Abstract
This article presents the results of a study on the effect of fluorine pollution of soil, including soil amendments, on the concentration of sodium and sulphur in six species of crops. The experiment consisted of six pot trials performed in a greenhouse. Two factors were taken into consideration: (I) Increasing doses of fluorine (0, 20, 40, and 60 mg F kg−1 of soil for narrow-leaf lupine and 0, 100, 200, and 300 mg F kg−1 of soil for yellow lupine, winter oilseed rape, spring triticale, black radish, and phacelia) and (II) neutralizing substances (lime–1 HAC, charcoal and loam–3% relative to the soil mass). The highest sodium content was in t roots of winter oilseed rape, and the lowest was in the grain of spring triticale. Large differences were detected in the Na content between the analyzed organs of phacelia. The roots of this plant contained 16-fold more sodium than aerial parts. The highest average content of sulphur was in yellow lupine aerial parts, and the lowest was in spring triticale aerial biomass. The increasingly severe soil pollution with fluorine, in most cases, led to a decline in the sodium and sulphur content in the analyzed crops. The highest degree of soil contamination with fluorine had the strongest negative effect in this regard. The content of the analyzed macronutrients concerned the species and organs of a crop, the dose of the tested xenobiotic, and the substance applied to soil for the inactivation of fluorine. A decrease in the sodium content in most of the tested crops could be attributed to the fact that fluorine ‘reacts’, i.e., binds sodium into compounds that are hardly soluble in water, thereby limiting sodium phytovailability.
1. Introduction
Fluorine belongs to earth elements present in high quantities with approximately 950 mg F kg−1 of the earth’s crust (13th most abundant element) [1,2]. In nature, this element mainly occurs in deposits of fluorite, cryolite, topaz, and fluorapatite. The world resources of fluorspar are estimated at approximately 230 million tons, with China, Mongolia, Mexico, and South Africa having almost half of the resources of this mineral [3]. No economically significant fluorspar deposits have been identified in Poland and therefore it has never been mined in our country. Insignificant amounts of synthetic fluorspar are obtained by Chemical Plant Siarkopol Tarnobrzeg Sp. z o.o. (Tarnobrzeg, Poland) during the production of phosphoric acid from phosphorites. The total Polish demand for this mineral is provided by importation, primarily from Mexico, Germany, and the Czech Republic. When it comes to phosphorite deposits, there is currently no mining of this deposit in Poland, and the entire demand for this raw material is covered by imports from other countries, primarily from Algeria, Morocco, Egypt, and Syria. The national consumption of phosphate rock is determined by the needs of agriculture and limited by the production capacity of large fertilizer industry plants, which include: Chemical Plant Police S.A., GZNF Fosfory Sp. z o.o. (AZOTY Group), Luvena S.A., Chemical Plant Siarkopol Tarnobrzeg Sp. z o.o., Fosfan S.A. There are a number of environmental problems associated with the processing of phosphate deposits. This applies especially to the so-called phosphogypsum, generated as waste in huge amounts in the wet production of phosphoric acid (Chemical Plant Police S.A., GZNF Fosfory Sp. z o.o. (AZOTY Group), and Chemical Plant Wizów). Phosphogypsum contains significant amounts of fluorine, uranium, and a number of other trace elements that should be recovered or neutralized, otherwise they pose a significant threat to the natural environment [4]. Santos et al. [5] found during their research that phosphogypsum, in addition to gypsum and basanite (~95%), contains fluorine (0.5–1.5%), orthophosphates, phosphates, sulphates, and elements such as Cd, Cr, Pb, Hg, As, B, Cu, Mg, Mo, Sc, Hf, Zn, Sr, and Ba, as well as elements from the lanthanide family (Ce, Eu, La, Lu, Nd, Sm, Tb, and Yb) and compounds of radioactive elements such as U, Th, Ra, and Po. In order to imagine the scale of the problem, it is worth providing the example of one of the largest heaps of phosphogypsum in Poland, located near the village of Wiślinka near Gdańsk. The landfill covers an area of 36 ha, where over 16 million tons of phosphogypsum have been deposited over 30 years, and the height of the heap reaches 40 m. After the end of the operation, attempts were made to reclaim the heap by covering it with dry sewage sludge and then sowing appropriate plant species to protect the surface against erosion [6]. The impact of such a heap on the natural environment is very unfavorable, mainly due to the constant dusting from the surface of the heap, the emissions released into the atmosphere of all types of toxic compounds including fluoride, and their penetration into the deeper layers of soil, surface, and ground waters. Reclamation of this type of landfill is very problematic, primarily due to the unfavorable physicochemical properties of the soil, the complete absence of organic matter, and a very low reaction. The selection of appropriate plant species also poses a serious problem and is usually performed via the trial method [7].
In soil, fluorine binds to other soil native elements, such as aluminum (Al), calcium (Ca), iron (Fe), or siltstone minerals, forming stable fluorine compounds. Factors that considerably alter the mobility of fluorine are the soil reaction and the presence of aluminum and calcium complexes in the soil. Romar et al. [8] claimed that fluorine in calcium-rich soils binds to insoluble compounds, such as CaF2, or apatite compounds of approximately the same composition, thus limiting fluorine availability to plants. Kau et al. [9,10,11] indicated that fluorine has a high affinity to aluminum in loam. Fluorine is easily bound to loam via an anion exchange process. Fluorine is bound by magnesium and calcium, forming hardly soluble compounds. Magnesium and calcium are found in large amounts in loam. The stress induced by the presence of fluorine compounds manifests itself as changes in the physicochemical properties of soil, which in turn influences the chemical composition of crops [12]. Fluorine acts as a xenobiotic even when present in low concentrations, and in areas where fluorine emissions are elevated, e.g., in the vicinity of phosphate plants, aluminum plants, steelworks, glassworks, earthenware plants, and municipal heat and power plants [13], this element accumulates heavily in soils and plants, reducing the yields and yield quality of crops [14,15,16,17,18]. It is therefore essential to constantly monitor the content of this element in agricultural products, especially when crops are grown in such areas as mentioned above. The relevant references still lack sufficient amounts of data on the effect of fluorine on the content of macronutrients in plants, hence this study was conducted to explore the influence of soil contamination with fluorine on the content of sodium and sulphur in crops under the conditions of lime, charcoal, and loam applications. The results presented in this study are a continuation of previously published works from widely undertaken research on the simulation of soil contamination with fluoride and its impact, among others on the content of this contaminant in plants [2], the yield development and content of nitrogen components [19], the content of calcium and magnesium [20], or publications on the impact of fluoride on the content of endo- and exogenous amino acids in maize and yellow lupine [21].
