The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism
Abstract
:1. Introduction
2. Materials and Methods
2.1. Source Materials
2.2. Experimental Programme
2.2.1. Specimens Preparation and Test Procedure
2.2.2. Test Procedures
3. Results and Discussion
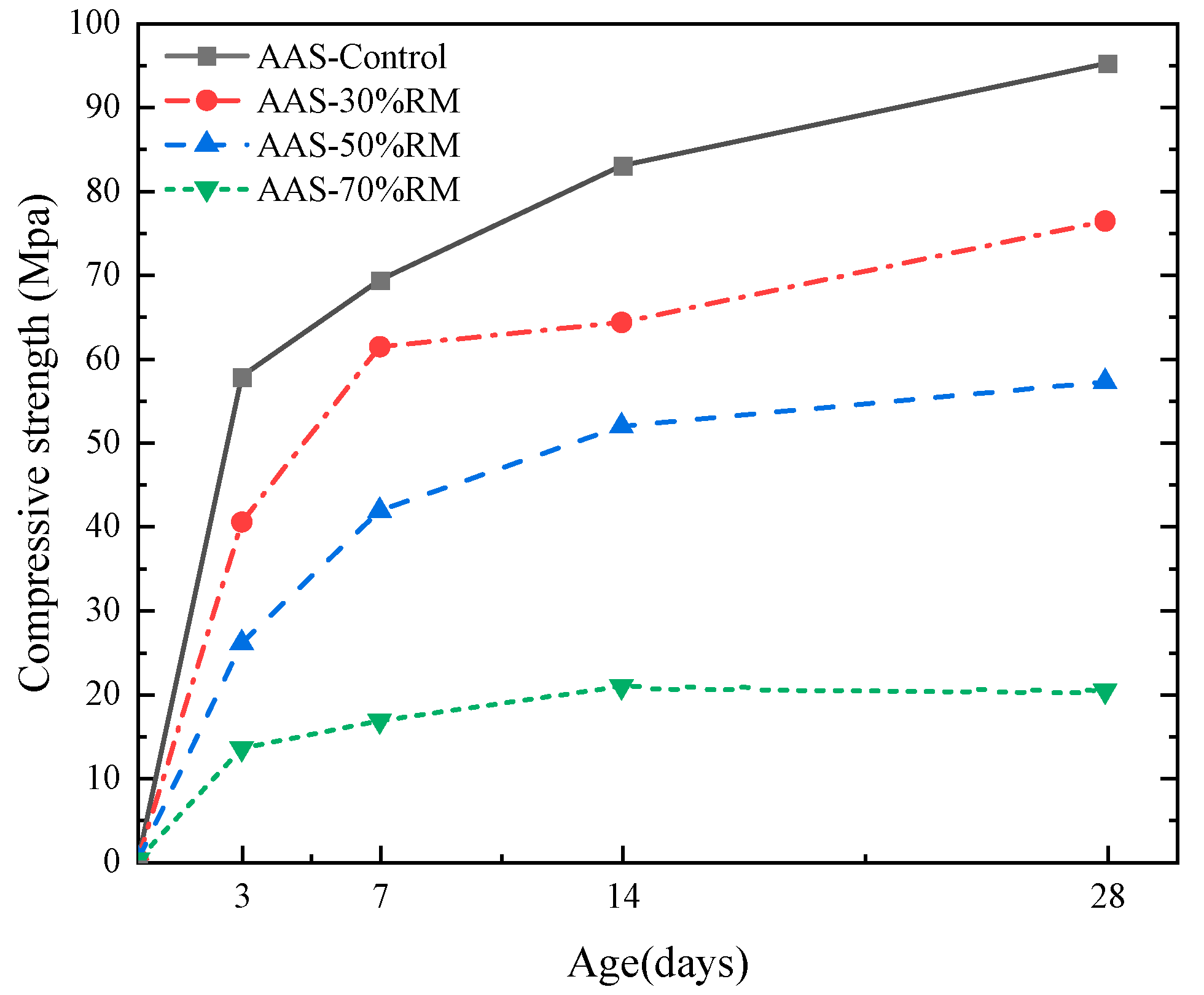
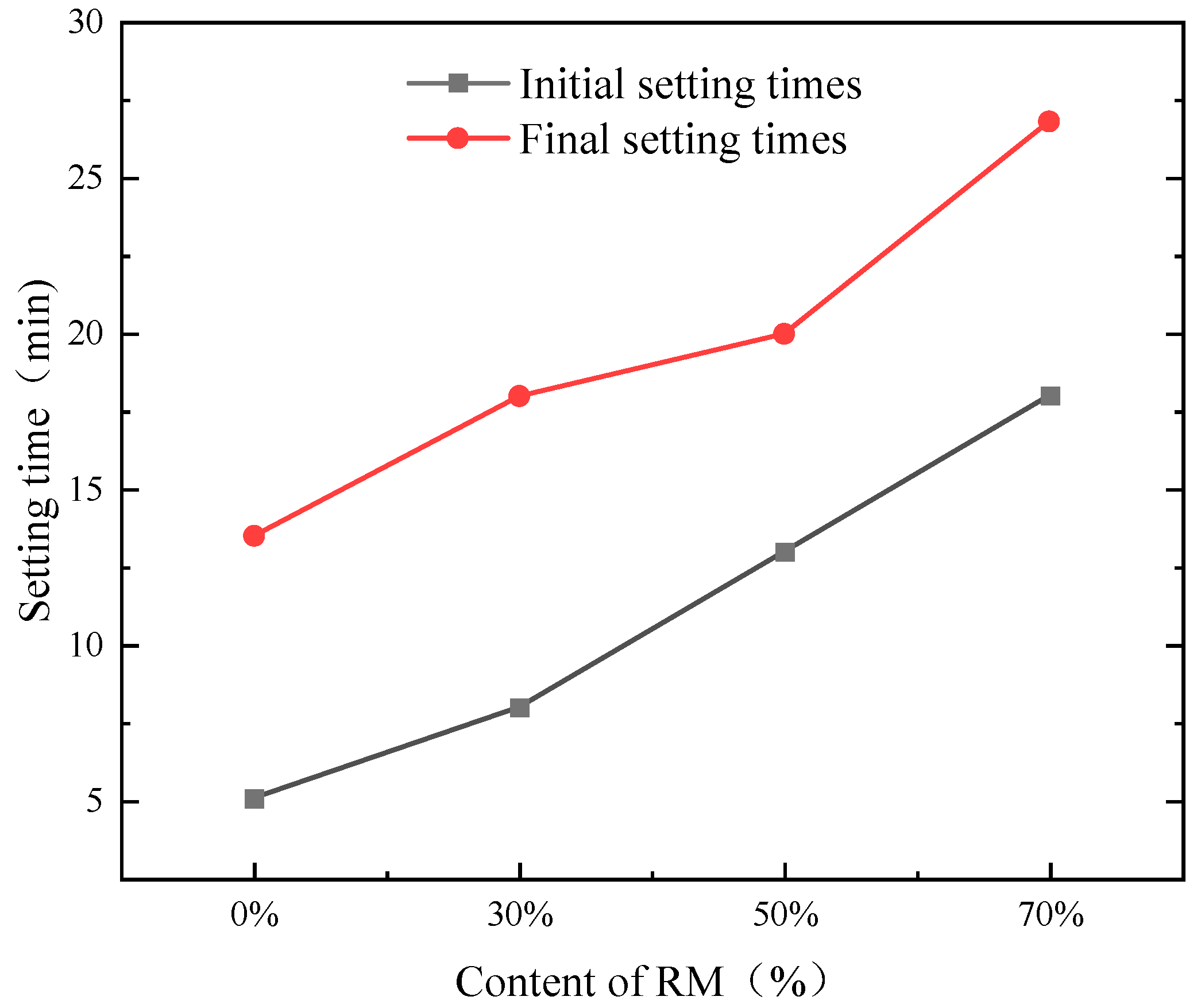
3.1. Compressive Strength and Setting Time of AASR
3.2. The Mechanism of Bayer Red Mud Admixture on the Compressive Strength of AASR
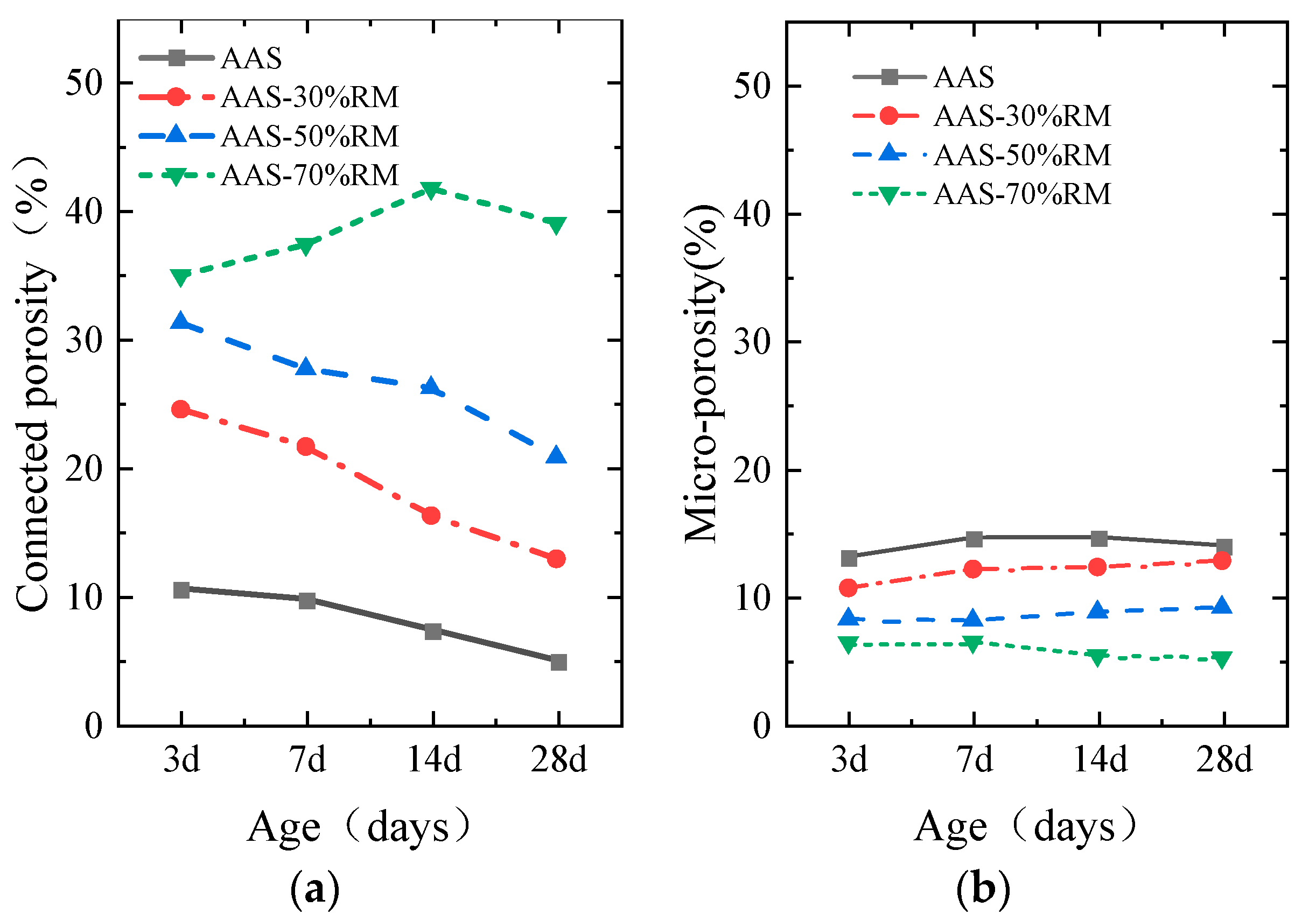
3.2.1. Porosity
