Demonstration of Fat Properties in Diagnostic Ultrasound Images through the Development of a Modular Phantom
Abstract
:1. Introduction
2. Materials and Methods
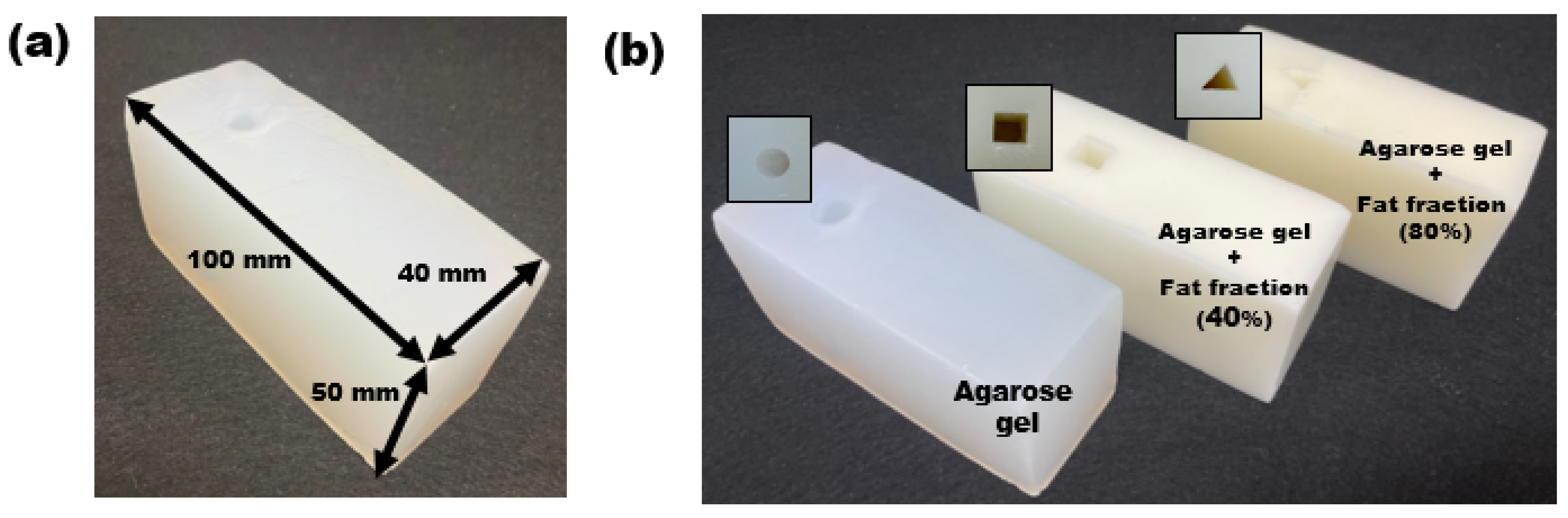
2.1. Fabrication of the Emulsion Phantom
