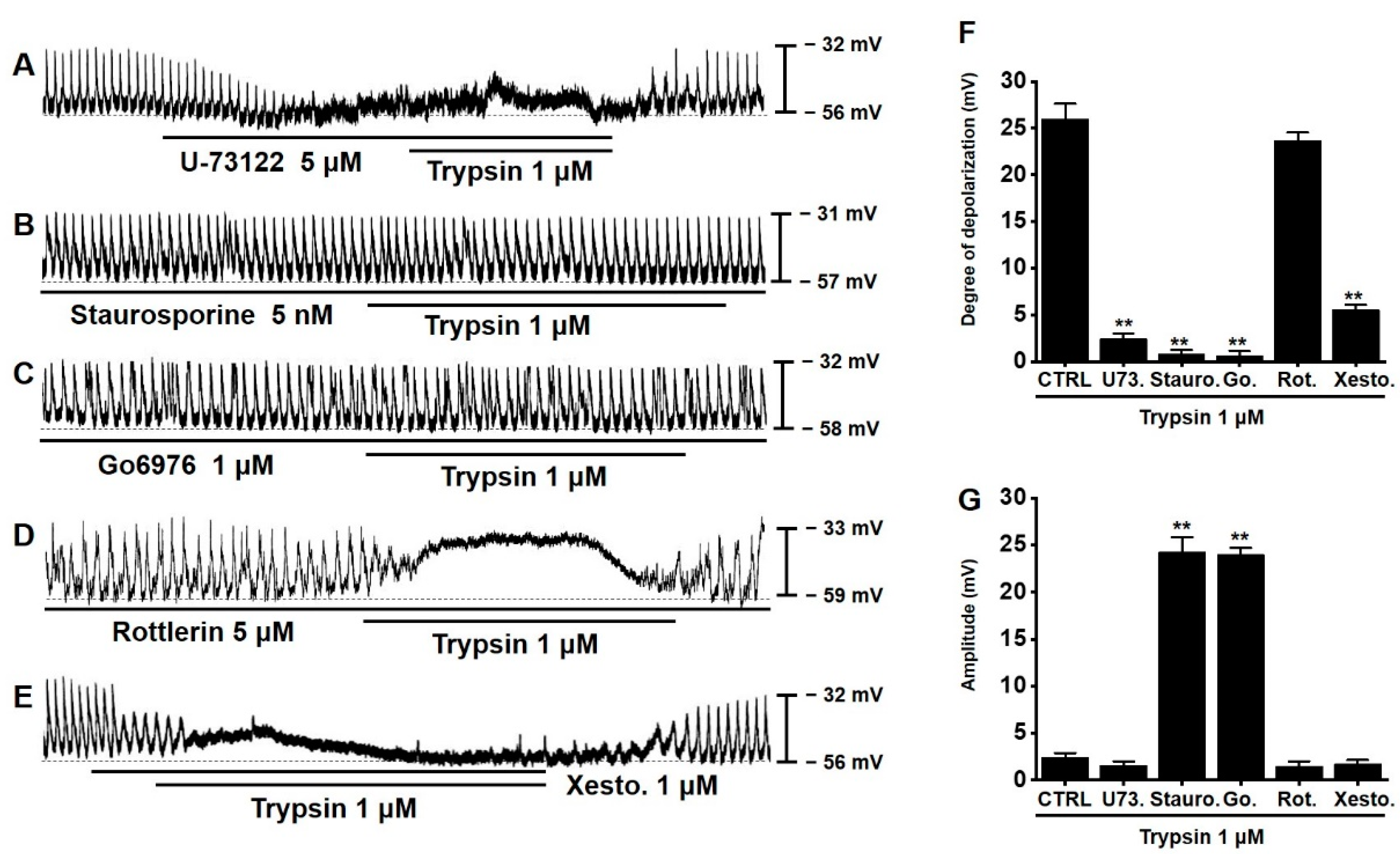
3.6. Involvement of PLC, PKC, and Inositol Triphosphate (IP3) Pathways in Trypsin-Induced Pacemaker Potential Depolarization in Murine Small Intestinal ICCs
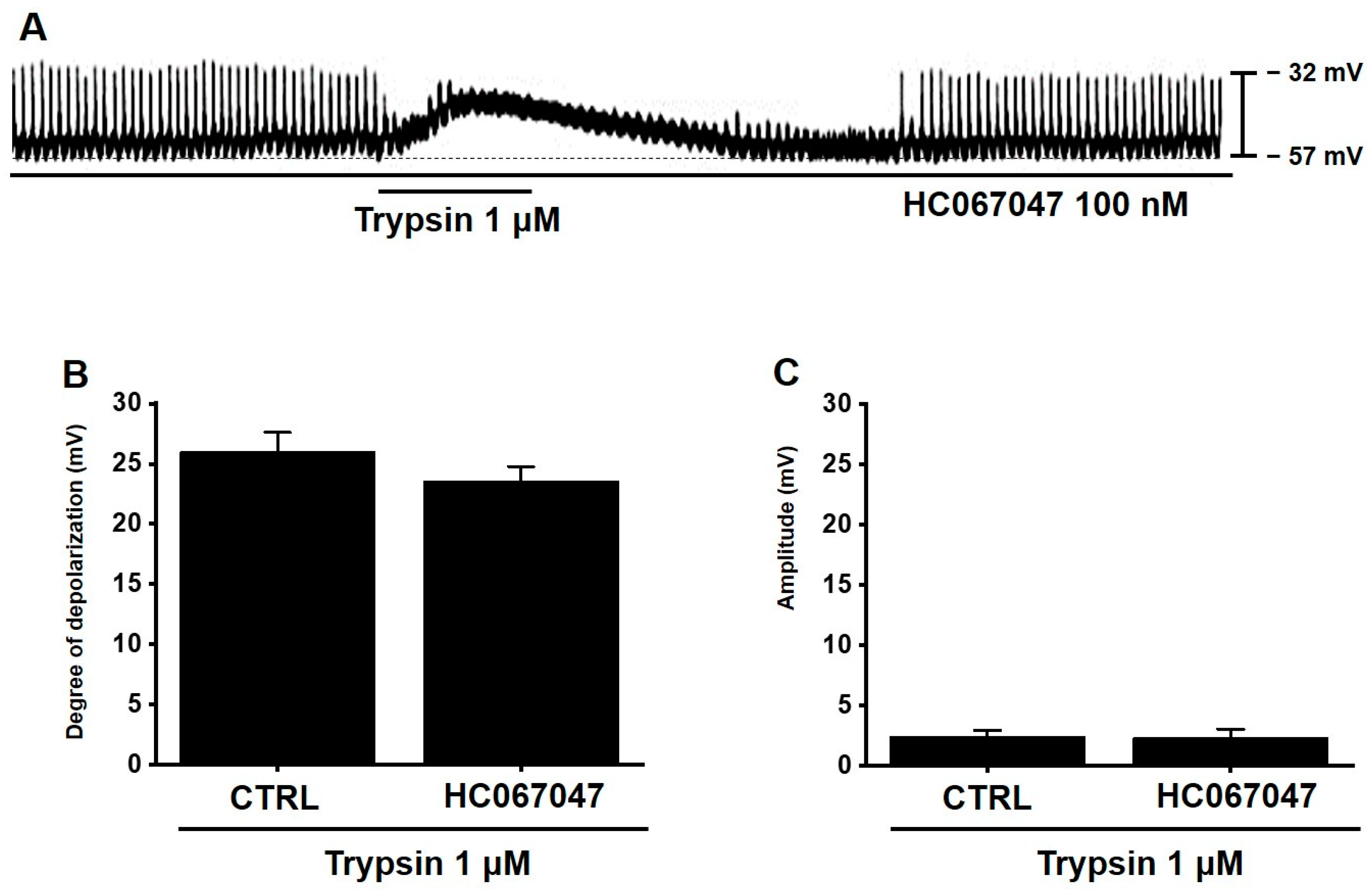
3.7. TRPV4 Is Not Involved in Trypsin-Induced Pacemaker Potential Depolarization in Murine Small Intestinal ICCs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, K.K.; Turner, R.; Khazan, N.; Kodza, A.; Jones, A.; Singh, R.K.; Moore, R.G. Role of trypsin and protease-activated receptor-2 in ovarian cancer. PLoS ONE 2020, 15, e0232253. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, S.R.; Seatter, M.J.; Kanke, T.; Hunter, G.D.; Plevin, R. Proteinase-activated receptors. Pharmacol. Rev. 2001, 53, 245–282. [Google Scholar] [PubMed]
- Darmoul, D.; Gratio, V.; Devaud, H.; Laburthe, M. Protease-activated receptor 2 in colon cancer: Trypsin induced MAPK phosphorylation and cell proliferation are mediated by epidermal growth factor receptor transactivation. J. Biol. Chem. 2004, 279, 20927–20934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vergnolle, N. Review article: Proteinase-activated receptors—novel signals for gastrointestinal pathophysiology. Aliment. Pharmacol. Ther. 2000, 14, 257–266. [Google Scholar] [CrossRef]
- Amadesi, S.; Bunnett, N. Protease-activated receptors: Protease signaling in the gastrointestinal tract. Curr. Opin. Pharmacol. 2004, 4, 551–556. [Google Scholar] [CrossRef]
- Bohm, S.K.; Kong, W.; Bromme, D.; Smeekens, S.P.; Anderson, D.C.; Connolly, A.; Kahn, M.; Nelken, N.A.; Coughlin, S.R.; Payan, D.G.; et al. Molecular cloning, expression and potential functions of the human proteinase-activated receptor-2. Biochem. J. 1996, 314, 1009–1016. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.P.; Bhagat, L.; Navina, S.; Sharif, R.; Dawra, R.; Saluja, A.K. PAR-2 protects against pancreatitis by stimulating exocrine secretion. Gut 2007, 56, 958–964. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, A.; Matsunami, M.; Tsutsumi, M.; Ishiki, T.; Fukushima, O.; Sekiguchi, F.; Kawao, N.; Minami, T.; Kanke, T.; Saito, N. Suppression of pancreatitis-related allodynia/hyperalgesia by proteinase-activated receptor-2 in mice. Br. J. Pharmacol. 2006, 148, 54–60. [Google Scholar] [CrossRef] [Green Version]