Sulphur is an important nutrient for plants, and cruciferous plants including rapeseed, radish, cabbage, and legumes show a particularly high demand for this element. The most important organic compounds of sulphur include sulphuric amino acids, i.e., cystine and cysteine, which are the redox system of plants. They play a significant role in the nitrogen management of plants. Plants uptake sulphur from the soil in the form of SO42− and SO2 ions from the air. With a sulphur deficiency, plants have small, pale green leaves and thin stems with heavily woody strengthening tissue. Adequate sulphur levels not only increase yields but also improve their quality by reducing the content of nitrates and increasing the number of carotenes in feed and oil in oilseeds. Well-met needs of plants regarding this ingredient increase their resistance to frost and drought. In turn, excess sulphur in the environment deteriorates soil properties and leads to plant poisoning. This occurs especially in industrial districts, which should be remembered when selecting mineral fertilizers for the plants grown there.
In turn, on one hand, sodium does not fulfill the criterion of a plant nutrient and is classified rather as a minor component. On the other hand, it is a valuable component of plants used as animal feed. Therefore, sodium is regarded as a useful element. Adequate sodium in feed is an important factor in determining animal performance. For example, to cover the daily sodium requirement of dairy cows, approximately 2 g per kg should be added to basic feed. Very often, however, the average sodium level in the biomass of grassland significantly deviates from the required standard and ranges between 0.1 and 1 g per kg of dry matter. Its deficiency leads to a loss of appetite, loss of milk yield, and weight loss, and even deterioration of health and fertility. Sodium has a beneficial effect not only on the physiology of nutrition, but it also increases the taste of green fodder.
In the undertaken research, it was hypothesized that increasing doses of fluoride reduce the sodium and sulphur supply in plants, which may be limited by the substance applications (lime, charcoal, and loam) in the soil.
2. Materials and Methods
2.1. Pot Experiment
The research was based on six pot experiments that were carried out in a greenhouse belonging to the University of Warmia and Mazury in Olsztyn 53.78° N/20.49° E (north-eastern Poland) in 2009–2011. The pots were filled with soil in the amount of 9 kg, gathered from the humus layer of brown soil with a loamy sand textural composition (<0.002 mm–1.89%; 0.002–0.050 mm–18.61%; >0.050 mm–79.50%) [22]. The soil reaction was 5.89 in H2O and 4.43 in 1 M KCl dm−3, while its hydrolytic acidity (HAC) was 30.7 mM(+) kg−1 dry matter (DM). The content of available forms of macroelements in the soil was 43.2 mg P, 124.5 mg K, and 30.0 mg Mg kg−1 DM of soil. In addition, the soil contained: 6.0 g kg−1 DM of organic carbon, 0.62 g kg−1 DM of total nitrogen, and 125 mg kg−1 DM of total fluorine. Lime, charcoal, and loam were used to immobilize fluorine in soil. The specification of the basic chemical properties of lime, charcoal, and loam are presented in Table 1.

Table 1.
Chemical composition of substances added to soil in order to inactivate fluorine.
2.2. Plant Material
The test plants were yellow lupine (Lupinus luteus L.), winter oilseed rape (Brassica napus L.), spring triticale (Triticosecale Wittm.), narrow-leaf lupine (Lupinus angustifolius L.), black radish (Raphanus sativus), and phacelia (Phacelia Juss.).
Two factors were considered in the research. The first-order factor was soil contamination with fluorine, applied as potassium fluoride (commercial form), while the second factor consisted of the comparison of three substances (lime, charcoal, and loam) added to soil to alleviate the fluorine pollution of soil.
The soil pollution with fluorine, depending on the sensitivity of the test plants, was:
- 0, 20, 40, and 60 mg F kg−1 of soil for narrow-leaf lupine.
- 0, 100, 200, and 300 mg F kg−1 of soil for yellow lupine, winter oilseed rape, spring triticale, black radish, and phacelia.
The choice of these doses was based on the average total fluorine content in Polish soils according to Kabata-Pendias [23]. With respect to narrow-leaf lupine, the soil contamination with fluorine was lower than in the other trials because papilionaceous plants are more sensitive to the presence of different xenobiotics in the soil. In addition, the sensitivity of plants to soil fluoride contamination was estimated from preliminary studies conducted prior to setting up this pot experiment. Another plant sensitive to fluorine soil pollution was yellow lupine. In this case, the residual effect of fluorine soil pollution was investigated.
The materials neutralizing the fluorine soil contamination were lime (in an equivalent dose of 1 hydrolytic acidity–HAC of soil) and charcoal and loam in amounts of 3% relative to the mass of soil in a pot. Additionally, NPK mineral fertilization was added to the soil in identical doses for all pots to cover the demand for plant nutrients. Nitrogen was applied in a dose of 11 mg N (urea), phosphorus of 48 mg P (triple superphosphate 46%), and potassium of 111 mg K kg−1 of soil (potassium salt 57%). Before setting up the experiment, the soil was dried and sieved (1 cm sieve diameter). A batch of soil weighing 9.0 kg was thoroughly mixed with the mineral fertilizers and with fluorine and neutralizing substances and transferred to adequately labelled pots. Each object was performed in triplicate. Next, the test crops were sown, finally leaving 13 plants per pot except for the trial with black radish, where 8 plants per pot were left to grow. During the plant growth and development, the soil moisture in the pots was constant (60% of capillary water capacity). Yellow lupine and narrow-leaf lupine were harvested at the flowering stage (BBCH 65), winter oilseed rape at the flowering stage (BBCH 65), spring triticale at the stage of full grain maturity (BBCH 89), phacelia at the stage of full flowering (BBCH 65), and black radish at the final stage of root development (BBCH 49).
2.3. Methods of Laboratory Analysis
During the harvest, samples of plants’ aerial parts and roots for laboratory analyses were collected. These samples were cut and dried at a temperature of 60 °C. After drying, the plant material was milled.
Soil analysis before the start of the experiment was performed using the following methods and the apparatus listed below:
- Texture composition—laser diffraction apparatus Mastersizer 2000 Hydro G dispersion unit–Malvern, UK [24].
- pHKCl—potentiometric method [25].
- Hydrolytic acidity (HAC)—the Kappen method [25].
- Total organic carbon (TOC)—Shimadzu TOC-L CSH/CNS analyzer (Kyoto, Japan) [26].
- Total nitrogen—the Kjeldahl method [27].
- Available phosphorus and potassium—the Egner–Riehm method [28].
- Available magnesium—the Shachtschabel method [28].
- Total fluorine—the X-ray fluorescence spectrometry method (XRF), Philips WD-XRF PW 2004 (Philips Research Corporation, Eindhoven, The Netherlands).
The content of sodium was determined by atomic emission spectrometry (AES) using SpectrAA-240FS (Varian Inc., Mulgrave, Australia) and detected at a wavelength of 589.6 nm. The plant samples were previously wet—ashed in 95% concentrated sulphuric acid with the addition of 30% H2O2. The optimal range for sodium at a wavelength of 589.6 nm was 0.01 mg L−1 (limit of detection) to 2 mg L−1 (limit of quantification). The maximal limit of quantification SpectrAA-240FS for sodium was 400 mg L−1 [28]. The total sulphur content was analyzed with the nephelometric method based on measurements of the turbidity of a solution in which sulphur was precipitated by barium chloride. The Cecil CE 7400 Double Beam UV/VIS spectrophotometer (Cecil Instruments Ltd., Cambridge, UK) was used in the determinations at a wavelength of 425 nm [28]. The limits of detection and quantification for sulphur were 0.1 mg L−1 and 35 mg L−1, respectively. The content of sulphur in the plants was analyzed in the aerial biomass of yellow lupine, winter oilseed rape, narrow-leaf lupine, and phacelia and the grain of spring triticale.
