Abstract
In addition to producing bioenergy and molecules with high added value, microalgae have been recognized as an efficient microorganism for wastewater treatment. However, a major obstacle preventing its widespread use is the high energy cost of pretreatment, cultivation and downstream processes. Different types of wastewaters have been tested as culture mediums for microalgal biorefinery system. This review gives a summary of the most used microalgae strains for wastewater treatment, as well as information on the physical and chemical characteristics of domestic, agricultural, and industrial wastewaters. It also discusses wastewater pretreatment techniques, nutrient uptake and removal, biomass production and biomolecules productivities. There is also discussion on how microalgae remove contaminants from wastewater. Additionally, the problems and restrictions of microalgae-based wastewater treatment are explored, and recommendations are made for additional study and advancement. This literature review demonstrates that microalgae monoculture systems have proven to be beneficial as an innovative wastewater treatment technology, due to its high efficiencies in pollutant removals and biomolecule production; however, the upstream and downstream treatment pose a limit to industrialize the process. Until now, there has been no conventional design of the wastewater treatment process using microalgae in the biorefinery system, which constitutes a huge gap to assess a real life cycle assessment (LCA) and techno economic analysis (TEA).
1. Introduction
Nowadays, planet earth is dealing with two significant issues: energy and water crises. According to [1], between 2015 and 2040, the global energy consumption is expected to rise by 28%, while the global use of petroleum-based fossil fuels would rise from 190 to 230 quadrillion British thermal unit. Additionally, excessive fossil fuel use not only depletes natural resources but causes continual increases in the carbon dioxide emissions, which contributes toward global warming [2]. Biodiesel from microalgae has been proposed as an alternative renewable fuel source [3], but the cost of production (160–480 USD BBL−1) is more than the cost of crude oil and over 50% of the overall price is often attributed to the price of freshwater, fertilizers and the harvesting procedure [4].
Freshwater represents only 0.5% of the global water on planet earth, and its consumption increases with the increasing population and industrial activity, which generate 380 trillion L y−1 of wastewater in the world [5]. In addition, by 2050, more than 50% of the planet’s population is projected to face chronic water shortages, hence the need for wastewater recycling [6]. As reported in this review, domestic, agricultural and industrial wastewaters contain significant concentrations of nutrients, including phosphorus, carbon and nitrogen, which are required for the growth of microalgae. Microalgae-based biorefineries for biofuels and wastewaters treatment have received a lot of interest to tackle the energy and water scarcity [1,5,7]. Consequently, researchers have frequently used wastewater from different sources for microalgae cultivation, in order to determine the potential of microalgae for simultaneous wastewater treatment. Recently, Chlorella pyrenoidosa (No. FACHB-863) was employed for its wastewater treatment ability to eliminate contaminants from synthetic tobacco wastewater [8]. The study’s findings demonstrated that Chlorella pyrenoidosa may grow in this kind of effluent, with a maximum biomass of 540.24 mg L−1. Arthrospira has been cultivated in seawater with the addition of anaerobic animal waste effluent to produce biofuels (biogas, biodiesel and biohydrogen) and high added value materials [7]. Although it has been widely studied in previous research, the use of wastewater for a biorefinery system at a low cost is rare on an industrial scale, because this cultivation method still has challenges that limit its application; however, several pieces of research are now underway for its implementation in the future.
In order to identify prospective directions for future research on microalgae-based bioremediation, this review intends to highlight the advances and existing work concerning the use of microalgae in wastewater treatment for a biorefinery system.
Following these guidelines, the available literature was assessed to accomplish the purpose of this review:
- Wastewater treatment by microalgae: In this section we present the physicochemical properties of domestic wastewater that have been used in domestic wastewater treatment by microalgae, followed by the pretreatment of domestic wastewater before microalgae cultivation, as well as pollutant removals such as chemical oxygen demand (COD), total nitrate (TN) and total phosphate (TP) removals, biomass generation and the productivity of biomolecules after microalgae cultivation, using different species that have been tested in the literature. The same parameters have been explored for both agricultural wastewater and industrial wastewater.
- Microalgal mechanisms for wastewater bioremediation: As the biodegradation response of microalgae to contaminants varies from one pollutant to another, we focus on the following mechanisms in this section: CO2 fixation, nitrogen sources assimilation, phosphate sources assimilation and heavy metals biodegradation mechanisms.
- Economic analysis of microalgae wastewater treatment technologies: In this section we compared microalgae wastewater treatment technologies with co-culture technologies such as microalgae-bacteria, microalgae-fungi, microalgae-yeast and microalgae-Nanoparticle. Furthermore, we discussed a life cycle assessment (LCA) and a techno economic assessment (TEA) of microalgae-based wastewater treatment process.
- Challenges and prospects: We have proposed strategies to overcome the challenges that the microalgae-based wastewater treatment process is facing.
2. Wastewater Treatment by Microalgae
Different species of microalgae have been used to remediate various types of wastewaters in recent times. In terms of the wastewater employed, Table 1, Table 2, Table 3, Table 4, Table 5, Table 6, Table 7, Table 8, Table 9, Table 10, Table 11 and Table 12 describe the culture conditions, treatment efficiency, final biomass generated and its biochemical composition.
2.1. Domestic Wastewater
2.1.1. Physicochemical Properties
Domestic effluent is defined as water that has been used by a population and contains all the materials that have been added to it throughout its usage [9]. It is generally classified into two types (Figure 1): black-water and grey-water, and is mostly made up of discharge from domestic and home activities [10]. In some part of the world, this type of wastewater is disposed into drainage systems, lakes and rivers without any previous treatment, posing challenges to the enhancement of the community’s living conditions, because untreated wastewater has a significant negative impact on the environment and human health [9,11]. Black-water may consist of urine, flash water, fecal matter, toilet paper and others water, such as water from cleaning activities [10], and grey-water is wastewater that comes from kitchen sinks, laundromats and showers, or any other domestic activity other than toilet waste [11]. Kitchen wastewater is a type of wastewater that includes a lot of ammonium due to food protein degradation, and its excessive thickness supplements a terrible odor signal that must be treated. The treatment of kitchen wastewater by microalgae has been explored and it appeared that the collected kitchen wastewater had a high organic content due to the existence of oil, grease and food products and was acidic, with chemical oxygen demand (COD) levels ranging from 800 mg L−1 to 1400 mg L−1, compared to raw sewage wastewater which has COD levels ranging from 250 mg L−1 to 800 mg L−1 [12]. To solve this problem and make the kitchen wastewater acceptable for microalgal development, it should be diluted by about 1:1 with water [12].