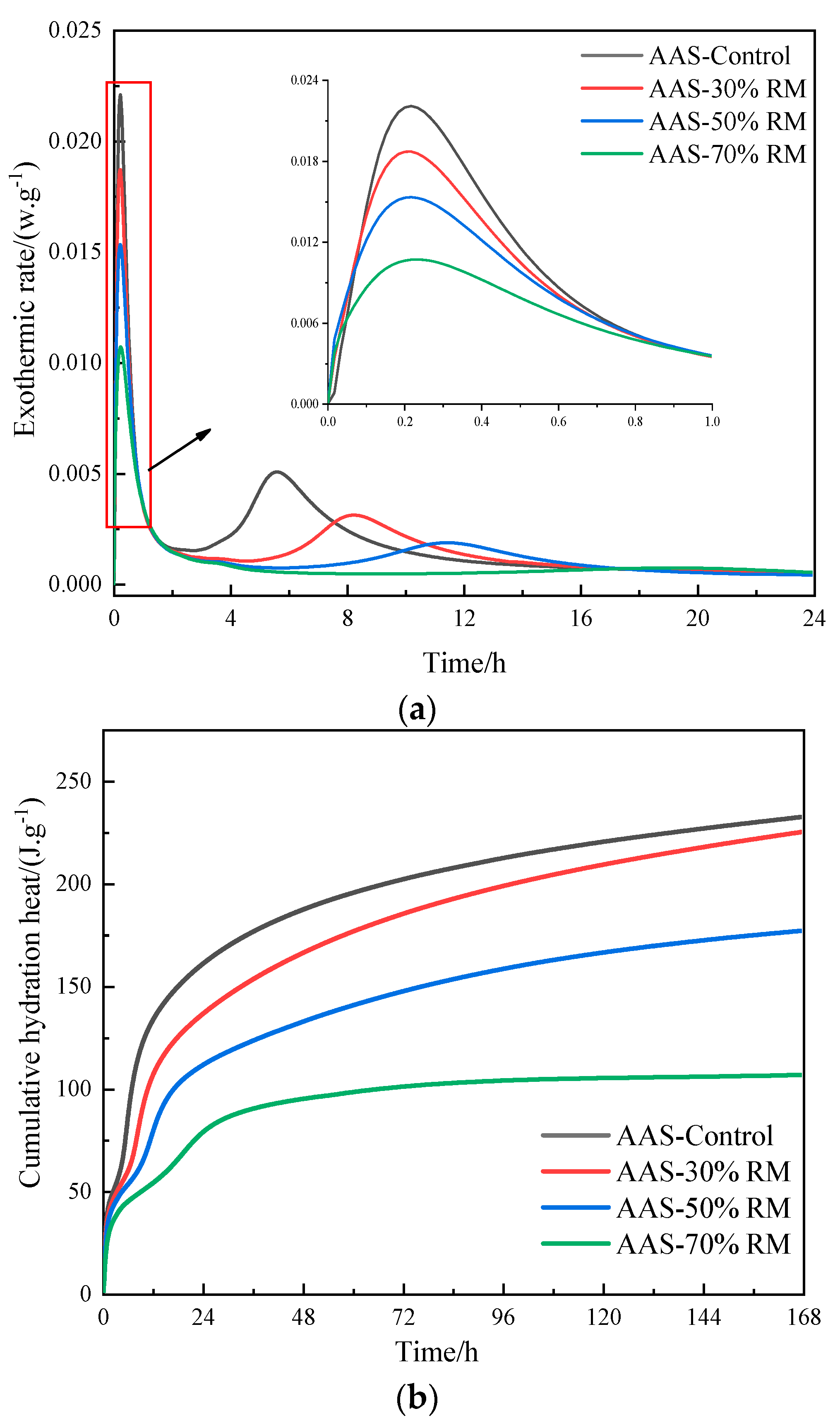
3.2.2. Hydration Process
3.2.3. Phase Composition
- XRD: The XRD patterns of all samples at 28 days are shown in Figure 7. It can be seen that the products of the control sample are mainly C-A-S-H gels with a disordered tholeite structure [10,27]. The main minerals of the AASR materials are hydrated calcium aluminosilicate (CaAl2Si2O8-4H2O), calcite (CaCO3), hematite (Fe2O3), hard alumina monohydrate (AlO(OH)), and calcium chalcocite (Al6CCa0.4H4.4Na7.6O29.2Si6), which