- Matej, R.; Housa, D.; Olejar, T. Acute pancreatitis: Proteinase activated receptor-2 as Dr. Jekyll and Mr. Hyde. Physiol. Res. 2006, 55, 467–474. [Google Scholar] [CrossRef]
- Kawabata, A. PAR-2: Structure, function and relevance to human diseases of the gastric mucosa. Expert. Rev. Mol. Med. 2002, 2002, 1–17. [Google Scholar] [CrossRef]
- Ossovskaya, V.S.; Bunnett, N.W. Protease-activated receptors: Contribution to physiology and disease. Physiol. Rev. 2004, 84, 579–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kawabata, A.; Kuroda, R.; Nishikawa, H.; Kawai, K. Modulation by protease-activated receptors of the rat duodenal motility in vitro: Possible mechanisms underlying the evoked contraction and relaxation. Br. J. Pharmacol. 1999, 128, 865–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sekiguchi, F.; Hasegawa, N.; Inoshita, K.; Yonezawa, D.; Inoi, N.; Kanke, T.; Saito, N.; Kawabata, A. Mechanisms for modulation of mouse gastrointestinal motility by proteinase-activated receptor (PAR)-1 and -2 in vitro. Life Sci. 2006, 78, 950–957. [Google Scholar] [CrossRef] [PubMed]
- Cocks, T.M.; Sozzi, V.; Moffatt, J.D.; Selemidis, S. Protease activated receptors mediate apamin-sensitive relaxation of mouse and guinea pig gastrointestinal smooth muscle. Gastroenterology 1999, 116, 586–592. [Google Scholar] [CrossRef]
- Vergnolle, N.; Bunnett, N.W.; Sharkey, K.A.; Brussee, V.; Compton, S.J.; Grady, E.F.; Cirino, G.; Gerard, N.; Basbaum, A.I.; Andrade-Gordon, P.; et al. Proteinase-activated receptor-2 and hyperalgesia: A novel pain pathway. Nat. Med. 2001, 7, 821–826. [Google Scholar] [CrossRef]
- Kawao, N.; Ikeda, H.; Kitano, T.; Kuroda, R.; Sekiguchi, F.; Kataoka, K.; Kamanaka, Y.; Kawabata, A. Modulation of capsaicin-evoked visceral pain and referred hyperalgesia by protease-activated receptors 1 and 2. J. Pharmacol. Sci. 2004, 94, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Huizinga, J.D.; Thuneberg, L.; Kluppel, M.; Malysz, J.; Mikkelsen, H.B.; Bernstein, A. W/kit gene required for interstitial cells of Cajal and for intestinal pacemaker activity. Nature 1995, 373, 347–349. [Google Scholar] [CrossRef]