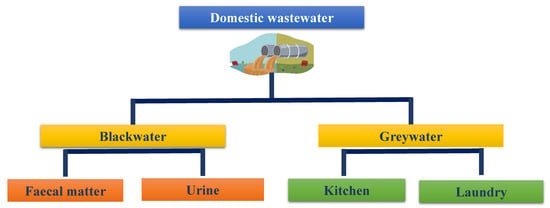
Figure 1.
Domestic wastewater types.
The composition of different domestic wastewaters, which have been used in the literature for microalgae culture, is given in Table 1. As described in this table, sewage effluent has a pH of 6.6 to 8.81, a chemical oxygen demand (COD) of 97 mg L−1 to 1100 mg L−1, a total nitrate content (TN) of 0.22 mg L−1 to 265 mg L−1, a total phosphate concentration (TP) of 0.6 mg L−1 to 170 mg L−1 and other constituents such as chloride and potassium. Kitchen wastewater has a COD content of 560 mg L−1, whereas toilet wastewater has a COD value of 506.8 mg L−1, with TN and TP levels of 203.6 mg L−1 and 22.3 mg L−1, respectively. As a reason, using domestic wastewater for microalgae growth is a suitable choice for both wastewater treatment and the generation of biodiesel and other chemicals from the produced biomass.

Table 1.
Domestic wastewater composition.
Table 1.
Domestic wastewater composition.
| Type | pH | TSS (mg L−1) | TOC (mg L−1) | COD (mg L−1) | NO3-N (mg L−1) | NO2-N (mg L−1) | NH4-N (mg L−1) | NH3-N (mg L−1) | PO4-P (mg L−1) | TN (mg L−1) | TP (mg L−1) | References |
| Secondarily Treated Sewage | 7.63 | - | 5.5 | - | 7.67 | 0.01 | 0.17 | - | 0.02 | 8.9 | 0.04 | [13] |
| 7.2 | 352 | - | 328 | - | - | - | - | - | 18 | 7 | [14] | |
| Raw Sewage | 8.77 | 740 | - | 784 | 30.25 | - | - | - | 1.7 | - | - | [12] |
| - | - | 310–560 | 1000–1100 | - | - | - | - | - | 230–260 | 15 | [15] | |
| 7.80 | - | - | 702 | 10.72 | - | - | 33.1 | 2.25 | - | - | [16] | |
| 7.1 | - | - | 252 | - | - | 205 | - | - | 265 | 17.1 | [17] | |
| 7.8 | - | - | 104 | - | - | 26.3 | - | - | 33 | 0.6 | [18] | |
| 6.6–7.6 | - | - | 190–230 | - | - | - | 20–35 | - | 40–60 | 4.5–5.6 | [19] | |
| 7.82 | - | - | 426 | 1.156 | - | 46.2 | - | - | - | 3.22 | [20] | |
| 7.7 | 3500 | - | 286 | 197 | - | 992 | - | - | - | 286 | [21] | |
| 7.86 | - | - | 618 | - | - | 54 | - | - | 80 | 4.2 | [22] | |
| 7.56 | - | 154 | 496 | 2.45 | - | 12.5 | - | 7.1 | 24.45 | 9.6 | [23] | |
| - | - | 208.15 | 446.25 | 16.58 | - | 37.64 | - | - | 61.47 | 7.42 | [24] | |
| 6.9–7.5 | - | - | - | 2–5 | - | 36–47 | - | - | - | 12–19 | [25] | |
| 7.9–8.2 | - | - | 296–858 | 1.5–5.6 | - | 58.2–136.9 | - | - | - | 7.9–27.7 | [26] | |
| 8.81 | - | 20.58 | - | 0.07 | - | 30.02 | - | - | 30.46 | 2.6 | [27] | |
| 7.2 | - | - | 129 | - | - | - | 37 | - | 48 | 9 | [28] | |
| 7.4–7.6 | 95.8 | 201–311 | 190–310 | - | - | 45.6 | - | - | 57.9 | 4.4 | [29] | |
| 8.2 | 32 | 22.1 | 97 | 10 | - | 136 | - | 62 | 146 | - | [30] | |
| - | - | - | - | - | - | - | - | - | 315 | 10.15 | [31] | |
| 8.55 | - | 343.07 | - | - | - | - | - | - | 0.22 | 2.28 | [32] | |
| Kitchen | 6.85 | 980 | - | 560 | 52.962 | - | - | - | 2.037 | - | - | [12] |
| Toilet | 7.1 | - | - | 506.8 | - | - | 157.5 | - | 16.4 | 203.6 | 22.3 | [33] |
| Type | Chloride (mg L−1) | Na (mg L−1) | F (mg L−1) | Fe (mg L−1) | Cu (mg L−1) | Zn (mg L−1) | Mg (mg L−1) | Ca (mg L−1) | K (mg L−1) | Pb (mg L−1) | Turbidity NTU | References |
| Secondarily Treated Sewage | - | 65 | - | - | - | 0.05 | 3 | - | 27 | - | - | [14] |
| Sewage | 58.25 | 110 | - | - | - | - | - | 160 | 135 | - | - | [12] |
| 1200 | - | - | - | <0.005 | - | 14.8 | - | - | - | - | [17] | |
| 268 | - | - | - | - | - | - | - | - | - | 379 | [21] | |
| - | - | - | - | - | - | - | - | - | - | 182 | [23] | |
| 173–190 | - | - | - | - | - | 108–144 | 300–400 | - | - | - | [25] | |
| 410–435 | 222.5–312.1 | - | - | - | 0.1–0.18 | 52.1–65.7 | 31.1–31.9 | 8.4–9.8 | - | - | [26] | |
| - | - | - | - | - | - | - | - | - | - | - | [27] | |
| - | - | - | - | - | - | - | - | - | - | - | [28] | |
| - | - | - | - | - | - | - | - | - | - | 140–160 | [29] | |
| - | - | - | 0.098 | 0.0321 | 0.0218 | - | 52.2 | 12.16 | 0.1707 | - | [31] | |
| Kitchen | 92.08 | 130 | - | - | - | - | - | 180 | 148 | - | - | [12] |
| Toilet | - | - | - | - | - | - | 8.6 | 23.8 | 98.2 | - | - | [33] |
TSS (total suspended solids), TOC (total organic carbon), COD (chemical oxygen demand), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), NH3-N (ammonia-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate), Na (sodium), F (fluorine), Fe (iron), Cu (copper), Zn (zinc), Mg (magnesium), Ca (calcium), K (potassium), Pb (lead), NTU (nephelometric turbidity units) and nr (not reported).