2.4. Statistical Analysis
Research results were processed statistically calculated using a Statistica software package, according to ANOVA variance two-factorial analysis and least significant differences (LSD) [29]. Relationships between fluorine contamination of soil and the content of sodium and sulphur in plants were determined using multinomial regression equations, Pearson’s simple correlations, and the principal component analysis (PCA) [29].
3. Results and Discussion
The content of macronutrients in plants is affected by many factors, of which the most important are the species, variety, and organs of a plant. This was confirmed in our study, in which—apart from the above factors—an important role in the shaping of the content of the two analyzed macronutrients was played by the level of soil contamination with fluorine and the soil application of substances immobilizing this xenobiotic (Table 2, Table 3, Table 4 and Table 5).

Table 2.
Sodium content in yellow lupine, winter oilseed rape, and narrow-leaf lupine in g Na kg−1 DM.

Table 3.
Sodium content in black radish, phacelia, and spring triticale in g Na kg−1 DM.

Table 4.
Sulphur content in yellow lupine, winter oilseed rape, and narrow-leaf lupine aerial parts in g S kg−1 DM.

Table 5.
Sulphur content in phacelia aerial parts and spring triticale seed, in g S kg−1 DM.
The data reported from this experiment (Table 2, Table 3, Table 4 and Table 5) show that the increasingly high fluorine soil pollution had a significant effect on the content of sodium and sulphur in all organs of all plant species. This is verified by the two-factorial analysis of variance performed and the calculated coefficients of Pearson’s simple correlation.
Based on the statistical analysis of the research data, highly statistically significant relationships were demonstrated between the fluorine soil pollution and the sodium content in the test plants (Table 2 and Table 3). In the series without any fluorine-immobilizing substances, a statistically significant decrease in the sodium content was recorded in the aerial parts (r = −0.83 **) and the roots of yellow lupine (r = −0.99 **), in the aerial parts (r = −0.79 **) and roots of winter oilseed rape (r = −0.98 **), in the roots of narrow-leaf lupine (r = −0.96 **), in the aerial parts (r = −0.90 **) and roots of black radish (r = −0.88 **), and in the roots of phacelia (r = −0.95 **). The decreased sodium content in most of the test plants was likely a consequence of fluorine ‘entering’ a reaction, i.e., the binding of sodium into complexes hardly soluble in water, thus making this element less available to plants. Fung and Wong [30] also reported a negative correlation between the presence of fluorine in soil and the content of sodium in seedlings of Camellia sinensis. An opposite tendency, that is, a statistically higher content of sodium in response to the growing fluorine soil contamination, was noted in the case of phacelia aerial parts (r = 0.93 **) and all organs of spring triticale, i.e., grain (r = 0.77 **), straw (r = 0.86 **), and roots (r = 0.74 **). A positive correlation between fluorine soil pollution and the sodium content in plants was found by Arnesen [31] in a study on Lolium multiflorium. In their experiment on Salicornia brachiata, Reddy and Kaur [32] proved that the highest fluorine dose (150 mM NaF) caused a three-fold increase in sodium content compared to the control. In this experiment, the highest average sodium content was determined in winter oilseed rape roots, at 9.5 g Na kg−1 DM, while the lowest was detected in spring triticale grains at 0.02 g Na kg−1 DM. A considerable difference in this element content between plant organs was noted in phacelia. The roots of this plant contained as much as 16-fold more sodium than its aerial parts. However, in most of the analyzed plants, their aerial parts contained more sodium than the roots.
Aslam et al. [33] in an experiment with Zea mays L. noted a high concentration of sodium in both tested varieties of this plant, with the highest concentration of this component observed at a dose of 200 mg L−1 NaF. According to Yamada et al. [34], the significant accumulation of sodium in tissues causes a number of unfavorable changes in cellular metabolism, mainly inhibiting the synthetic activity of plants. Research by Ranjith et al. [35] showed a significantly positively correlated relationship between soil bioavailable fluoride and exchangeable sodium (0.462, p = 0.05). Moreover, Anbuvel et al. [36] reported a significantly positive correlation between fluoride and exchangeable sodium. According to Shekhar et al. [37], the reported positive relationship between the content of fluorine and sodium is likely explained by the accumulation of a large amount of minerals in the soil in the form of cryolite (Na3AlF6).
A general overview of the research results reveals more distinct differences in the shaping of two analyzed macronutrients content under the impact of soil pollution with fluoride for sodium rather than sulphur. Thus, the largest average decrease in the sodium content was noted in the roots of narrow lupine and the roots of winter oilseed rape, where it reached 49 and 32%, respectively, in comparison with the series not polluted with fluoride.
The fluoride soil contamination and the applied neutralizing substances only slightly differentiated the sulphur content in the test plants (Table 4 and Table 5). The highest average sulphur content was determined in the aerial parts of yellow lupine, with 3.8 g S kg−1 DM, while the lowest was in the aerial parts of spring triticale, with 1.3 g S kg−1 DM. Dąbrowski and Seniczak [38] recorded the highest content of sulphur in lichens growing in the nearest proximity to the Luboń Chemical Plant. However, Zbierska [39] did not observe any influence of the distance to the Phosphate Fertilizer Manufacturing Plant in Luboń on the content of sulphur in the tested plants.
The statistical analysis of the research results elucidated a highly significant negative impact of increasing fluorine soil pollution on the sulphur content in the aerial parts of yellow lupine (r = −0.82 **), the aerial parts (r = −0.90 **) and roots of winter oilseed rape (r = −0.82 **), and the aerial parts of phacelia (r = −0.81 **). In contrast, the fluorine soil contamination contributed to a highly significant increase in the sulphur content in black radish roots (r = 0.84 **) and spring triticale aerial parts (r = 0.93 **).
The obtained results confirm the thesis available in the literature that the presence of fluoride in the soil can change the quality of crops [14,15,16,17,18] and their feed suitability.
Statistical processing of the experimental data also demonstrated a significant effect of the soil application of materials immobilizing fluorine on the plant content of both sodium and sulphur (Table 2, Table 3, Table 4 and Table 5).
