Application of Infrared Spectroscopy and Thermal Analysis in Explaining the Variability of Soil Water Repellency
Abstract
:1. Introduction
2. Materials and Methods
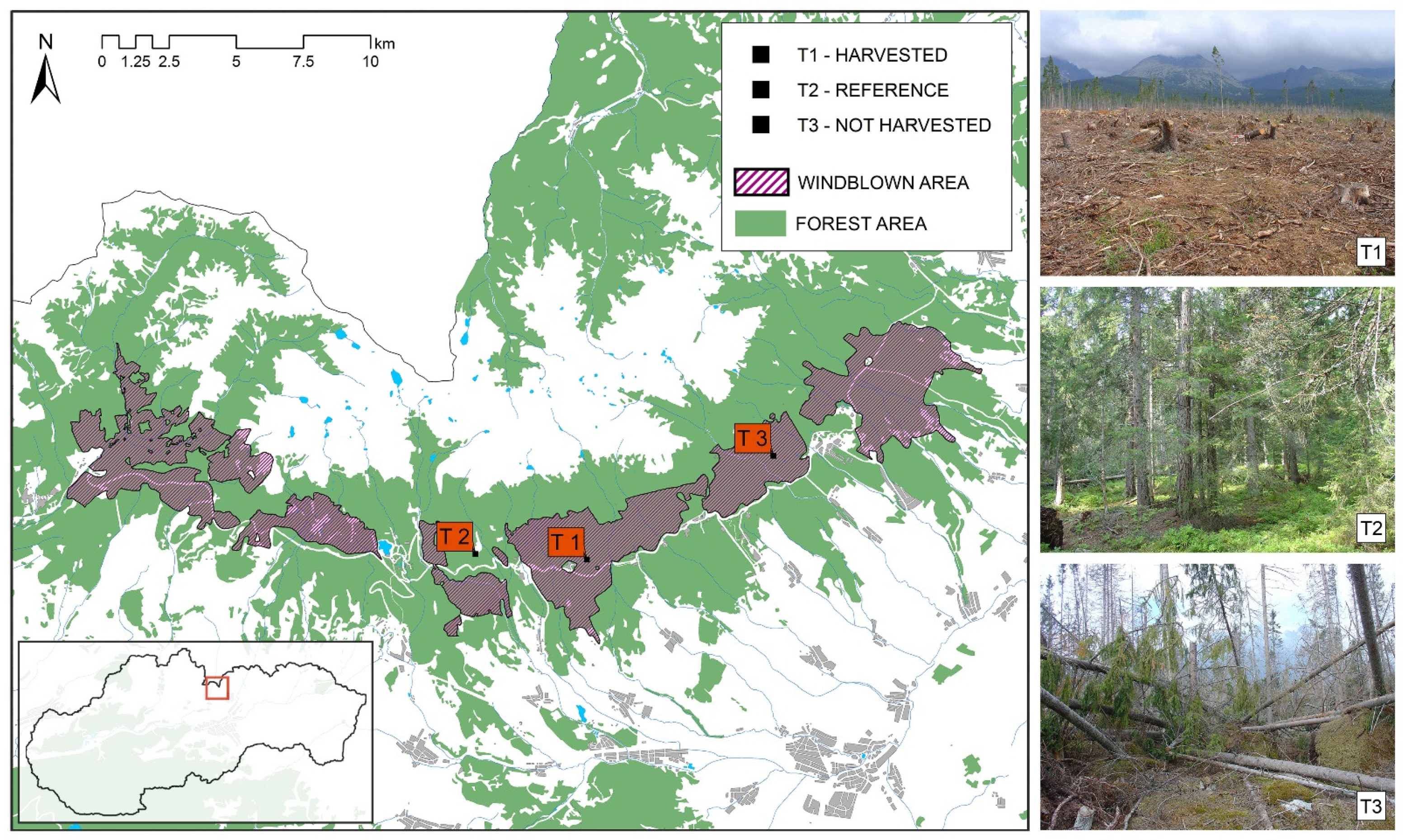
2.1. Studied Area and Soil Sampling
2.2. Sample Preparation and Analysis of Basic Soil Properties
2.3. Soil Water Repellency Measurements
2.4. FTIR Spectroscopy
2.5. Thermal Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Basic Soil Properties
3.2. Soil Water Repellency
3.3. Effect of Basic Soil Properties on WDPT and MED Values
3.4. Functional Composition of SOM
3.5. Thermal Stability of SOM
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Morley, C.P.; Mainwaring, K.A.; Doerr, S.H.; Douglas, P.; Llewellyn, C.T.; Dekker, L.W. Organic compounds at different depths in a sandy soil and their role in water repellency. Aust. J. Soil Res. 2005, 43, 239–249. [Google Scholar] [CrossRef]
- Dekker, L.W.; Ritsema, C.J. Uneven moisture patterns in water repellent soils. Geoderma 1996, 70, 87–99. [Google Scholar] [CrossRef]
- Roper, M.M.; Davies, S.L.; Blackwell, P.S.; Hall, D.J.M.; Bakker, D.M.; Jongepier, R.; Ward, P.R. Management options for water-repellent soils in Australian dryland agriculture. Soil Res. 2015, 53, 786–806. [Google Scholar] [CrossRef]
- Shakesby, R.A.; Doerr, S.H. Wildfire as a hydrological and geomorphological agent. Earth-Sci. Rev. 2006, 74, 269–307. [Google Scholar] [CrossRef]
- Certini, G. Effects of fire on properties of forest soils: A review. Oecologia 2005, 143, 1–10. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Gnatowski, T.; Oleszczuk, R. Effects of Soil Water Repellency on Moisture Patterns in a Degraded Sapric Histosol. Land Degrad. Dev. 2016, 27, 955–964. [Google Scholar] [CrossRef]
- Bayer, J.; Schaumann, G.E. Development of soil water repellency in course of isothermal drying and upon pH changes in two urban soils. Hydrol. Process. 2007, 21, 2266–2275. [Google Scholar] [CrossRef]
- Goebel, M.-O.; Bachmann, J.; Woche, S.K.; Fischer, W.R.; Horton, R. Water potential and aggregate size effects on contact angle and surface energy. Soil Sci. Soc. Am. J. 2004, 68, 383–393. [Google Scholar] [CrossRef]
- Täumer, K.; Stoffregen, H.; Wessolek, G. Determination of repellency distribution using soil organic matter and water content. Geoderma 2005, 125, 107–115. [Google Scholar] [CrossRef]