2.1.2. Pretreatment
Table 2 shows three pretreatment procedures that have been mostly used for domestic wastewater: filtration, autoclaving and centrifugation. These pretreatments can remove suspended solids, debris and colloidal particles, which can limit light penetration and microbial contamination from wastewater. According to [33], all of their wastewater samples included significant levels of suspended particles, which might limit photosynthetic performance. As a result, they centrifuged them for 15 min at 5000 rpm before filtering the liquids through 0.45 µm polyester filters. They further sterilized the filtrated samples in an autoclave for 20 min at 121 °C to guarantee that they were axenic [33]. Other authors have filtered and sterilized the wastewater by autoclaving at 15 psi for 30 min to remove particles and microorganisms such as bacteria, fungi and microalgae [34]. The autoclaving has reduced the biological oxygen demand from 159.63 mg L−1 to 93 mg L−1 and the ammonium concentration from 31.38 mg L−1 to 17.19 mg L−1. In contrast, the total phosphate concentration increased from 9.24 mg L−1 to 12.8 mg L−1 and the nitrate concentration increased from 40.02 mg L−1 to 45.1 mg L−1 [34]. Even if the centrifugation and sterilization of wastewater is feasible for microalgae culture in the laboratory, it would be impossible to use it on a massive scale due to the increased costs of production, and the most significant barriers being the energy usage and time consumed [1].

Table 2.
Pretreatment methods of domestic wastewater before microalgae cultivation.
Table 2.
Pretreatment methods of domestic wastewater before microalgae cultivation.
| Wastewater | Pretreatment Method | Microalgae | References |
|---|---|---|---|
| Domestic | Filtered (Whatman filter paper, grade 1), autoclaved | Chaetoceros sp. and Isochrysis sp. | [35] |
| Municipal | Settled in flask for two hours and pretreated by passing Whatman filter paper | Chlorella minutissima | [23] |
| Domestic | Filtered using a 0.2 μm nylon membrane filter | Scenedesmus sp. | [36] |
| Domestic | Filtered through a mesh sieve (100 µm) | Chlorella sp. and Scenedesmus sp. | [24] |
| Domestic | Pre-filtered using filter cloth (nylon monofilament, pore size 25 μm) and autoclaved at 121 °C for 20 min | Chlorella variabilis TH03 | [37] |
| Septic tank | Centrifuged at 5000 rpm for 15 min, filtered by 0.45 μm polyester filters and sterilized by an autoclave for 20 min at 121 °C | Chlorella pyrenoidosa (FACHB-9) | [33] |
| Municipal | Filtered using LLG-filter papers (pore size 5 µm to 13 µm) | Chlorella sorokiniana (UTEX 1230) | [22] |
| Municipal | Filtered with a 2 μm filter | Phaeodactylum tricornutum | [28] |
| Domestic | Filtered using nylon mesh with pore size ∼50 μm | Botryococcus sp. | [14] |
| Domestic | Filtered | Chlorella sp. | [12] |
| Domestic | Autoclaved at 121 °C for 30 min | Chlorella pyrenoidosa | [19] |
| Municipal | Filtered and autoclaved at 121 °C for 20 min | Chlorella zofingiensis | [31] |
| Municipal | Filtered through glass microfiber filters with 0.6-μm pores and autoclaved at 121 °C under 15 psi for 20 min | Dunaliella salina | [38] |
| Domestic | Autoclaved at 121 °C for 30 min | Chlorella pyrenoidosa | [20] |
| Municipal | Filtered and sterilized using vacuum filtration unit and autoclaved at 121 °C for 15 min | Nannochloropsis oceanica | [32] |
| Domestic | Filtered and sterilized by autoclaving at 15 psi for 30 min | Chlorella vulgaris and Nannochloropsis oculata | [34] |
2.1.3. Microalgae Treatment of Domestic Wastewater
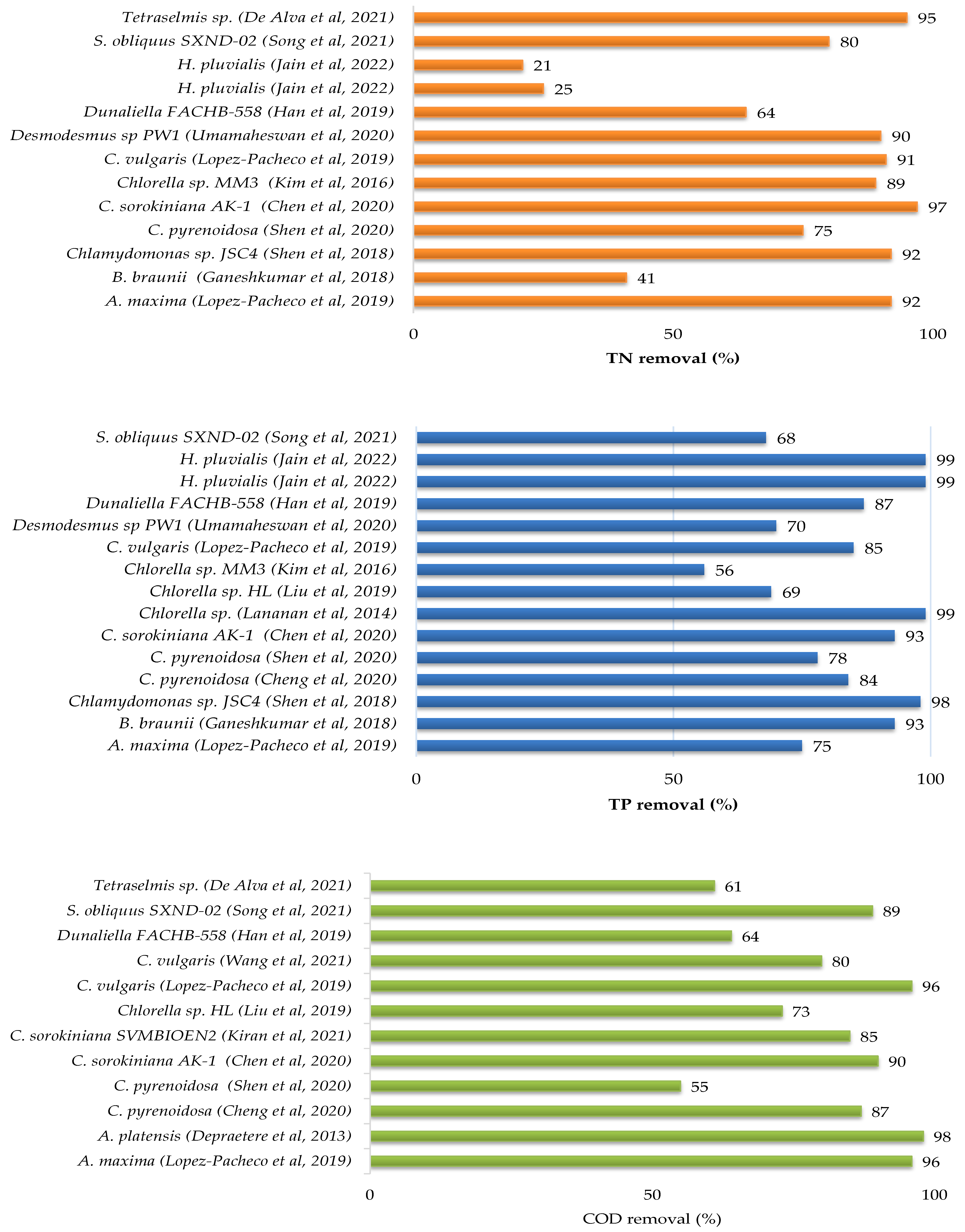
There have been several pieces of research published in the literature that have investigated the feasibility of treating sewage, kitchen and toilet wastewaters with or without dilution using microalgae. Culture conditions, initial nutrients concentrations and removal rates have been gathered in Table 3 for different microalgae and domestic wastewater treatments, while the growth parameters and metabolites production of microalgae culture in domestic wastewater are provided in Table 4. Figure 2 showed that each species of microalgae has a different capacity to reduce pollutants from domestic wastewater, and this ability varies from one wastewater to another.