- Kim, B.J.; Lim, H.H.; Yang, D.K.; Jun, J.Y.; Chang, I.Y.; Park, C.S.; So, I.; Stanfield, P.R.; Kim, K.W. Melastatin-type transient receptor potential channel 7 is required for intestinal pacemaking activity. Gastroenterology 2005, 129, 1504–1517. [Google Scholar] [CrossRef]
- Yin, J.; Chen, J.D.Z. Roles of interstitial cells of Cajal in regulating gastrointestinal motility: In Vitro versus in vivo studies. J. Cell. Mol. Med. 2008, 12, 1118–1129. [Google Scholar] [CrossRef] [Green Version]
- Sung, T.S.; Kim, H.U.; Kim, J.H.; Lu, H.; Sanders, K.M.; Koh, S.D. Protease-activated receptors modulate excitability of murine colonic smooth muscles by differential effects on interstitial cells. J. Physiol. 2015, 593, 1169–1181. [Google Scholar] [CrossRef] [Green Version]
- Shin, D.H.; Kim, M.W.; Choi, S.; Zuo, D.C.; Park, C.G.; Kim, Y.D.; Lee, J.; Cho, J.Y.; So, I.; Jun, J.Y. Regulation of the Pacemaker Activity of Colonic Interstitial Cells of Cajal by Protease-Activated Receptors: Involvement of Hyperpolarization-Activated Cyclic Nucleotide Channels. Pharmacology 2016, 98, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Hong, N.R.; Park, H.S.; Ahn, T.S.; Kim, H.J.; Ha, K.T.; Kim, B.J. Ginsenoside Re inhibits pacemaker potentials via adenosine triphosphate-sensitive potassium channels and the cyclic guanosine monophosphate/nitric oxide-dependent pathway in cultured interstitial cells of Cajal from mouse small intestine. J. Ginseng Res. 2015, 39, 314–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moon, S.B.; Choi, N.R.; Kim, J.N.; Kwon, M.J.; Kim, B.S.; Ha, K.T.; Lim, E.Y.; Kim, Y.T.; Kim, B.J. Effects of black garlic on the pacemaker potentials of interstitial cells of Cajal in murine small intestine in vitro and on gastrointestinal motility in vivo. Anim. Cells. Syst. 2022, 26, 37–44. [Google Scholar] [CrossRef] [PubMed]
- Seitzberg, J.G.; Knapp, A.E.; Lund, B.W.; Bertozzi, S.M.; Currier, E.A.; Ma, J.; Sherbukhin, V.; Burstein, E.S.; Olsson, R. Discovery of potent and selective small-molecule PAR-2 agonists. J. Med. Chem. 2008, 51, 5490–5493. [Google Scholar] [CrossRef]
- Nieuwmeyer, F.; Ye, J.; Huizinga, J.D. Ava[l-Pro9,N-MeLeu10] substance P(7-11) (GR 73632) and Sar9, Met(O2)11 increase distention-induced peristalsis through activation of neurokinin-1 receptors on smooth muscle and interstitial cells of cajal. J. Pharmacol. Exp. Ther. 2006, 317, 439–445. [Google Scholar] [CrossRef]