2.2. MRI Image Acquisition for Phantom Verification
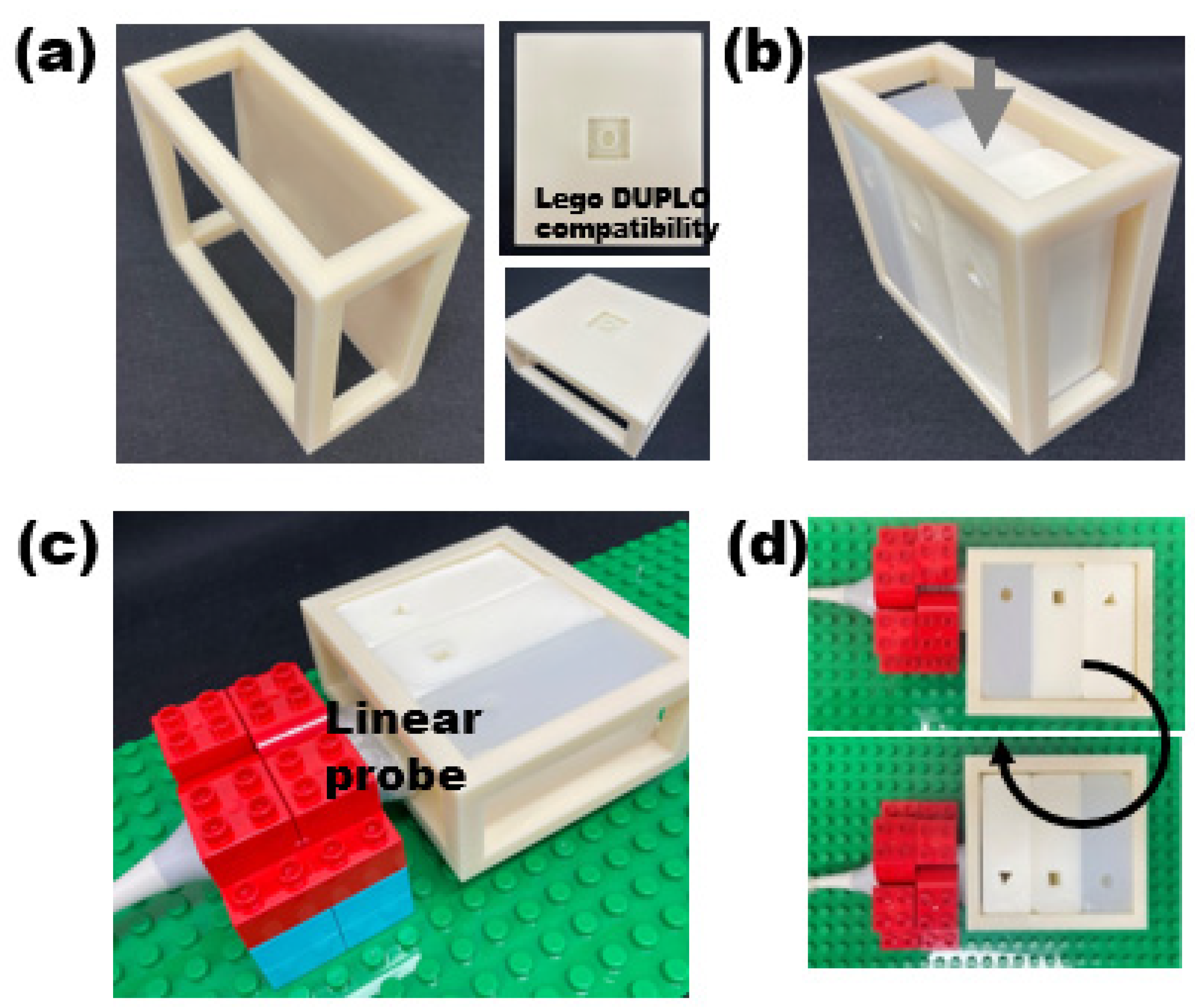
2.3. LEGO-Compatible Modular Frame for Reproducible Image Acquisiton
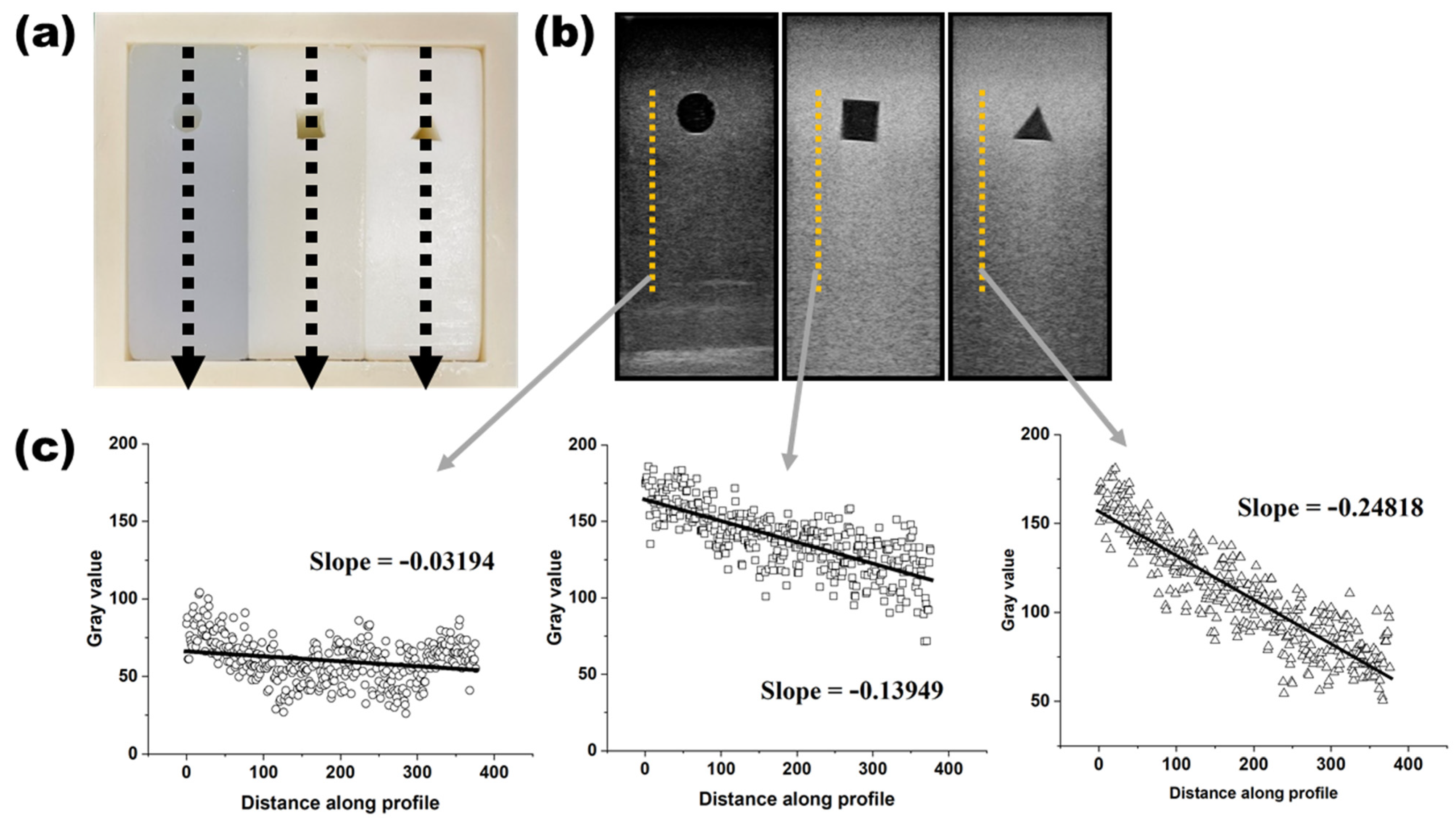
2.4. Acquisition of Ultrasound Image and Analysis