The soil application of lime as a substance immobilizing fluorine, in most cases, contributed to a decrease in the average sodium content in the tested crops relative to the control series, i.e., not contaminated with this xenobiotic (Table 2 and Table 3). The highest decrease in the content of this macronutrient was noted in the aerial parts of phacelia (−22%), the aerial parts of black radish (−21%), and the roots of narrow-lupine (−16%). As for the content of sulphur in this series of experiments, it was similar or identical to the control series, that is, the unpolluted one. On the other hand, it is worth noting that the application of lime did not limit the impact of fluorine on the sodium content in plants, which in most cases was negative. The calculated correlation coefficients implicate a significant increase in the content of this macronutrient alongside the increasing fluorine soil pollution only in the case of the aerial parts of phacelia (r = 0.76 **) and the grain (r = 0.67 *), straw (r = 0.86 **)m and roots (r = 0.76 **) of spring triticale. Ciećko et al. [40] observed that the lime and charcoal application to soil has most contributed to the reduction of sodium content in phacelia, especially in the roots. Different results were obtained in other studies [41]. In this research, the sodium content in Zea Mays L. increased from a few to over a dozen percent after calcium oxide application compared to the objects without neutralizing substances.
Charcoal, similar to lime, did not limit the adverse effect of fluorine on shaping the sodium content in plants. In this series, the average content of sodium was lower in the vast majority of the test plants and in their organs, except the aerial parts of narrow-leaf lupine and spring triticale. The highest decline in the average content of sodium relative to the control was marked in the aerial parts and roots of yellow lupine (by 28 and 40%, respectively), the aerial parts (−24%) and roots of winter oilseed rape (−35%), and the aerial parts of phacelia (−30%). Trends in the concentration of sulphur in plants in this series of trials were similar and showed no significant differences in comparison with the series unpolluted with fluorine. Kosiorek and Wyszkowski [42] showed that the addition of charcoal to the soil did not limit the negative effects of this contaminant on sodium content in white mustard. This decrease was 11% in leaves and 13% in the roots. Lime also limited the sodium content in the aerial parts and the roots of this plant species.