- Lewis, G.; Christiansen, L.; McKenzie, J.; Luo, M.; Pasackow, E.; Smurnyy, Y.; Harrington, S.; Gregory, P.; Veres, G.; Negre, O.; et al. Staurosporine Increases Lentiviral Vector Transduction Efficiency of Human Hematopoietic Stem and Progenitor Cells. Mol. Ther. Methods Clin. Dev. 2018, 9, 313–322. [Google Scholar] [CrossRef] [Green Version]
- Grandage, V.L.; Everington, T.; Linch, D.C.; Khwaja, A. Gö6976 is a potent inhibitor of the JAK 2 and FLT3 tyrosine kinases with significant activity in primary acute myeloid leukaemia cells. Br. J. Haematol. 2006, 135, 303–316. [Google Scholar] [CrossRef]
- Gschwendt, M.; Müller, H.J.; Kielbassa, K.; Zang, R.; Kittstein, W.; Rincke, G.; Marks, F. Rottlerin, a novel protein kinase inhibitor. Biochem. Biophys. Res. Commun. 1994, 199, 93–98. [Google Scholar] [CrossRef]
- Everaerts, W.; Zhen, X.; Ghosh, D.; Vriens, J.; Gevaert, T.; Gilbert, J.P.; Hayward, N.J.; McNamara, C.R.; Xue, F.; Moran, M.M.; et al. Inhibition of the cation channel TRPV4 improves bladder function in mice and rats with cyclophosphamide-induced cystitis. Proc. Natl. Acad. Sci. USA 2010, 107, 19084–19089. [Google Scholar] [CrossRef] [Green Version]
- Ding, W.X.; Ni, H.M.; Gao, W.; Hou, Y.F.; Melan, M.A.; Chen, X.; Stolz, D.B.; Shao, Z.M.; Yin, X.M. Differential effects of endoplasmic reticulum stress-induced autophagy on cell survival. J. Biol. Chem. 2007, 282, 4702–4710. [Google Scholar] [CrossRef] [Green Version]
- Leitner, M.G.; Michel, N.; Behrendt, M.; Dierich, M.; Dembla, S.; Wilke, B.U.; Konrad, M.; Lindner, M.; Oberwinkler, J.; Oliver, D. Direct modulation of TRPM4 and TRPM3 channels by the phospholipase C inhibitor U73122. Br. J. Pharmacol. 2016, 173, 2555–2569. [Google Scholar] [CrossRef] [PubMed]
- Saleem, H.; Tovey, S.C.; Molinski, T.F.; Taylor, C.W. Interactions of antagonists with subtypes of inositol 1,4,5-trisphosphate (IP3) receptor. Br. J. Pharmacol. 2014, 171, 3298–3312. [Google Scholar] [CrossRef] [PubMed]
- Komori, S.; Kawai, M.; Takewaki, T.; Ohashi, H. GTP-binding protein involvement in membrane currents evoked by carbachol and histamine in guinea-pig ileal muscle. J. Physiol. 1992, 450, 105–126. [Google Scholar] [CrossRef] [Green Version]
