Development of Multi-Cation-Doped M-Type Hexaferrite Permanent Magnets
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
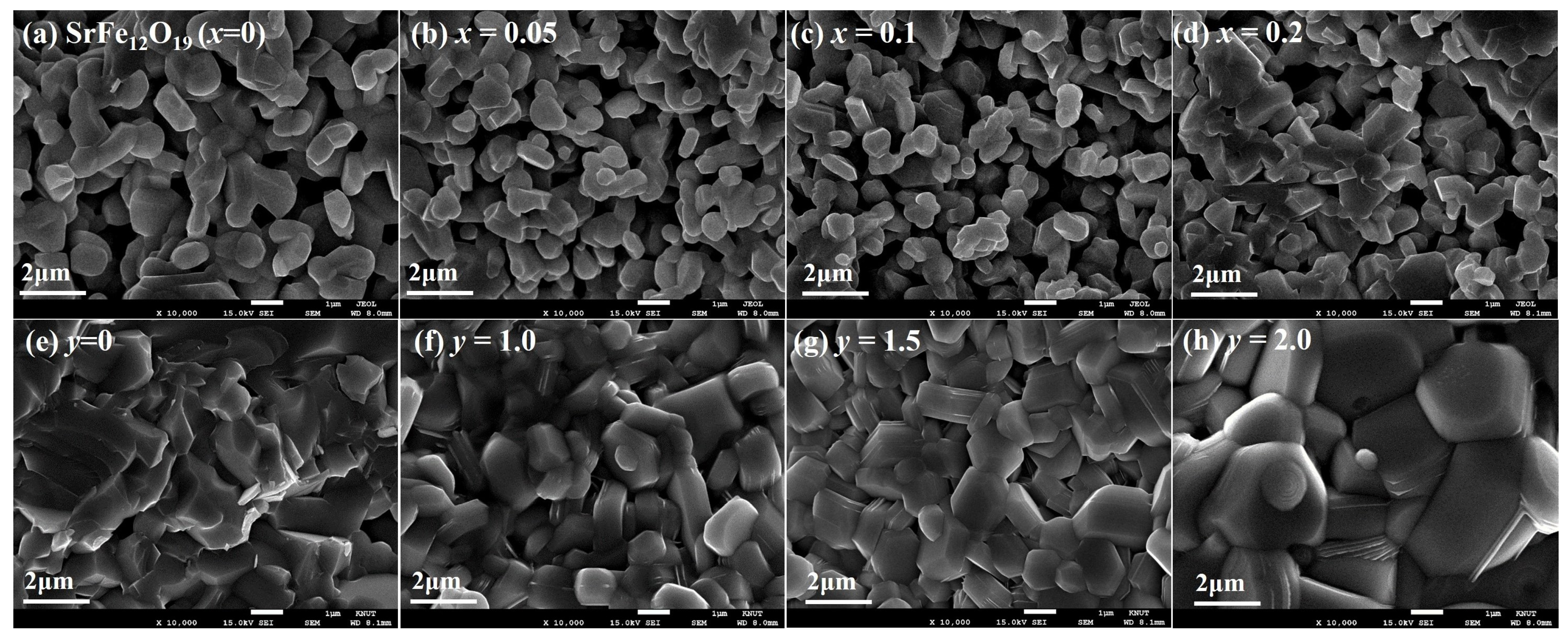
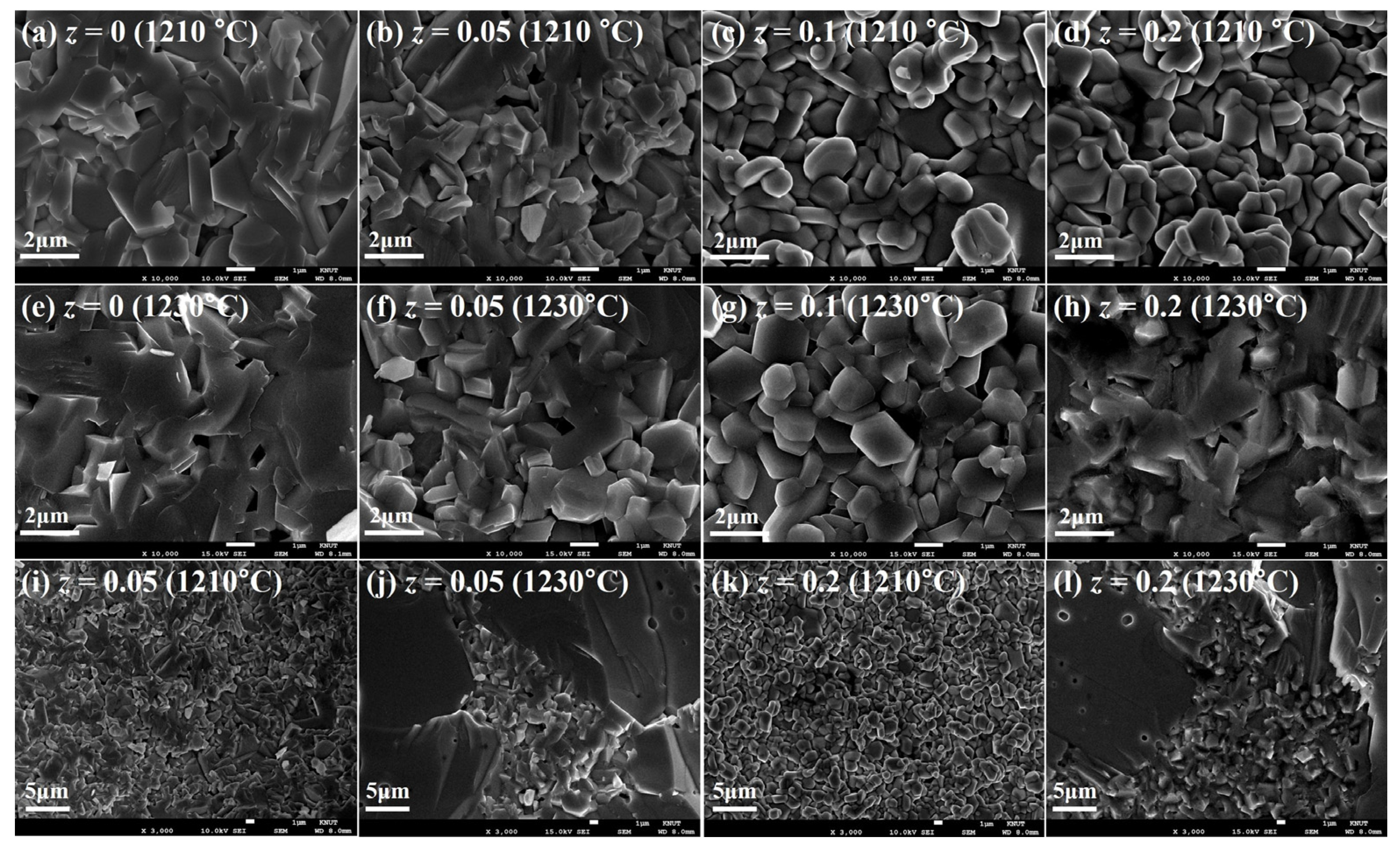
3.1. Crystalline Structure and Microstructure Analysis
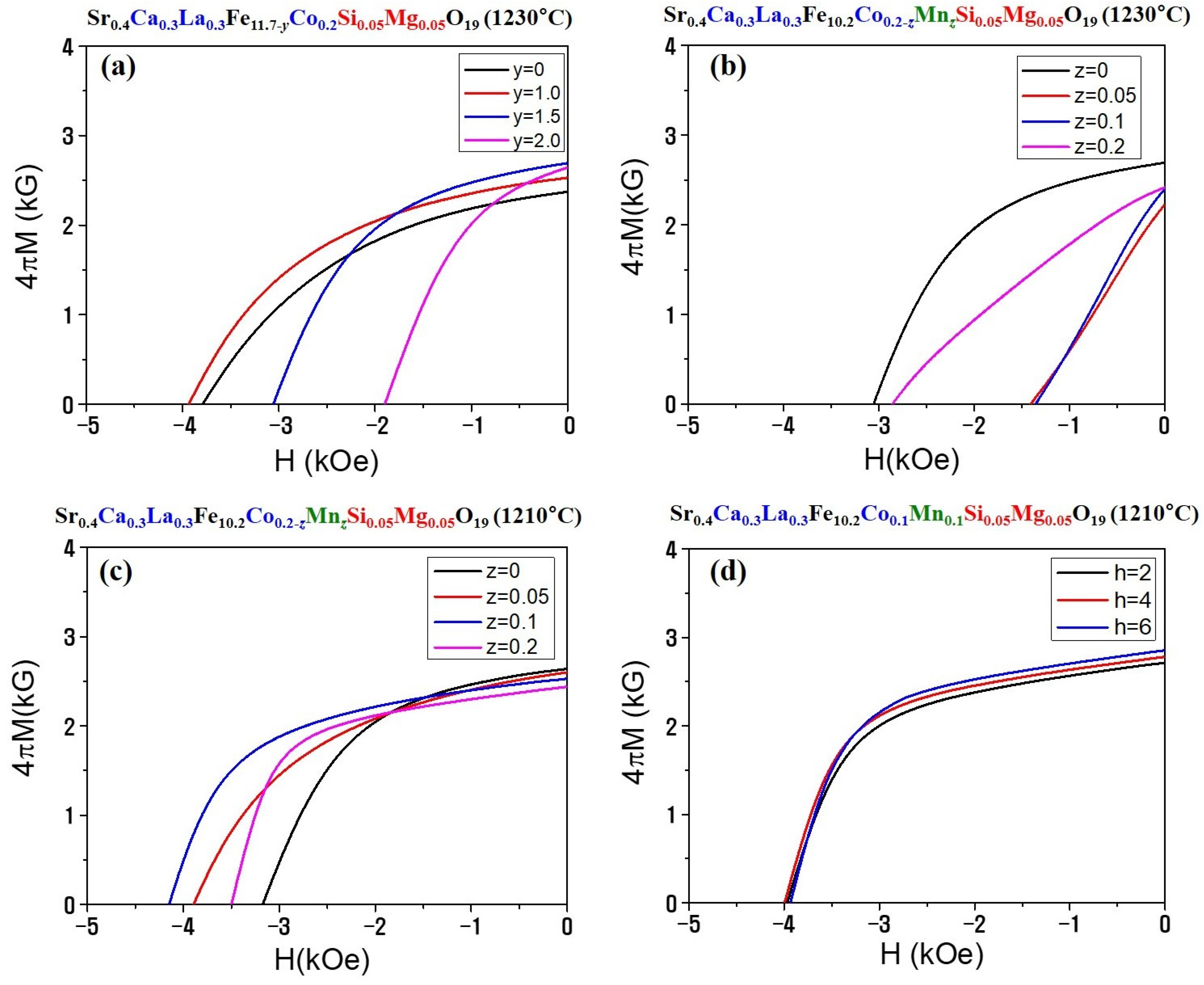
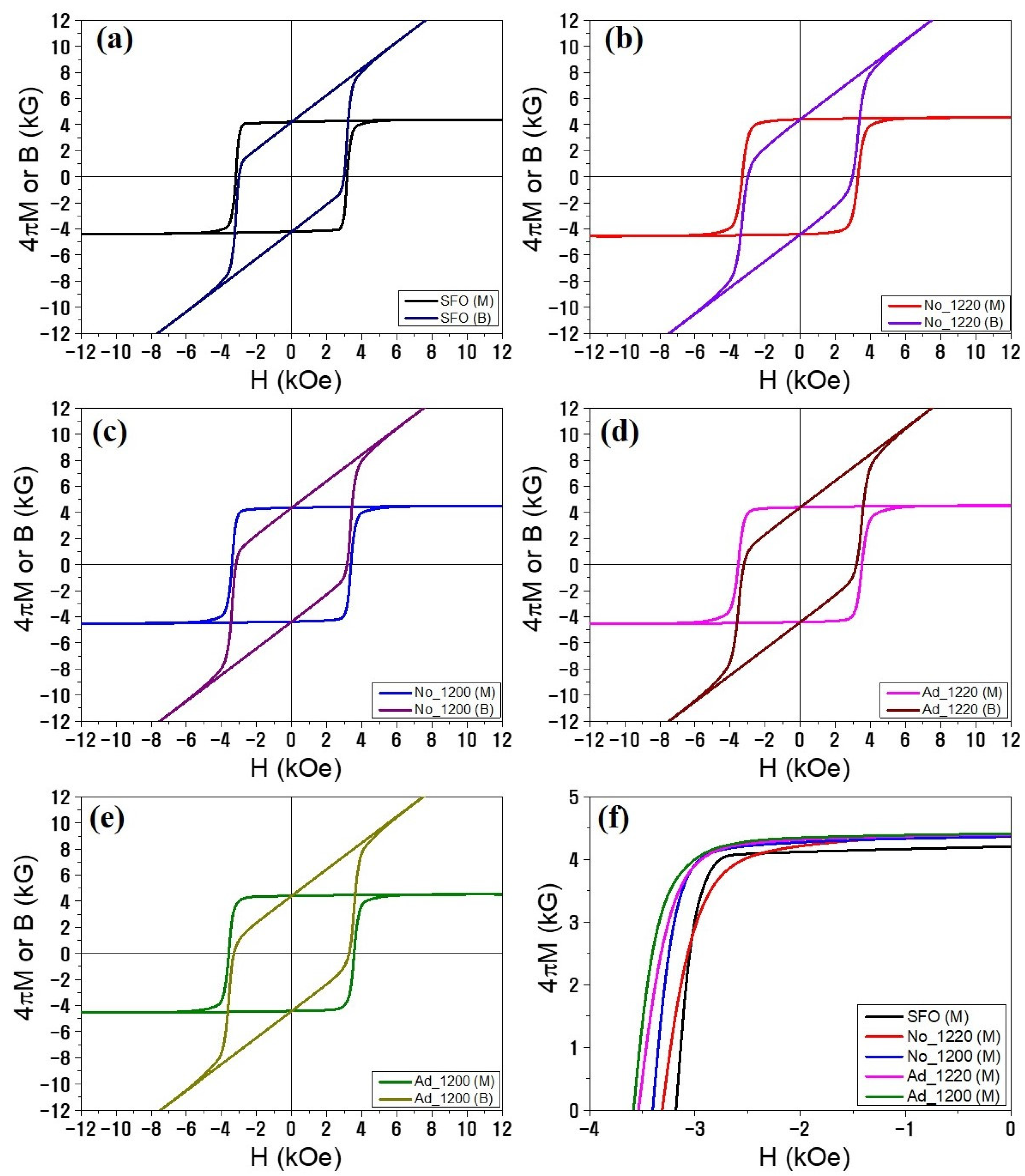
3.2. Magnetic Properties and Magnet Performances
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pullar, R.C. Hexagonal ferrites: A review of the synthesis, properties and applications of hexaferrite ceramics. Prog. Mater. Sci. 2012, 57, 1191–1334. [Google Scholar] [CrossRef]
- Smit, J.; Wijn, H.P.J. Ferrites: Physical Properties of Ferromagnetic Oxides in Relation to Their Technical Applications; Philips Technical Library: Eindhoven, The Netherlands, 1959. [Google Scholar]
- Taguchi, H. Recent improvements of ferrite magnets. Phys. IV Fr. 1997, 7, C1-299–C1-302. [Google Scholar] [CrossRef]