- Kořenková, L.; Šimkovic, I.; Dlapa, P.; Juráni, B.; Matúš, P. Identifying the origin of soil water repellency at regional level using multiple soil characteristics: The White Carpathians and Myjavska pahorkatina Upland case study. Soil Water Res. 2015, 10, 78–89. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Near infrared spectroscopy for determination of various physical, chemical and biochemical properties in Mediterranean soils. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Summers, D.; Lewis, M.; Ostendorf, B.; Chittleborough, D. Visible near-infrared reflectance spectroscopy as a predictive indicator of soil properties. Ecol. Indic. 2011, 11, 123–131. [Google Scholar] [CrossRef]
- Hermansen, C.; Knadel, M.; Moldrup, P.; Greve, M.H.; Karup, D.; de Jonge, L.W. Complete Soil Texture is Accurately Predicted by Visible Near-Infrared Spectroscopy. Soil Sci. Soc. Am. J. 2017, 81, 758–769. [Google Scholar] [CrossRef]
- Negri, S.; Stanchi, S.; Celi, L.; Bonifacio, E. Simulating wildfires with lab-heating experiments: Drivers and mechanisms of water repellency in alpine soils. Geoderma 2021, 402, 115357. [Google Scholar] [CrossRef]
- Šimkovic, I.; Dlapa, P.; Šimonovičová, A.; Ziegler, W. Water Repellency of Mountain Forest Soils in Relation to Impact of the Katabatic Windstorm and Subsequent Management Practices. Pol. J. Environ. Stud. 2009, 18, 443–454. [Google Scholar]
- Soil Survey Staff. Keys to Soil Taxonomy, 10th ed.; NRCS: Washington, DC, USA, 2006. [Google Scholar]
- FAO. Guidelines for Soil Description, 4th ed.; Food and Agriculture Organization of the United Nations: Rome, Italy, 2006. [Google Scholar]
- Doerr, S.H. On standardizing the Water Drop Penetration Time and the Molarity of an Ethanol Droplet technique to classify soil hydrophobicity: A case study using medium textured soils. Earth Surf. Process. Landf. 1998, 23, 663–668. [Google Scholar] [CrossRef]
- Dekker, L.W.; Doerr, S.H.; Oostindie, K.; Ziogas, A.K.; Ritsema, C.J. Water repellency and critical soil water content in a dune sand. Soil Sci. Soc. Am. J. 2001, 65, 1667–1674. [Google Scholar] [CrossRef]
- Doerr, S.H.; Shakesby, R.A.; Dekker, L.W.; Ritsema, C.J. Occurrence, prediction and hydrological effects of water repellency amongst major soil and land-use types in a humid temperate climate. Eur. J. Soil Sci. 2006, 57, 741–754. [Google Scholar] [CrossRef]