are consistent with the main mineral composition of the original red mud. Furthermore, as the content of RM increased, the intensity of diffraction peaks gradually became stronger, indicating that the red mud hardly participated in the alkali activated reaction, which corresponds well to the results from isothermal calorimetry.
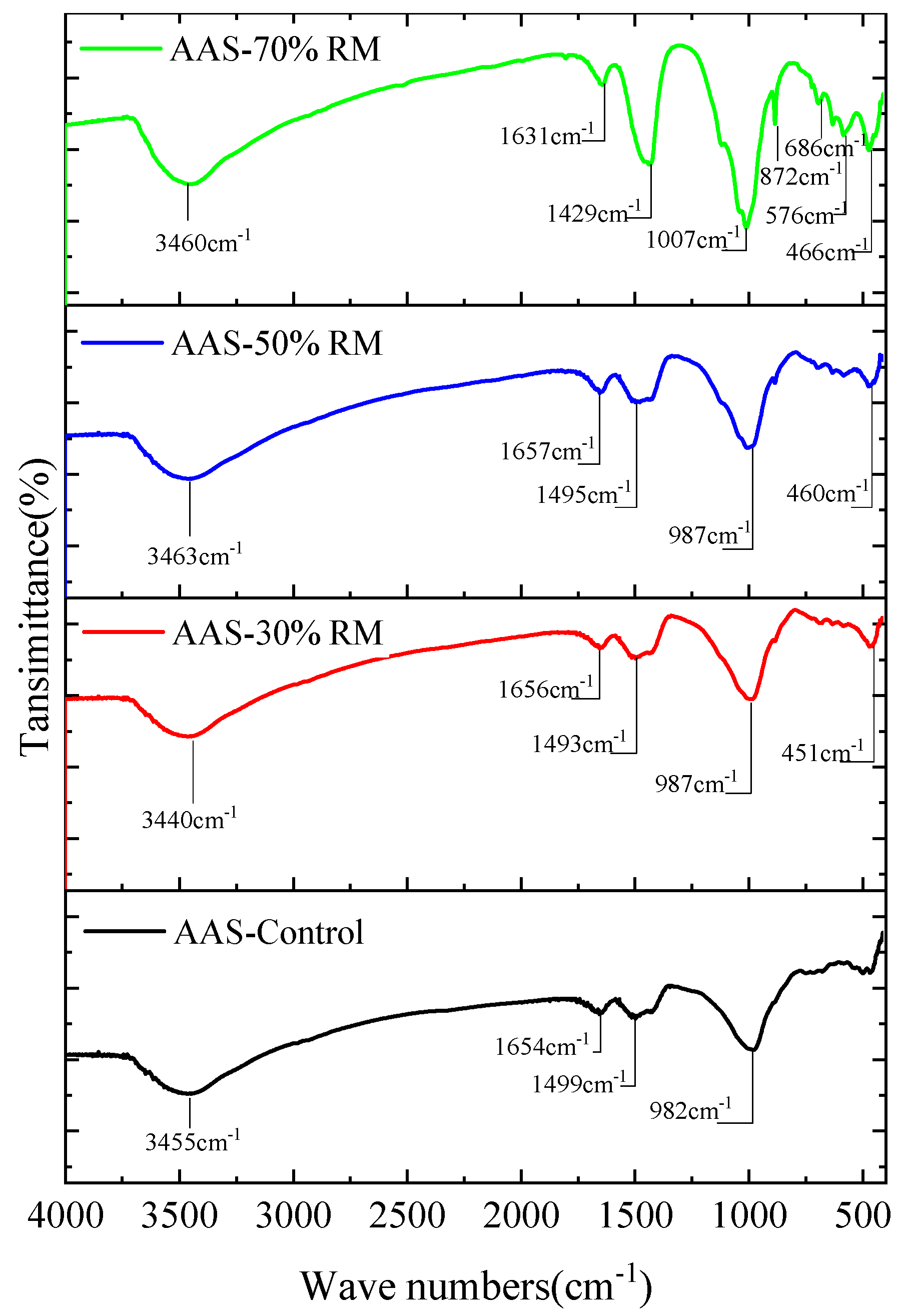
- FTIR: Due to the poor crystallinity of most of the AASR products, which was difficult to detect by the XRD, the FTIR test of each group of paste specimens