- Bai, J.; Liu, X.; Xie, T.; Wei, F.; Yang, Z. The effects of La–Zn substitution on the magnetic properties of Sr-magnetoplumbite ferrite nano-particles. Mater. Sci. Eng. B 2000, 68, 182–185. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Kwon, Y.-H.; Kim, M.-H.; Lee, D.-Y. Enhancement of magnetic properties in Mn-Zn substituted M-type Sr-hexaferrites. J. Magn. Magn. Mater. 2015, 382, 10–14. [Google Scholar] [CrossRef]
- Kang, Y.-M. High saturation magnetization in La–Ce–Zn–doped M-type Sr-hexaferrites. Ceram. Int. 2015, 41, 4354–4359. [Google Scholar] [CrossRef]
- Moon, K.-S.; Kang, Y.-M. Structural and magnetic properties of Ca-Mn-Zn-substituted M-type Sr-hexaferrites. J. Eur. Ceram. Soc. 2016, 36, 3383–3389. [Google Scholar] [CrossRef]
- Godara, S.K.; sneh; Kaur, V.; Malhi, P.S.; Ahmed, J.; Alshehri, S.M.; Singh, M.; Verma, S.; Singh, C.; Maji, P.K.; et al. Sol-gel auto-combustion synthesis of double metal-doped barium hexaferrite nanoparticles for permanent magnet applications. J. Sol. Stat. Chem. 2022, 312, 123215. [Google Scholar] [CrossRef]