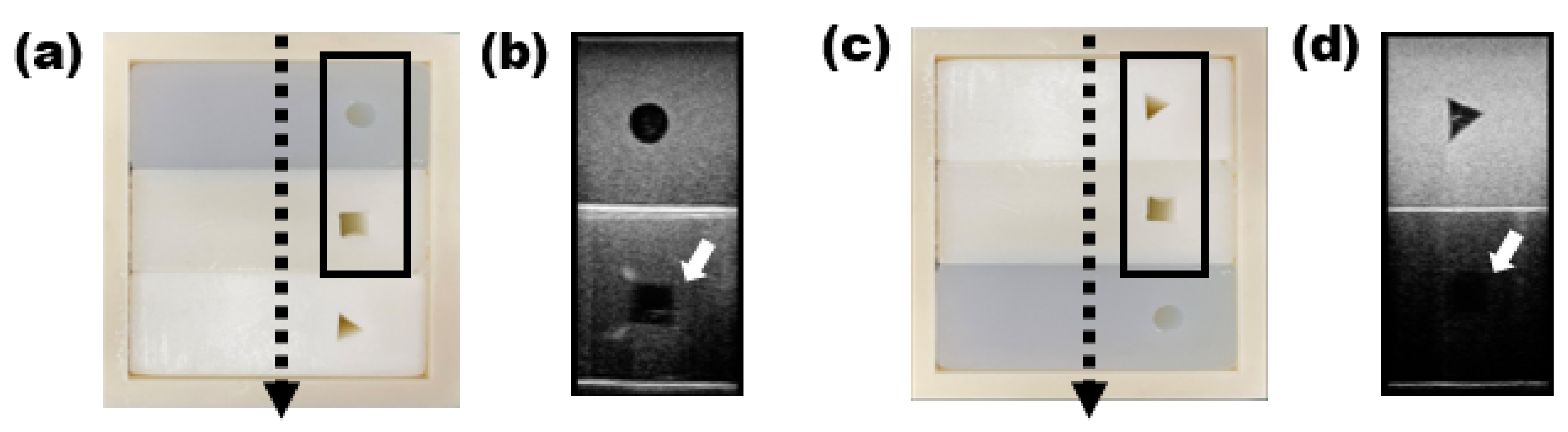
3. Results
4. Discussion
- (1)
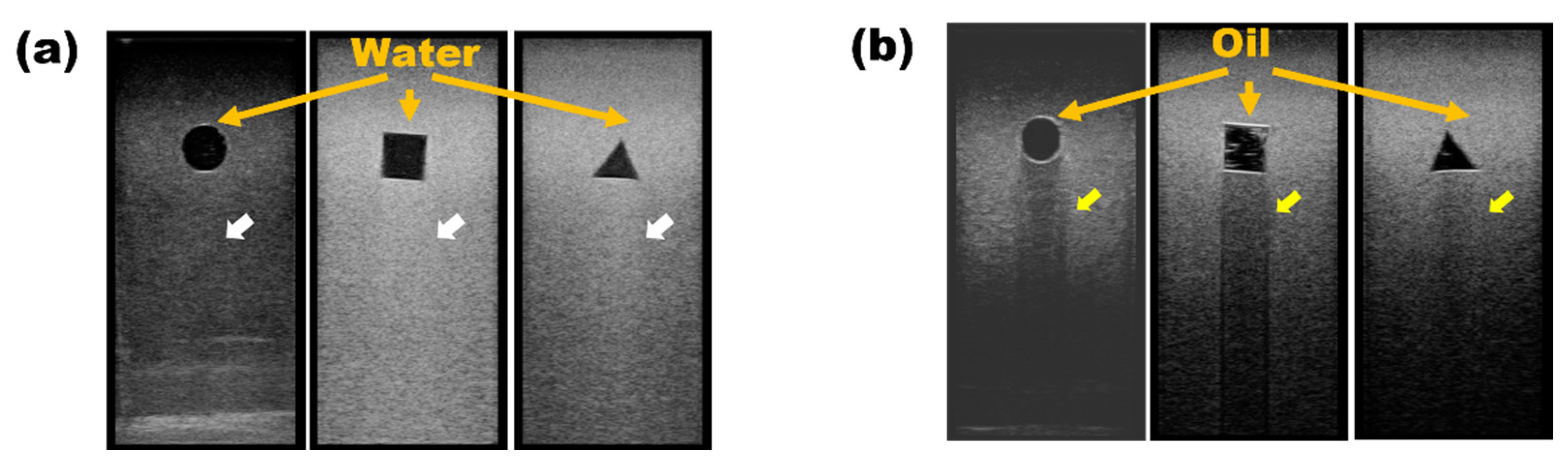
- The brightness and attenuation of fat content in the phantom were similar to those seen in mild, moderate, and severe fatty livers;
- (2)
- Posterior acoustic enhancement, which is mainly seen in anechoic cysts with clear boundaries, was also observed, along with the posterior acoustic shadowing caused by high attenuation such as oil cyst of the breast [23].