- Ogata, R.; Inoue, Y.; Nakano, H.; Ito, Y.; Kitamura, K. Oestradiol-induced relaxation of rabbit basilar artery by inhibition of voltage-dependent Ca channels through GTP-binding protein. Br. J. Pharmacol. 1996, 117, 351–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koh, S.D.; Sanders, K.M.; Ward, S.M. Spontaneous electrical rhythmicity in cultured interstitial cells of Cajal from the murine small intestine. J. Physiol. 1998, 513, 203–213. [Google Scholar] [CrossRef]
- Boichot, E.; Germain, N.; Emonds-Alt, X.; Advenier, C.; Lagente, V. Effects of SR 140333 and SR 48968 on antigen and substance P-induced activation of guinea-pig alveolar macrophages. Clin. Exp. Allergy 1998, 28, 1299–1305. [Google Scholar] [CrossRef]
- Kirchhoff, P.; Geibel, J.P. Role of calcium and other trace elements in the gastrointestinal physiology. World J. Gastroenterol. 2006, 12, 3229–3236. [Google Scholar] [CrossRef]
- Grant, A.D.; Cottrell, G.S.; Amadesi, S.; Trevisani, M.; Nicoletti, P.; Materazzi, S.; Altier, C.; Cenac, N.; Zamponi, G.W.; Bautista-Cruz, F.; et al. Protease-activated receptor 2 sensitizes the transient receptor potential vanilloid 4 ion channel to cause mechanical hyperalgesia in mice. J. Physiol. 2007, 578, 715–733. [Google Scholar] [CrossRef]
- Sipe, W.E.; Brierley, S.M.; Martin, C.M.; Phillis, B.D.; Cruz, F.B.; Grady, E.F.; Liedtke, W.; Cohen, D.M.; Vanner, S.; Blackshaw, L.A.; et al. Transient receptor potential vanilloid 4 mediates protease activated receptor 2-induced sensitization of colonic afferent nerves and visceral hyperalgesia. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G1288–G1298. [Google Scholar] [CrossRef]
- Poole, D.P.; Amadesi, S.; Veldhuis, N.A.; Abogadie, F.C.; Lieu, T.M.; Darby, W.; Liedtke, W.; Lew, M.J.; McIntyre, P.; Bunnett, N.W. Protease-activated receptor 2 (PAR2) protein and transient receptor potential vanilloid 4 (TRPV4) protein coupling is required for sustained inflammatory signaling. J. Biol. Chem. 2013, 288, 5790–5802. [Google Scholar] [CrossRef] [Green Version]