- Kang, Y.-M.; Moon, K.-S. Magnetic properties of Ce-Mn substituted M-type Sr-hexaferrites. Ceram. Inter. 2015, 41, 12828–12834. [Google Scholar] [CrossRef]
- Yasmin, N.; Mirza, M.; Muhammad, S.; Zahid, M.; Ahmad, M.; Awan, M.S.; Muhammad, A. Influence of samarium substitution on the structural and magnetic properties of M-type hexagonal ferrites. J. Magn. Magn. Mater. 2018, 446, 276–281. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, F.; Shao, J.; Huang, D.; He, H.; Trukhanov, A.V.; Trukhanov, S.V. Influence of Nd-NbZn co-substitution on structural, spectral and magnetic properties of M-type calcium-strontium hexaferrites Ca0.4Sr0.6-xNdxFe12.0-x(Nb0.5Zn0.5)xO19. J. Alloy. Compd. 2018, 765, 616–623. [Google Scholar] [CrossRef]
- Huang, K.; Yu, J.; Zhang, L.; Xu, J.; Yang, Z.; Liu, C.; Wang, W.; Kan, X. Structural and magnetic properties of Gd–Zn substituted M-type Ba–Sr hexaferrites by sol-gel auto-combustion method. J. Alloy. Compd. 2019, 803, 971–980. [Google Scholar] [CrossRef]