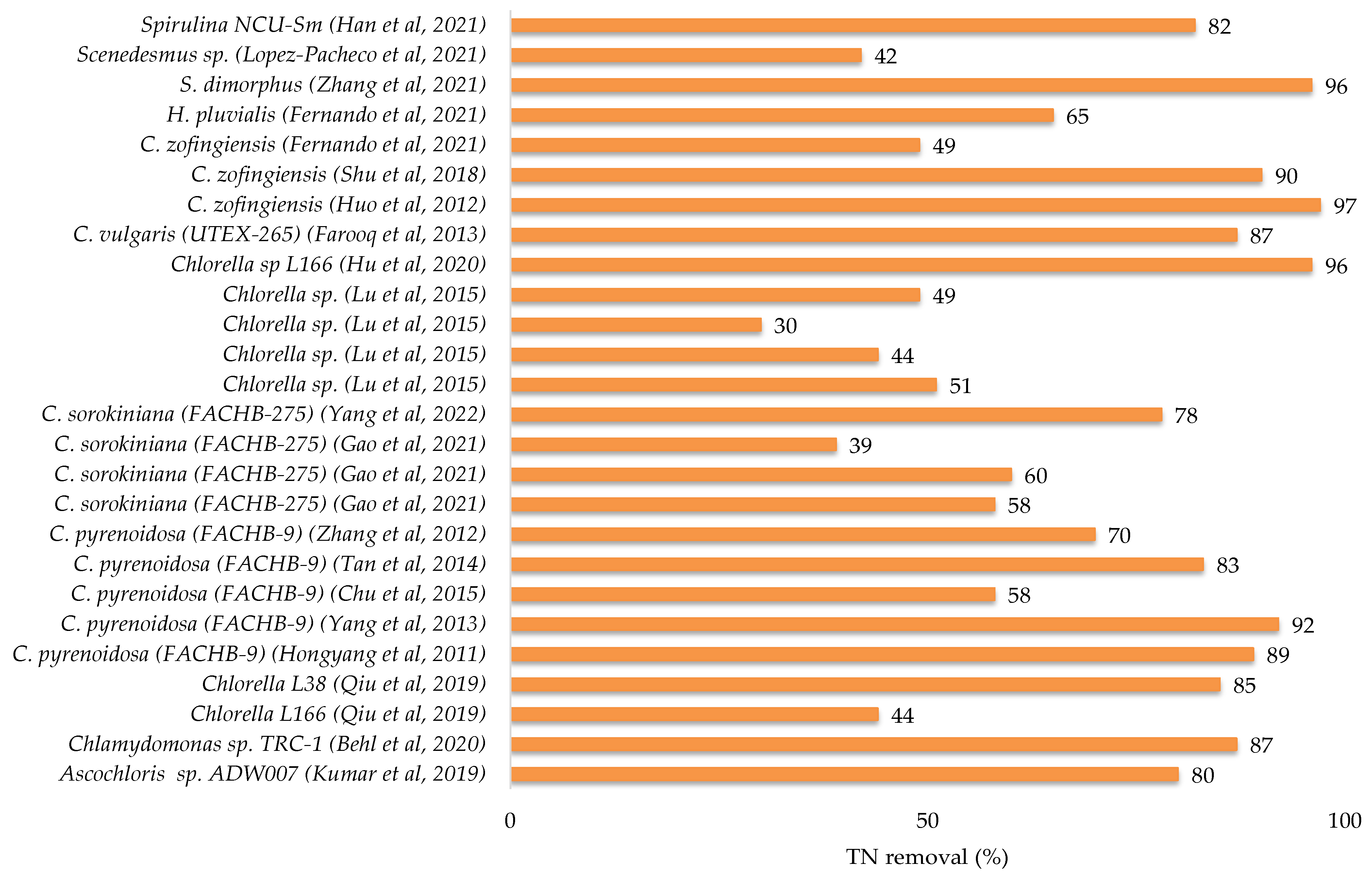
The maximum potential for total nitrogen (TN) and total phosphate (TP) removals from municipal wastewater were found for Chlorella variabilis TH03 at a 96.8% and 100% removal rate [37]. The other microalgae removed between 28% and 95% of total nitrogen and between 12% and 99% of total phosphate. The removal efficiency of chemical oxygen demand was not assessed in the majority of these studies.
