Optimization of Image Quality in Digital Mammography with the Response of a Selenium Detector by Monte Carlo Simulation
Abstract
:1. Introduction
2. Materials and Method
2.1. Simulation
2.1.1. Monte Carlo Software
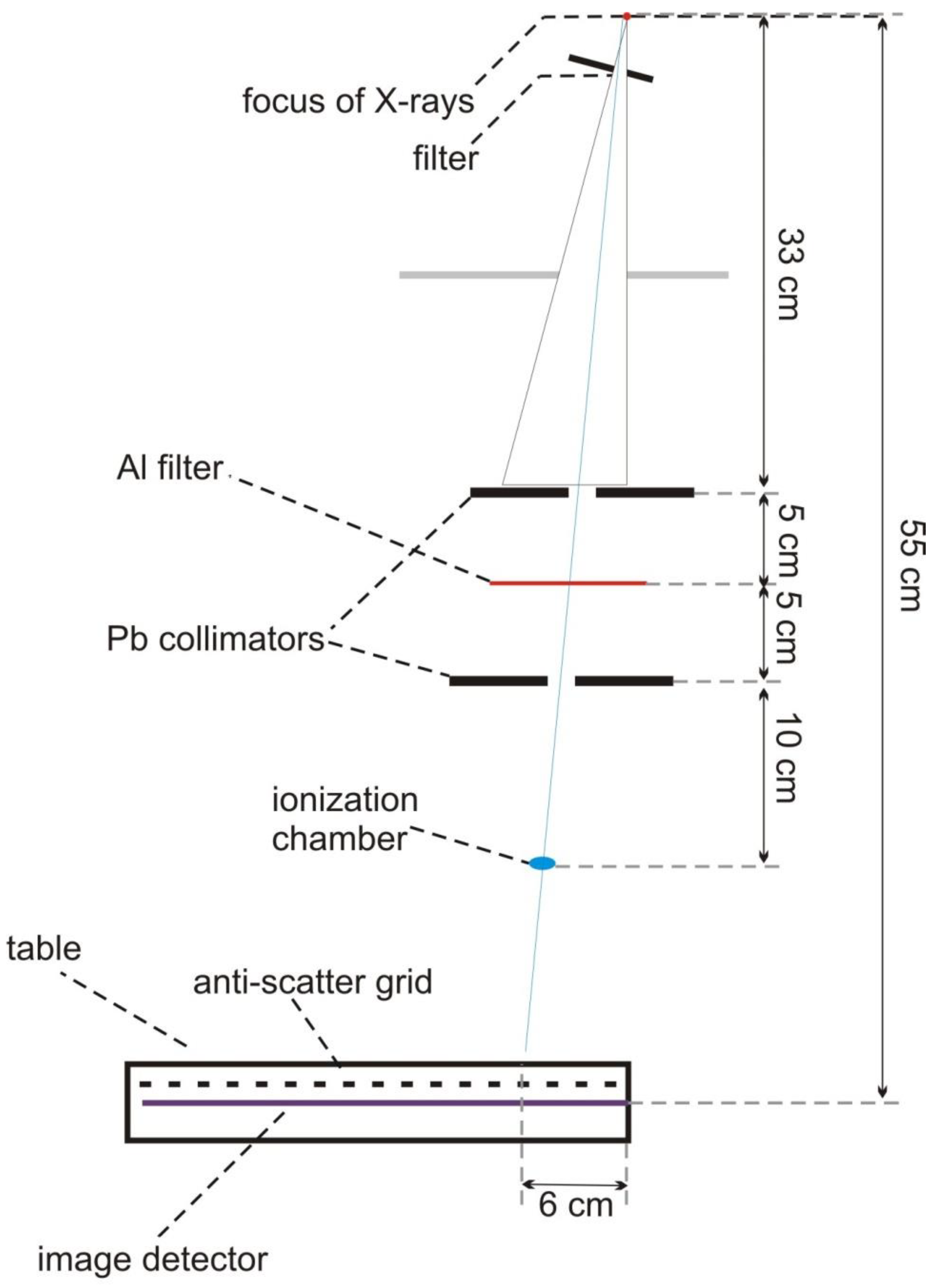
2.1.2. The Simulated System
2.1.3. The Simulation of the X-ray Source
2.1.4. Collimator
2.1.5. Table with an Anti-Scatter Grid
