Lithium Metal: The Key to Green Transportation
Abstract
:1. Introduction
1.1. Impact of Fossil Fuels on the Climate
1.2. Lithium Properties and General Applications
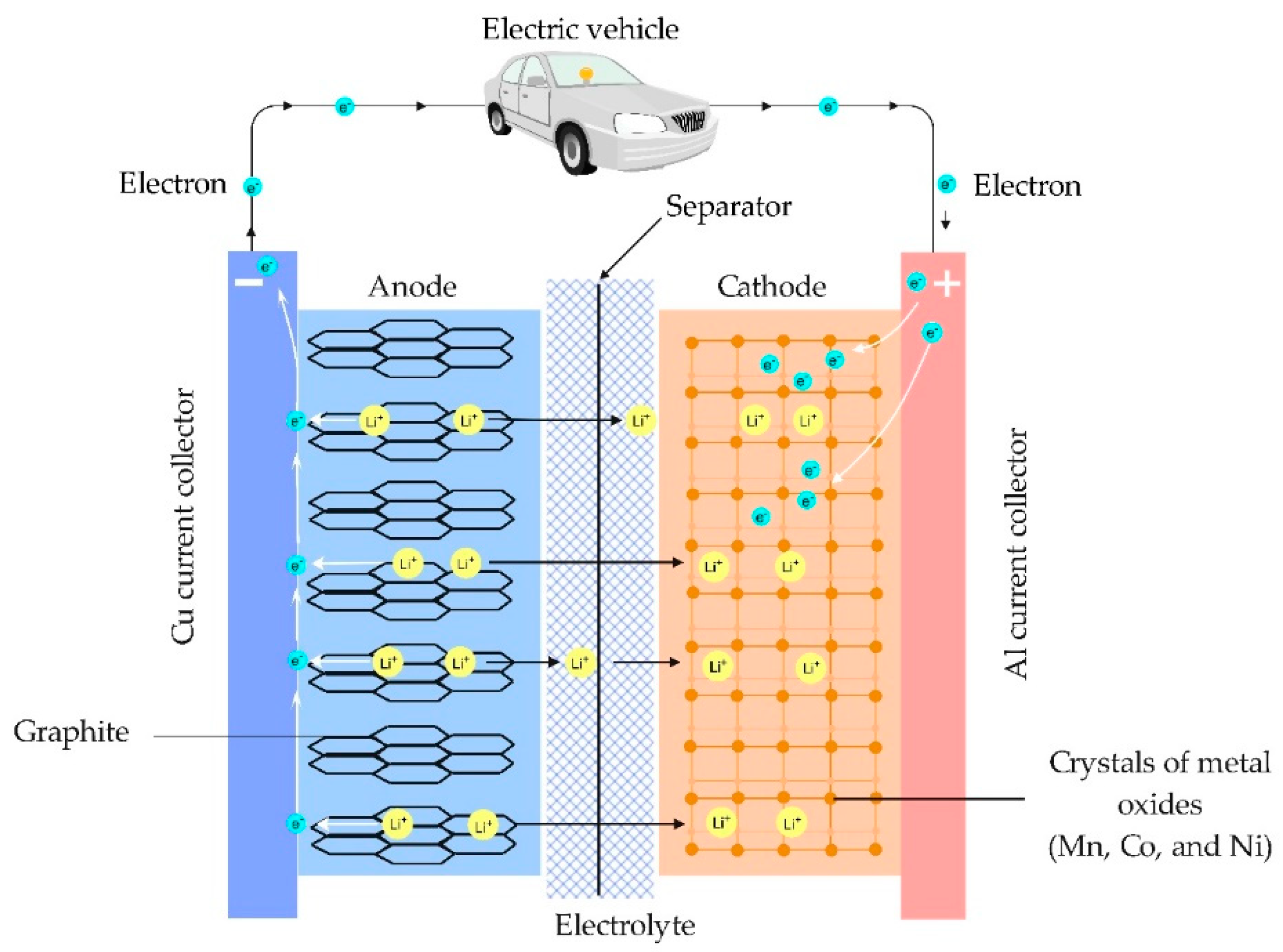
1.3. Properties of Lithium towards Lithium-Ion Battery
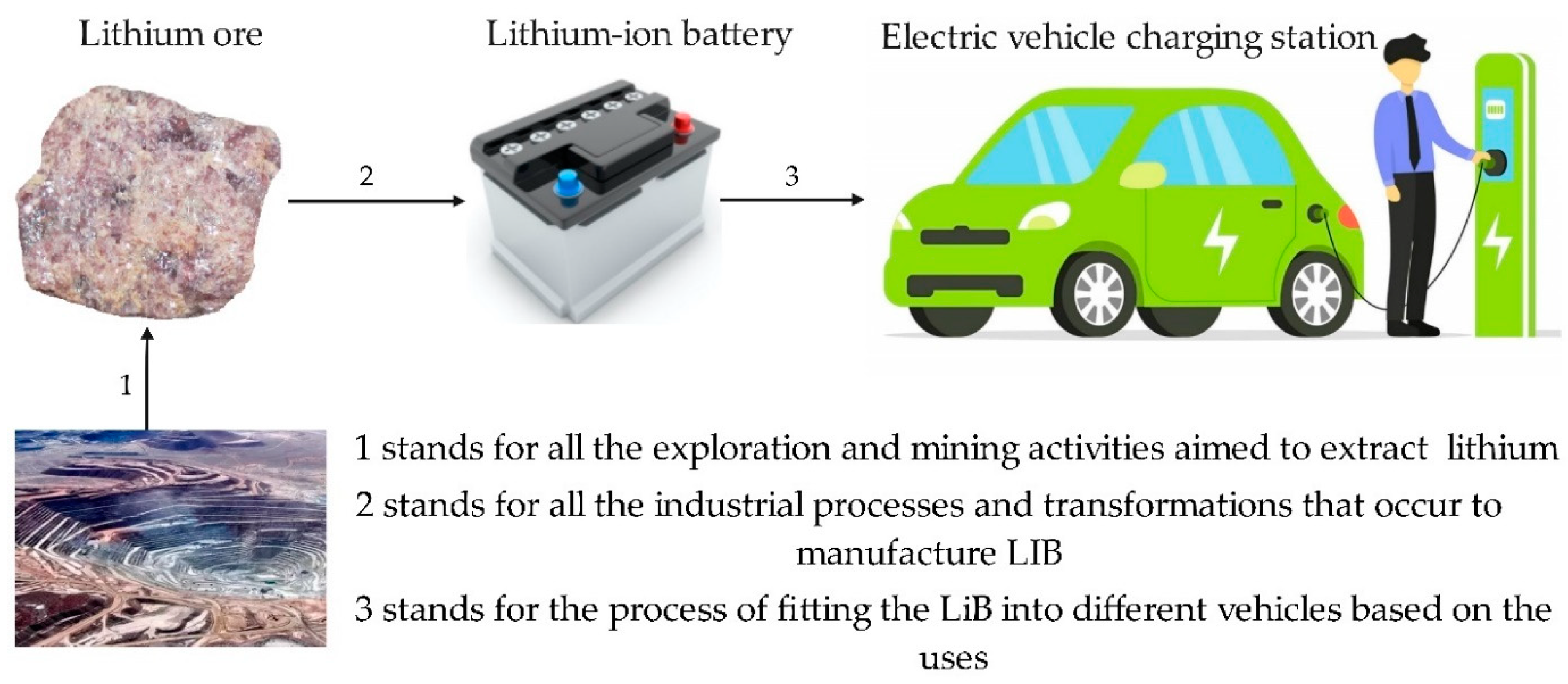
1.4. Lithium in Electric Vehicles
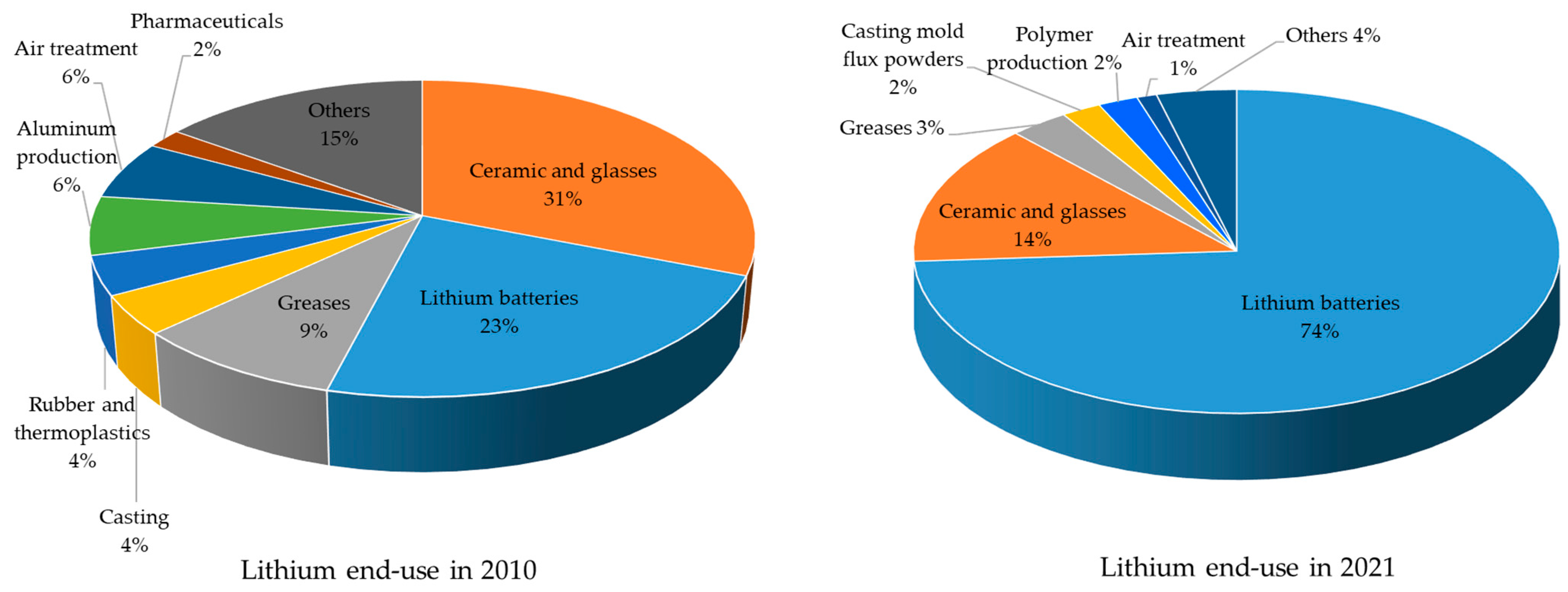
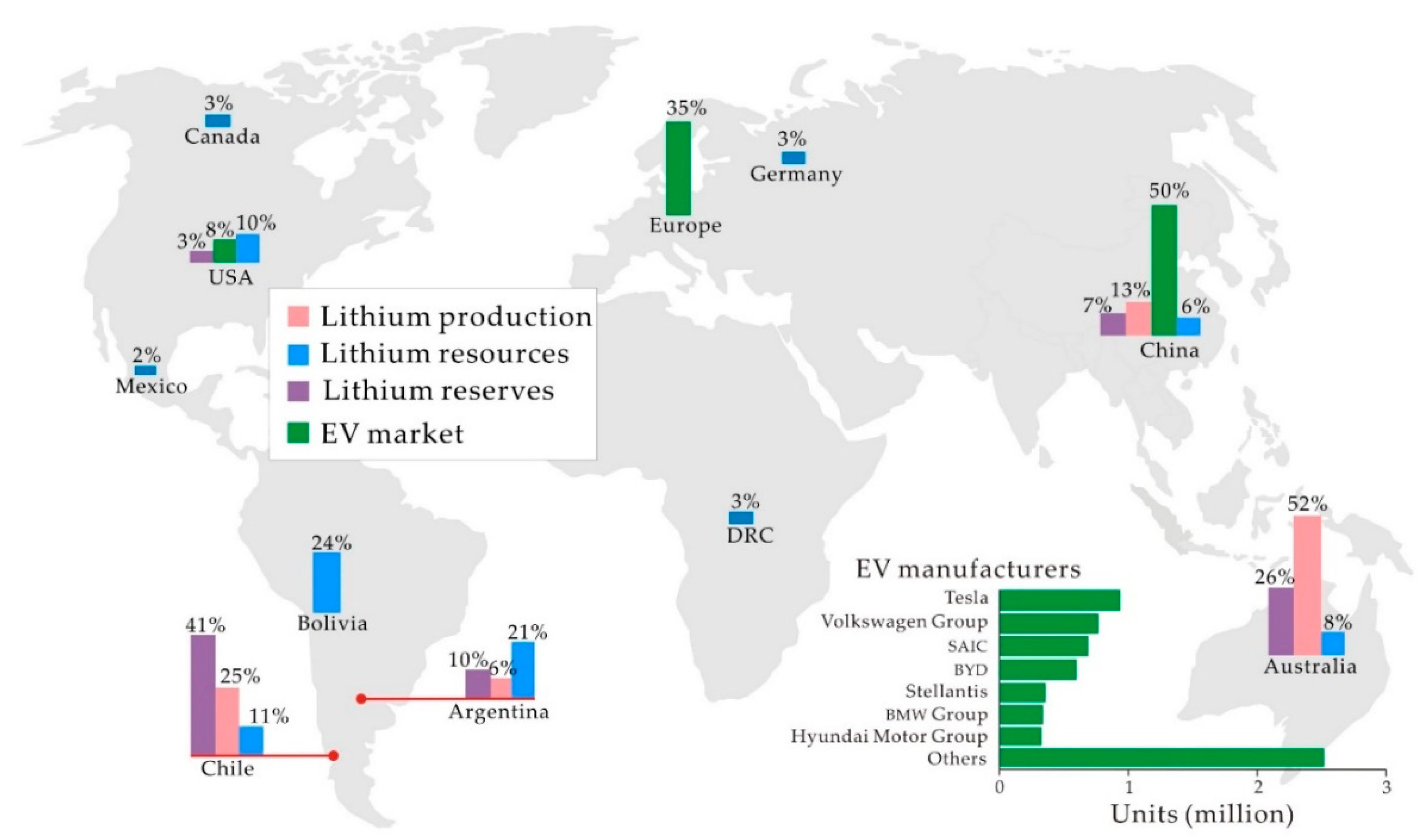
1.5. Lithium Sources
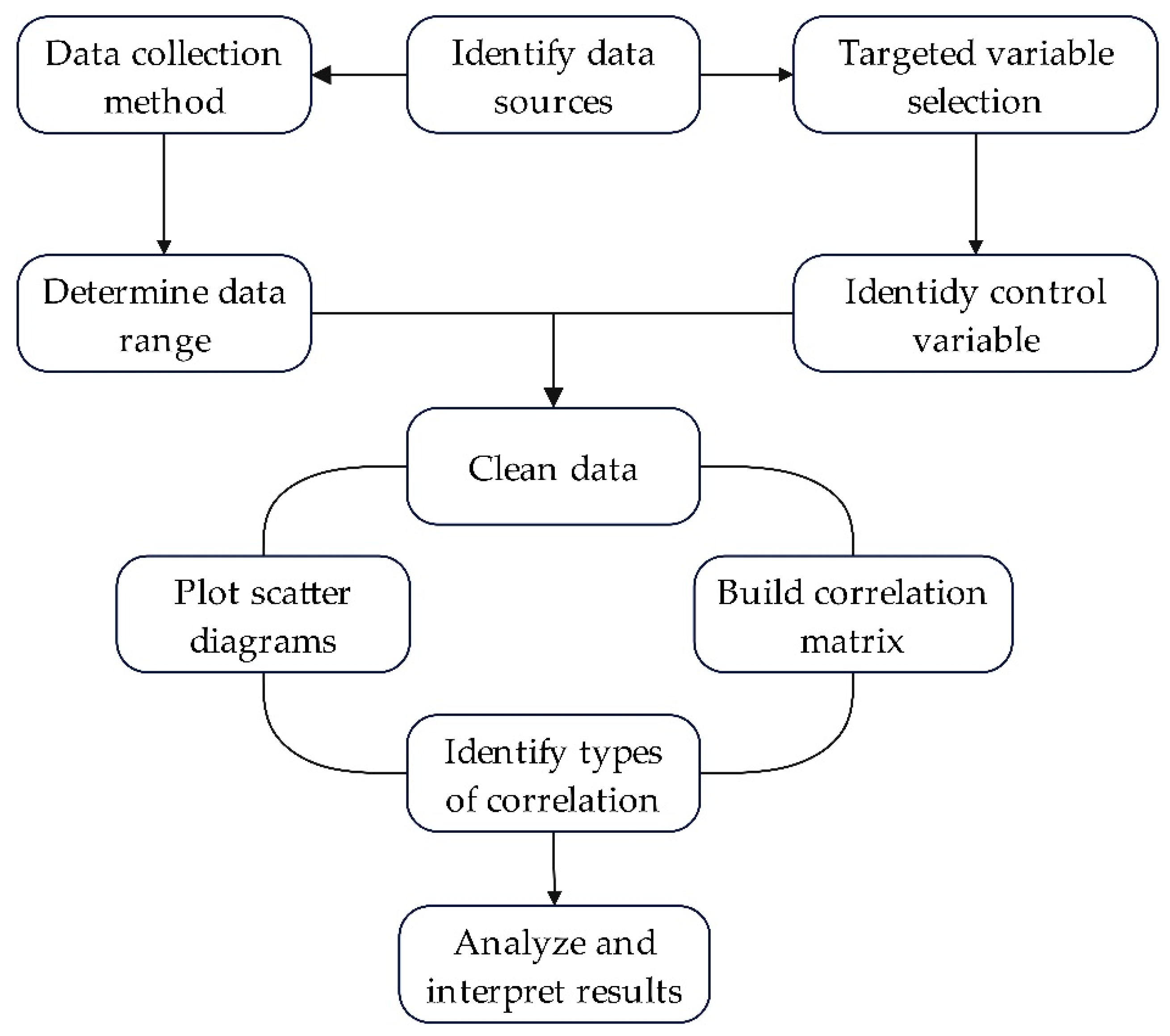
2. Data and Methods
| Algorithm 1. Global impact of electric vehicle market demand on lithium production | ||||||