In the series with loam as a soil amendment, there was a tendency towards decreasing concentrations of sodium in most plants under the impact of fluorine soil contamination. A positive correlation between the fluorine dose and the sodium content of a plant was only determined for the grain (r = 0.77 *), straw (r = 0.99 **), and roots (r = 0.75 **) of spring triticale (r = 0.80 **). The biggest decrease in sodium content relative to the control treatment was noted to have occurred under the influence of the most severe soil pollution with the xenobiotic. Moreover, a larger decrease appeared in the roots than in the aerial parts of the test crops. Thus, the highest decrease of over 30% compared to the control was determined in the roots of winter oilseed rape (−37%), the roots of phacelia (−35%), and the roots of yellow lupine (−31%). A similar tendency was recorded by Żołnowski et al. [43]. In their research, the lime and loam application significantly reduced the sodium content in Brassica nigra L. Koch. In our own research, a tendency to decrease the sodium content was generally observed as a result of soil contamination with fluorine. Loam formations are generally characterized by a higher content of fluorine in contrast to soils, e.g., sandy. At the same time, in this type of soil, we observed higher contents of other elements such as aluminum, calcium, magnesium, or sodium [44]. This is also confirmed by our results regarding the chemical composition of the additives used (Table 1). Therefore, the decrease in sodium content in most of the plants tested in this series of experiments is likely due to the fact that fluorine has a strong affinity for these elements and forms sparingly soluble compounds with them, thus contributing to their limited uptake by plants. The application of loam in some cases contributed to a slight increase in the sodium content in relation to analogous objects without additives. Such a relationship was observed in the roots of winter rape, the aerial parts of narrow-leaved lupine, the aerial parts and roots of black radish, both organs of phacelia, and the straw of spring triticale. Similar relationships were shown by Ogundola et al. [45]. They attempted to estimate the uptake and accumulation of selected nutrients and heavy metals by Solanum nigrum grown on different types of soil. The results of their research showed that the highest content of sodium was found in plants grown on loamy soil. Regarding sulphur, its plant content in the series with loam tended to be approximately the same as its concentrations in the series without fluorine pollution. Loam is rich in humic acids. Humic acids can reduce the sulphur content in plants, e.g., in the aerial parts of maize [46]. In the research of other authors [42], lime application caused a reduction in sodium content in the grain and straw of oats, while loam had such an effect only in the straw of oats. The opposite impact was obtained in the straw of this plant. The lime application to soil leads to an ion exchange with sodium in a sorption complex. High sodium content in a soil solution may cause an increase in the sodium content in the organs of some plant species [47].