- Almessiere, M.A.; Slimani, Y.; Güner, S.; van Leusen, J.; Baykal, A.; Kögerler, P. Effect of Nb3+ ion substitution on the magnetic properties of SrFe12O19 hexaferrites. J. Mater. Sci. Mater. Electron. 2019, 30, 11181–11192. [Google Scholar] [CrossRef]
- Todkar, G.B.; Kunale, R.A.; Kamble, R.N.; Batoo, K.M.; Ijaz, M.F.; Imran, A.; Hadi, M.; Raslan, E.H.; Shirsath, S.E.; Kadam, R.H. Ce–Dy substituted barium hexaferrite nanoparticles with large coercivity for permanent magnet and microwave absorber application. J. Phys. D. Appl. Phys. 2021, 54, 294001. [Google Scholar] [CrossRef]
- Shirsath, S.E.; Kadam, R.H.; Batoo, K.M.; Wang, D.; Li, S. Co–Al-substituted strontium hexaferrite for rare earth free permanent magnet and microwave absorber application. J. Phys. D Appl. Phys. 2021, 54, 024001. [Google Scholar] [CrossRef]
- Han, G.; Sui, R.; Yu, Y.; Wang, L.; Li, M.; Li, J.; Liu, H.; Yang, W. Structure and magnetic properties of the porous Al-substituted barium hexaferrites. J. Magn. Magn. Mater. 2021, 528, 167824. [Google Scholar] [CrossRef]