| Let n be the number of EV in EV stock and C be the threshold value for allowed CO2 | ||||||
| Input:n, EV market demand | ||||||
| Output: Correlation between EV sales, EV stock, LIB production, CO2, CS and LRM | ||||||
| 1 | Initialization of variables: assign n to EV stock | |||||
| 2 | Procedure(EV market demand) | |||||
| 3 | EV market demand → EV sales // | |||||
| 4 | EV sales → EV stock | |||||
| 5 | while (Li resources! = Ø)do: | |||||
| 6 | for eachyeardo: | |||||
| 7 | measure CO2 | |||||
| 8 | check n in EV stock | |||||
| 9 | if n ≤ minimumANDCO2 < Cdo: | |||||
| 10 | extractLRM | |||||
| 11 | produceLIB | |||||
| 12 | ifEV demand > EV stockdo: | |||||
| 13 | increase charging stations | |||||
| 14 | end if | |||||
| 15 | end if | |||||
| 16 | end for | |||||
| 17 | end while | |||||
| 18 | End procedure | |||||
3. Results
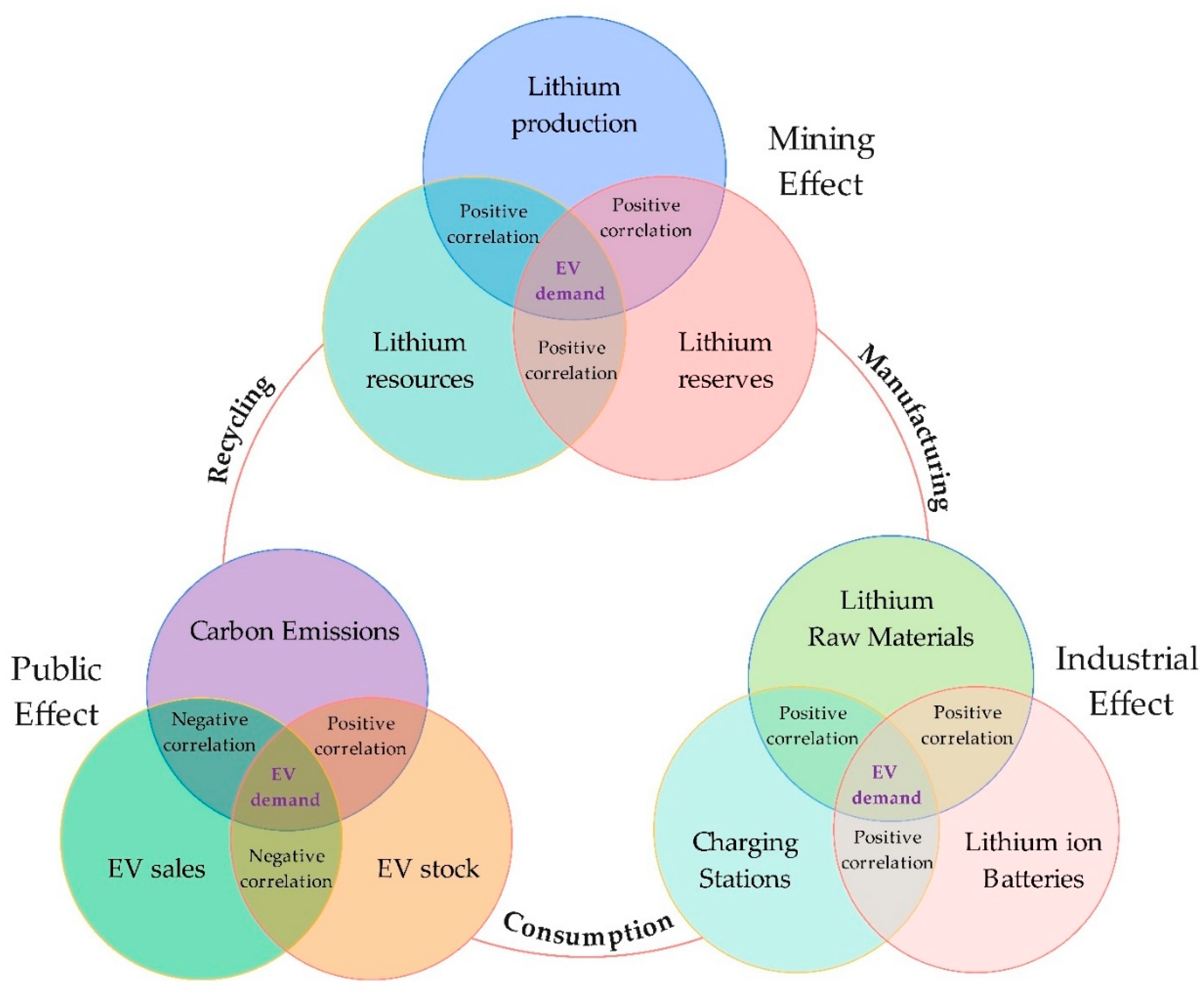
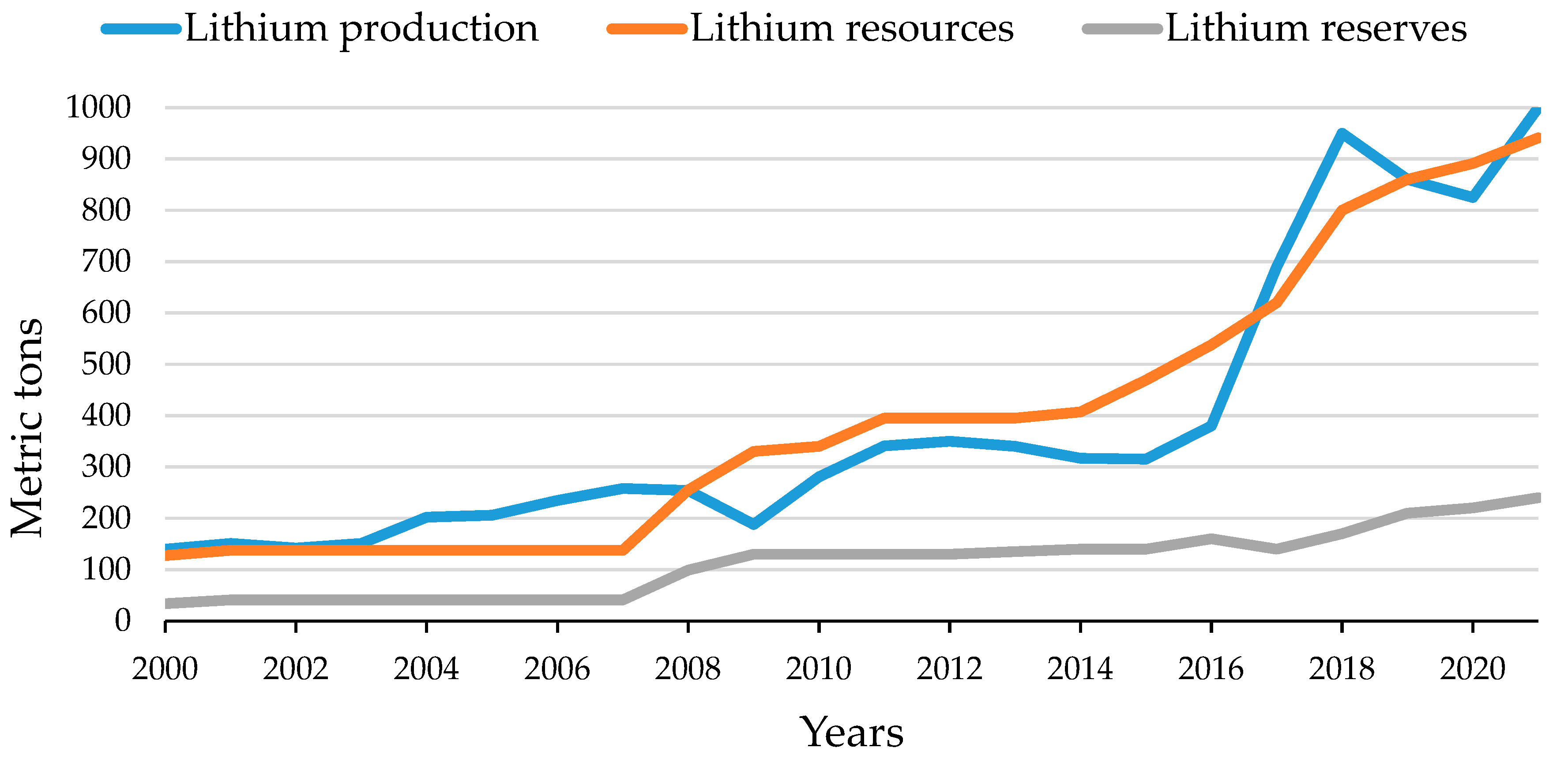
3.1. First Group: “Mining Effect”
3.2. Second Group: “Industrial Effect”
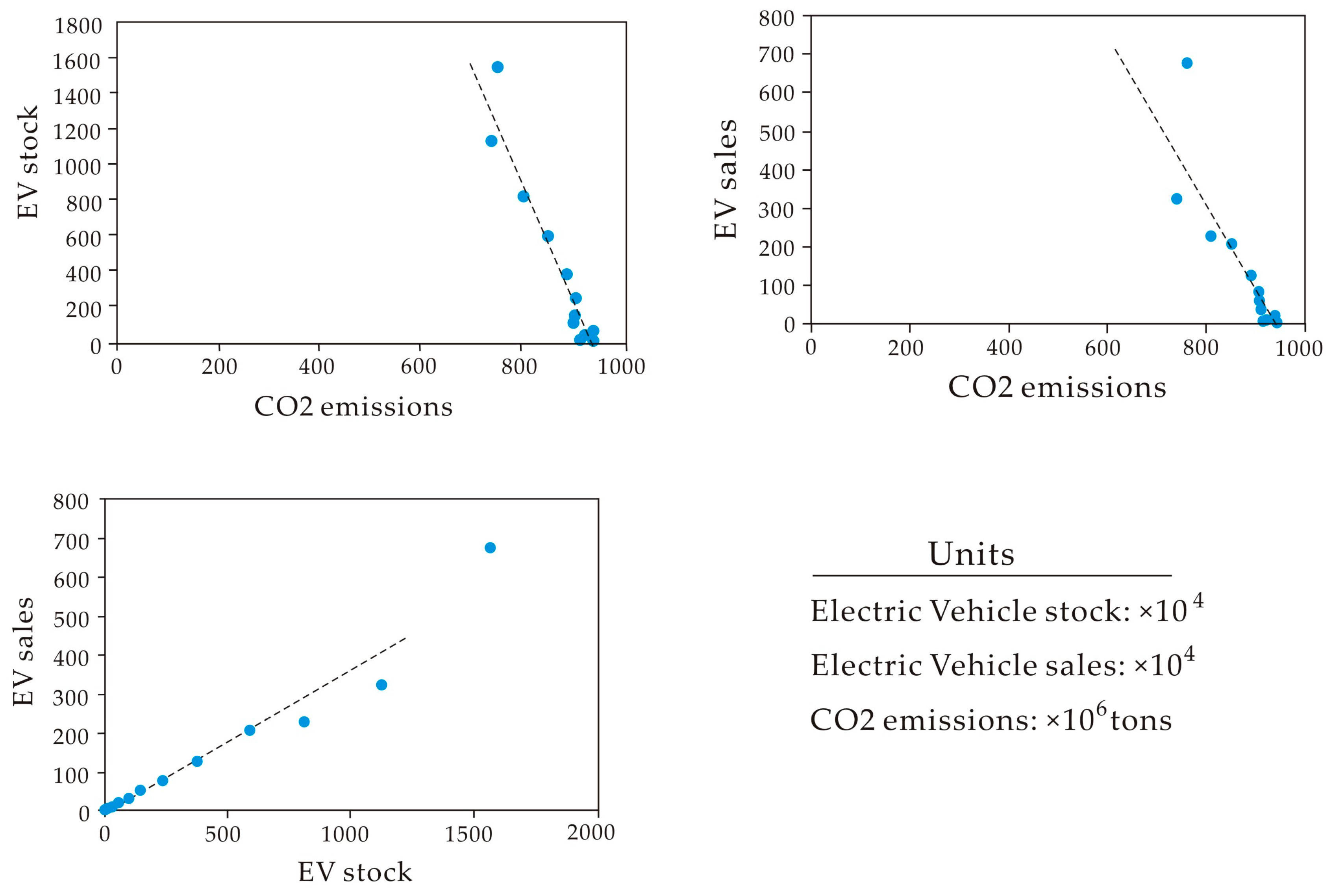
3.3. Third Group: “Public Effect”
4. Discussion
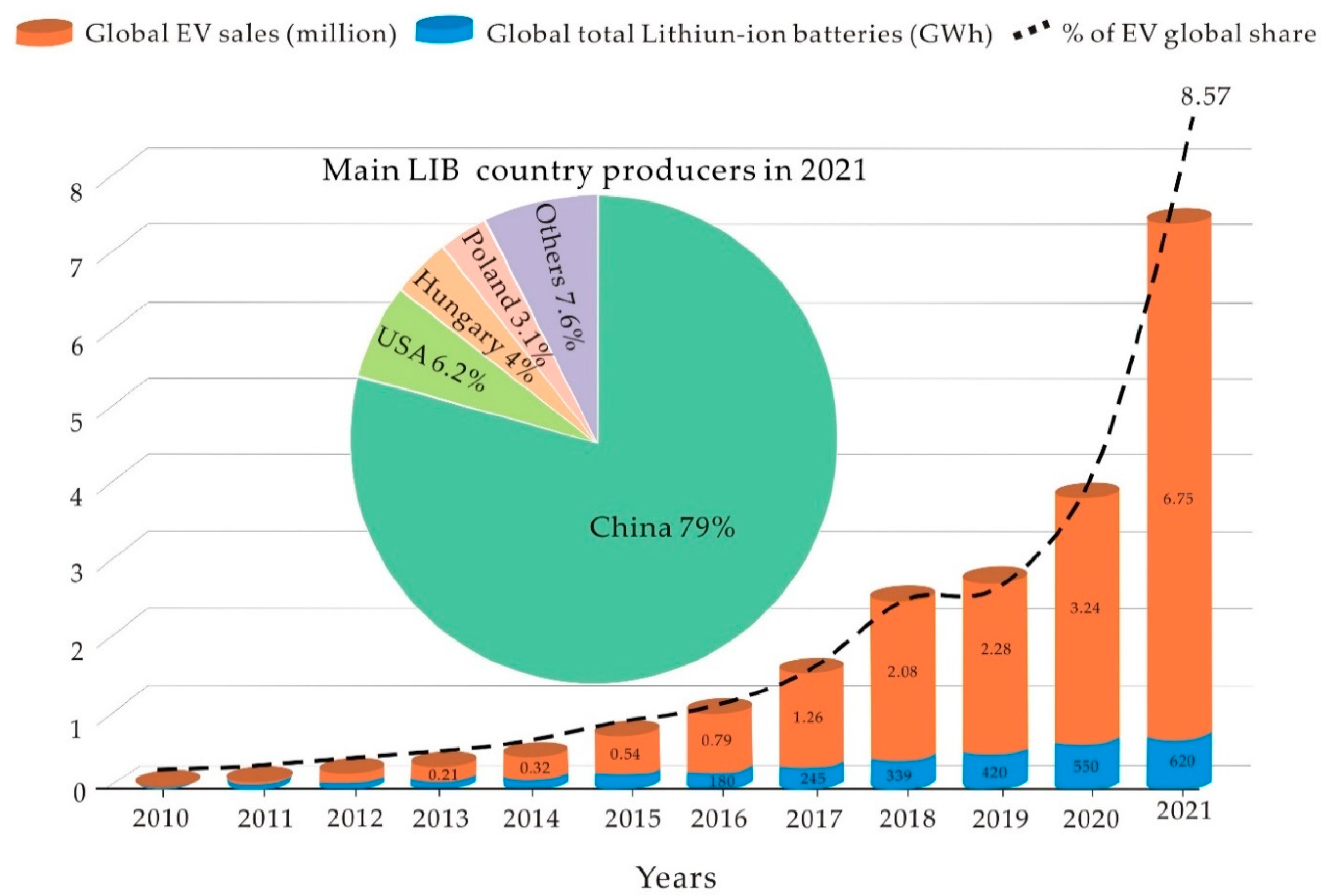
4.1. Manufacturing of Lithium-Ion Battery and Demand
4.2. Advantages of Lithium-Ion Batteries
4.3. Lithium and Electric Vehicles Market