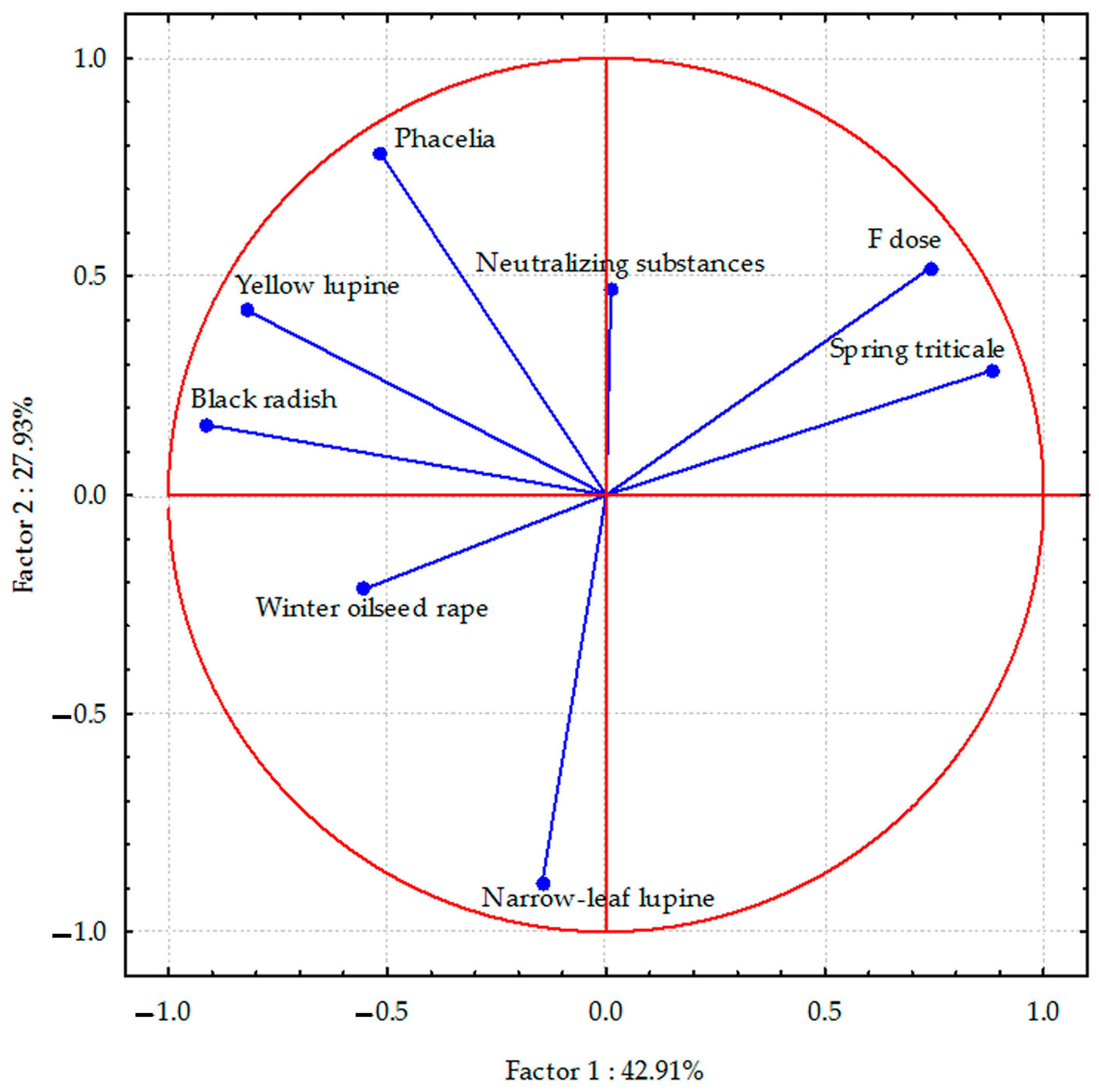
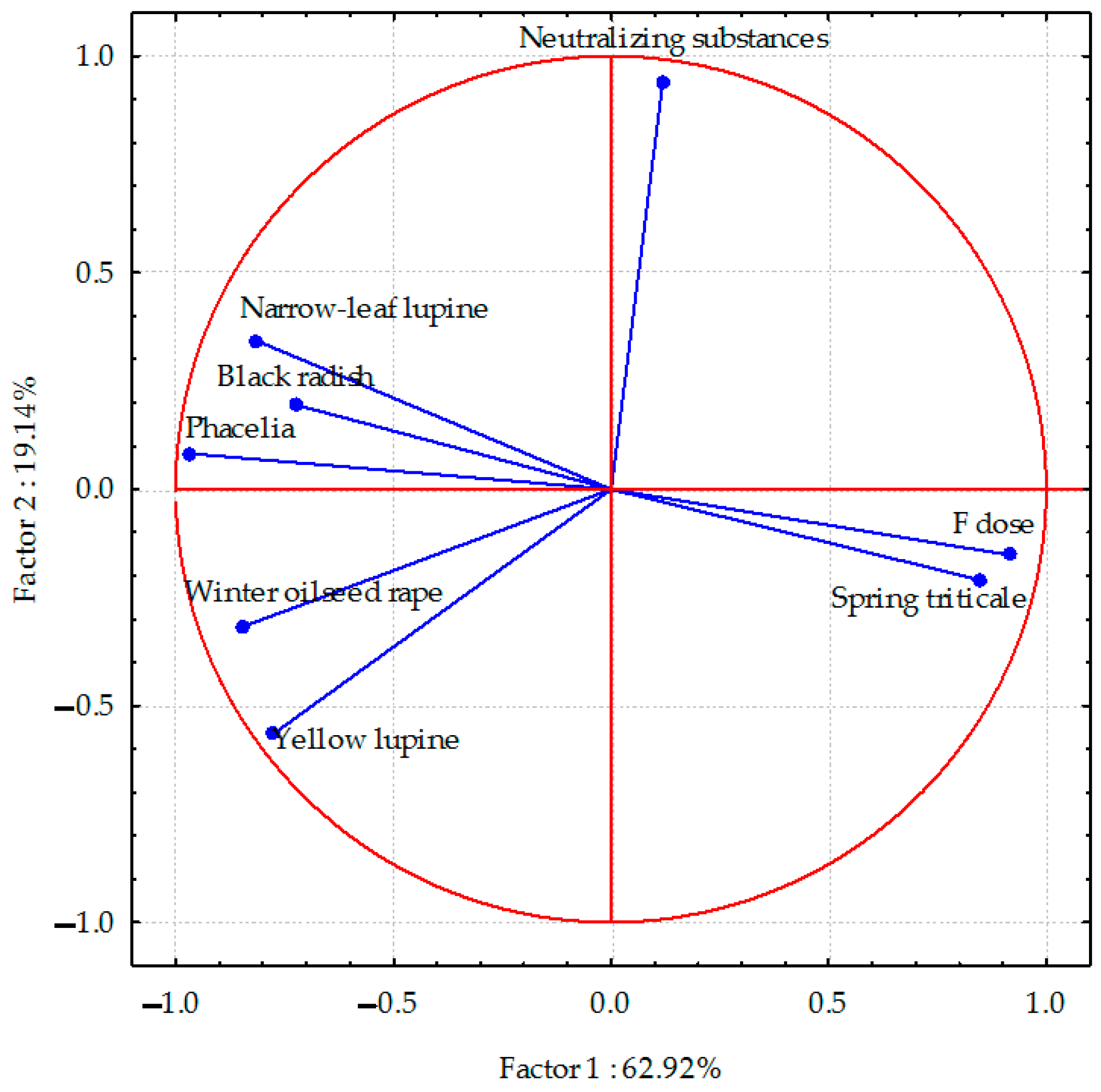
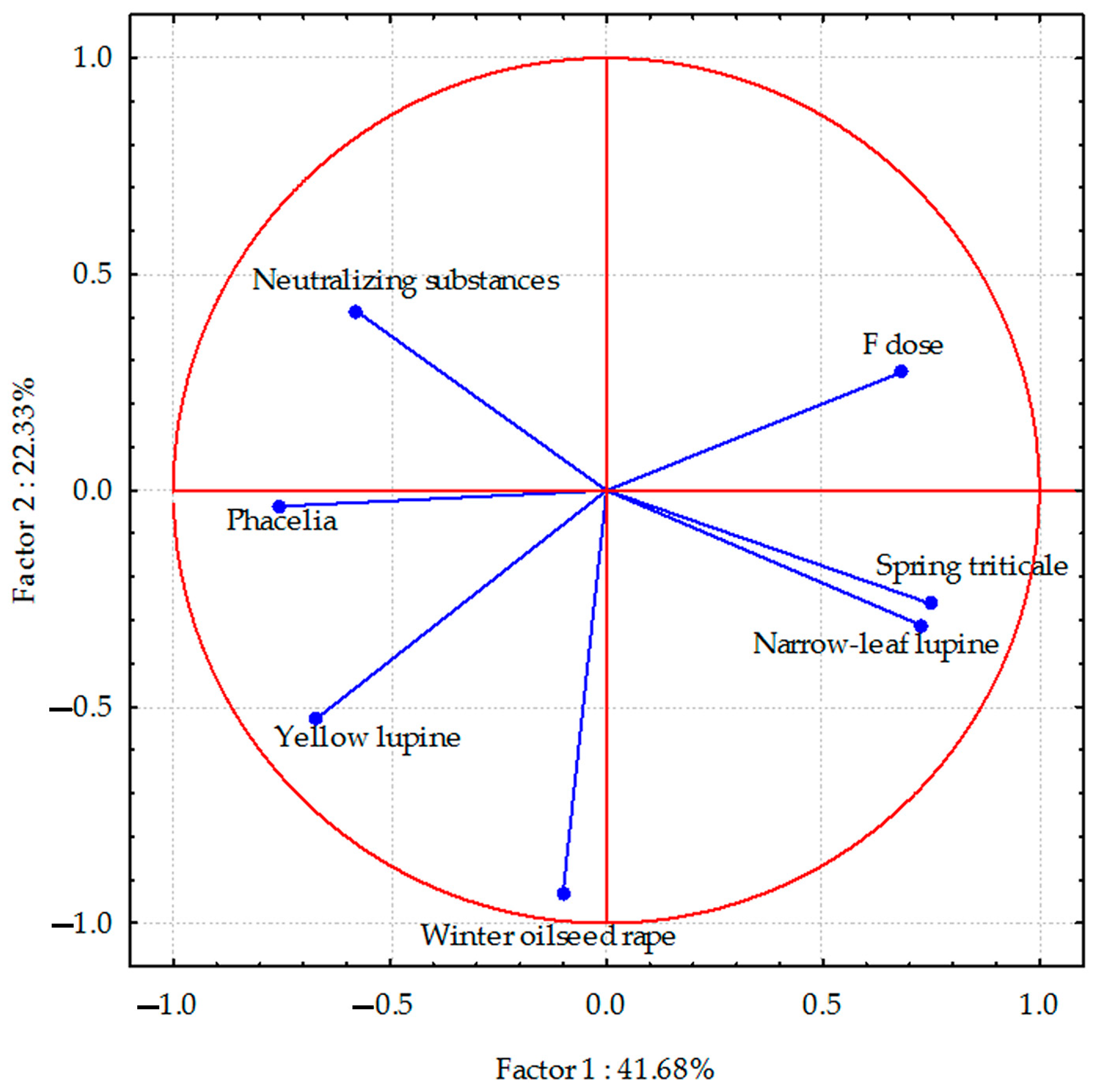
PCA analyses indicated that the total parameters describing the sodium content in the aerial parts of plants accounted for 70.84% (Figure 1), the sodium content in the plant species’ roots was 82.06% (Figure 2), and the sulphur content in spring triticale seeds and parts of other plant species was 64.01% (Figure 3) of the correlation of the dataset. Vectors of sodium content in the aerial parts of most plant species were of similar length, which indicates a similar range of impact. The sodium content in the aerial parts of black radish and narrow-leaf lupine showed the highest correlation, and in yellow lupine and winter oilseed rape, a smaller negative degree of correlation with the dose of fluorine was observed. In the case of spring triticale, positive correlations were found. Negative correlations were also found between the sodium content in winter oil-seed rape and narrow-leaf lupine (as opposed to phacelia) and the application of neutralizing substances. In the case of phacelia, positive correlations were found.
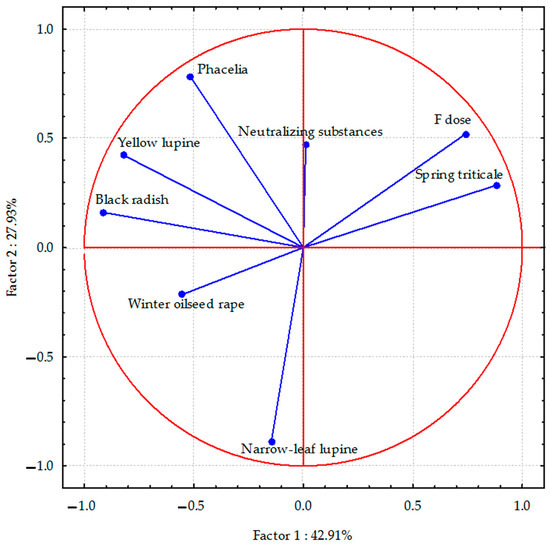
Figure 1.
Sodium content in aerial parts of plant species calculated with PCA method. Vectors represent sodium content in aerial parts of plant species versus fluorine dose and neutralizing substances application.
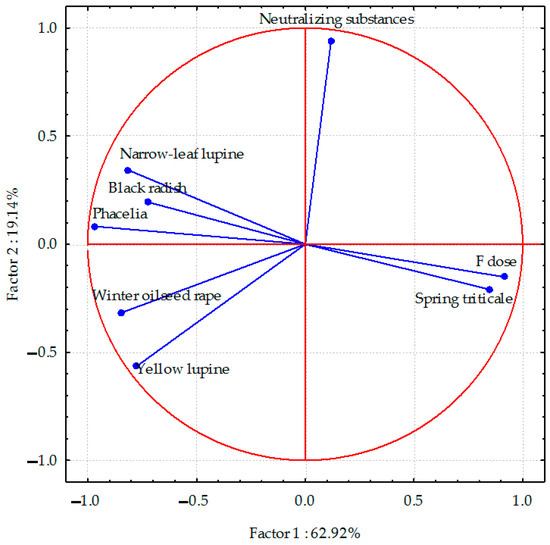
Figure 2.
Sodium content in roots of plant species calculated with PCA method. Vectors represent sodium content in roots of plant species versus fluorine dose and neutralizing substances application.
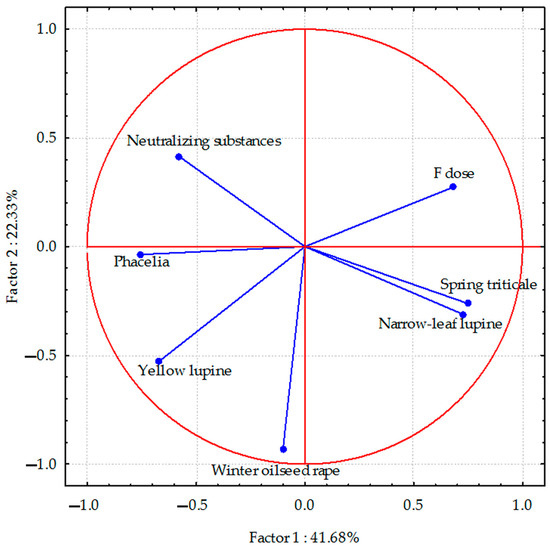
Figure 3.
Sulphur content in aerial parts of plant species calculated with PCA method. Vectors represent sodium content in aerial parts of plant species versus fluorine dose and neutralizing substances application.
The sodium content in the roots of all plant species (except spring triticale), and especially in phacelia and narrow-leaf lupine, was negatively correlated with soil contamination with fluorine. Negative correlations between the sodium content and the application of neutralizing substances were found in yellow lupine and winter oilseed rape.
Correlations between the sulphur content in plants and the studied parameters were relatively weak. However, the sodium content showed a negative correlation in yellow lupine and a positive correlation in spring triticale (weaker in narrow-leaf lupine) with the dose of fluorine. Negative correlations also occurred between the sulphur content in winter triticale and narrow-leaf lupine and the application of neutralizing substances. The sulphur content in phacelia and yellow lupine was positively correlated with these substances.