- (3)
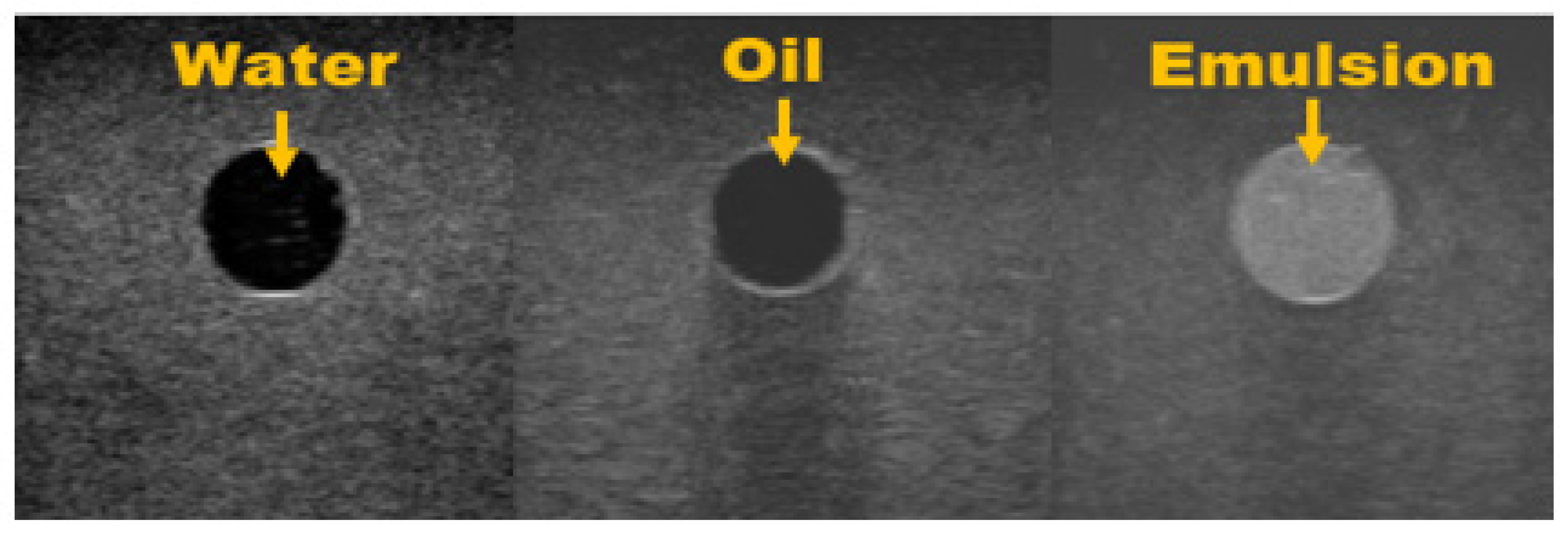
- Pure water and fat appeared to be echo-free, while an emulsion of water and fat showed a remarkably hyperechoic pattern [22].
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dragoo, J.L.; Shapiro, S.A.; Bradsell, H.; Frank, R.M. The essential roles of human adipose tissue: Metabolic, thermoregulatory, cellular, and paracrine effects. J. Cartil. Jt. Preserv. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Luo, L.; Liu, M. Adipose tissue in control of metabolism. J. Endocrinol. 2016, 231, R77–R99. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.S.; Huh, J.Y.; Hwang, I.J.; Kim, J.I.; Kim, J.B. Adipose Tissue Remodeling: Its Role in Energy Metabolism and Metabolic Disorders. Front. Endocrinol. 2016, 7, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, S.C.; Heba, E.; Wolfson, T.; Ang, B.; Gamst, A.; Han, A.; Erdman, J.W., Jr.; O’Brien, W.D., Jr.; Andre, M.P.; Sirlin, C.B.; et al. Non-invasive diagnosis of non-alcoholic fatty liver disease and quantification of liver fat using a new quantitative ultrasound technique. Clin. Gastroenterol. Hepatol. 2015, 13, 1337–1345.e6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stefan, N.; Häring, H.U.; Cusi, K. Non-alcoholic fatty liver disease: Causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019, 7, 313–324. [Google Scholar] [CrossRef] [PubMed]
- Brunt, E.M.; Wong, V.W.S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef]
- Kramer, H.; Pickhardt, P.J.; Kliewer, M.A.; Hernando, D.; Chen, G.-H.; Zagzebski, J.A.; Reeder, S.B. Accuracy of Liver Fat Quantification with Advanced CT, MRI, and Ultrasound Techniques: Prospective Comparison with MR Spectroscopy. Am. J. Roentgenol. 2017, 208, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Chowdhury Aqib, B.; Mehta Kosha, J. Liver biopsy for assessment of chronic liver diseases: A synopsis. Clin. Exp. Med. 2022, 1–13. [Google Scholar] [CrossRef]
- Schwenzer, N.F.; Springer, F.; Schraml, C.; Stefan, N.; Machann, J.; Schick, F. Non-invasive assessment and quantification of liver steatosis by ultrasound, computed tomography and magnetic resonance. J. Hepatol. 2009, 51, 433–445. [Google Scholar] [CrossRef]
- Troelstra, M.A.; Witjes, J.J.; van Dijk, A.M.; Mak, A.L.; Gurney-Champion, O.; Runge, J.H.; Zwirs, D.; Stols-Gonçalves, D.; Zwinderman, A.H.; ten Wolde, M.; et al. Assessment of imaging modalities against liver biopsy in nonalcoholic fatty liver disease: The Amsterdam NAFLD-NASH cohort. J. Magn. Reson. Imaging 2021, 54, 1937–1949. [Google Scholar] [CrossRef]
- Pirmoazen, A.M.; Khurana, A.; El Kaffas, A.; Kamaya, A. Quantitative ultrasound approaches for diagnosis and monitoring hepatic steatosis in nonalcoholic fatty liver disease. Theranostics 2020, 10, 4277–4289. [Google Scholar] [CrossRef] [PubMed]