Recently, Kumar et al. 2019 [12] explored the treatment of kitchen wastewater (Phosphates: 2.037 mg L−1; Nitrates: 52.962 mg L−1; COD: 560 mg L−1) and sewage wastewater (Phosphates: 1.7 mg L−1; Nitrates: 30.25 mg L−1; COD: 784 mg L−1) by the species Chlorella sp. in culture flasks in the laboratory. The nitrate and phosphate removals were 38% and 75%, respectively, for kitchen wastewater, and 67% and 88% for sewage wastewater. The COD content in kitchen wastewater was gradually reduced in the first four days, after which it was kept around the same value and varied in a modest range throughout the culture period. COD reduction efficiency was 32.14% on the final day of culture [12]. In this study, the color of the kitchen wastewater was black during microalgal culture, and they supposed that it was due to the high concentration of ammonia and high turbidity and then concluded that these conditions were not conducive to microalgal development. To solve this problem and make the kitchen wastewater acceptable for microalgal development, they diluted it by about 1:1 with water [12]. Tan et al. 2021 [33] reported that septic tank wastewater can be utilized for microalgae cultivation. They tested diverse types of wastewaters for Chlorella pyrenoidosa (FACHB-9) cultivation and demonstrated that the toilet wastewater (TN: 203.6 mg L−1; NH4-N: 157.5 mg L−1; TP: 22.3 mg L−1; COD: 5200 mg L−1) was the great medium for biomass (1.68 g L−1) and nutrient removal efficiencies (NH4-N: 90.8%; TP: 62.9%; COD: 61.3%). According to the same study, discharging kitchen or laundry wastewater decreased the biomass production by 50.5–79%, and should be isolated from toilet wastewater. Kumar et al. 2021 [35] compared the remediation capability of the marine diatom Chaetoceros with the haptophyte Isochrysis microalgae when grown in urban wastewater. Their results demonstrated that the two strains can successfully remediate NO2-N of 0.63% from 10% of domestic wastewater by Chaetoceros and 5.57% from 30% of domestic wastewater by Isochrysis, as well as a total phosphorus removal of 83–84 and 84–94% by Chaetoceros and Isochrysis, respectively. Chemical oxygen demand was reduced more efficiently in 40% of domestic wastewater by Chaetoceros (157 mg L−1) and in 100% of domestic wastewater by Isochrysis (93%). According to [35], the use of domestic wastewater as a culture medium for Chaetoceros sp. and Isochrysis sp. has shown their effectiveness as a sustainable food source and a source of biofuels due to their production of total polyunsaturated fatty acids (PUFAs) from 33.5% to 71.6% in the case of Chaetoceros and from 20% to 63.4% in Isochrysis.
The effectiveness of autotrophic and heterotrophic metabolisms in different microalgae species for wastewater bioremediation and the generation of sustainable microalgal products/coproducts was also evaluated using various culture regimes [27,34,39,40]. For example, Leong et al. 2022 [39] assessed the photoperiod-induced mixotrophic metabolism in Chlorella vulgaris. They measured cell biomass growth and lipid content of Chlorella vulgaris and conducted organic nutrient removals from municipal wastewater (COD: 145 mg L−1; NH4-N: 48 mg L−1) with distinct light: dark photoperiod cycles. In their results, the 16:8 (light: dark) photoperiod was shown to be appropriate for producing high biomass (0.89 g L−1) and lipid production (0.16 g L−1), while also removing chemical oxygen demand and ammonium-nitrogen from municipal wastewater with near–complete removal (>94%).
The removal of nutrients from municipal wastewater by Chlorella vulgaris and Nannochloropsis oculata was examined utilizing mixotrophic culture with glycerol (0 g L−1 to 5 g L−1) [34]. The addition of 2 g L−1 of glycerol in the municipal wastewater as a medium for Chlorella vulgaris enhanced the biomass production to 56 mg L−1 d−1 and had a total nitrate removal of 64%. However, for N. oculata, the best nitrogen removal (80.62%) was obtained with 3 g L−1 of glycerol, with COD and TP removals of 96.3 and 60.72%, respectively, using 1 g L−1 and 5 g L−1 of glycerol. C. vulgaris had the highest lipid content (15.11%) when municipal wastewater was supplemented with 5 g L−1 of glycerol compared to wastewater without glycerol (7.72%). In the same way, N. oculata had a high lipid content (8.91%) with 2 g L−1 of glycerol compared to the culture without glycerol (4.59%) [34].
Similar to this finding, [40] demonstrated that combining a municipal wastewater with glycerol for boosting the mixotrophic culture of C. vulgaris Wu-G22 was a viable option for integrating wastewater treatment with energy production from algal biomass. They found that this microalga had the highest removals for COD, TN and PO4-P from 6195.6 mg L−1 to 448.47 mg L−1, from 46.78 mg L−1 to 3.39 mg L−1 and from 9.79 mg L−1 to 0.75 mg L−1, respectively, when domestic wastewater was supplemented with 50 mM of glycerol [40]. In addition, C. vulgaris Wu-G22 produced a high lipid content of 15.7% and produced contents of other compounds such as carbohydrates and proteins by about 7.3% and 70%, respectively [40].
A recent study used sodium acetate (NaAc) to enhance the removal efficiency of nutrients from municipal wastewater [27]. S. obliquus cultivated in the municipal wastewater grew faster and accumulated more lipids than those cultivated in the BG11 synthetic medium, indicating that the domestic wastewater might be used to replace the synthetic medium for microalgae growth [27]. Furthermore, introducing exogenous NaAc to mixotrophic cultivation significantly increased the algal growth and lipid synthesis [27]. The growth of Scenedesmus obliquus was 2.40 times greater (from 0.2 mg L−1 to 0.48 mg L−1) with the addition of 1 g L−1 of sodium acetate than that in the municipal wastewater without supplement. Furthermore, this concentration enhanced the microalga removals of nitrogen and phosphorus by 1.75 (from 46.85% to 82.2%) and 2.23 times (from 34.18% to 76.35%), respectively, accompanied with a high lipid productivity of 22.08 mg L−1 d−1 [27].
The dilution approach was investigated as a strategy for improving Scenedesmus sp. nutrient recovery and biomass. The author of [41] investigated the impact of different dilutions of fresh leachate (5%, 10%, 15%, 20% and 25%) using treated municipal wastewater on the growth and nutrient recovery potential of Scenedesmus sp. They found the highest removal efficiencies of 100%, 94% and 96% in 15% of fresh leachate for nitrite, ammonium, and phosphorus removals, respectively. Musetsho et al. research [42] on Acutodesmus obliquus revealed that poultry litter extract (2 g) diluted in municipal wastewater (100 mL) has the potential to be utilized as a nutrient and water source for this species. A. obliquus produced the most biomass (1.90 g/L) and had the greatest NO3-N, NH4-N and PO4-P removal rates (79.51%, 81.82%, and 80.52%, respectively). Furthermore, raw poultry litter extract had a slightly greater COD recovery efficiency of 50.80% when compared to poultry litter diluted in municipal wastewater (40.47%), which might be attributed to the proliferation of heterotrophic microorganisms/bacteria in the raw poultry litter extract [42].