The sulphur content in plants is usually positively correlated with other elements, most often with nitrogen and protein [46]. This has a direct impact on the growth and development of plants. Positive correlations of sodium with other elements in plants are less frequently found. Some authors have found positive correlations between sodium and phosphorus content in both aerial parts and plant roots, e.g., spring barley, oat, and white mustard [42,48], and weaker negative correlations between potassium and sodium [48].
The study presented in this paper verifies that the impact of fluorine soil pollution on the plant content of macronutrients depends on many factors, of which the most important ones are plant species and soil conditions. When planning the remediation of soil polluted with this xenobiotic, both these factors should be given careful consideration.
4. Conclusions
The impact of increasingly severe fluorine soil contamination on the sodium and sulphur content of the test plants depended on the organs and plant species, the dose of the analyzed xenobiotic, and the substance applied to immobilize fluorine. According to the assumed hypothesis, increasing fluorine soil pollution in most plants resulted in a decrease in the concentrations of sodium and sulphur in the analyzed plant parts. It is worth noting that the highest degree of fluorine soil pollution exhibited the most adverse consequences in this regard. A decline in the sodium content in most of the test plants was likely due to the fact that fluorine ‘entered’ a reaction whereby sodium was bound into compounds hardly soluble in water, thus becoming less available to plants. The materials added to soil in order to immobilize fluorine in most cases decreased the sodium content but did not have any significant effect on the sulphur content in plants. The observed negative changes in sodium concentrations in plants, considering the three fluorine-immobilizing substances added to soil, were most evident in soil remediated with charcoal, being slightly smaller after the application of loam, whereas the soil amendment with lime had the most beneficial influence on the sodium content in crops.
Author Contributions
Conceptualization and methodology, Z.C.; investigation, R.S.; writing—review and editing, R.S. and E.R.; visualization, R.S. and M.W.; supervision, Z.C., M.W. and E.R.; funding acquisition, R.S. and M.W.; M.W., corresponding author. All authors have read and agreed to the published version of the manuscript.
Funding
The results presented in this paper were obtained as part of a comprehensive study financed by the University of Warmia and Mazury in Olsztyn, Faculty of Environmental Management and Agriculture, Department of Environmental Chemistry (grant No. 528-1004-0881). The project was financially supported by the Minister of Education and Science in the range of the program entitled “Regional Initiative of Excellence” for the years 2019–2022, Project No. 010/RID/2018/19, amount of funding 12,000,000 PLN.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available by contacting the authors.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Banerjee, A.; Roychoudhury, A. Fluorine: A biohazardous agent for plants and phytoremediation strategies for its removal from the environment. Biol. Plant. 2019, 63, 104–112. [Google Scholar] [CrossRef]
- Szostek, R.; Ciećko, Z. Content of fluorine in biomass of crops depending on soil contamination by this element. Fluoride 2014, 47, 294–306. [Google Scholar]
- Technavio. Global Fluorochemicals Market for 2016–2020; Infiniti Research Limited: London, UK, 2016. [Google Scholar]
- Polish Geological Institute. The Balance of Mineral Resources Deposits in Poland and World 2013; Polish Geological Institute-National Research Institute: Warsaw, Poland, 2015. (In Polish) [Google Scholar]
- Santos, A.J.G.; Mazzilli, B.P.; Favaro, D.I.T.; Silvap, S.C. Partitioning of radionuclides and trace elements in phosphogypsum and its source materials based on sequential extraction methods. J. Environ. Radioact. 2006, 87, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, A.; Brown, J.E.; Szymańska, M.; Ciupek, K. Application of an environmental impact assessment methodology for areas exhibiting enhanced levels of NORM in Norway and Poland. Radioprotection 2011, 46, 759–764. [Google Scholar] [CrossRef]
- Nowak, W. Biological reclamation of a phosphogypsum dump at the Chemical Plant “Wizów” S. A. Sci. J. Univ. Life Sci. Wroc. 2006, 545, 195–203. (In Polish) [Google Scholar]
- Romar, A.; Gago, C.; Fernandez-Marcos, L.M.; Alvarez, E. Influence of fluoride addition on the composition of solutions in Equilibrium with acid soils. Pedosphere 2009, 19, 60–70. [Google Scholar] [CrossRef]
- Kau, P.M.H.; Smith, D.W.; Binning, P. Fluoride retention by kaolin loam. J. Contam. Hydrol. 1997, 28, 267–288. [Google Scholar] [CrossRef]
- Kau, P.M.H.; Smith, D.W.; Binning, P. Experimental sorption of fluoride by kaolinite and bentonite. Geoderma 1998, 84, 89–108. [Google Scholar] [CrossRef]
- Kau, P.M.H.; Binning, P.; Hitchcock, P.W.; Smith, D.W. Experimental analysis of fluoride diffusion and sorption in loam. J. Contam. Hydrol. 1999, 36, 131–151. [Google Scholar] [CrossRef]
- Telesiński, A.; Biczak, R.; Stręk, M.; Płatkowski, M.; Pawłowska, B.; Emin, N. A study on the fluoride content and the enzymatic activity in soil exposed to inorganic ammonium salt and quaternary ammonium salts with hexafluorophosphate anions. Fluoride 2018, 51, 206–219. [Google Scholar]
- Yu, L.; Zhang, J.; Du, C.; Yang, H.; Ye, B.-C. Distribution and pollution evaluation of fluoride in a soil–water–plant system in Shihezi, Xinjiang, China. Hum. Ecol. Risk Assess. Int. J. 2018, 24, 445–455. [Google Scholar] [CrossRef]
- Chakrabarti, S.; Patra, P.K.; Mandal, B.; Mahato, D. Effect of sodium fluoride on germination, seedling growth and biochemistry of bengal gram (Cicer arietinum). Fluoride 2012, 45, 257–262. [Google Scholar]
- Joshi, M.; Bhardwaj, N. Effect of fluoride on growth parameters and its accumulation in Triticum aestivum var. Raj. 3675. Fluoride 2012, 45, 297–301. [Google Scholar]
- Saini, P.; Khan, S.; Baunthiyal, M.; Sharma, V. Organ-wise accumulation of fluoride in Prosopis juliflora and its potential for phytoremediation of fluoride contaminated soil. Chemosphere 2012, 89, 633–635. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Kumari, B.; Sinam, G.; Kriti; Kumar, N.; Mallick, S. Fluoride distribution and contamination in the water, soil and plants continuum and its remedial technologies, an Indian perspective—A review. Environ. Pollut. 2018, 239, 95–108. [Google Scholar] [CrossRef]
- Yadu, B.; Chandrakar, V.; Keshavkant, S. Responses of plants to fluoride: An overview of oxidative stress and defense mechanism. Fluoride 2016, 49, 293–302. [Google Scholar]
- Szostek, R.; Ciećko, Z. Effect of soil contamination with fluorine on the yield and content of nitrogen forms in the biomass of crops. Environ. Sci. Pollut. Res. 2017, 24, 8588–8601. [Google Scholar] [CrossRef]
- Szostek, R.; Ciećko, Z. The effect of soil contamination with fluorine on the contents of calcium and magnesium in the biomass of crop plants. Fluoride 2017, 50, 41–58. [Google Scholar]
- Szostek, R.; Ciećko, Z.; Rolka, E.; Wyszkowski, M. Content of amino acids in maize and yellow lupine after fluorine application to soil. Agriculture 2021, 11, 1120–1131. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014; World Soil Resources Report. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps. Update 2015; World Soil Resources; Reports No. 106; FAO: Rome, Italy, 2015; p. 192. Available online: https://www.fao.org/3/i3794en/I3794en.pdf (accessed on 10 November 2022).