- Madejová, J.; Komadel, P. Baseline studies of the Clay Minerals Society source clays: Infrared methods. Clays Clay Min. 2001, 49, 410–432. [Google Scholar] [CrossRef]
- Stuard, B. Infrared Spectroscopy: Fundamentals and Applications; John Wiley & Sons: Hoboken, NJ, USA, 2004. [Google Scholar]
- Stevenson, F.J. Humus Chemistry. Genesis, Composition, Reactions, 2nd ed.; Wiley: New York, NY, USA, 1994. [Google Scholar]
- Capriel, P.; Beck, T.; Borchert, H.; Gronholz, J.; Zachmann, G. Hydrophobicity of organic matter in arable soils. Soil Biol. Biochem. 1995, 28, 1453–1458. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H.; Bachmann, J.; Goebel, M.-O. Composition of organic matter fractions for explaining wettability of three forest soils. Soil Sci. Soc. Am. J. 2005, 69, 57–66. [Google Scholar] [CrossRef]
- Filip, Z.; Pechner, W.; Berthelin, J. Microbial utilization and transformation of humic acids extracted from different soils. J. Plant Nutr. Soil Sci. 1999, 162, 215–222. [Google Scholar] [CrossRef]
- Ribeiro, J.S.; Sonay, S.O.; Garrigues, S.; de la Guardia, M. FTIR tentative characterization of humic acids extracted from organic materials. Spectr. Lett. 2001, 34, 179–190. [Google Scholar] [CrossRef]
- Smidt, E.; Eckhardt, K.U.; Lechner, P.; Schulten, H.R.; Leinweber, P. Characterization of different decomposition stages of biowaste using FT-IR spectroscopy and pyrolysis-field ionization mass spectrometry. Biodegradation 2005, 16, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Pietikäinen, J.; Hiukka, R.; Fritze, H. Does short-term heating of forest humus change its properties as a substrate for microbes? Soil Biol. Biochem. 2000, 32, 277–288. [Google Scholar] [CrossRef]
- Tatzber, M.; Stemmer, M.; Spiegel, H.; Katzlberger, C.; Haberhauer, G.; Mentler, A.; Gerzabek, M.H. FTIR-spectroscopic characterization of humic acids and humin fractions obtained by advanced NaOH, Na4P2O7, and Na2CO3 extraction procedures. J. Plant Nutr. Soil Sci. 2007, 170, 522–529. [Google Scholar] [CrossRef]
- Carballo, T.; Gil, M.V.; Gómez, X.; González-Andréz, F.; Morán, A. Characterization of different compost extracts using Fourier-transform infrared spectroscopy (FTIR) and thermal analysis. Biodegradation 2008, 19, 815–830. [Google Scholar] [CrossRef]
- Hollas, J.M. Modern Spectroscopy, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2004; p. 452. [Google Scholar]