4.4. Electric Vehicles vs. Total Vehicle Vehicles
4.5. Lithium Availability and Demand
4.6. Demand for the Two and Three-Wheelers
4.7. LIB Swapping Stations
4.8. Lithium Recycling
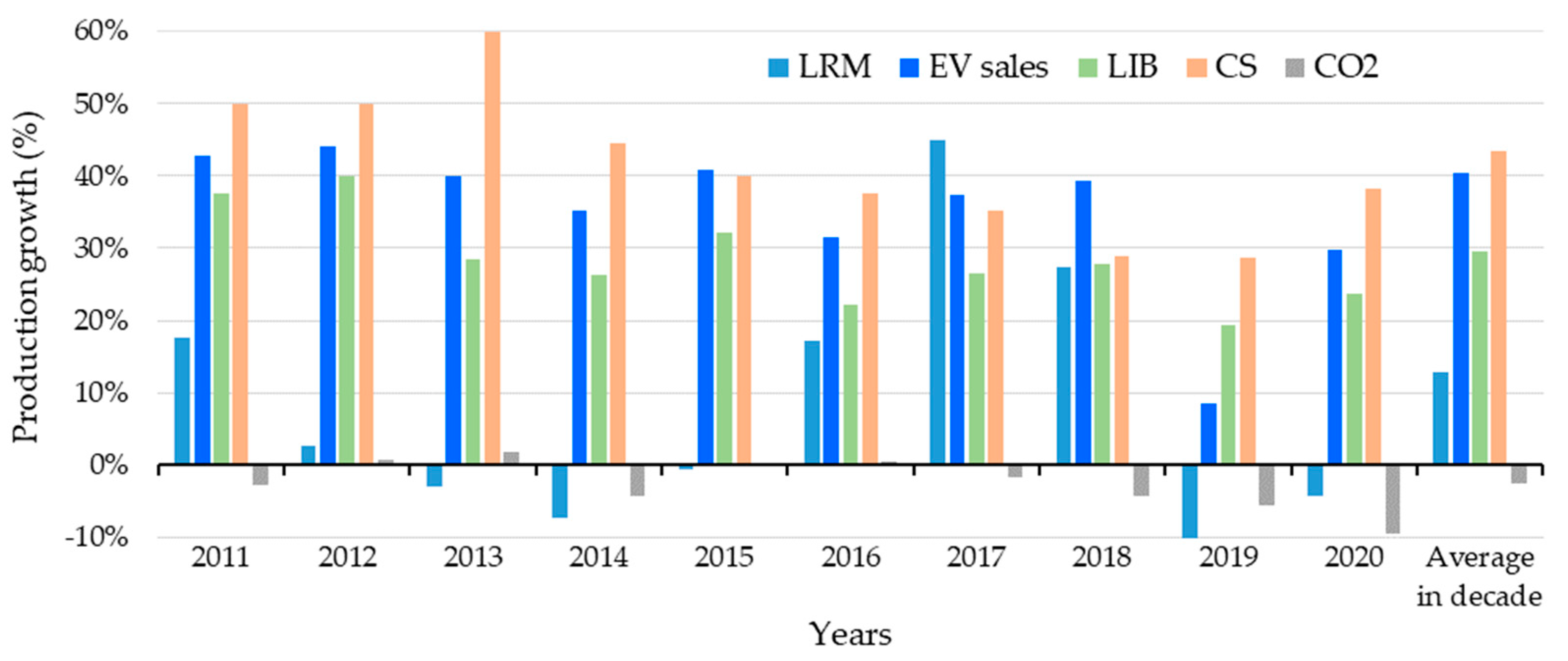
4.9. Production Growth
4.10. Challenges Linked to LIB
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Goodenough, J.B.; Park, K. The Li-Ion Rechargeable Battery: A Perspective. J. Am. Chem. Soc. 2013, 135, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- United Nations. United Nations Framework Convention on Climate Change. Available online: https://unfccc.int/process-and-meetings (accessed on 15 September 2022).