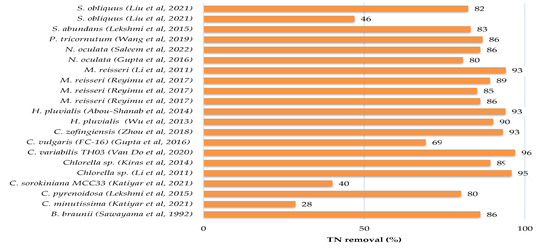
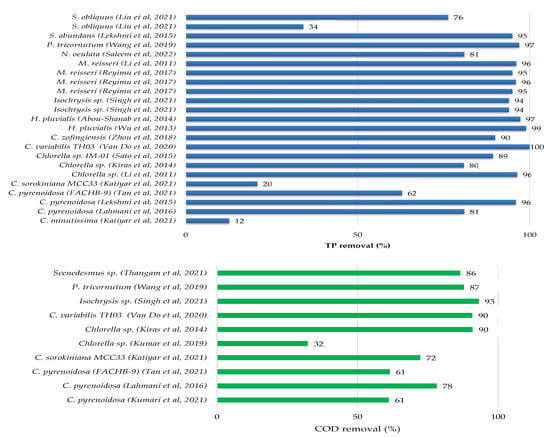
Figure 2.
Nutrients and COD removals from domestic wastewater by microalgae [12,13,16,20,21,23,25,27,28,31,33,34,35,37,41,42,43,44,45,46,47,48,49,50,51].
Figure 2.
Nutrients and COD removals from domestic wastewater by microalgae [12,13,16,20,21,23,25,27,28,31,33,34,35,37,41,42,43,44,45,46,47,48,49,50,51].
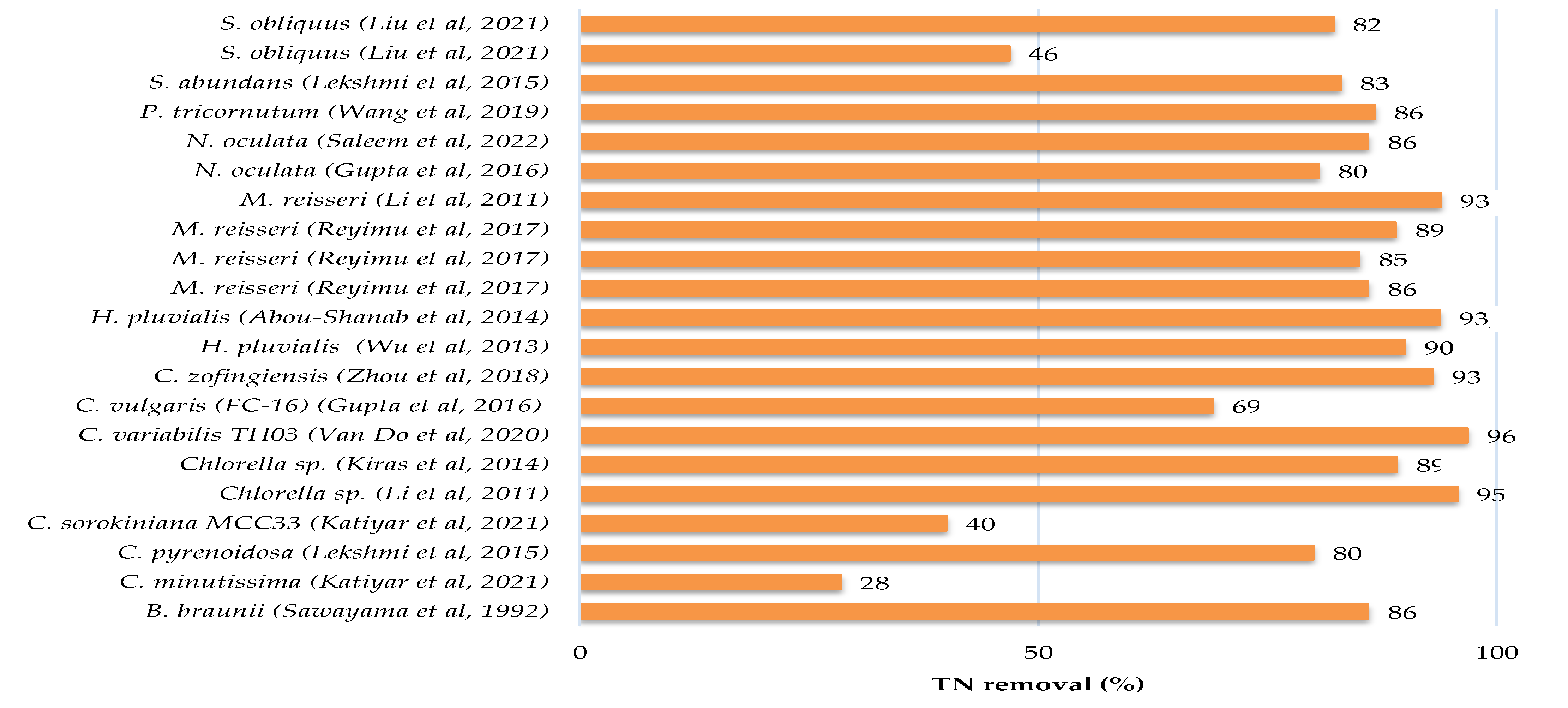
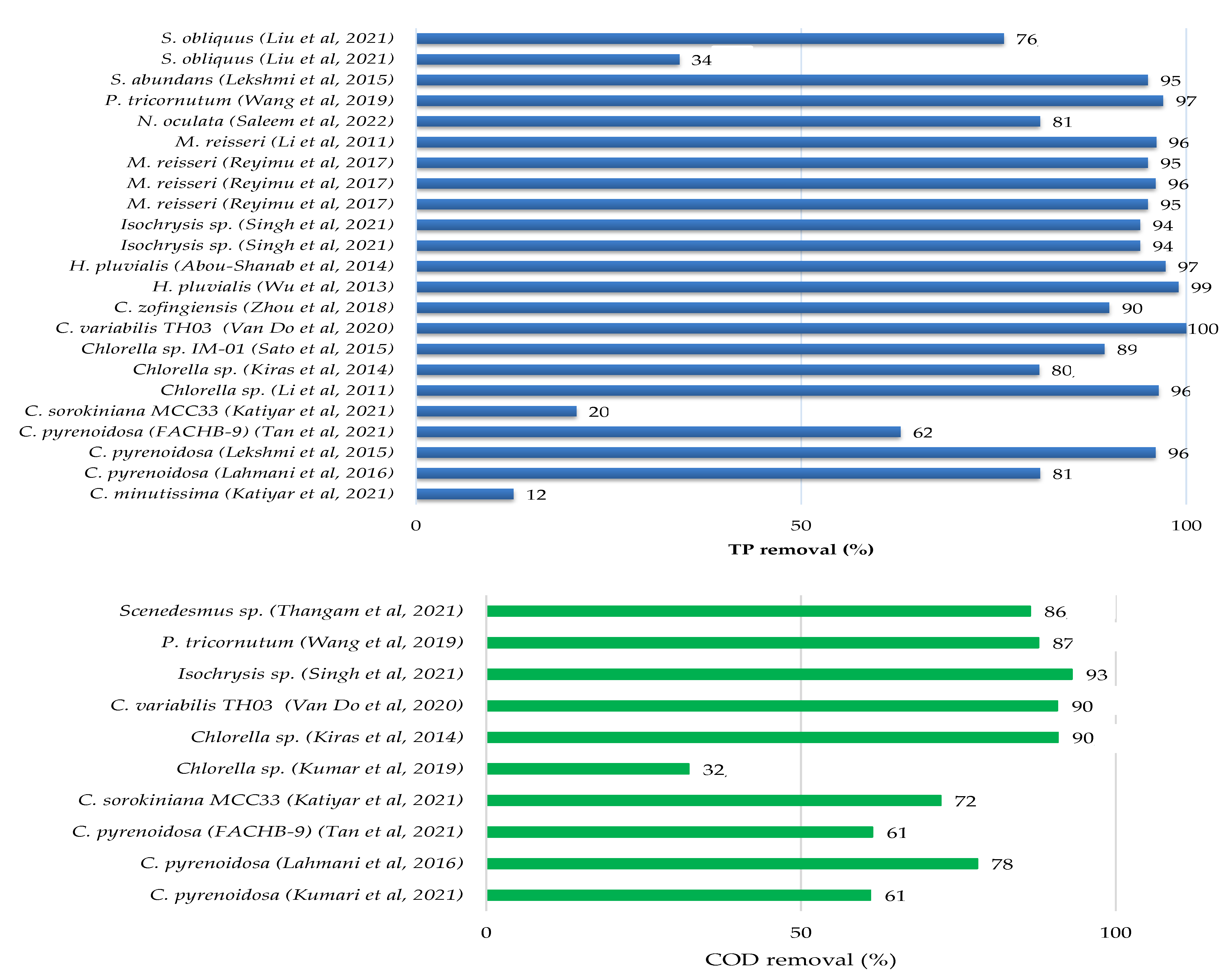

Table 3.