- Artz, R.R.E.; Chapman, S.J.; Robertson, A.H.J.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.-R.; Francez, A.-J. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds, 7th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2005; p. 502. [Google Scholar]
- Bisdom, E.B.A.; Dekker, L.W.; Schoute, J.F.T. Water repellency of sieve fractions from sandy soils and relationships with organic material and soil structure. Geoderma 1993, 56, 105–118. [Google Scholar] [CrossRef]
- Ma’shum, M.; Farmer, V.C. Origin and assessment of water-repellency of a sandy Australian soil. Aust. J. Soil Res. 1985, 23, 623–626. [Google Scholar] [CrossRef]
- Bowman, W.D.; Cleveland, C.C.; Halada, Ľ.; Hreško, J.; Baron, J.S. Negative impact of nitrogen deposition on soil buffering capacity. Nat. Geosci. 2008, 1, 767–770. [Google Scholar] [CrossRef]
- Prietzel, J.; Stetter, U.; Klemmt, H.-J.; Rehfuess, K.E. Recent carbon and nitrogen accumulation and acidification in two Scots pine ecosystems in Southern Germany. Plant Soil 2006, 289, 153–170. [Google Scholar] [CrossRef]
- Mataix-Solera, J.; Arcenegui, V.; Guerrero, C.; Mayoral, A.M.; Morales, J.; González, J.; García-Orenes, F.; Gómez, I. Water repellency under different plant species in a calcareous forest soil in a semiarid Mediterranean environment. Hydrol. Process. 2007, 21, 2300–2309. [Google Scholar] [CrossRef]
- Diehl, D.; Bayer, J.V.; Woche, S.K.; Bryant, R.; Doerr, S.H.; Schaumann, G.E. Reaction of soil water repellency on artificially induced changes in soil pH. Geoderma 2010, 158, 375–384. [Google Scholar] [CrossRef]
- Gondar, D.; Lopez, R.; Fiol, S.; Antelo, J.M.; Arce, F. Characterization and acid-base properties of fulvic and humic acids isolated from two horizons of an ombrotrophic peat bog. Geoderma 2005, 126, 367–374. [Google Scholar] [CrossRef]
- Dell’Abate, M.T.; Canali, S.; Trinchera, A.; Benedetti, A.; Sequi, P. Thermal method of organic matter maturation monitoring during composting process. J. Therm. Anal. Calorim. 2000, 61, 389–396. [Google Scholar] [CrossRef]
- Plante, A.F.; Pernes, M.; Chenu, C. Changes in clay-associated organic matter quality in a C depletion sequence as measured by differential thermal analyses. Geoderma 2005, 129, 186–199. [Google Scholar] [CrossRef]
- Dell’Abate, M.T.; Canali, S.; Trinchera, A.; Benedetti, A.; Sequi, P. Thermal analysis in the evaluation of compost stability: A comparison with humification parameters. Nutr. Cycl. Agroecosystems 1998, 51, 217–224. [Google Scholar] [CrossRef]