- Manglam, M.K.; Shukla, A.; Mallick, J.; Yadav, M.K.; Kumari, S.; Zope, M.; Kar, M. Enhancement of coercivity of M-type barium hexaferrite by Ho doping. Mater. Today Proc. 2022, 59, 149–152. [Google Scholar] [CrossRef]
- Kools, F.; Morel, A.; Grössinger, R.; Le Breton, J.M.; Tenaud, P. LaCo-substituted ferrite magnets, a new class of high-grade ceramic magnets; intrinsic and microstructural aspects. J. Magn. Magn. Mater. 2002, 242–245, 1270–1276. [Google Scholar] [CrossRef]
- Ogata, Y.; Takami, T.; Kubota, Y. Development of La-Co Substituted Ferrite Magnets. J. Jpn. Soc. Powder Powder Metall. 2003, 50, 636–641. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, Y.; Hosokawa, S.; Oda, E.; Toyota, S. Magnetic properties and composition of Ca-La-Co M-type ferrites. J. Jpn. Soc. Powder Powder Metall. 2008, 55, 541–546. [Google Scholar] [CrossRef] [Green Version]
- Yoo, J.-Y.; Lee, K.-H.; Kang, Y.-M.; Yoo, S.-I. Enhancement of the magnetic properties in Si4+-Li+-substituted M-type hexaferrites for permanent magnets. Appl. Sci. 2022, 12, 12295. [Google Scholar] [CrossRef]