Treatment of domestic wastewater by the cultivation of microalgae.
Table 3.
Treatment of domestic wastewater by the cultivation of microalgae.
| Algae Used | Wastewater Type | Conditions of Culture | Nutrient Concentration | Average Nutrient Removal Rate | References |
|---|---|---|---|---|---|
| Acutodesmus obliquus | Municipal and poultry litter | 25 °C; 80 μE m−2 s−1; 16:8 light: dark | PO4-P: 21.05 mg L−1; NO3-N: 13.25 mg L−1; NH4-N: 108.0 mg L−1; | NO3-N: 79.51%; NH4-N: 81.82%; PO4-P: 80.52% | [42] |
| Botryococcus Braunii | Secondary treated sewage | 25 °C; 3000 lx; 1% CO2 (50 mL min−1) | TN: 8.9 mg L−1; TP: 0.04 mg L−1 | TN:86%; TP:50% | [13] |
| Botryococcus sp. | Domestic | 25 °C; 30 μE m−2 s−1; 16:8 light: dark | TN:18 mg L−1; COD: 328 mg L−1; PO4-P: 7 mg L−1 | nr | [14] |
| Chaetoceros sp. | Municipal | 25 °C; 100 μE m−2 s−1; 12:l2 light: dark | nr | NO2-N: 0.63%; TP: 83–84%; COD: 157 mg L−1 | [35] |
| Chlorella minutissima | Municipal | 24 °C | COD: 496.0 mg L−1; TN: 24.45 mg L−1; TP: 9.6 mg L−1 | TN: 28.46%; TP: 12.68%; COD: 61.69% | [23] |
| Chlorella pyrenoidosa | Sewage treatment plant | 26 °C; 1500 lx | PO4-P: 2.25 mg L−1; NO3-N: 10.72 mg L−1; COD: 702 mg L−1 | NO3-N: 99.2%; PO4-P: 70.1%; COD: 61.0% | [16] |
| Domestic | Open pond systems using direct sunlight: 18–31 °C (day) and 6–15 °C (night); the insolation 9 h a day | NH4-N: 46.2 mg L−1; TP: 3.22 mg L−1; COD: 426 mg L−1 | NH4-N: 95%; TP: 81%; COD: 78% | [20] | |
| Raw domestic | Continuous illumination of 1800 lx | TN: 197 mg L−1; NH4-N: 992 mg L−1; TP: 286 mg L−1 | NH4-N: 99%; TP: 96%; TN: 80% | [21] | |
| Chlorella pyrenoidosa (FACHB-9) | Municipal | 23.2 °C; 4000 lx; 24:0 light: dark | TN: 33 g L−1; TP: 0.6 g L−1; COD: 104 g L−1 | nr | [18] |
| Municipal | nr | TN: 50 mg L−1; NH4– N: 40 mg L−1; TP: 5 mg L−1; COD: 240 mg L−1 | nr | [43] | |
| Septic tank effluents (toilet) | 25 °C, 260 μE m−2 s−1; 12:12 light: dark | TN: 203.6 mg L−1; NH4-N: 157.5 mg L−1; TP: 22.3 mg L−1; COD: 5200 mg L−1 | NH4-N: 90.8%; TP: 62.9%; COD: 61.3% | [33] | |
| Chlorella Sorokiniana MCC33 | Municipal | 25 °C; | COD: 496.0 mg L−1; TN: 24.45 mg L−1; TP: 9.6 mg L−1 | TN: 40%; TP: 20.83%; COD: 72.17% | [23] |
| Chlorella sp. | Kitchen | 26 °C; 4000 lx; 12:12 light: dark | PO4-P: 2.037 mg L−1; NO3-N: 52.962 mg L−1; COD: 560 mg L−1 | NO3-N: 38%; PO4-P: 75%; COD: 32.14% | [12] |
| Sewage | 26 °C; 4000 lx; 12:12 light: dark | PO4-P: 1.7 mg L−1; NO3-N: 30.25 mg L−1; COD: 784 mg L−1 | NO3-N: 67%; PO4-P: 88% | [12] | |
| Primary effluent treatement plant | 20 °C; 16:8 light: dark; 60 μE m−2 s−1 | COD: 93 mg L−1; TN: 36.1 mg L−1; TP: 4.0 mg L−1 | TN: 95.7%; TP: 96.4% | [44] | |
| Raw centrate from municipal wastewater | 25 °C; 50 μE m−2 s−1 | COD: 2304 mg L−1; TN: 116.1 mg L−1; TP: 212.0 mg L−1 | TN: 89.1%; TP: 80.9%; COD: 90.8% | [45] | |
| Chlorella sp. IM-01 | Municipal | 27 °C; 2000 lx | NO2-N 1222.1 mg L−1; NO3-N: 112.7 mg L−1; NH4-N: 282.4 mg L−1; TP: 1.51 mg L−1 | NO2-N: 70.42%; NO3-N: 97.81%; NH4-N: 98.35%; TP: 89.39% | [46] |
| Chlorella variabilisTH03 | Domestic | 25.5–35 °C; 12,670 lx to 107,695 lx (outdoor) | nr | COD: 64.7% to 90.7%; TN: 85.1–96.8%; TP: 99.7% to 100% | [37] |
| Chlorella vulgaris | Municipal | 1200 lx; 16:8 light: dark | COD: 145 mg L−1; NH4-N: 48 mg L−1 | (>94% of COD and NH4-N) | [39] |
| Chlorella vulgaris (FC-16) | Municipal + glycerol | 25 °C, 100 μE m−2 s−1; 12:12 light: dark | TN: 45.1 mg L−1 | TN: 69.04% | [34] |