- Rovira, P.; Kurz-Besson, C.; Couteaux, M.M.; Vallejo, V.R. Changes in litter properties during decomposition: A study by differential thermogravimetry and scanning calorimetry. Soil Biol. Biochem. 2008, 40, 172–185. [Google Scholar] [CrossRef]
- Simkovic, I.; Dlapa, P.; Doerr, S.H.; Mataix-Solera, J.; Sasinkova, V. Thermal destruction of soil water repellency and associated changes to soil organic matter as observed by FTIR spectroscopy. Catena 2008, 74, 205–211. [Google Scholar] [CrossRef]
- Gressel, N.; Inbar, Y.; Singer, A.; Chen, Y. Chemical and spectroscopic properties of leaf litter and decomposed organic matter in the Carmel Renge, Israel. Soil Biol. Biochem. 1995, 27, 23–31. [Google Scholar] [CrossRef]
- Madari, B.E.; Reeves, J.B.; Machado, P.L.O.A.; Guimaraes, C.M.; Torres, E.; McCarty, G.W. Mid- and near-infrared spectroscopic assessment of soil compositional parameters and structural indices in two Ferralsols. Geoderma 2006, 136, 245–259. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Leifeld, J. Application of thermal analysis techniques in soil science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Siewert, C. Rapid Screening of Soil Properties using Thermogravimetry. Soil Sci. Soc. Am. J. 2004, 68, 1656–1661. [Google Scholar] [CrossRef]
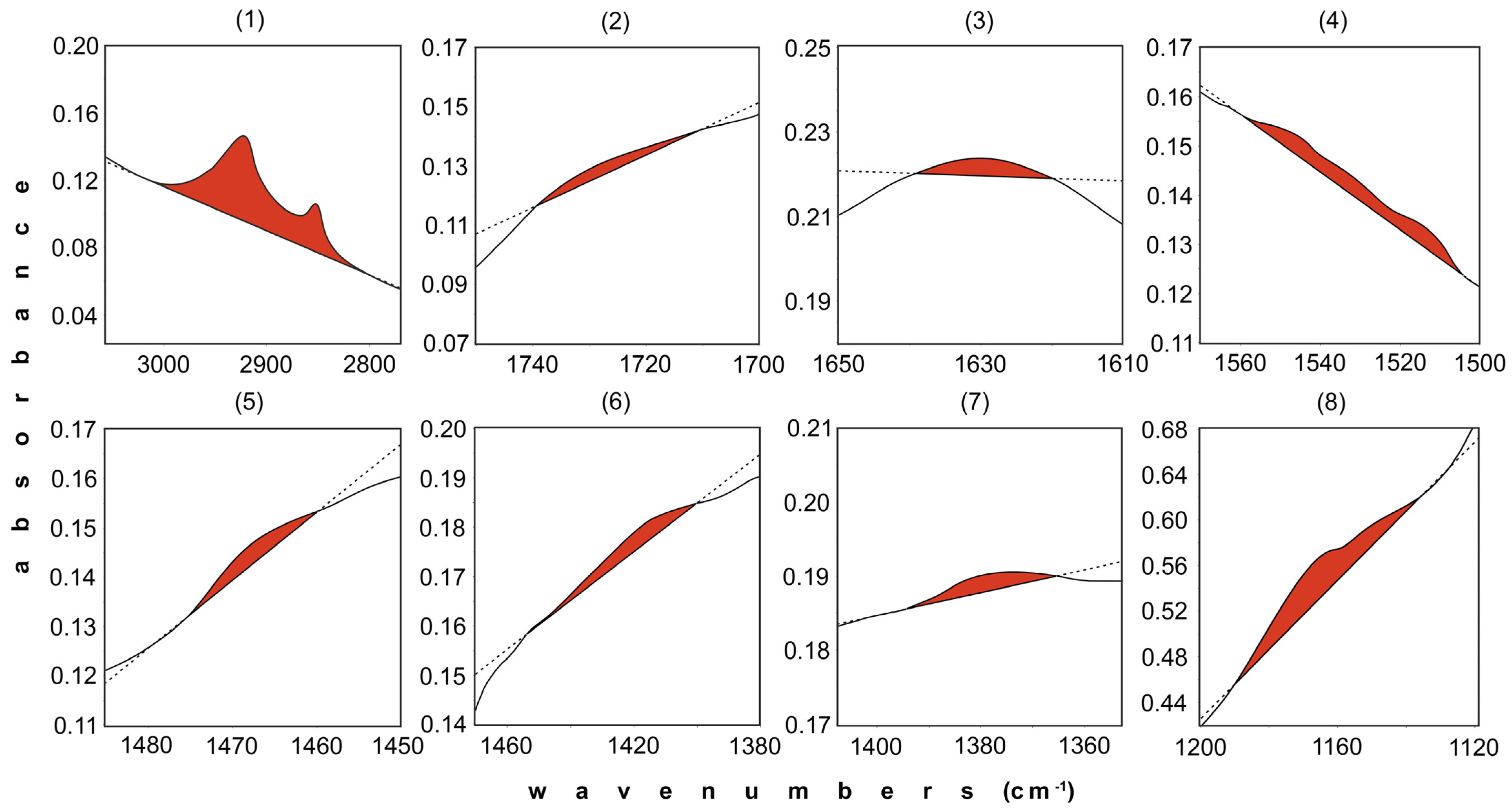


| Band No. | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 |
|---|---|---|---|---|---|---|---|---|
| Wavenumbers | 3020–2800 cm−1 | 1740–1710 cm−1 | 1640–1620 cm−1 | 1560–1505 cm−1 | 1475–1460 cm−1 | 1455–1400 cm−1 | 1395–1365 cm−1 | 1190–1135 cm−1 |
| Assignment | 2960 cm−1: methyl symmetric C-H stretching; 2930 cm−1: methylene asymmetric C-H stretching; 2870 cm−1: methyl asymmetric C-H stretching; 2850 cm−1: methylene symmetric C-H stretching | C=O stretching of carboxyl and carbonyl group contained in carboxylic acids, aldehydes, ketones, esters | primary amide NH2 bending plus C=O stretch in amides, quinones, and/or H-bonded conjugated ketones, carboxylates, C=N, and aromatic C=C cannot be ruled out, lignocellulose | 1560–1530 cm−1: secondary amide N-H bending (NH2 or NH bending), C-N stretching, 1590–1517 cm−1: C=O in COO−, 1520 cm−1: C=O of amide, 1650–1550 cm−1: aromatic C | methylene scissoring and methyl asymmetrical CH bending | 1430 cm−1: C-O-H in-plane bending, 1426 cm−1: symmetric C-O stretch