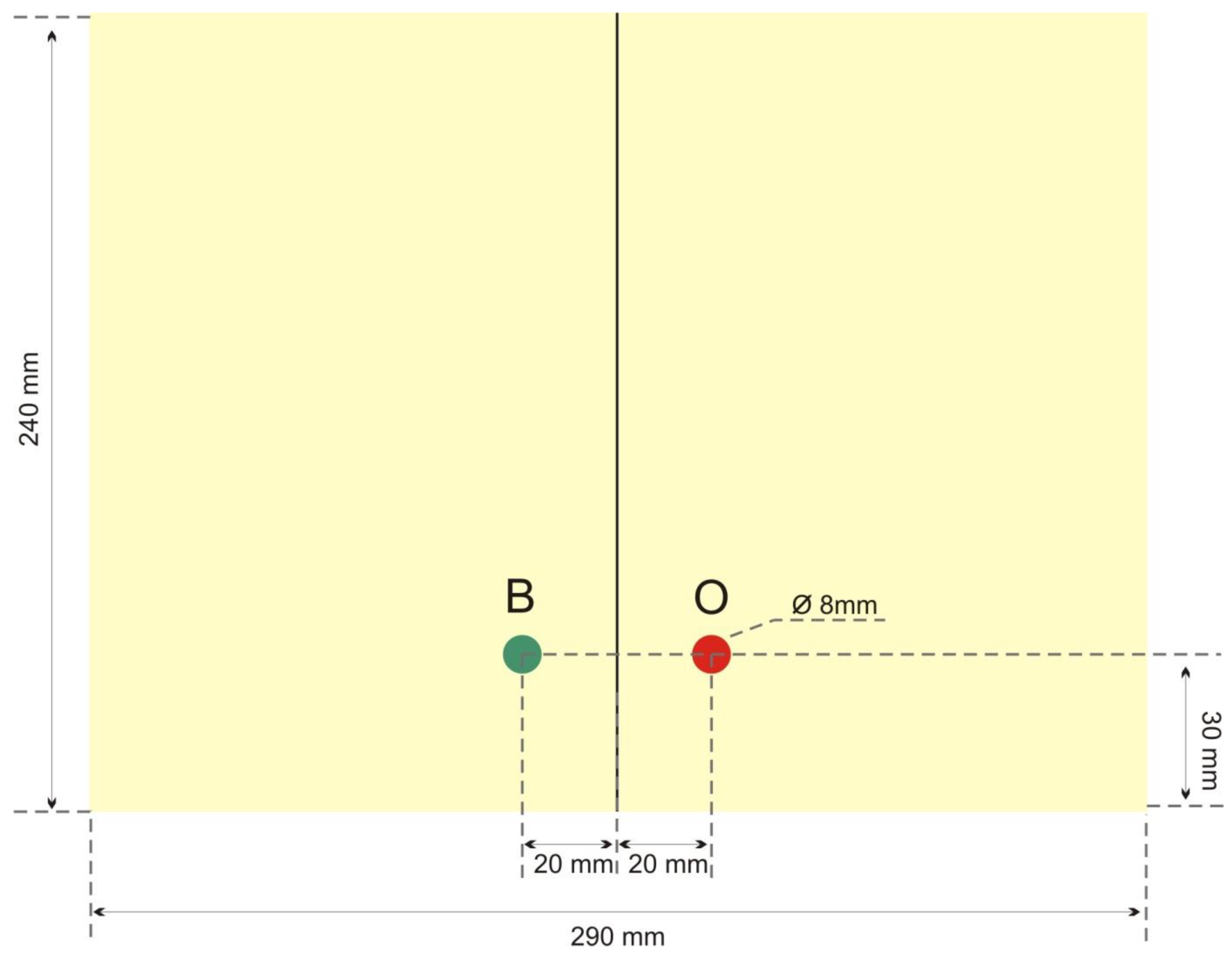
2.1.6. Image Detector
2.1.7. Breast Model
2.2. Experimental Verification of Simulations
3. Results
3.1. Simulation Verification
3.2. Image Quality Optimization
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO. 2021. Available online: https://www.who.int/news-room/fact-sheets/detail/breast-cancer (accessed on 20 November 2022).