- Tabelin, C.B.; Dallas, J.; Casanova, S.; Pelech, T.; Bournival, G.; Saydam, S.; Canbulat, I. Towards a low-carbon society: A review of lithium resource availability, challenges and innovations in mining, extraction and recycling, and future perspectives. Miner. Eng. 2021, 163, 106743. [Google Scholar] [CrossRef]
- Lindagato, P.; Li, Y.; Yang, G. Save the giants: Demand beyond production capacity of tantalum raw materials. Miner. Econ. 2022. [Google Scholar] [CrossRef]
- Winter, M.; Brodd, R.J. What Are Batteries, Fuel Cells, and Supercapacitors? Am. Chem. Soc. 2004, 104, 4245–4269. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Kim, K.C.; Lee, B.; Chen, Z.; Noda, S.; Jang, S.S.; Lee, S.W. Self-polymerized dopamine as an organic cathode for Li- and Na-ion batteries. Energy Environ. Sci. 2017, 10, 205–215. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.G.; Liu, H.; Liu, J.; Qiao, S.; Lu, G.M.; Munroe, P. Mesoporous LiFePO 4/C Nanocomposite Cathode Materials for High Power Lithium Ion Batteries with Superior Performance. Adv. Mater. 2010, 22, 4944–4948. [Google Scholar] [CrossRef]
- Yoo, E.; Kim, J.; Hosono, E.; Zhou, H.; Kudo, T.; Honma, I. Large Reversible Li Storage of Graphene Nanosheet Families for Use in Rechargeable Lithium Ion Batteries. Nano Lett. 2008, 8, 2277–2282. [Google Scholar] [CrossRef]
- Wu, Z.; Sun, Y.; Tan, Y.; Yang, S.; Feng, X. Three-Dimensional Graphene-Based Macro- and Mesoporous Frameworks for High-Performance Electrochemical Capacitive Energy Storage. J. Am. Chem. Soc. 2012, 134, 19532–19535. [Google Scholar] [CrossRef]
- Wu, Z.; Parvez, K.; Feng, X.; Mu, K. Graphene-based in-plane micro-supercapacitors with high power and energy densities. Nat. Commun. 2013, 4, 2487. [Google Scholar] [CrossRef] [Green Version]
- Zhong, C.; Deng, Y.; Hu, W.; Qiao, J. A review of electrolyte materials and compositions for electrochemical supercapacitors. Chem. Soc. Rev. 2015, 44, 7484–7539. [Google Scholar] [CrossRef]
- Choi, B.G.; Yang, M.; Hong, W.H.; Choi, J.W.; Huh, Y.S. 3D Macroporous Graphene Frameworks for Supercapacitors with High Energy and Power Densities. ACS Nano 2012, 6, 4020–4028. [Google Scholar] [CrossRef] [PubMed]
- Fetcenko, M.A.; Ovshinsky, S.R.; Reichman, B.; Young, K.; Fierro, C.; Koch, J.; Zallen, A.; Mays, W.; Ouchi, T. Recent advances in NiMH battery technology. J. Power Sources 2007, 165, 544–551. [Google Scholar] [CrossRef]
- Ovshinsky, S.R.; Fetcenko, M.A.; Ross, J. A Nickel Metal Hydride Battery for Electric Vehicles. Science 1993, 260, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Sood, P.; Kim, K.C.; Jang, S.S. Electrochemical Properties of Boron-Doped Fullerene Derivatives for Lithium-Ion Battery Applications. ChemPhysChem 2018, 19, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Kushnir, D.; Sandén, B.A. The time dimension and lithium resource constraints for electric vehicles. Resour. Policy 2012, 37, 93–103. [Google Scholar] [CrossRef]
- Kosai, S.; Takata, U.; Yamasue, E. Natural resource use of a traction lithium-ion battery production based on land disturbances through mining activities. J. Clean. Prod. 2021, 280, 124871. [Google Scholar] [CrossRef]
- Vikström, H.; Davidsson, S.; Höök, M. Lithium availability and future production outlooks. Appl. Energy 2013, 110, 252–266. [Google Scholar] [CrossRef] [Green Version]
- Yin, W.; Grimaud, A.; Lepoivre, F.; Yang, C.; Tarascon, J.M. Chemical vs. Electrochemical Formation of Li2CO3 as a Discharge Product in Li-O2/CO2 Batteries by Controlling the Superoxide Intermediate. J. Phys. Chem. Lett. 2017, 8, 214–222. [Google Scholar] [CrossRef]
- Tarascon, J.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367. [Google Scholar] [CrossRef]
- Van Noorden, R. The rechargeable revolution: A better battery. Nature 2014, 507, 26–28. [Google Scholar] [CrossRef]
- Miedema, J.H.; Moll, H.C. Lithium availability in the EU27 for battery-driven vehicles: The impact of recycling and substitution on the confrontation between supply and demand until2050. Resour. Policy 2013, 38, 204–211. [Google Scholar] [CrossRef]
- Xie, F.; Czogalla, O.; Naumann, S. Lithium battery model development and application in simulation of the energy consumption of electric bus running. IFAC-PapersOnLine 2020, 53, 14230–14235. [Google Scholar] [CrossRef]
- Jaskula, B.W. Mineral Commodity Summary: Lithium; U.S. Geological Survey: Washington, DC, USA, 2017; pp. 100–101. [CrossRef]
- Zeng, X.; Li, J.; Liu, L. Solving spent lithium-ion battery problems in China: Opportunities and challenges. Renew. Sustain. Energy Rev. 2015, 52, 1759–1767. [Google Scholar] [CrossRef]
- Talens Peiró, L.; Villalba Méndez, G.; Ayres, R.U. Lithium: Sources, production, uses, and recovery outlook. JOM 2013, 65, 986–996. [Google Scholar] [CrossRef] [Green Version]
- Gates, B. How to Avoid a Climate Disaster: The Solutions We Have and the Breakthroughs We Need, 1st ed.; Alfred, A. Knopf: New York, NY, USA; Toronto, Canada, 2021; ISBN 9780385546140. [Google Scholar]
- Gil-Alana, L.A.; Monge, M. Lithium: Production and estimated consumption. Evidence of persistence. Resour. Policy 2019, 60, 198–202. [Google Scholar] [CrossRef]
- Jahangir, H.; Golkar, M.A.; Ahmadian, A.; Elkamel, A. Why Electric Vehicles? In Electric Vehicles in Energy Systems: Modelling, Integration, Analysis, and Optimization; Ahmadian, A., Elkamel, A., Behnam, M., Eds.; Springer Nature: Gewerbestrasse, Switzerland, 2020; p. 392. ISBN 9783030344481. [Google Scholar]
- Birol, F. World Energy Outlook; Zoerł, W., Ed.; Global Energy Trends: Paris, France, 2010; ISBN 978 92 64 08624 1. [Google Scholar]
- Zeng, W.; Miwa, T.; Morikawa, T. Prediction of vehicle CO2 emission and its application to eco-routing navigation. Transp. Res. Part C 2016, 68, 194–214. [Google Scholar] [CrossRef]
- EPA. Impacts of Climate Change. Available online: https://www.epa.gov/climatechange-science/impacts-climate-change (accessed on 8 December 2022).
- International Energy Agency. World Energy Outlook; U.S. Geological Survey: Reston, VA, USA, 2013. [Google Scholar]
- Wu, Y.; Shi, X.; Hu, C. Per capita CO2 emissions divergence influenced by bilateral trade with china under the belt and road initiative. Sustain. Prod. Consum. 2021, 27, 1589–1601. [Google Scholar] [CrossRef]
- Ritchie, H. Global CO2 Emisions from Transport. Available online: https://ourworldindata.org/co2-emissions-from-transport (accessed on 15 October 2022).
- Tiseo, I. Distribution of Carbon Dioxide Emissions Produced by the Transportation Sector Worldwide in 2020, by Subsector. Available online: https://www.statista.com/statistics/1185535/transport-carbon-dioxide-emissions-breakdown/ (accessed on 15 October 2022).
- Xiao, H.; Zhou, Y.; Zhang, N.; Wang, D.; Shan, Y.; Ren, J. CO2 emission reduction potential in China from combined effects of structural adjustment of economy and efficiency improvement. Resour. Conserv. Recycl. 2021, 174, 105760. [Google Scholar] [CrossRef]
- Khaligh, A.; Li, Z. Battery, ultracapacitor, fuel cell, and hybrid energy storage systems for electric, hybrid electric, fuel cell, and plug-in hybrid electric vehicles: State of the art. IEEE Trans. Veh. Technol. 2010, 59, 2806–2814. [Google Scholar] [CrossRef]
- Sun, X.; Hao, H.; Zhao, F.; Liu, Z. Tracing global lithium flow: A trade-linked material flow analysis. Resour. Conserv. Recycl. 2017, 124, 50–61. [Google Scholar] [CrossRef]
- Martin, G.; Rentsch, L.; Höck, M.; Bertau, M. Lithium market research—Global supply, future demand and price development. Energy Storage Mater. 2017, 6, 171–179. [Google Scholar] [CrossRef]
- Jaskula, B.W. Mineral Commodity Summary: Lithium; U.S. Geological Survey: Washington, DC, USA, 2022; pp. 100–101. [CrossRef]
- Jaskula, B.W. Mineral Commodity Summary: Lithium; U.S. Geological Survey: Washington, DC, USA, 2011; pp. 94–95. [CrossRef]
- Boxall, N.J.; King, S.; Cheng, K.Y.; Gumulya, Y.; Bruckard, W.; Kaksonen, A.H. Urban mining of lithium-ion batteries in Australia: Current state and future trends. Miner. Eng. 2018, 128, 45–55. [Google Scholar] [CrossRef]
- Kahl, M.; Pavón, S.; Bertau, M. Recycling of Primary Lithium Batteries Production Residues. ChemPhysChem 2021, 22, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Jeppson, D.W.; Ballif, J.L.; Yuan, W.W.; Chou, B. Lithium Literature Review: Lithium’s Properties and Interactions; Engineering Development Laboratory: Richland, WA, USA, 1978. [Google Scholar]
- Gratz, E.; Sa, Q.; Apelian, D.; Wang, Y. A closed loop process for recycling spent lithium ion batteries. J. Power Sources 2014, 262, 255–262. [Google Scholar] [CrossRef]
- Reddy, M.V.; Mauger, A.; Julien, C.M.; Paolella, A.; Zaghib, K. Brief history of early lithium-battery development. Materials 2020, 13, 1884. [Google Scholar] [CrossRef] [Green Version]
- Nelson, P.A.; Webster, W.H.; Shimotake, H. Batteries for Electric Vehicles. New Mater. New Process. 2019, 2, 362–364. [Google Scholar] [CrossRef] [Green Version]
- Munk, L.A.; Hynek, S.A.; Bradley, D.C.; Boutt, D.; Labay, K.; Jochens, H. Lithium Brines: A Global Perspective. In Rare Earth and Critical Elements in Ore Deposits; Verplanck, P.L., Hitzman, M.W., Eds.; Society of Economic Geologists: Littleton, CO, USA, 2016; Volume 18, pp. 339–365. ISBN 9781629490922. [Google Scholar]
- Risacher, F.; Alonso, H.; Salazar, C. The origin of brines and salts in Chilean salars: A hydrochemical review. Earth-Sci. Rev. 2003, 63, 249–293. [Google Scholar] [CrossRef]
- Cabello, J. Lithium brine production, reserves, resources and exploration in Chile: An updated review. Ore Geol. Rev. 2021, 128. [Google Scholar] [CrossRef]
- Gruber, P.W.; Medina, P.A.; Keoleian, G.A.; Kesler, S.E.; Everson, M.P.; Wallington, T.J. Global lithium availability: A constraint for electric vehicles? J. Ind. Ecol. 2011, 15, 760–775. [Google Scholar] [CrossRef]
- Yu, J.Q.; Gao, C.L.; Cheng, A.Y.; Liu, Y.; Zhang, L.; He, X.H. Geomorphic, hydroclimatic and hydrothermal controls on the formation of lithium brine deposits in the Qaidam Basin, northern Tibetan Plateau, China. Ore Geol. Rev. 2013, 50, 171–183. [Google Scholar] [CrossRef]
- Yaksic, A.; Tilton, J.E. Using the cumulative availability curve to assess the threat of mineral depletion: The case of lithium. Resour. Policy 2009, 34, 185–194. [Google Scholar] [CrossRef]
- Tahil, W. The Trouble with Lithium: Implications of Future PHEV Production for Lithium Demand; Meridian International Research: Martainville, France, 2007. [Google Scholar]
- Yoshizuka, K.; Kitajou, A.; Holba, M. Selective recovery of lithium from seawater using a novel MnO2 type adsorbent III—Benchmark evaluation. ARS Sep. Acta 2006, 4, 78–85. [Google Scholar]
- Jaskula, B.W. Mineral Commodity Summaries: Lithium; U.S. Geological Survey: Washington, DC, USA, 2013. [CrossRef]
- Kunasz, I.A. Brines Resources and Reserves. Analysis of and Practical Recommendations for CIM’s Publication “Best Practices for Resource and Reserve Estimation for Lithium Brines”. Tucson Arizona, USA. TRU Group. 2013; p. 7. Available online: https://trugroup.com/whitepapers/Kunasz-CIM-Lithium-Best-Practice-TRU-2013-01-25.pdf (accessed on 13 November 2022).