from COO− or stretch and OH deformation of carboxylates, 1450 cm−1: aliphatic C-H of CH2 | symmetric and in-plane bending of CH3 group at around 1380 cm−1, 1388 cm−1: OH deformation and C-O stretch (phenolics) | 1000–1260 cm−1: C-O stretching vibration in alcohols and phenols; 1150–1085 cm−1: C-O-C stretching in ethers and polysaccharides (1160 cm−1); 1100–1300 cm−1: C-O stretching in anhydrides; 1100–1300 cm−1: aliphatic C-O stretching in esters |
| Reference | [22,23,24,25] | [26,27,28] | [22,26,27,29] | [22,26,27,28,29,30,31] | [22,23,26] | [22,28,30,31,32,33] | [22,26,32] | [22,27,28,34] |
| The effect on soil wettability | hydrophobic | hydrophilic | hydrophilic | rather hydrophilic | hydrophobic | rather hydrophilic | rather hydrophobic | hydrophilic |
| Sample No. | WDPTA s | WR Classification | WDPTP s | MED mol L−1 | wF % | SOM g kg−1 | SOC g kg−1 | N g kg−1 | Sand % | Silt % | Clay % | pH |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | <1 | wettable | 45 | 0.87 | 70.97 | 170.3 | 81.9 | 4.74 | 59.90 | 31.70 | 8.40 | 3.75 |
| 2 | 2 | wettable | 933 | 2.11 | 36.93 | 140.4 | 67.3 | 3.18 | 69.29 | 22.47 | 8.24 | 3.83 |
| 3 | 200 | strongly WR | 1413 | 2.48 | 38.41 | 173.0 | 86.9 | 3.70 | 71.21 | 21.98 | 6.81 | 3.69 |
| 4 | 42,658 | extremely WR | 43,652 | 3.21 | 26.76 | 162.7 | 78.6 | 3.65 | 66.69 | 23.15 | 10.16 | 3.70 |
| 5 | 18,197 | extremely WR | 12,023 | 2.48 | 20.65 | 96.1 | 48.4 | 1.87 | 64.74 | 24.65 | 10.61 | 3.58 |
| 6 | <1 | wettable | 14 | 0.52 | 48.90 | 167.9 | 79.9 | 4.47 | 62.12 | 25.97 | 11.91 | 3.83 |
| 7 | 28 | slightly WR | 1549 | 2.11 | 39.16 | 198.7 | 103.5 | 5.57 | 65.26 | 24.00 | 10.74 | 3.85 |
| 8 | <1 | wettable | 195 | 1.22 | 47.36 | 168.6 | 82.6 | 4.43 | 66.00 | 25.02 | 8.98 | 3.73 |
| 9 | 4365 | extremely WR | 3020 | 1.75 | 44.55 | 120.2 | 56.2 | 2.72 | 70.87 | 19.21 | 9.92 | 3.93 |
| 10 | <1 | wettable | 72 | 1.04 | 57.43 | 181.9 | 91.6 | 4.81 | 60.35 | 31.31 | 8.34 | 3.56 |
| 11 | <1 | wettable | 191 | 1.40 | 44.05 | 154.1 | 74.4 | 4.14 | 63.85 | 24.97 | 11.18 | 3.73 |
| 12 | <1 | wettable | <1 | 0 | 26.14 | 90.1 | 40.2 | 1.52 | 63.49 | 25.12 | 11.39 | 3.88 |
| 13 | 43,652 | extremely WR | 43,652 | 2.84 | 26.62 | 136.7 | 68.4 | 3.40 | 61.98 | 26.07 | 11.95 | 3.87 |
| 14 | <1 | wettable | 3 | 0.17 | 48.84 | 118.4 | 60.0 | 2.85 | 68.58 | 23.72 | 7.70 | 3.75 |
| 15 | <1 | wettable | <1 | 0 | 30.87 | 84.5 | 39.3 | 1.50 | 69.97 | 20.36 | 9.67 | 4.08 |