- Vogelstein, B.; Papadopoulos, N.; Velculescu, V.E.; Zhou, S.; Diaz, L.A.; Kinzler, K.W. Cancer Genome Landscapes. Science 2013, 339, 1546–1558. [Google Scholar] [CrossRef] [PubMed]
- Herrmann, C.; Vounatsou, P.; Thürlimann, B.; Probst-Hensch, N.; Rothermundt, C.; Ess, S. Impact of mammography screening programmes on breast cancer mortality in Switzerland, a country with different regional screening policies. BMJ Open 2018, 8, e017806. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, Y.; Kaucher, S.; Lorenz, E.; Barnighausen, T.; Winkler, V. Development of breast cancer mortalityconsidering the implementation of mammography screening programs-acomparison of western European countries. BMC Public Health 2019, 19, 823. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berry, D.A.; Cronin, K.A.; Plevritis, S.K.; Fryback, D.G.; Clarke, J.; Zelen, M.; Mandelblatt, J.S.; Yakovlev, A.Y.; Habbema, J.D.F.; Feuer, E.J. Effect of screening and adjuvant therapy on mortality from breast cancer. N. Engl. J. Med. 2005, 353, 1784–1792. [Google Scholar] [CrossRef] [PubMed]
- The Swedish Organised Service Screening Evaluation Group. Reduction in breast cancer mortality from organized service screening with mammography: 1. Further confirmation with extended data. Cancer Epidemiol. Biomark. Prev. 2006, 15, 45–51. [Google Scholar] [CrossRef] [Green Version]
- Preston, D.L.; Mattsson, A.; Holmberg, E.; Shore, R.; Hildreth, N.G.; Boice, J.D., Jr. Radiation effects on breast cancer risk: A pooled analysis of eight cohorts. Radiat. Res. 2002, 158, 220–235. [Google Scholar] [CrossRef]
- Pauwels, E.K.; Foray, N. Bourguignon MH. Breast cancer induced by X-ray mammography screening? A review based on recent understanding of low-dose radiobiology. Med. Princ. Pract. 2016, 25, 101–109. [Google Scholar] [CrossRef]
- Zewde, N.; Ria, F.; Rehani, M.M. Organ doses and cancer risk assessment in patients exposed to high doses from recurrent CT exams. Eur. J. Radiol. 2022, 149, 110224. [Google Scholar] [CrossRef]
- International Atomic Energy Agency, IAEA. Human Health Series 17. Quality Assurance Programme for Digital Mammography, Vienna. 2011. Available online: https://www.iaea.org/publications/8560/quality-assurance-programme-for-digital-mammography (accessed on 1 November 2022).
- Dalmazo, J.; Elias, J.J.; Brocchi, M.A.C. Radiation dose optimization in routine computed tomography: A study of feasibility in a University Hospital. Radiol. Bras. 2010, 43, 241–248. [Google Scholar] [CrossRef] [Green Version]
- Oduko, J.M.; Young, K.C.; Burch, A.; Castellano, E.; Kulama, E.; Lawinski, C.; Marshall, N. Review of Measurements on Full Field Digital Mammography Systems—NHSBSP Equipment Report 0901; NHS Cancer Screening Programmes: London, UK, 2009. [Google Scholar]
- Young, K.C.; Oduko, J.M.; Woolley, L. Technical Evaluation of the Hologic Selenia Full Field Digital Mammography System—NHSBSP Equipment Report 0701; NHS Cancer Screening Programmes: London, UK, 2007. [Google Scholar]
- Bernhardt, P.; Mertelmeier, T.; Hoheisel, M. X-ray spectrum optimization of full-field digital mammography: Simulation and phantom study. Med. Phys. 2010, 33, 4337–4349. [Google Scholar] [CrossRef]
- Hoye, J.; Sharma, S.; Zhang, Y.; Fu, W.; Ria, F.; Kapadia, A.; Segars, W.P.; Wilson, J.; Samei, E. Organ doses from CT localizer radiographs: Development, validation, and application of a Monte Carlo estimation technique. Med. Phys. 2019, 46, 5262–5272. [Google Scholar] [CrossRef]