were then carried out, and the results are shown in Figure 8. The absorption peaks, at around 3460 cm−1 and 1631 cm−1, can be assigned to the stretching vibration of [O-H] from water molecules [28]. One important feature in Figure 8 is that the bending vibration absorption peaks and the stretching vibration absorption peaks, at around 982–1007 cm−1 and 466 cm−1, which are assigned to the [Si-O] from C-A-S-H [29], became narrower with the increase of red mud content, indicating that the formation of the C-A-S-H gel is reduced. Moreover, bands at 1495 cm−1 and 872 cm−1 could be found in all specimens, which can be attributed to the anti-symmetric stretching and out-of-plane bending modes of CO32− ions, indicating the presence of calcite (originated from raw material of red mud). After adding red mud, the intensity of these bands became stronger, and the more red mud that was introduced, the stronger the intensity of these bands. This indicates that the geopolymerization reaction is less as increase of red mud, which is in agreement with the XRD results.
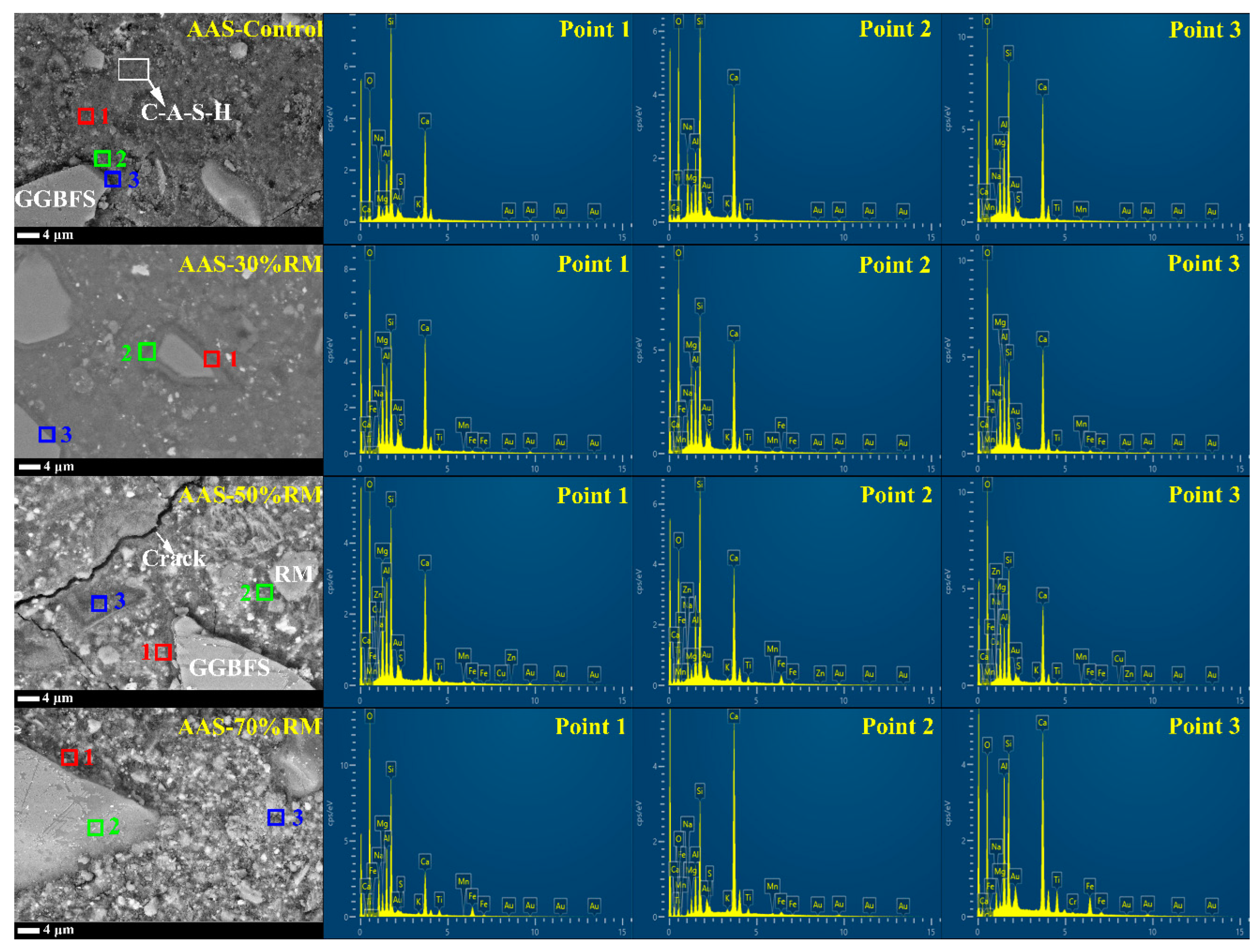
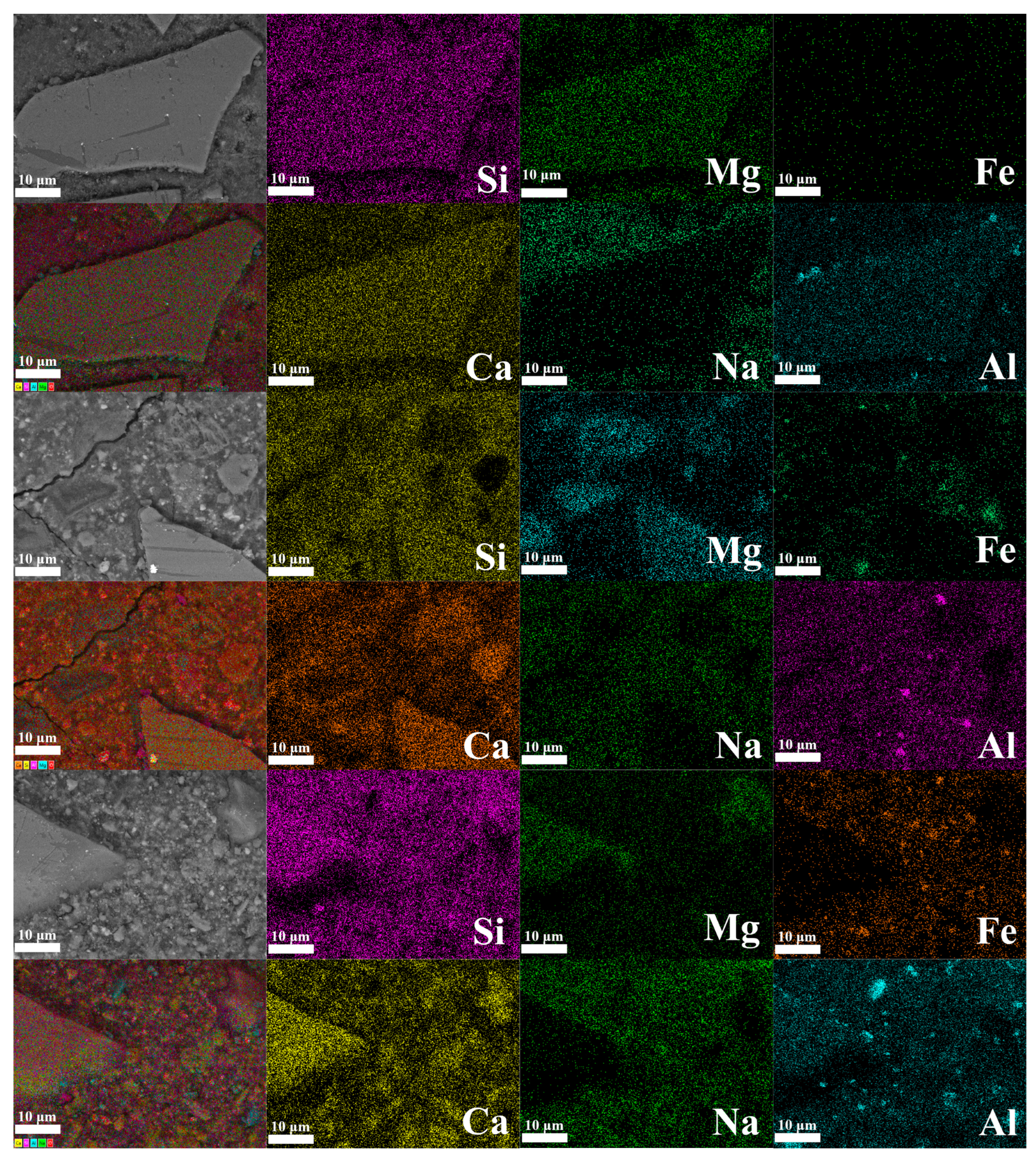
3.2.4. Micromorphology Analysis
4. Conclusions
- The addition of the proper amount of red mud makes the alkalinity of the AASR system increase, which is beneficial to the development of AASR strength, while when the amount of red mud is too great, the compressive strength at the same age decreases with the increase of red mud, and the 28-day compressive strength decreases by 78.8% compared with the baseline group.