- Lopez-Otin, C.; Bond, J.S. Proteases: Multifunctional enzymes in life and disease. J. Biol. Chem. 2008, 283, 30433–30437. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidlin, F.; Bunnett, N.W. Protease-activated receptors: How proteases signal to cells. Curr. Opin. Pharmacol. 2001, 1, 575–582. [Google Scholar] [CrossRef]
- Steinhoff, M.; Corvera, C.U.; Thoma, M.S.; Kong, W.; McAlpine, B.E.; Caughey, G.H.; Ansel, J.C.; Bunnett, N.W. Proteinase activated receptor-2 in human skin: Tissue distribution and activation of keratinocytes by mast cell tryptase. Exp. Dermatol. 1999, 8, 282–294. [Google Scholar] [CrossRef] [PubMed]
- Sabri, A.; Muske, G.; Zhang, H.; Pak, E.; Darrow, A.; Andrade Gordon, P.; Steinberg, S.F. Signaling properties and functions of two distinct cardiomyocyte protease-activated receptors. Circ. Res. 2000, 86, 1054–1061. [Google Scholar] [CrossRef] [Green Version]
- Shintani, Y.; Hirano, K.; Nakayama, T.; Nishimura, J.; Nakano, H.; Kanaide, H. Mechanism of trypsin-induced contraction in the rat myometrium: The possible involvement of a novel member of protease-activated receptor. Br. J. Pharmacol. 2001, 133, 1276–1285. [Google Scholar] [CrossRef] [Green Version]
- Cocks, T.M.; Moffatt, J.D. Protease-activated receptor-2 (PAR2) in the airways. Pulm. Pharmacol. Ther. 2001, 14, 183–191. [Google Scholar] [CrossRef]
- Kong, W.; McConalogue, K.; Khitin, L.M.; Hollenberg, M.D.; Payan, D.G.; Bohm, S.K.; Bunnett, N.W. Luminal trypsin may regulate enterocytes through proteinase-activated receptor 2. Proc. Natl. Acad. Sci. USA 1997, 94, 8884–8889. [Google Scholar] [CrossRef] [Green Version]
- Mall, M.; Gonska, T.; Thomas, J.; Hirtz, S.; Schreiber, R.; Kunzelmann, K. Activation of ion secretion via proteinaseactivated receptor-2 in human colon. Am. J. Physiol. Gastrointest. Liver Physiol. 2002, 282, G200–G210. [Google Scholar] [CrossRef] [Green Version]
- Corvera, C.U.; Dery, O.; McConalogue, K.; Bohm, S.K.; Khitin, L.M.; Caughey, G.H.; Payan, D.G.; Bunnett, N.W. Mast cell tryptase regulates rat colonic myocytes through proteinase-activated receptor 2. J. Clin. Investig. 1997, 100, 1383–1393. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, A.; Kuroda, R.; Nagata, N.; Kawao, N.; Masuko, T.; Nishikawa, H.; Kawai, K. In vivo evidence that proteaseactivated receptors 1 and 2 modulate gastrointestinal transit in the mouse. Br. J. Pharmacol. 2001, 133, 1213–1218. [Google Scholar] [CrossRef] [Green Version]