- Dance, D.R.; Thilander, A.K.; Sandborg, M.; Skinner, C.L.; Castellano, I.A.; Carlsson, G.A. Infuence of anode/filter material and tube potential on contrast, signal-to-noise ratio and average absorbed dose in mammography: A Monte Carlo study. Br. J. Radiol. 2000, 73, 1056–1067. [Google Scholar] [CrossRef] [PubMed]
- Villarreal, O.A.M.; Velasco, F.G.; Fausto, A.M.F.; Milian, F.M.; Mol, A.W.; Capizzi, K.R.; Ambrosio, P. Optimization of the exposure parameters in digital mammography for diverse glandularities using the contrast-detail metric. Phys. Med. 2022, 101, 112–119. [Google Scholar] [CrossRef]
- Yaffe, M.J.; Boone, J.M.; Packard, N.; Alonzo-Proulx, O.; Huang, S.Y.; Peressotti, C.L.; Al-Mayah, A.; Brock, K. The myth of the 50-50 breast. Med. Phys. 2009, 36, 5437–5443. [Google Scholar] [CrossRef] [PubMed]
- Cullen, D.E. EPICS2014: Electron Photon Interaction Cross Sections; Documentation Series of the IAEA Nuclear Data Section; The Nuclear Energy Agency: Paris, France, 2015. [Google Scholar]
- Lechner, A.; Ivanchenko, V.N.; Knobloch, J. Validation of recent Geant4 physics models for application in carbon ion therapy. Nucl. Instrum. Methods B 2010, 268, 2343–2354. [Google Scholar] [CrossRef]
- Cirrone, G.A.P.; Cuttone, G.; Di Rosa, F.; Pandola, L.; Romano, F.; Zhang, Q. Validation of the Geant4 electromagnetic photon cross sections for elements and compounds. Nucl. Instrum. Methods Phys. Res. A 2010, 618, 315–322. [Google Scholar]
- Kadri, O.; Ivanchenko, V.N.; Gharbi, F.; Trabelsi, A. GEANT4 simulation of electron energy deposition in extended media. Nucl. Instrum. Methods Phys. Res. B 2007, 258, 381–387. [Google Scholar]
- Boone, J.M.; Fewell, T.R.; Jennings, R.J. Molybdenium, rhodium andtungsten anode spectral models using interpolationg polynomials with application to mammography. Med. Phys. 1997, 24, 1863–1874. [Google Scholar] [CrossRef]
- Dance, D.R. Monte Carlo calculation of conversion factors for the estimation of mean glandular breast dose. Phys. Med. Biol. 1990, 35, 1211–1219. [Google Scholar] [CrossRef]
- Dance, D.R.; Skinner, C.L.; Young, K.C.; Beckett, J.R.; Kotre, C.J. Additional factors for the estimation of mean glandular breast dose using the UK mammography dosimetry protocol. Phys. Med. Biol. 2000, 45, 3225–3240. [Google Scholar] [CrossRef]
- Dance, D.R.; Young, K.C.; van Engen, R.E. Further factors for the estimation of mean glandular dose using the United Kingdom, European and IAEA breast dosimetry protocols. Phys. Med. Biol. 2009, 54, 4361–4372. [Google Scholar] [CrossRef] [PubMed]
- Jansen, J.T.; Veldkamp, W.J.; Thijssen, M.A.; van Woudenberg, S.; Zoetelief, J. Method for determination of the mean fraction of glandular tissue in individual female breasts using mammography. Phys. Med. Biol. 2005, 50, 5953–5967. [Google Scholar] [CrossRef] [PubMed]
- Boone, J.M. Glandular breast dose for monoenergetic and high-energy X-raybeams: Monte Carlo assessment. Radiology 1999, 213, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Sarno, A.; Mettivier, G.; Di Lillo, F.; Bliznakova, K.; Sechopoulos, I.; Russo, P. Homogeneous vs. patient specific breast models for Monte Carlo evaluation of mean glandular dose in mammography. Phys. Med. 2018, 51, 56–63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hammerstein, G.R.; Miller, D.W.; White, D.R.; Masterson, M.E.; Woodard, H.Q.; Laughlin, J.S. Absorbed radiation dose in mammography. Radiology 1979, 130, 485–491. [Google Scholar] [CrossRef] [PubMed]
- Johns, P.C.; Yaffe, M.J. X-ray characterization of normal and neoplastic breast tissues. Phys. Med. Biol. 1987, 32, 675–695. [Google Scholar] [CrossRef] [PubMed]
- Ullman, G.; Sandborg, M.; Hunt, R.; Dance, D.R.; Carlsson, G.A. Implementation of Pathologies in the Monte Carlo Model in Chest and Breast Imaging; Report 94; Institutionen för Radiologi, Universitetet in Linköping: Linköping, Sweden, 2003; pp. 1–12. [Google Scholar]