- U.S. Geological Survey Mineral Commodity Summaries 1994–2022. Available online: https://www.usgs.gov/centers/national-minerals-information-center/mineral-commodity-summaries (accessed on 20 June 2022).
- Jaskula, B.W. 2017 Minerals Yearbook: Lithium; U.S. Geological Survey: Reston, VA, USA, 2020. [Google Scholar]
- Goonan, T.G. Lithium Use in Batteries [Circular 1371]; U.S. Geological Survey: Washington, DC, USA, 2012.
- Emmanuel, L.; Ben, K. Lithium Supply Is Set to Triple by 2025. Will it Be Enough? Available online: https://www.spglobal.com/en/research-insights/articles/lithium-supply-is-set-to-triple-by-2025-will-it-be-enough (accessed on 21 April 2022).
- Ritchie, H.; Roser, M.; Rosado, P. CO2 and Greenhouse Gas Emissions. Available online: https://ourworldindata.org/co2-and-other-greenhouse-gas-emissions (accessed on 23 May 2022).
- EPA Global Greenhouse Gas Emissions Data. Available online: https://www.epa.gov/ (accessed on 15 October 2022).
- Bamisile, O.; Obiora, S.; Huang, Q.; Yimen, N. Impact of economic development on CO2 emission in Africa: The role of BEVs and hydrogen production in renewable energy integration. Int. J. Hydrogen Energy 2022, 46, 2755–2773. [Google Scholar] [CrossRef]
- Tucki, K.; Orynycz, O. Perspectives for Mitigation of CO2 Emission due to Development of Electromobility in Several Countries. Energies 2020, 13, 4127. [Google Scholar] [CrossRef]
- Birol, F. World Energy Outlook; International Energy Agency: Paris, France, 2013; ISBN 9789264201309. [Google Scholar]
- Canalys Global Electric Vehicle Sales up 109% in 2021, with Half in Mainland China. Available online: https://www.canalys.com/newsroom/global-electric-vehicle-market-2021 (accessed on 21 July 2022).
- International Energy Agency. Global EV Outlook: Entering the Decade of Electric Drive; OECD Publishing: Paris, France, 2020. [Google Scholar]
- Mandys, F. Electric vehicles and consumer choices. Renew. Sustain. Energy Rev. 2021, 142, 110874. [Google Scholar] [CrossRef]
- Muratori, M.; Alexander, M.; Arent, D.; Bazilian, M.; Cazzola, P.; Dede, E.M.; Farrell, J.; Gearhart, C.; Greene, D.; Jenn, A.; et al. The rise of electric vehicles—2020 status and future expectations. Prog. Energy 2021, 3, 022002. [Google Scholar] [CrossRef]
- Bernard, M.R.; Hall, D.; Lutsey, N. Update on Electric Vehicle Uptake in European Cities; International Council on Clean Transportation: Washington, DC, USA, 2021. [Google Scholar]
- BloombergNEF Electric Vehicle Outlook. 2021. Available online: https://energydata.info/organization/bloomberg-new-energy-finance (accessed on 21 June 2022).
- Irle, R. EV Volume Global EV Sales for 2022. Available online: https://www.ev-volumes.com/country/total-world-plug-in-vehicle-volumes/ (accessed on 21 June 2022).
- IEA. Global EV Outlook 2021: Accelerating Ambitions Despite the Pandemic; International Energy Agency: Paris, France, 2021. [Google Scholar]
- Umwelt Bundesamt Total Greenhouse Gas Emissions Per Year. Available online: https://www.umweltbundesamt.de/en (accessed on 30 April 2022).
- Dyatkin, B.; Meng, Y.S. COVID-19 disrupts battery materials and manufacture supply chains, but outlook remains strong. MRS Bull. 2020, 45, 700–702. [Google Scholar] [CrossRef]
- Jaskula, B.W. Mineral Commodity Summary: Lithium; U.S. Geological Survey: Washington, DC, USA, 2021. [CrossRef]
- Yu, J.J.; Tang, C.S.; Li, M.K.; Shen, Z.J.M. Coordinating Installation of Electric Vehicle Charging Stations between Governments and Automakers. Prod. Oper. Manag. 2022, 31, 681–696. [Google Scholar] [CrossRef]
- Tyson, M.; Charlie, B. Breakthrough Batteries: Powering the Era of Clean Electrification. Rocky Mt. Inst. 2019, 2, 1–84. [Google Scholar]
- Alice, Y.; Sumangil, M. Top Electric Vehicle Markets Dominate Lithium-Ion Battery Capacity Growth. Available online: https://www.spglobal.com/marketintelligence/en/news-insights/blog/top-electric-vehicle-markets-dominate-lithium-ion-battery-capacity-growth (accessed on 12 February 2022).
- Gao, S.; Gong, X.; Liu, Y.; Zhang, Q. Energy consumption and carbon emission analysis of natural graphite anode material for lithium batteries. Mater. Sci. Forum 2018, 913, 985–990. [Google Scholar] [CrossRef]
- Xiao, M. General Motors and LG Chem Joint Venture—How Does This Differ From Tesla and Panasonic? Available online: https://www.interactanalysis.com/general-motors-and-lg-chem-joint-venture-how-does-this-differ-from-tesla-and-panasonic/ (accessed on 30 April 2022).
- Kromer, M.A.; Heywood, J. Electric Powertrains: Opportunities and Challenges in the US Light-Duty Vehicle Fleet. 2007. Available online: http://hdl.handle.net/1721.1/40372 (accessed on 30 April 2022).
- Hacker, F.; Harthan, R.; Matthes, F.; Zimmer, W. Environmental impacts and impact on the electricity market of a large scale introduction of electric cars in Europe: Critical Review of Literature. ETC/ACC Tech. Pap. 2009, 4, 169. [Google Scholar]
- Gaines, L.; Nelson, P. Lithium-Ion Batteries: Possible Materials Issues. In Proceedings of the 13th International Battery Materials Recycling Seminar and Exhibit, Argonne, IL, USA, 18 March 2009; U.S. Department of Transportation, Broward County Convention Center: Fort Lauderdale, FL, USA, 2009; pp. 1–16. [Google Scholar]
- Paoli, L.; Gül, T. Electric Cars Fend Off Supply Challenges to More than Double Global Sales. Available online: https://www.iea.org/commentaries/electric-cars-fend-off-supply-challenges-to-more-than-double-global-sales?utm_content=bufferd90dd&utm_medium=social&utm_source=twitter.com&utm_campaign=buffer (accessed on 16 March 2022).
- Julien, C.; Nazri, G.-A. Solid State Batteries: Materials Design and Optimization, 1st ed.; Springer New York: New York, NY, USA, 1994; ISBN 978-1-4615-2704-6. [Google Scholar]
- Jiang, F.; Peng, P. Elucidating the Performance Limitations of Lithium-ion Batteries due to Species and Charge Transport through Five Characteristic Parameters. Sci. Rep. 2016, 6, 32639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, D.; Li, S. Regulating the Performance of Lithium-Ion Battery Focus on the Electrode-Electrolyte Interface. Front. Chem. 2020, 8, 821. [Google Scholar] [CrossRef] [PubMed]
- Chalk, S.G.; Miller, J.F. Key challenges and recent progress in batteries, fuel cells, and hydrogen storage for clean energy systems. J. Power Sources 2006, 159, 73–80. [Google Scholar] [CrossRef]
- Hou, J.; Wang, X.; Su, Y.; Yang, Y.; Gao, T. Parameter Identification of Lithium Battery Model Based on Chaotic Quantum Sparrow Search Algorithm. Appl. Sci. 2022, 12, 7332. [Google Scholar] [CrossRef]
- Kavanagh, L.; Keohane, J.; Cabellos, G.G.; Lloyd, A.; Cleary, J. Global lithium sources-industrial use and future in the electric vehicle industry: A review. Resources 2018, 7, 57. [Google Scholar] [CrossRef] [Green Version]
- Ziemann, S.; Müller, D.B.; Schebek, L.; Weil, M. Modeling the potential impact of lithium recycling from EV batteries on lithium demand: A dynamic MFA approach. Resour. Conserv. Recycl. 2018, 133, 76–85. [Google Scholar] [CrossRef]
- Trading Economics Lithium Carbonate Prices in China. Available online: https://tradingeconomics.com/commodity/lithium (accessed on 25 March 2022).