- Moon, K.-S.; Lim, E.-S.; Kang, Y.-M. Effect of Ca and La substitution on the structure and magnetic properties of M-type Sr-hexaferrites. J. Alloy. Compd. 2019, 771, 350–355. [Google Scholar] [CrossRef]
- Yu, P.-Y.; Kim, M.-H.; Kang, Y.-M. Development of a high-performance ferrite magnet fabrication process without sintering additives. Korean J. Met. Mater. 2021, 59, 551–559. [Google Scholar] [CrossRef]
- Moon, K.-S.; Yu, P.-Y.; Kang, Y.-M. Microstructure and magnetic properties of La-Ca-Co substituted M-type Sr-hexaferrites with controlled Si diffusion. Appl. Sci. 2020, 10, 7570. [Google Scholar]
- Cullity, B.D.; Graham, C.D. Introduction to Magnetic Materials, 2nd ed.; Hohn Wiley & Sons: Hoboken, NJ, USA, 2009; p. 13. [Google Scholar]
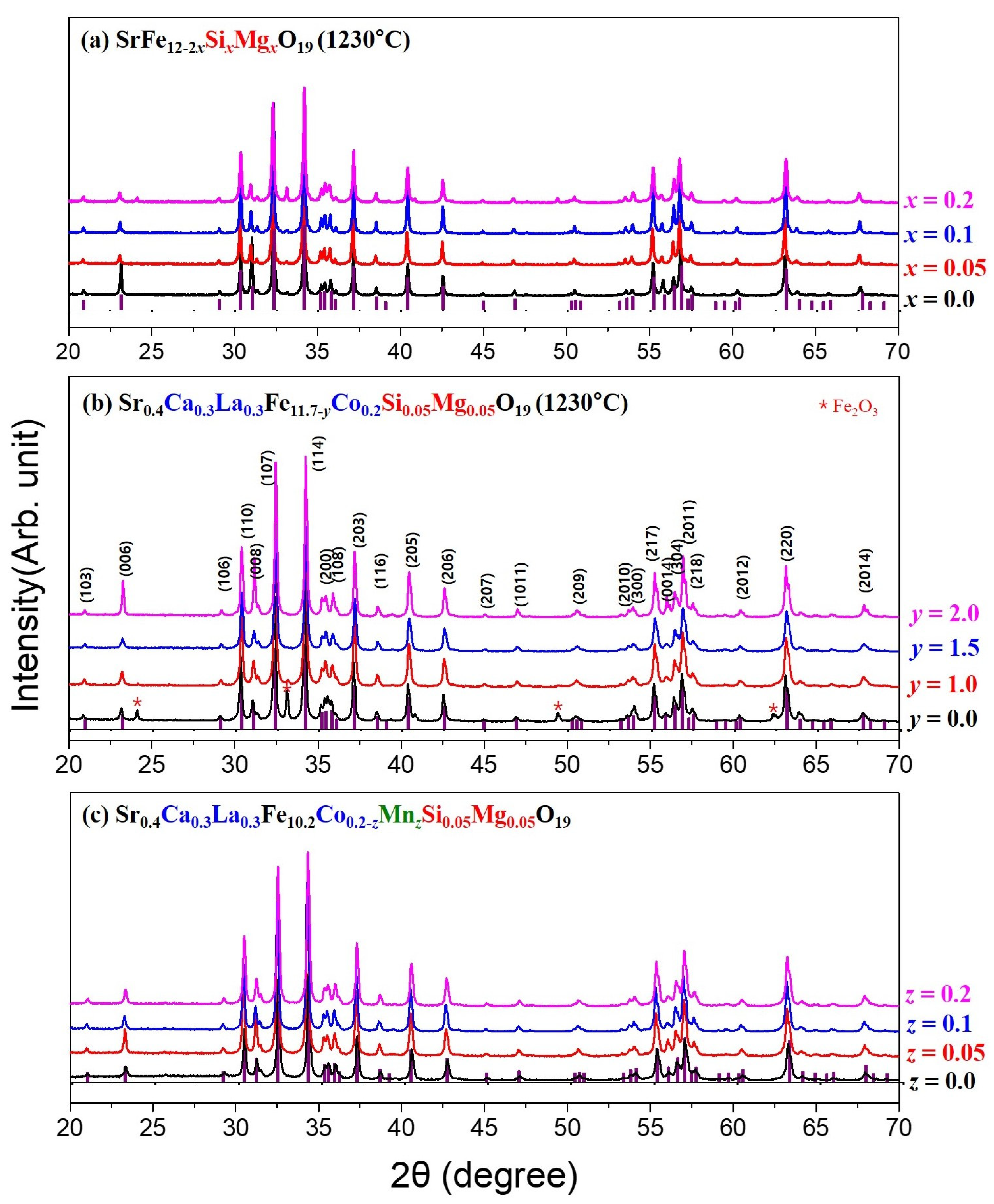
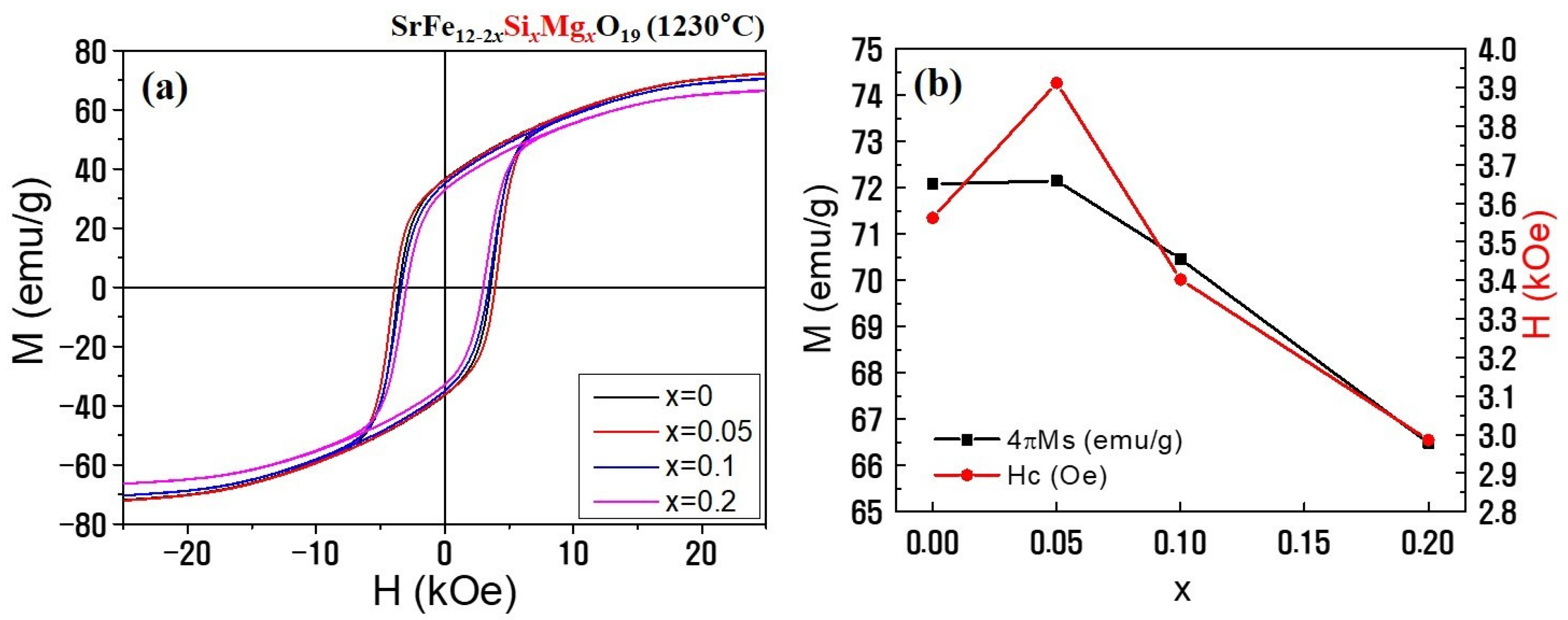

| Cation Composition | x | ρ (g/cm3) | MS (emu/g) | HC (Oe) |
|---|---|---|---|---|
| SrFe12−2xSixMgx | 0.0 | 2.75 | 72.08 | 3562 |
| 0.05 | 2.87 | 72.15 | 3912 | |
| 0.1 | 2.91 | 70.46 | 3402 | |