- Festi, D.; Schiumerini, R.; Marzi, L.; Di Biase, A.R.; Mandolesi, D.; Montrone, L.; Scaioli, E.; Bonato, G.; Marchesini-Reggiani, G.; Colecchia, A. The diagnosis of non-alcoholic fatty liver disease—Availability and accuracy of non-invasive methods. Aliment. Pharmacol. Ther. 2013, 37, 392–400. [Google Scholar] [CrossRef] [PubMed]
- Bierig, S.M.; Jones, A. Accuracy and cost comparison of ultrasound versus alternative imaging modalities, including CT, MR, PET, and angiography. J. Diagn. Med. Sonogr. 2009, 25, 138–144. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, P.A.; Antunes, B.D.M.M.; Silveira, L.S.; Christofaro, D.G.D.; Fernandes, R.A.; Freitas, I.F. Body composition variables as predictors of NAFLD by ultrasound in obese children and adolescents. BMC Pediatr. 2014, 14, 25. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.N.; Fowler, K.J.; Hamilton, G.; Cui, J.Y.; Sy, E.Z.; Balanay, M.; Hooker, J.C.; Szeverenyi, N.; Sirlin, C.B. Liver fat imaging—A clinical overview of ultrasound, CT, and MR imaging. Br. J. Radiol. 2018, 91, 20170959. [Google Scholar] [CrossRef] [Green Version]
- Spencer, G.M.; Rubens, D.J.; Roach, D.J. Hypoechoic fat: A sonographic pitfall. Am. J. Roentgenol. 1995, 164, 1277–1280. [Google Scholar] [CrossRef] [Green Version]
- Venta, L.A.; Dudiak, C.M.; Salomon, C.G.; Flisak, M.E. Sonographic evaluation of the breast. Radiographics 1994, 14, 29–50. [Google Scholar] [CrossRef] [Green Version]
- Andre, M.P.; Han, A.; Heba, E.; Hooker, J.; Loomba, R.; Sirlin, C.B.; Erdman, J.W.; O’Brien, W.D. Accurate diagnosis of nonalcoholic fatty liver disease in human participants via quantitative ultrasound. In Proceedings of the 2014 IEEE International Ultrasonics Symposium, Chicago, IL, USA, 3–6 September 2014; IEEE: Piscataway, NJ, USA, 2014. [Google Scholar]
- Leivas, G.; Maraschin, C.K.; Blume, C.A.; Telo, G.H.; Trindade, M.R.; Trindade, E.N.; Diemen, V.V.; Cerski, C.T.S.; Schaan, B.D. Accuracy of ultrasound diagnosis of nonalcoholic fatty liver disease in patients with classes II and III obesity: A pathological image study. Obes. Res. Clin. Pract. 2021, 15, 461–465. [Google Scholar] [CrossRef]
- Keenan, K.E.; Wilmes, L.J.; Aliu, S.O.; Newitt, D.C.; Jones, E.F.; Boss, M.A.; Stupic, K.F.; Russek, S.E.; Hylton, N.M. Design of a breast phantom for quantitative MRI. J. Magn. Reson. Imaging 2016, 44, 610–619. [Google Scholar] [CrossRef] [Green Version]
- Fornage, B.D.; Tassin, G.B. Sonographic appearances of superficial soft tissue lipomas. J. Clin. Ultrasound 1991, 19, 215–220. [Google Scholar] [CrossRef]
- Inampudi, P.; Jacobson, J.; Fessell, D.P.; Carlos, R.C.; Patel, S.V.; Delaney-Sathy, L.O.; van Holsbeeck, M. Soft-Tissue Lipomas: Accuracy of Sonography in Diagnosis with Pathologic Correlation. Radiology 2004, 233, 763–767. [Google Scholar] [CrossRef] [PubMed]