- Corby, S. How Many Electric Cars Are There in the World? Available online: https://www.carsguide.com.au/ev/advice/how-many-electric-cars-are-there-in-the-world-85961 (accessed on 30 April 2022).
- Placek, M. Worldwide Motor Vehicle Production 2000–2021. Available online: https://www.statista.com/statistics/262747/worldwide-automobile-production-since-2000/ (accessed on 12 December 2022).
- Carlier, M. Motor Vehicle Sales Worldwide 2005–2021. Available online: https://www.statista.com/statistics/265859/vehicle-sales-worldwide/ (accessed on 12 December 2022).
- European Commission. European Green Cars Initiative. Available online: http://www.green-cars-initiative.eu/ (accessed on 20 April 2021).
- European Commission. Clean Urban Transport. Electric Vehicles. Available online: http://ec.europa.eu/transport/urban/vehicles/%20road/electric_en.htm (accessed on 22 November 2021).
- European Commission. Clean Vehicles Directive. Available online: https://transport.ec.europa.eu/transport-themes/clean-transport-urban-transport/clean-and-energy-efficient-vehicles/clean-vehicles-directive_en (accessed on 12 October 2022).
- U.S. Geological Survey. Minerals Yearbook: Metals and Minerals; U.S. Geological Survey: Washington, DC, USA, 2010.
- Akcil, A.; Sun, Z.; Panda, S. COVID-19 disruptions to tech-metals supply are a wake-up call. Nature 2020, 587, 365–367. [Google Scholar] [CrossRef]
- Arroyo, F.A.; Vesin, V.; Menéndez, A.; Bonneau, P.X. The Electrification of Two- and Three-Wheelers in the Sahel—Four Questions to Understand (and Guide) the Transition. Available online: https://blogs.worldbank.org/transport/electrification-two-and-three-wheelers-sahel-four-questions-understand-and-guide (accessed on 8 December 2022).
- Maddumage, W.; Perera, M.; Attalage, R.; Kelly, P. Power management strategy of a parallel hybrid three-wheeler for fuel and emission reduction. Energies 2021, 14, 1833. [Google Scholar] [CrossRef]
- Astute Analytica. Global Electric Two & Three-Wheeler Market, by Vehicle Type, by Usage, by End-User, Estimation & Forecast, 2017–2030; Research and Markets: Dublin, Ireland, 2022. [Google Scholar]
- Reference revised as Majumdar, D.; Jash, T. Merits and Challenges of E-Rickshaw as An Alternative form of Public Road Transport System: A Case Study in the State of West Bengal in India. Energy Procedia 2015, 79, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Mi, C.C.; Xu, J.; Gong, X.; You, C. Energy management for a power-split plug-in hybrid electric vehicle based on dynamic programming and neural networks. IEEE Trans. Veh. Technol. 2014, 63, 1567–1580. [Google Scholar] [CrossRef]
- McKerracher, C.; O’Donovan, A.; Soulopoulos, N.; Grant, A.; Mi, S.; Doherty, D.; Fisher, R.; Cantor, C.; Lyu, J.; Ampofo, K.; et al. Electric Vehicle Outlook 2022. BloombergNEF 2022, 38, 1–11. [Google Scholar]
- Coltura Gasoline Vehicle Phase-Out Advances around the World. Available online: https://www.coltura.org/world-gasoline-phaseouts (accessed on 7 December 2022).
- Wu, Q.; Sun, S. Energy and Environmental Impact of the Promotion of Battery Electric Vehicles in the Context of Banning Gasoline Vehicle Sales. Energies 2022, 15, 8388. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2018—Towards Cross-Modal Electrification; International Energy Agency: Paris, France, 2018. [Google Scholar]
- Murray, A. Will swapping out electric car batteries catch on? BBCNews 2022, 11, 78. [Google Scholar]
- Zheng, Y.; Dong, Z.Y.; Xu, Y.; Meng, K.; Zhao, J.H.; Qiu, J. Electric vehicle battery charging/swap stations in distribution systems: Comparison study and optimal planning. IEEE Trans. Power Syst. 2014, 29, 221–229. [Google Scholar] [CrossRef]
- Zeng, B.; Luo, Y.; Zhang, C.; Liu, Y. Assessing the impact of an EV battery swapping station on the reliability of distribution systems. Appl. Sci. 2020, 10, 8023. [Google Scholar] [CrossRef]
- Hanley, S. NIO Completes More Than 500,000 Battery Swaps. Available online: https://cleantechnica.com/2020/05/31/nio-completes-more-than-500000-battery-swaps/ (accessed on 28 November 2022).
- Adegbohun, F.; von Jouanne, A.; Lee, K.Y. Autonomous battery swapping system and methodologies of electric vehicles. Energies 2019, 12, 667. [Google Scholar] [CrossRef] [Green Version]
- Toll, M. Gogoro Named Global Leader in Light Electric Vehicle Battery Swapping, Passes 200 Million Swaps. Available online: https://electrek.co/2021/08/30/gogoro-named-global-leader-in-light-electric-vehicle-battery-swapping-passes-200-million-swaps/ (accessed on 28 November 2022).
- Zhong, L.; Pei, M. Optimal design for a shared swap charging system considering the electric vehicle battery charging rate. Energies 2020, 13, 1213. [Google Scholar] [CrossRef] [Green Version]
- Feil, C. What is EV Range Anxiety and how Can We Overcome It? Available online: https://www.geotab.com/blog/range-anxiety/ (accessed on 29 November 2022).
- Zeng, X.; Li, J.; Singh, N. Recycling of spent lithium-ion battery: A critical review. Crit. Rev. Environ. Sci. Technol. 2014, 44, 1129–1165. [Google Scholar] [CrossRef]
- Alfaro-Algaba, M.; Ramirez, F.J. Techno-economic and environmental disassembly planning of lithium-ion electric vehicle battery packs for remanufacturing. Resour. Conserv. Recycl. 2020, 154, 104461. [Google Scholar] [CrossRef]
- Anju, M.; Banerjee, D.K. Associations of cadmium, zinc, and lead in soils from a lead and zinc mining area as studied by single and sequential extractions. Environ. Monit. Assess. 2011, 176, 67–85. [Google Scholar] [CrossRef]
- Winslow, K.M.; Laux, S.J.; Townsend, T.G. A review on the growing concern and potential management strategies of waste lithium-ion batteries. Resour. Conserv. Recycl. 2018, 129, 263–277. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B.; Fukushima, T.; Park, I.; Jeon, S.; Igarashi, T.; Sato, T.; Nyambe, I.; et al. Detoxification of lead-bearing zinc plant leach residues from Kabwe, Zambia by coupled extraction-cementation method. J. Environ. Chem. Eng. 2020, 8, 104197. [Google Scholar] [CrossRef]
- Silwamba, M.; Ito, M.; Hiroyoshi, N.; Tabelin, C.B. Recovery of Lead and Zinc from Zinc Plant Leach Residues by Concurrent Dissolution-Cementation. Metals 2020, 10, 531. [Google Scholar] [CrossRef] [Green Version]
- Christmann, P.; Gloaguen, E.; Labbé, J.F.; Melleton, J.; Piantone, P. Global Lithium Resources and Sustainability Issues. In Lithium Process Chemistry: Resources, Extraction, Batteries, and Recycling; Chagnes, A., Światowska, J., Eds.; Elsevier: San Diego, CA, USA, 2015; pp. 1–40. ISBN 9780128014172. [Google Scholar]
- Casals, L.C.; García, B.A.; Aguesse, F.; Iturrondobeitia, A. Second life of electric vehicle batteries: Relation between materials degradation and environmental impact. Int. J. Life Cycle Assess. 2017, 22, 82–93. [Google Scholar] [CrossRef]
- Department of Industry, Science and Resources. Resources and Energy Quarterly. Available online: https://www.industry.gov.au/publications/resources-and-energy-quarterly (accessed on 26 November 2022).
- Reck, B.K.; Graedel, T.E. Challenges in metal recycling. Science 2012, 337, 690–695. [Google Scholar] [CrossRef] [PubMed]
- Metalary Lithium Price. Available online: https://www.metalary.com/lithium-price/ (accessed on 28 November 2022).
- Batteries News Lithium Price Forecast: Will The Price Keep its Bull Run? Available online: https://batteriesnews.com/lithium-price-forecast-price-keep-bull-run/ (accessed on 28 November 2022).
- Castelvecchi, D. Electric cars and batteries: How will the world produce enough? Nature 2021, 596, 336–339. [Google Scholar] [CrossRef] [PubMed]
- Song, J.; Yan, W.; Cao, H.; Song, Q.; Ding, H.; Lv, Z.; Zhang, Y.; Sun, Z. Material flow analysis on critical raw materials of lithium-ion batteries in China. J. Clean. Prod. 2019, 215, 570–581. [Google Scholar] [CrossRef]
- Financial Times Electric Cars: China Powers the Battery Supply Chain. Available online: https://www.ft.com/content/455fe41c-7185-11e9-bf5c-6eeb837566c5 (accessed on 21 June 2022).