| 0.2 | 4.07 | 66.49 | 2986 |
| Cation Composition | TS (°C) | y, z, t | ρ (g/cm3) | Br (kG) | HC (kOe) |
|---|---|---|---|---|---|
| Sr0.4Ca0.3La0.3Fe11.7-yCo0.2Si0.05Mg0.05 | 1230 | y = 0.0 | 4.86 | 2.37 | 3.78 |
| 1230 | y = 1.0 | 4.82 | 2.53 | 3.91 | |
| 1230 | y = 1.5 | 4.89 | 2.69 | 3.05 | |
| 1230 | y = 2.0 | 5.00 | 2.64 | 1.89 | |
| Sr0.4Ca0.3La0.3Fe10.2Co0.2−zMnzSi0.05Mg0.05 | 1230 | z = 0.0 | 4.89 | 2.69 | 3.05 |
| 1230 | z = 0.05 | 4.94 | 2.25 | 1.41 | |
| 1230 | z = 1.0 | 4.93 | 2.40 | 1.35 | |
| 1230 | z = 2.0 | 4.76 | 2.42 | 2.85 | |
| Sr0.4Ca0.3La0.3Fe10.2Co0.2−zMnzSi0.05Mg0.05 | 1210 | z = 0 | 4.77 | 2.64 | 3.17 |
| 1210 | z = 0.05 | 4.76 | 2.60 | 3.89 | |
| 1210 | z = 1.0 | 4.73 | 2.53 | 4.15 | |
| 1210 | z = 2.0 | 4.64 | 2.44 | 3.50 | |
| Sr0.4Ca0.3La0.3Fe10.2Co0.1Mn0.1Si0.05Mg0.05 (z = 1.0) | 1210 | t = 2 h | 4.81 | 2.49 | 3.97 |
| 1210 | t = 4 h | 4.88 | 2.53 | 4.00 | |
| 1210 | t = 6 h | 4.92 | 2.60 | 3.95 |
| Magnet ID | Sintering Additives | TS (°C) | ρ (g/cm3) | 4πMS (kG) | iHC (kOe) | bHC (kOe) | Br (kG) | BHmax (M·G·Oe) |