- Zho, A.; Shea-Donohue, T. PAR-2 agonists induce contraction of murine small intestine through neurokinin receptors. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 285, G696–G703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lavin, S.T.; Southwell, B.R.; Murphy, R.; Jenkinson, K.M.; Furness, J.B. Activation of neurokinin 1 receptors on interstitial cells of Cajal of the guinea-pig small intestine by substance P. Histochem. Cell Biol. 1998, 110, 263–271. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.Y.; Choi, S.; Yeum, C.H.; Chang, I.Y.; You, H.J.; Park, C.K.; Kim, M.Y.; Kong, I.D.; Kim, M.J.; Lee, K.P.; et al. Substance P induces inward current and regulates pacemaker currents through tachykinin NK1 receptor in cultured interstitial cells of Cajal of murine small intestine. Eur. J. Pharmacol. 2004, 495, 35–42. [Google Scholar] [CrossRef] [PubMed]
- Luttrell, L.M. Location, location, location: Activation and targeting of MAP kinases by G protein-coupled receptors. J. Mol. Endocrinol. 2003, 30, 117–126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Swystun, V.; Chen, L.; Factor, P.; Siroky, B.; Bell, P.D.; Matalon, S. Apical trypsin increases ion transport and resistance by a phospholipase C-dependent rise of Ca2+. Am. J. Physiol. Lung Cell. Mol. Physiol. 2005, 288, L820–L830. [Google Scholar] [CrossRef] [Green Version]
- Basbaum, A.I.; Bautista, D.M.; Scherrer, G.; Julius, D. Cellular and molecular mechanisms of pain. Cell 2009, 139, 267–284. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, N.R.; Kim, J.N.; Kim, B.J. Trypsin Depolarizes Pacemaker Potentials in Murine Small Intestinal Interstitial Cells of Cajal. Appl. Sci. 2022, 12, 4755. https://doi.org/10.3390/app12094755
Choi NR, Kim JN, Kim BJ. Trypsin Depolarizes Pacemaker Potentials in Murine Small Intestinal Interstitial Cells of Cajal. Applied Sciences. 2022; 12(9):4755. https://doi.org/10.3390/app12094755
Chicago/Turabian StyleChoi, Na Ri, Jeong Nam Kim, and Byung Joo Kim. 2022. "Trypsin Depolarizes Pacemaker Potentials in Murine Small Intestinal Interstitial Cells of Cajal" Applied Sciences 12, no. 9: 4755. https://doi.org/10.3390/app12094755