- Gmar, S.; Muhr, L.; Lutin, F.; Chagnes, A. Lithium-Ion Battery Recycling: Metal Recovery from Electrolyte and Cathode Materials by Electrodialysis. Metals 2022, 12, 1859. [Google Scholar] [CrossRef]


| Type | Applications | Estimated Global Market Share (%) |
|---|---|---|
| Primary Lithium | They are single-use LIB for electronics, that range from button cells to car batteries. | n/a |
| Lithium cobalt oxide (LiCoO2) | They have the high energy storage density required for portable electronics. Thus, used in portable electronic devices (e.g., phones, laptops, tablets, cameras, etc.). | 37.2 |
| Lithium nickel manganese cobalt oxide (NMC) (LiNiMnCoO2) | They are used in power tools, EVs, energy storage, and medical devices. | 29 |
| Lithium manganese oxide (LiMn2O4) | It has a shorter life than others and has a high discharge or recharge with better thermal stability. It is used in power tools, EVs, and medical devices. | 21.4 |
| Lithium nickel oxide (LiNiO2) | They are used in EVs. | 7.2 |
| Lithium iron phosphate (LiFePO4) | Not thermally stable as other cathodes. They are used in energy storage tools, EVs, and medical devices | 5.2 |
| Types of Electric Vehicles | Descriptions |
|---|---|
| All-electric Vehicles | All-electric vehicles are the first generation of EVs that use the energy stored in batteries to power electric motors and provide propulsion power. They are called non-polluting vehicles or zero pollution vehicles. They are plug-in electric vehicles (PEVs). Although they have the advantage of being charged to the power grid either at home or in public places, their batteries are of limited capacity and cannot run long distances. They do not give off any greenhouse gases at all and can be charged with energy from renewable sources. |
| Hybrid Electric Vehicles (HEVs) | They have both a fuel engine and an electric motor. Their battery capacity is sufficient to save energy from the fuel engine and brakes. About 1.5 million HEVs have been sold in the last decade. However, they are dependent on the fossil fuel consumption engine. |
| Plug-in Hybrid Electric Vehicles (PHEVs) | They are combinations of Hybrid Electric Vehicles (HEVs) and All-Electric Vehicles. Their batteries require more capacity than HEVs because they are rechargeable from the power grid. The PHEV battery must be capable of fast discharge and fast recharge. It is therefore possible to travel longer distances. |
| Country | Main Mineral | Formula | Lithium Content (%) |
|---|---|---|---|
| Afghanistan | spodumene | LiAlSi2O6 | 3.73 |
| Australia | spodumene | LiAlSi2O6 | 3.73 |
| Austria | spodumene | LiAlSi2O6 | 3.73 |
| Brazile | Petalite | LiAlSi4O10 | 2.09 |
| spodumene | LiAlSi2O6 | 3.73 | |
| Canada | spodumene | LiAlSi2O6 | 3.73 |
| pegmatites | Unspecified | 0.49 | |
| petalite | LiAlSi4O10 | 2.09 | |
| China | Lepidolite | KLi2Al(Al,Si)3O10(F,OH)2 | 3.58 |
| spodumene | LiAlSi2O6 | 3.73 | |
| petalite | LiAlSi4O10 | 2.09 | |
| DRC | spodumene | LiAlSi2O6 | 3.73 |
| Finland | spodumene | LiAlSi2O6 | 3.73 |
| Mali | Amblygonite | (Li,Na)AlPO4(F,OH) | 3.44 |
| Portugal | Petalite | LiAlSi4O10 | 2.09 |
| Namibia | Petalite | LiAlSi4O10 | 2.09 |
| Russia | pegmatites | not specified | 0.49 |
| Lepidolite | KLi2Al(Al,Si)3O10(F,OH)2 | 3.58 | |
| spodumene | LiAlSi2O6 | 3.73 | |
| Serbia | Jadarite | LiNaSiB3O7(OH) | 3.16 |
| Spain | Lepidolite | KLi2Al(Al,Si)3O10(F,OH)2 | 3.58 |
| Sweden | spodumene | LiAlSi2O6 | 3.73 |
| USA | spodumene | LiAlSi2O6 | 3.73 |
| pegmatites | Not specified | 0.49 | |
| Hectorite | Na0.3(Mg,Li)3Si4O10(OH)2 | 0.53 | |
| Zimbabwe | pegmatites | Not specified | 0.49 |
| spodumene | LiAlSi2O6 | 3.73 |
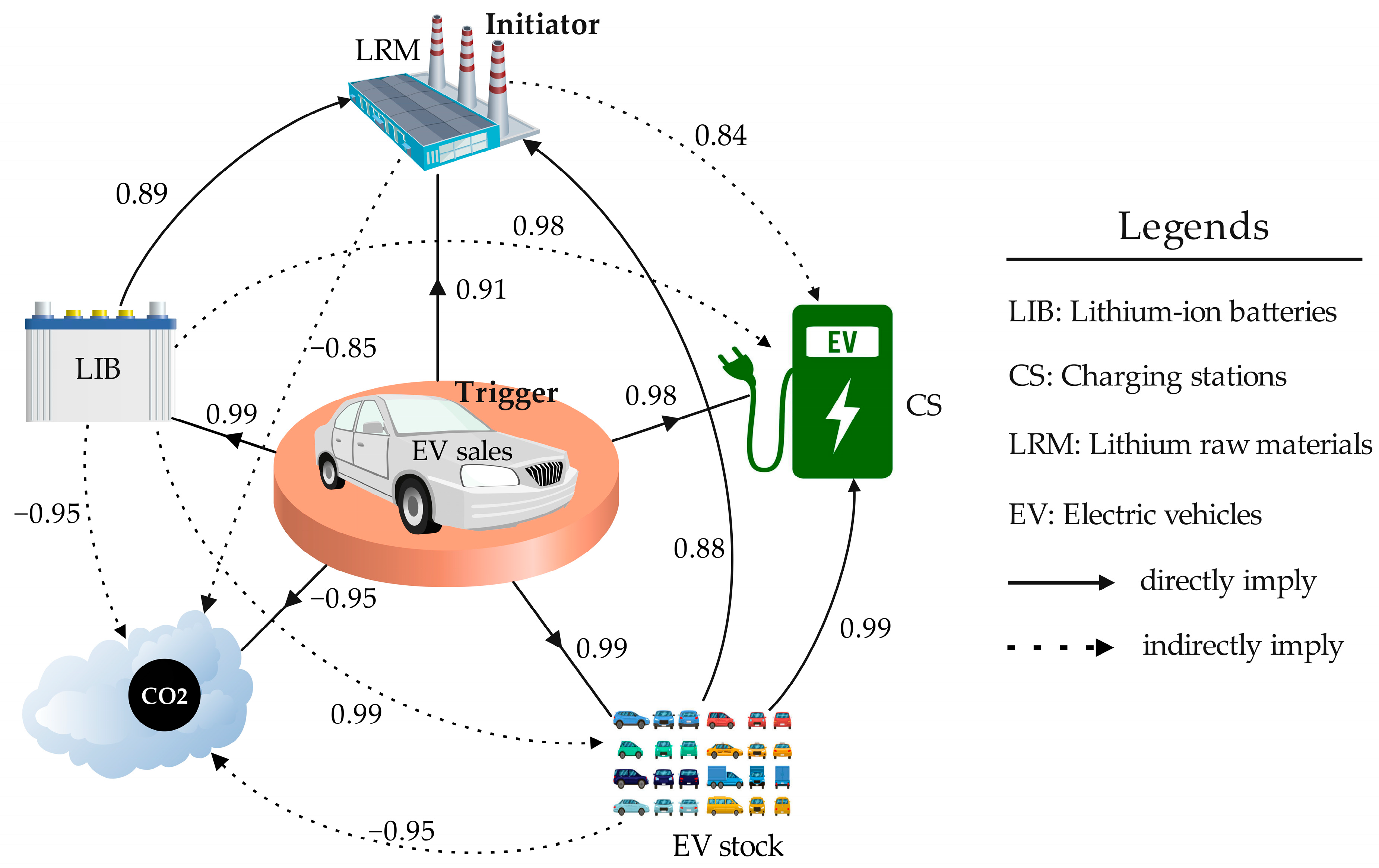
| Parameters | LRM | CS | LIB | EV Sales | EV Stock | CO2 |
|---|---|---|---|---|---|---|
| LRM | 1 | |||||
| CS | 0.84 | 1 | ||||
| LIB | 0.89 | 0.98 | 1 | |||
| EV sales | 0.91 | 0.99 | 0.99 | 1 | ||
| EV Stock | 0.88 | 1.00 | 0.99 | 0.99 | 1 | |
| CO2 | −0.80 | −0.98 | −0.96 | −0.96 | −0.97 | 1 |
| Parameters | Lithium Production | Lithium Resources | Lithium Reserves |
|---|---|---|---|
| Lithium Production | 1 | ||
| Lithium Resources | 0.958 | 1 | |
| Lithium Reserves | 0.87 | 0.959 | 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindagato, P.; Li, Y.; Macháček, J.; Yang, G.; Mungwarakarama, I.; Ndahimana, A.; Ntwali, H.P.K. Lithium Metal: The Key to Green Transportation. Appl. Sci. 2023, 13, 405. https://doi.org/10.3390/app13010405
Lindagato P, Li Y, Macháček J, Yang G, Mungwarakarama I, Ndahimana A, Ntwali HPK. Lithium Metal: The Key to Green Transportation. Applied Sciences. 2023; 13(1):405. https://doi.org/10.3390/app13010405
Chicago/Turabian StyleLindagato, Philemon, Yongjun Li, Jan Macháček, Gaoxue Yang, Irénée Mungwarakarama, Anastase Ndahimana, and Henri Patrick Kanimba Ntwali. 2023. "Lithium Metal: The Key to Green Transportation" Applied Sciences 13, no. 1: 405. https://doi.org/10.3390/app13010405
APA StyleLindagato, P., Li, Y., Macháček, J., Yang, G., Mungwarakarama, I., Ndahimana, A., & Ntwali, H. P. K. (2023). Lithium Metal: The Key to Green Transportation. Applied Sciences, 13(1), 405. https://doi.org/10.3390/app13010405