- The mechanism of the effect of red mud content on the compressive strength of AASR indicates that it is difficult to leach the silicon and aluminum components in red mud, as only a small part of red mud participates in hydration, the degree of hydration reaction of the system was low, and the formation of hydration products makes the strength of the system develop slowly.
- Due to the difficulty of red mud dissolution, it slows down the hydration process of the AASR system, which makes the initial and final setting time longer.
- The red mud content does not change the type of hydration products of the AASR cementitious material, but with the increase of red mud content, the amount of hydration generation decreases, which is one of the reasons for the reduction of the AASR system’s compressive strength.
- The addition of red mud introduces more harmful pores in the specimen, and when the content of red mud is 70%, the 28-day connected porosity reaches 39.05%; at the same time, the filling and refinement effect of the reaction products on the pores is small, resulting in a loose paste structure and reduced strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gräfe, M.; Power, G.; Klauber, C. Bauxite residue issues: III. Alkalinity and associated Chemistry. Hydrometallurgy 2011, 108, 60–79. [Google Scholar] [CrossRef]
- Occhicone, A.; Vukčević, M.; Bosković, I.; Mingione, S.; Ferone, C. Alkali-activated red mud and construction and demolition waste-based components: Characterization and environmental assessment. Materials 2022, 15, 1617. [Google Scholar] [CrossRef]
- Oprčkal, P.; Mladenovič, A.; Zupančič, N.; Ščančar, J.; Milačič, R.; Serjun, V.Z. Remediation of contaminated soil by red mud and paper Ash. J. Clean. Prod. 2020, 256, 120440. [Google Scholar] [CrossRef]
- Li, X.; Zhang, Q.; Mao, S. Investigation of the bond strength and microstructure of the interfacial transition zone between cement paste and aggregate modified by Bayer red Mud. J. Hazard. Mater. 2021, 403, 123482. [Google Scholar] [CrossRef]
- Occhicone, A.; Vukčević, M.; Bosković, I.; Ferone, C. Red Mud-Blast Furnace Slag-Based Alkali-Activated Materials. Sustainability 2021, 13, 11298. [Google Scholar] [CrossRef]
- Duxson, P.; Provis, J.L.; Lukey, G.C.; Van Deventer, J.S. The role of inorganic polymer technology in the development of ‘green concrete’. Cem. Concr. Res. 2007, 37, 1590–1597. [Google Scholar] [CrossRef]
- Zhang, J.; Li, S.; Li, Z.; Liu, C.; Gao, Y. Feasibility study of red mud for geopolymer preparation: Effect of particle size Fraction. J. Mater. Cycles Waste Manag. 2020, 22, 1328–1338. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, J.; Li, S.; Gao, Y.; Liu, C.; Qi, Y. Effect of different gypsums on the workability and mechanical properties of red Mud-slag based grouting Materials. J. Clean. Prod. 2020, 245, 118759. [Google Scholar] [CrossRef]
- Chen, X.; Guo, Y.; Ding, S.; Zhang, H.; Xia, F.; Wang, J.; Zhou, M. Utilization of red mud in Geopolymer-based pervious concrete with function of adsorption of heavy metal Ions. J. Clean. Prod. 2019, 207, 789–800. [Google Scholar] [CrossRef]
- Lemougna, P.N.; Wang, K.T.; Tang, Q.; Cui, X.M. Study on the development of inorganic polymers from red mud and slag system: Application in mortar and lightweight materials. Constr. Build. Mater. 2017, 156, 486–495. [Google Scholar] [CrossRef]
- Zhang, P. Optimized Design and Performance Study of Red Mud-Based Alkali-Inspired Cementitious Materials; South China University of Technology: Guangzhou, China, 2016. [Google Scholar]
- Garanayak, L. Strength effect of alkali activated red mud slag cement in ambient Condition. Mater. Today Proc. 2021, 44, 1437–1443. [Google Scholar] [CrossRef]
- Liang, X.; Ji, Y. Preparation sequences and pretreatment optimization of Alkali-activated red mud and blast furnace slag-based Materials. J. Mater. Cycles Waste Manag. 2021, 23, 259–271. [Google Scholar] [CrossRef]
- Gao, Y.; Li, Z.; Zhang, J.; Zhang, Q.; Wang, Y. Synergistic use of industrial solid wastes to prepare Belite-rich sulphoaluminate cement and its feasibility use in repairing Materials. Constr. Build. Mater. 2020, 264, 120201. [Google Scholar] [CrossRef]
- Tian, K.; Wang, Y.; Dong, B.; Fang, G.; Xing, F. Engineering and Micro-properties of alkali-activated slag pastes with Bayer red Mud. Constr. Build. Mater. 2022, 351, 128869. [Google Scholar] [CrossRef]
- GB/T 17671-1999; Method of Testing Cements-Determination of Strength. The State Bureau of Quality and Technical Supervision: Beijing, China, 1999; p. 9.