| 16 | 8511 | extremely WR | 13,183 | 2.84 | 57.69 | 276.0 | 160.9 | 8.58 | 70.99 | 22.14 | 6.87 | 3.59 |
| 17 | 43,652 | extremely WR | 43,652 | 3.21 | 19.76 | 164.9 | 67.9 | 3.00 | 67.00 | 22.93 | 10.07 | 3.72 |
| 18 | 20,417 | extremely WR | 32,359 | 3.96 | 43.00 | 219.0 | 113.9 | 5.19 | 64.17 | 25.05 | 10.78 | 3.35 |
| 19 | 6607 | extremely WR | 8511 | 3.40 | 48.91 | 244.6 | 120.7 | 5.90 | 60.51 | 27.70 | 11.79 | 3.25 |
| 20 | 43,652 | extremely WR | 38,019 | 3.96 | 25.25 | 210.4 | 78.1 | 3.59 | 65.77 | 23.65 | 10.58 | 3.49 |
| 21 | 43,652 | extremely WR | 26,915 | 3.58 | 34.15 | 230.5 | 123.4 | 5.62 | 66.19 | 24.88 | 8.93 | 3.31 |
| 22 | 22,387 | extremely WR | 1514 | 2.66 | 40.60 | 189.3 | 84.3 | 4.12 | 70.62 | 19.38 | 10.00 | 3.53 |
| 23 | 43,652 | extremely WR | 33,113 | 3.58 | 31.94 | 219.6 | 108.2 | 4.52 | 63.94 | 24.91 | 11.15 | 3.26 |
| 24 | 437 | strongly WR | 1820 | 2.84 | 33.99 | 165.1 | 83.7 | 4.19 | 75.33 | 17.32 | 7.35 | 3.41 |
| 25 | 955 | severely WR | 1514 | 2.84 | 55.09 | 289.4 | 155.9 | 6.73 | 61.65 | 26.29 | 12.06 | 3.21 |
| 26 | 724 | severely WR | 575 | 2.48 | 44.47 | 165.0 | 80.7 | 4.00 | 78.41 | 14.49 | 7.10 | 3.77 |
| 27 | 126 | strongly WR | 1514 | 2.11 | 37.36 | 161.1 | 77.4 | 3.19 | 79.20 | 15.29 | 5.51 | 3.75 |
| 28 | <1 | wettable | 3 | 0.17 | 54.11 | 208.9 | 104.0 | 5.34 | 78.45 | 16.65 | 4.90 | 3.77 |
| 29 | 257 | strongly WR | 933 | 2.48 | 58.03 | 213.4 | 104.6 | 4.90 | 81.53 | 11.88 | 6.59 | 3.53 |
| 30 | 3890 | extremely WR | 2754 | 2.84 | 42.12 | 205.6 | 98.3 | 5.13 | 68.63 | 23.68 | 7.69 | 3.52 |
| 31 | <1 | wettable | 28 | 0.52 | 71.85 | 197.4 | 102.0 | 5.55 | 68.18 | 21.86 | 9.96 | 3.77 |
| 32 | <1 | wettable | 8 | 0.34 | 70.25 | 240.8 | 90.5 | 4.09 | 58.70 | 29.48 | 11.82 | 3.44 |
| 33 | <1 | wettable | 6 | 0.34 | 59.45 | 147.3 | 73.4 | 3.43 | 69.01 | 21.35 | 9.64 | 3.73 |
| 34 | <1 | wettable | 14 | 0.69 | 50.63 | 144.4 | 70.8 | 3.31 | 75.61 | 17.97 | 6.42 | 3.88 |
| 35 | <1 | wettable | 282 | 1.40 | 107.4 | 146.7 | 75.3 | 3.10 | 63.03 | 27.28 | 9.69 | 3.75 |
| 36 | 21 | slightly WR | 832 | 2.11 | 16.48 | 207.1 | 106.3 | 5.74 | 72.50 | 18.55 | 8.95 | 3.62 |
| 37 | <1 | wettable | 9 | 0.34 | 64.76 | 236.4 | 100.4 | 4.89 | 61.68 | 28.11 | 10.21 | 3.40 |
| 38 | <1 | wettable | 26 | 1.40 | 44.18 | 205.1 | 105.6 | 5.65 | 71.88 | 19.60 | 8.52 | 3.78 |