|---|---|---|---|---|---|---|---|---|
| SFO | Optimized | 1230 | 4.92 | 4.39 | 3.18 | 3.00 | 4.21 | 4.24 |
| No_1220 | No additives | 1220 | 5.04 | 4.55 | 3.31 | 2.89 | 4.41 | 4.44 |
| No_1200 | 1200 | 5.01 | 4.51 | 3.40 | 3.17 | 4.37 | 4.55 | |
| Ad_1220 | SiO2 0.4wt% + CaCO3 0.8wt% | 1220 | 5.00 | 4.54 | 3.53 | 3.21 | 4.40 | 4.64 |
| Ad_1200 | 1200 | 5.01 | 4.54 | 3.57 | 3.29 | 4.42 | 4.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lim, J.-P.; Kang, M.-G.; Kang, Y.-M. Development of Multi-Cation-Doped M-Type Hexaferrite Permanent Magnets. Appl. Sci. 2023, 13, 295. https://doi.org/10.3390/app13010295
Lim J-P, Kang M-G, Kang Y-M. Development of Multi-Cation-Doped M-Type Hexaferrite Permanent Magnets. Applied Sciences. 2023; 13(1):295. https://doi.org/10.3390/app13010295
Chicago/Turabian StyleLim, Jun-Pyo, Min-Gu Kang, and Young-Min Kang. 2023. "Development of Multi-Cation-Doped M-Type Hexaferrite Permanent Magnets" Applied Sciences 13, no. 1: 295. https://doi.org/10.3390/app13010295