- Harvey, J.A.; Moran, R.E.; Maurer, E.J.; A DeAngelis, G. Sonographic features of mammary oil cysts. J. Ultrasound Med. 1997, 16, 719–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGarry, C.K.; Grattan, L.J.; Ivory, A.M.; Leek, F.; Liney, G.P.; Liu, Y.; Miloro, P.; Rai, R.; Robinson, A.; Shih, A.J.; et al. Tissue mimicking materials for imaging and therapy phantoms: A review. Phys. Med. Biol. 2020, 65, 23TR01. [Google Scholar] [CrossRef] [PubMed]
- Hines, C.D.; Yu, H.; Shimakawa, A.; McKenzie, C.A.; Brittain, J.H.; Reeder, S.B. T1 independent, T2* corrected MRI with accurate spectral modeling for quantification of fat: Validation in a fat-water-SPIO phantom. J. Magn. Reson. Imaging Off. J. Int. Soc. Magn. Reson. Med. 2009, 30, 1215–1222. [Google Scholar]
- Hernando, D.; Sharma, S.D.; Aliyari Ghasabeh, M.; Alvis, B.D.; Arora, S.S.; Hamilton, G.; Pan, L.; Shaffer, J.M.; Sofue, K.; Szeverenyi, N.M.; et al. Multisite, multivendor validation of the accuracy and reproducibility of proton-density fat-fraction quantification at 1.5 T and 3T using a fat–water phantom. Magn. Reson. Med. 2017, 77, 1516–1524. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.K.; Lee, S.S.; Kim, B.; Cho, E.S.; Kim, Y.J.; Byun, J.; Park, B.J.; Kim, S.Y.; Kim, J.H. Agreement and reproducibility of proton density fat fraction measurements using commercial MR sequences across different platforms: A multivendor, multi-institutional phantom experiment. Investig. Radiol. 2019, 54, 517–523. [Google Scholar] [CrossRef]
- Drakos, T.; Antoniou, A.; Evripidou, N.; Alecou, T.; Giannakou, M.; Menikou, G.; Constantinides, G.; Damianou, C. Ultrasonic attenuation of an agar, silicon dioxide, and evaporated milk gel phantom. J. Med. Ultrasound 2021, 29, 239. [Google Scholar]
- Ferraioli, G.; Kumar, V.; Ozturk, A.; Nam, K.; de Korte, C.L.; Barr, R.G. US Attenuation for Liver Fat Quantification: An AIUM-RSNA QIBA Pulse-Echo Quantitative Ultrasound Initiative. Radiology 2022, 302, 495–506. [Google Scholar] [CrossRef]
- Labyed, Y.; Milkowski, A. Novel Method for Ultrasound-Derived Fat Fraction Using an Integrated Phantom. J. Ultrasound Med. 2020, 39, 2427–2438. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, S.I.; Hong, C.; Lee, C.; Cho, H.-M. Demonstration of Fat Properties in Diagnostic Ultrasound Images through the Development of a Modular Phantom. Appl. Sci. 2023, 13, 432. https://doi.org/10.3390/app13010432
Lee SI, Hong C, Lee C, Cho H-M. Demonstration of Fat Properties in Diagnostic Ultrasound Images through the Development of a Modular Phantom. Applied Sciences. 2023; 13(1):432. https://doi.org/10.3390/app13010432
Chicago/Turabian StyleLee, Su In, Cheolpyo Hong, Changwoo Lee, and Hyo-Min Cho. 2023. "Demonstration of Fat Properties in Diagnostic Ultrasound Images through the Development of a Modular Phantom" Applied Sciences 13, no. 1: 432. https://doi.org/10.3390/app13010432
APA StyleLee, S. I., Hong, C., Lee, C., & Cho, H.-M. (2023). Demonstration of Fat Properties in Diagnostic Ultrasound Images through the Development of a Modular Phantom. Applied Sciences, 13(1), 432. https://doi.org/10.3390/app13010432