- Kabata-Pendias, A. Trace Elements in Soils and Plants, 4th ed; CRC Press: Boca Raton, FL, USA, 2011; p. 505. [Google Scholar]
- Research Procedure No. 29. Research Procedure. In Chemical-Agricultural Station Instruction, 2nd ed.; Chemical-Agricultural Station: Warsaw, Poland, 2008.
- Lityński, T.; Jurkowska, H.; Gorlach, E. Chemical and Agricultural Analysis; PWN Publishing House: Warsaw, Poland, 1976; pp. 129–132. [Google Scholar]
- Shimadzu. Shimadzu Analytical and Measuring Instruments; User’s Manual; Shimadzu Corporation: Kyoto, Japan, 2016. [Google Scholar]
- Bremner, J.M. Total nitrogen. In Methods of Soil Analysis, Part 2. Chemical and Microbiological Properties (Agronomy 9); Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Ostrowska, A.; Gawliński, S.; Szczubiałka, Z. Methods for Analysis and Assessment of Soil and Plant Properties; Institute of Environmental Protection: Warsaw, Poland, 1997; p. 334. (In Polish) [Google Scholar]
- StatSoft, Inc. STATISTICA Data Analysis Software System; Version 12.0; Statsoft Inc.: Tulsa, OK, USA, 2012; Available online: www.statsoft.com (accessed on 23 October 2021).
- Fung, K.F.; Wong, M.H. Effects of soil pH on the uptake of Al, F and other elements by tea plants. J. Sci. Food Agric. 2001, 82, 146–152. [Google Scholar] [CrossRef]
- Arnesen, M.K.A. Availability of fluoride to plants grown in contaminated soils. Plant Soil 1997, 191, 13–25. [Google Scholar] [CrossRef]
- Reddy, P.M.; Kaur, M. Sodium fluoride induced growth and metabolic changes in Salicornia brachiata Roxb. Water Air Soil Pollut. 2008, 188, 171–179. [Google Scholar] [CrossRef]
- Aslam, A.; Nawaz, H.; Khan, A.; Ghaffar, R.; Abbas, G. Effect of exogenous application of citric acid on growth of maize (Zea mays L.) under sodium fluoride stress. Fluoride. Available online: https://www.fluorideresearch.online/epub/files/188.pdf (accessed on 10 November 2022).
- Yamada, M.; Kuroda, C.; Fujiyama, H. Function of sodium and potassium in growth of sodium loving Amaranthaceae species. Soil Sci. Plant. Nutr. 2016, 62, 20–26. [Google Scholar] [CrossRef]
- Ranjith, M.; Sridevi, S.; Jeevanrao, K.; Tamesh, T.; Bhave, M.H.V. Fluoride content of agricultural soils and it’s relation with physicochemical properties in Kalwakurthy mandal, Mahabubnagar district, Telangana State. Int. J. Pure. Biosci. 2017, 5, 1588–1598. [Google Scholar] [CrossRef]
- Anbuvel, D.; Kumaresan, S.; Jothibai Margret, R. Fluoride analysis of soil in cultivated areas of Thovalai channel in Kanyakumari district, Tamilnadu, India: Correlation with physico-chemical parameters. Int. J. Basic. Appl. Chem. Sci. 2014, 4, 20–29. [Google Scholar]
- Shekhar, S.; Mohiddin, S.K.; Singh, P.N. Variation in concentration of fluoride in the groundwater of south-west district, NCT Delhi—A case study. In Assessment of Groundwater Resources and Management; Ramanathan, A.L., Bhattacharya, P., Keshari, A., Bundschuh, K., Chandrashekharan, D., Singh, S.K., Eds.; IK International: New Delhi, India, 2006; pp. 370–376. [Google Scholar]
- Dąbrowski, J.; Seniczak, S. The arboreal oribatid mites (Acari, Oribatida) of young Sots pine forests in the region polluted by the Luboń Chemical Factory near Poznań. Anim. Sci. 1965, 204, 77–85. [Google Scholar]
- Zbierska, J. The content of macroelements and fluorine in the grassland soil and sward in the region of a Phosphate Fertilizer Plant in Luboń. Work. Comm. Agric. Sci. Comm. For. Sci. 1996, 81, 227–234. [Google Scholar]
- Ciećko, Z.; Wyszkowski, M.; Rolka, E. Effect of cadmium soil contamination and addition of neutralizing substances on the sodium content in plants. Ecol. Chem. Eng. 2006, 13, 883–890. [Google Scholar]
- Sivitskaya, V.; Wyszkowski, M. Changes in the content of some macroelements in maize (Zea Mays L.) after application of fuel oil and different neutralizing substances to soil. J. Elem. 2013, 18, 706–714. [Google Scholar] [CrossRef]
- Kosiorek, M.; Wyszkowski, M. Macroelement content in plants after amendment application to cobalt-contaminated soil. J. Soils Sediments 2021, 21, 1769–1784. [Google Scholar] [CrossRef]
- Żołnowski, A.C.; Wyszkowski, M.; Rolka, E.; Sawicka, M. Mineral materials as a neutralizing agent used on soil contaminated with copper. Materials 2021, 14, 6830. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Sinha, R.; Sharma, P.K.; Ivy, N.; Kumar, P.; Kant, N.; Jha, A.; Jha, P.K.; Gupta, P.K.; Sharma, P.; et al. Bioaccumulation of fluoride in plants and its microbially assisted remediation: A review of biological processes and technological performance. Processes 2021, 9, 2154. [Google Scholar] [CrossRef]
- Ogundola, A.F.; Bvenura, C.; Afolayan, A.J. Nutrient and antinutrient compositions and heave metal uptake and accumulation in S. nigrum cultivated on different soil types. Sci. World. J. 2018, 2018, 5703929. [Google Scholar] [CrossRef] [PubMed]
- Brodowska, M.S.; Wyszkowski, M.; Kordala, N. Use of organic materials to limit the potential negative effect of nitrogen on maize in different soils. Materials 2022, 15, 5755. [Google Scholar] [CrossRef]
- International Plant Nutrition Institute. Calcium. Nutrifacts. Agronomic Fact Sheets on Crop Nutrients; North American Edition; International Plant Nutrition Institute: Norcross, GA, USA, 2015; Volume 5, pp. 1–2. [Google Scholar]
- Kosiorek, M.; Wyszkowski, M. Content of macronutrients in oat (Avena sativa L.) after remediation of soil polluted with cobalt. Environ. Mon. Assess. 2019, 191, 389. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).