- ASTM C642; Standard Test Method for Density, Absorption, and Voids in Hardened Concrete, Annual Book of ASTM Standards, 04.02. American Society for Testing and Materials: Philadelphia, PA, USA, 2002.
- Romer, M. Effect of moisture and concrete composition on the Torrent permeability Measurement. Mater. Struct. 2005, 38, 541–547. [Google Scholar] [CrossRef]
- Parrott, L.J. Moisture conditioning and transport properties of concrete test Specimens. Mater. Struct. 1994, 27, 460–468. [Google Scholar] [CrossRef]
- Collins, F.G.; Sanjayan, J.G. Capillary Shape: Influence on Water Transport within Unsaturated Alkali Activated Slag Concrete. J. Mater. Civ. Eng. 2010, 22, 260–266. [Google Scholar] [CrossRef]
- Li, Q.; Li, X.; Yang, K.; Zhu, X.; Gevaudan, J.P.; Yang, C.; Basheer, M. The Long-term failure mechanisms of alkali-activated slag mortar exposed to wet-dry cycles of sodium Sulphate. Cem. Concr. Compos. 2021, 116, 103893. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Yang, K.; Chao, Z.; Tang, D.; Tian, Y.; Wu, F.; Basheer, M.; Yang, C. The role of calcium stearate on regulating activation to form stable, uniform and flawless reaction products in alkali-activated slag Cement. Cem. Concr. Compos. 2019, 103, 242–251. [Google Scholar] [CrossRef]
- Wang, H. Research and Application of High Performance Water Glass Suspension Type Double Liquid Grouting Material; Wuhan University of Technology: Wuhan, China, 2007. [Google Scholar]
- Wu, Z. Green high performance concrete—The direction of concrete development. China Concr. Cem. Prod. 1998, 1, 3–6. [Google Scholar]
- Pacewska, B.; Wilińska, I. Usage of supplementary cementitious materials: Advantages and limitations: Part I. C–S–H, C–A–S–H and other products formed in different binding Mixtures. J. Therm. Anal. Calorim. 2020, 142, 371–393. [Google Scholar] [CrossRef]
- Zhang, J.; Li, G.; Ye, W.; Chang, Y.; Liu, Q.; Song, Z. Effects of ordinary Portland cement on the early properties and hydration of calcium sulfoaluminate Cement. Constr. Build. Mater. 2018, 186, 1144–1153. [Google Scholar] [CrossRef]
- Qaidi, S.M.; Tayeh, B.A.; Isleem, H.F.; de Azevedo, A.R.; Ahmed, H.U.; Emad, W. Sustainable utilization of red mud waste (bauxite residue) and slag for the production of geopolymer composites: A Review. Case Stud. Constr. Mater. 2022, 16, e00994. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland Cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Bayat, A.; Hassani, A.; Yousefi, A.A. Effects of red mud on the properties of fresh and hardened Alkali-activated slag paste and Mortar. Constr. Build. Mater. 2018, 167, 775–790. [Google Scholar] [CrossRef]




| Binder | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | SO3 | TiO2 |
|---|---|---|---|---|---|---|---|---|---|
| Slag | 30.79 | 13.56 | 0.96 | 8.18 | 41.59 | 0.40 | 0.46 | 2.12 | 0.94 |
| Red Mud | 13.49 | 20.39 | 30.58 | 0.32 | 15.82 | 9.15 | 0.19 | 0.66 | 7.80 |
| Binder | GGBFS/g | RM/g | WG/g | NaOH/g | Water/g |
|---|---|---|---|---|---|
| AAS-Control | 500 | / | 157.15 | 15.39 | 136.16 |
| AAS-30%RM | 350 | 150 | 157.15 | 15.39 | 136.16 |
| AAS-50%RM | 250 | 250 | 157.15 | 15.39 | 136.16 |
| AAS-70%RM | 150 | 350 | 157.15 | 15.39 | 136.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Li, Q.; Chen, P.; Yao, K.; Wang, P.; Ming, Y.; Yi, J.; Zhi, L. The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism. Appl. Sci. 2023, 13, 452. https://doi.org/10.3390/app13010452
Li J, Li Q, Chen P, Yao K, Wang P, Ming Y, Yi J, Zhi L. The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism. Applied Sciences. 2023; 13(1):452. https://doi.org/10.3390/app13010452
Chicago/Turabian StyleLi, Juntong, Qing Li, Ping Chen, Kai Yao, Penghuai Wang, Yang Ming, Jin Yi, and Lili Zhi. 2023. "The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism" Applied Sciences 13, no. 1: 452. https://doi.org/10.3390/app13010452
APA StyleLi, J., Li, Q., Chen, P., Yao, K., Wang, P., Ming, Y., Yi, J., & Zhi, L. (2023). The Effect of Bayer Red Mud Blending on the Mechanical Properties of Alkali-Activated Slag-Red Mud and the Mechanism. Applied Sciences, 13(1), 452. https://doi.org/10.3390/app13010452