| 39 | <1 | wettable | 5 | 0.34 | 46.88 | 158.1 | 69.6 | 3.00 | 63.59 | 28.75 | 7.66 | 3.67 |
| 40 | <1 | wettable | 3 | 0.34 | 54.92 | 173.3 | 80.6 | 3.39 | 57.33 | 32.49 | 10.18 | 3.56 |
| 41 | <1 | wettable | 13 | 0.52 | 84.79 | 178.3 | 93.9 | 5.00 | 66.36 | 26.01 | 7.63 | 3.44 |
| 42 | <1 | wettable | 21 | 0.69 | 97.00 | 215.9 | 116.6 | 6.36 | 73.37 | 18.32 | 8.31 | 3.51 |
| 43 | 89 | strongly WR | 1096 | 2.48 | 44.68 | 179.8 | 93.6 | 4.23 | 66.64 | 25.13 | 8.23 | 3.47 |
| 44 | <1 | wettable | 13 | 0.69 | 60.88 | 193.0 | 99.8 | 4.30 | 69.95 | 21.32 | 8.73 | 3.58 |
| 45 | <1 | wettable | 3 | 0.34 | 71.35 | 172.9 | 109.4 | 4.91 | 62.69 | 28.36 | 8.95 | 3.46 |
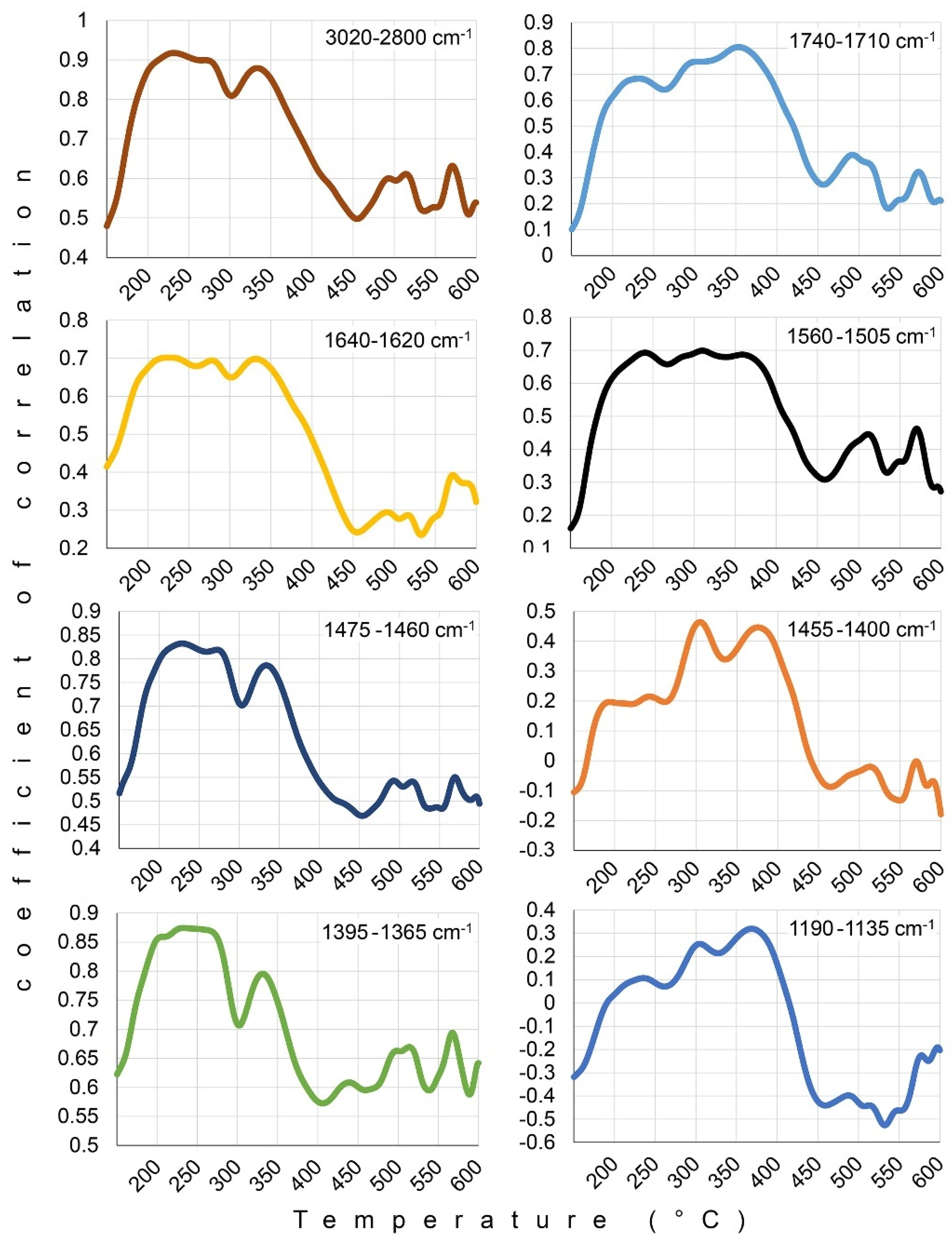
| 3020–2800 | 1740–1710 | 1640–1620 | 1560–1505 | 1475–1460 | 1455–1400 | 1395–1365 | 1190–1135 | |
|---|---|---|---|---|---|---|---|---|
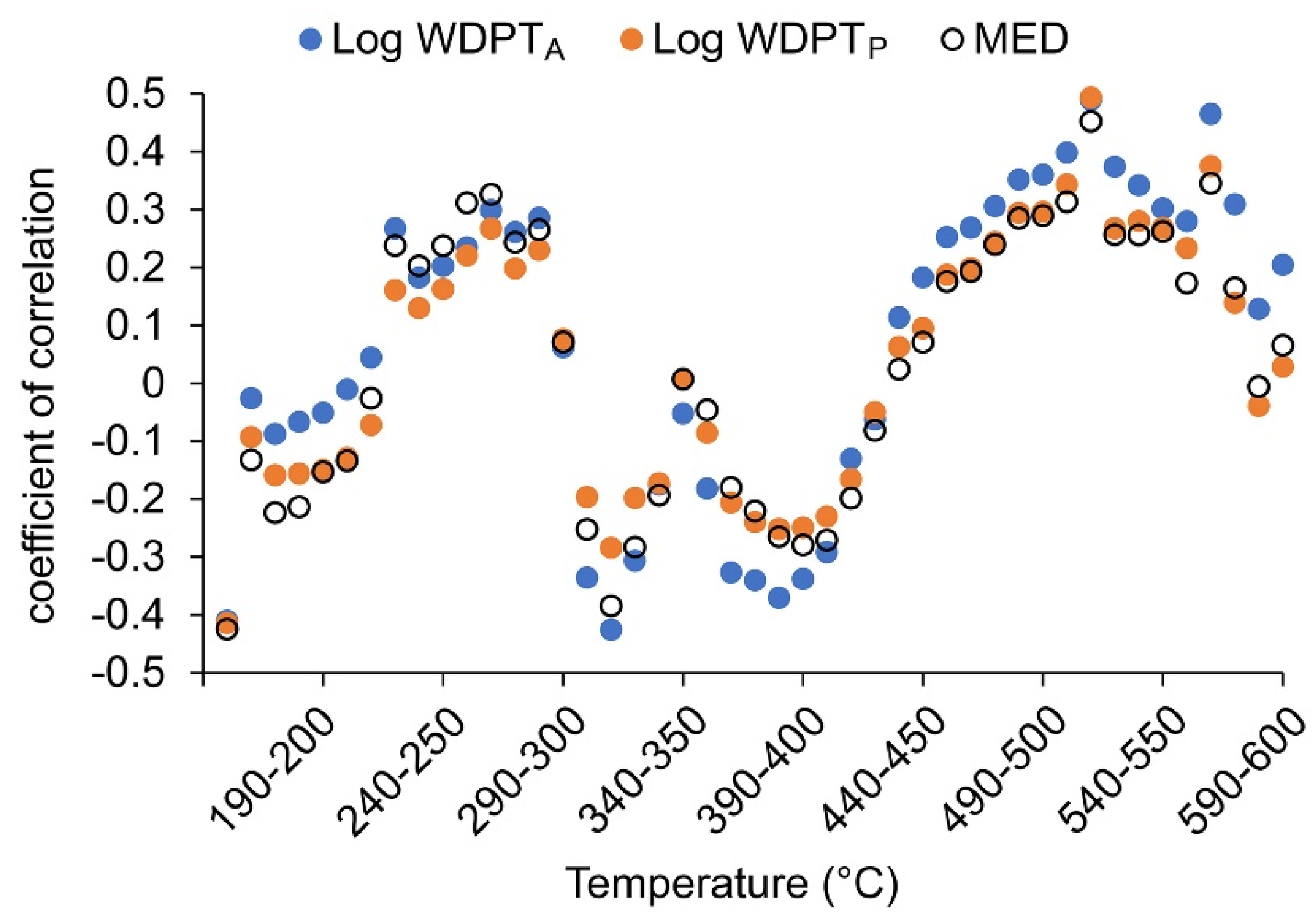
| Log WDPTA | 0.29 * | −0.02 | −0.04 | 0.06 | 0.31 * | −0.40 ** | 0.37 * | −0.51 *** |
| Log WDPTP | 0.26 | 0.00 | −0.13 | 0.14 | 0.21 | −0.26 | 0.35 * | −0.51 *** |
| MED | 0.34 * | 0.08 | −0.08 | 0.16 | 0.28 | −0.29 * | 0.44 ** | −0.48 *** |
| wF | 0.23 | 0.36 * | 0.25 | 0.24 | 0.07 | 0.37 * | 0.04 | 0.49 *** |
| SOM | 0.87 *** | 0.72 *** | 0.66 *** | 0.68 *** | 0.77 *** | 0.31 * | 0.81 *** | 0.12 |
| SOC | 0.81 *** | 0.65 *** | 0.50 *** | 0.75 *** | 0.67 *** | 0.41 ** | 0.68 *** | 0.18 |
| pH | −0.71 *** | −0.75 *** | −0.51 *** | −0.46 ** | −0.63 *** | −0.01 | −0.55 *** | −0.14 |
| Sand | −0.14 | −0.22 * | −0.25 * | −0.07 | −0.17 | −0.03 | −0.10 | −0.1 |
| Silt | 0.14 | 0.21 * | 0.28 | 0.08 | 0.15 | 0.05 | 0.08 | 0.17 |
| Clay | 0.11 | 0.15 | 0.07 | 0 | 0.14 | −0.03 | 0.11 | −0.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šimkovic, I.; Dlapa, P.; Feketeová, Z. Application of Infrared Spectroscopy and Thermal Analysis in Explaining the Variability of Soil Water Repellency. Appl. Sci. 2023, 13, 216. https://doi.org/10.3390/app13010216
Šimkovic I, Dlapa P, Feketeová Z. Application of Infrared Spectroscopy and Thermal Analysis in Explaining the Variability of Soil Water Repellency. Applied Sciences. 2023; 13(1):216. https://doi.org/10.3390/app13010216
Chicago/Turabian StyleŠimkovic, Ivan, Pavel Dlapa, and Zuzana Feketeová. 2023. "Application of Infrared Spectroscopy and Thermal Analysis in Explaining the Variability of Soil Water Repellency" Applied Sciences 13, no. 1: 216. https://doi.org/10.3390/app13010216