- Perry, N.; Broeders, M.; de Wolf, C.; Törnberg, S.; Holland, R.; von Karsa, L. European Guidelines for Quality Assurance in Breast Cancer Screening and Diagnosis, 4th ed.; Office for Official Publications of the European Communities: Luxembourg, 2006. [Google Scholar]
- Pietrzak, R.; Konefał, A.; Sokół, M.; Orlef, A. Comparison of depth-dose distributions of proton therapeutic beams calculated by means of logical detectors and ionization chamber modeled in Monte Carlo codes. Nucl. Instrum. Methods Phys. Res. A 2016, 826, 55–59. [Google Scholar] [CrossRef]
- Yaffe, M.J.; Rowlands, J.A. X-ray detectors for digital radiography. Phys. Med. Biol. 1997, 42, 1. [Google Scholar] [CrossRef] [PubMed]
- Pruszyński, B. Diagnostic Imaging—Theoretical Basis and Research Methodology; Wydawnictwo Lekarskie PZWL: Warsaw, Poland, 2007. (In Polish) [Google Scholar]




| Anode Material | Secondary Filter Material and Thickness |
|---|---|
| Mo | Mo 25 μm |
| Mo | Rh 25 μm |
| Rh | Rh 50 μm |
| W | Rh 50 μm |
| Thickness of Breast (mm) | Glandularity (%) |
|---|---|
| 32 | 67 |
| 45 | 41 |
| 53 | 29 |
| 60 | 20 |
| 75 | 9 |
| 90 | 4 |
| Element | Adipose Tissue ρa = 0.93 g/cm3 | Glandular Tissue ρg = 1.04 g/cm3 | Cancerous Tissue ρt = 1.058 g/cm3 |
|---|---|---|---|
| H | 0.112 | 0.102 | 0.102 |
| C | 0.619 | 0.184 | 0.184 |
| N | 0.017 | 0.032 | 0.032 |
| O | 0.251 | 0.677 | 0.677 |
| S | 0.00025 | 0.00125 | 0.00125 |
| P | 0.00025 | 0.00125 | 0.00125 |
| K | 0.00025 | 0.00125 | 0.00125 |
| Ca | 0.00025 | 0.00125 | 0.00125 |
| Anode–Filter | Mo-Rh | Mo-Mo | W-Rh | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Voltage (kV) | 24 | 28 | 32 | 24 | 28 | 32 | 24 | 28 | 32 |
| measurements | |||||||||
| HVL (mm) | 0.315 | 0.372 | 0.405 | 0.268 | 0.311 | 0.351 | 0.456 | 0.515 | 0.549 |
| Δ (mm) | 0.022 | 0.021 | 0.027 | 0.019 | 0.023 | 0.019 | 0.022 | 0.031 | 0.026 |
| simulations | |||||||||
| HVL (mm) | 0.324 | 0.340 | 0.372 | 0.257 | 0.311 | 0.337 | 0.433 | 0.494 | 0.572 |
| Δ (mm) | 0.015 | 0.014 | 0.018 | 0.010 | 0.010 | 0.015 | 0.013 | 0.040 | 0.009 |
| Thickness of Phantom | |||||||
|---|---|---|---|---|---|---|---|
| 32 mm | 45 mm | 53 mm | 60 mm | 75 mm | 90 mm | ||
| Phantom glandularity | |||||||
| 67% | 41% | 29% | 20% | 9% | 4% | ||
| Mo-Mo | voltage | 24 kV | 25 kV | 25 kV | 28 kV | 27 kV | 29 kV |
| Qmax | 0.991 | 1.959 | 2.029 | 1.171 | 1.312 | 1.313 | |
| Mo-Rh | voltage | 24 kV | 25 kV | 26 kV | 27 kV | 28 kV | 28 kV |
| Qmax | 1.278 | 2.084 | 2.103 | 2.131 | 1.503 | 1.889 | |
| Rh-Rh | voltage | 25 kV | 25 kV | 26 kV | 26 kV | 27 kV | 28 kV |
| Qmax | 1.957 | 2.273 | 2.471 | 2.476 | 1.832 | 2.150 | |
| W-Rh | voltage | 24 kV | 25 kV | 26 kV | 28 kV | 28 kV | 30 kV |
| Qmax | 1.026 | 3.589 | 5.374 | 2.965 | 2.109 | 3.200 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szewczuk, M.; Konefał, A. Optimization of Image Quality in Digital Mammography with the Response of a Selenium Detector by Monte Carlo Simulation. Appl. Sci. 2023, 13, 171. https://doi.org/10.3390/app13010171
Szewczuk M, Konefał A. Optimization of Image Quality in Digital Mammography with the Response of a Selenium Detector by Monte Carlo Simulation. Applied Sciences. 2023; 13(1):171. https://doi.org/10.3390/app13010171
Chicago/Turabian StyleSzewczuk, Marek, and Adam Konefał. 2023. "Optimization of Image Quality in Digital Mammography with the Response of a Selenium Detector by Monte Carlo Simulation" Applied Sciences 13, no. 1: 171. https://doi.org/10.3390/app13010171