| Chlorella vulgaris Wu-G22 | Domestic (unsterilized) + Glycerol (50 mM) | 25 °C; 174 μE m−2 s−1; 12:12 light: dark 2.5% CO2 | COD: 6195.6 mg L−1; TN: 46.78 mg L−1; PO4-P: 9.79 mg L−1 | COD: 448.47 mg L−1; TN: 3.39 mg L−1; PO4-P: 0.75 mg L−1 | [40] |
| Chlorella zofingiensis | Municipal + effluent from anaerobic digestion of piggery waste (92% + 8%) | 25 °C, 150 μE m−2 s−1; 12:12 light: dark; 5% CO2; (Indoor); 5–6% CO2 (Outdoor) | TN: 76.34 mg L−1; TP: 16.56 mg L−1 | TN: ~93%, TP: ~90% | [31] |
| Dunaliella salina | 75% Municipal + 25% saline water | 20 °C; 120 μE m−2 s−1 | NO3-N: 40.7 mg L−1; NH4-N: 0.95 mg L−1; PO4-P: 3.8 mg L−1 | NO3-N: 84.2%; NH4-N: 71.0%; PO4-P: 47.5% | [38] |
| Haematococcus pluvialis | Raw primary effluent | 25 °C, 3000 lx (Green stage)/35,400 lx (Red stage); 12:12 light: dark; 5% CO2 | TN: 20.1 mg L−1; TP: 2.2 mg L−1 | TN: 90%; TP: 99% | [47] |
| Domestic secondary effluent | 25 °C, 55–60 μE m−2 s−1; 14:10 light: dark | TN: 7.0 mg L−1; TP: 0.46 mg L−1 | TN: 93.8%; TP: 97.3% | [48] | |
| Isochrysis sp. | Municipal | 25 °C; 100 μE m−2 s−1; 12:12 light: dark | nr | NO2-N: 5.57%; TP: 84–94%; COD: 93% | [35] |
| 80% Municipal | 25 °C; 100 μE m−2 s−1; 12:12 light: dark | nr | TP: 94% | ||
| Micractinium reisseri | Influent (Municipal) | 27 °C; 40 μE m−2 s−1 | TN: 15 mg L−1; TP: 3 mg L−1 | TN: 86%; TP: 95% | [49] |
| Secondary effluent | 27 °C; 40 μE m−2 s−1 | TN: 13 mg L−1; TP: 2 mg L−1 | TN: 85%; TP: 96% | ||
| Tertiary effluent | 27 °C; 40 μE m−2 s−1 | TN: 11 mg L−1; TP: 1.6 mg L−1 | TN: 89%; TP: 95% | ||
| Micractinium sp. | Primary effluent | 20 °C; 60 μE m−2 s−1; 16:8 light: dark | TN: 36.1 mg L−1; TP: 4.0 mg L−1 | TN: 93.9%; TP: 96.1% | [44] |
| Nannochloropsis oceanica | Municipal sewage | 25 °C; 60 μE m−2 s−1 | TN:0.22 mg L−1; TP: 2.28 mg L−1 | nr | [32] |
| Nannochloropsis oculata | Municipal + glycerol | 25 °C; 100 μE m−2 s−1; 12:12 light: dark | TN: 45.1 mg L−1; | TN: 80.6% | [34] |
| 75% of treated municipal | 24 °C;150 rpm mixing; Continuous illumination | TN: 3.77 mg L−1; | nr | [50] | |
| Municipal | 70–100 μE m−2 s−1 | TP: 4.4 mg L−1; TN: 57.9 mg L−1; | TN: 86%; TP: 81% | [51] | |
| Phaeodactylum tricornutum | Municipal and seawater (1:1) | 18 °C; 120 μE m−2 s−1; 12:12 light: dark | COD: 129 mg L−1; TN: 48 mg L−1; TP: 9 mg L−1; NH4-N: 37 mg L−1 | COD: 87.7%; TN: 86.7%; TP: 97.0%; NH4-N: 84.2% | [28] |
| Scenedesmus abundans | Raw domestic | Continuous illumination of 1800 lx; | TN: 197 mg L−1; NH4-N: 992 mg L−1; TP: 286 mg L−1 | NH4-N: 98%; TP: 95%; TN: 83% | [21] |
| Scenedesmus obliquus | Municipal | 25 °C; 40 ± 10 μE m−2 s−1 | TN: 30.46 mg L−1; TP: 2.60 mg L−1 | TN: 46.85%; TP: 34.18% | [27] |
| Municipal + 1 g L−1 of sodium acetate | 25 °C; 40 ± 10 μE m−2 s−1 | TN: 30.46 mg L−1; TP: 2.60 mg L−1 | TN: 82.20%; TP: 76.35% | ||
| Scenedesmus sp. | 15% Fresh leachate from transfer station | 25 °C; 75 μE m−2 s−1; 14:10 light: dark | NH4-N: 507 mg L−1; PO4-P: 109 mg L−1; NO2-N: 0.9 mg L−1 | NH4-N: 94%; PO4-P: 96%; NO2-N: 100% | [41] |
| Domestic | 20 °C; 12:12 light: dark | NO3-N: 2.39 mg mL−1; PO4-P: 18.53 mg mL−1; COD: 257 mg mL−1 | NO3-N: 71.2%; PO4-P: 89.6%; COD: 86.38% | [25] | |
| Primary urban | nr | PO4-P: 7.9–27.7 mg L−1; NH4-N: 58.2–136.9 mg L−1 | PO4-P: 0.65 mg m−2 d−1; NH4-N: 99%; | [26] | |
| Domestic | 24 °C; 60 μE m−2 s−1; 12:12 light: dark | TP: 98.3 mg L−1; NO2-N: 303.3 mg L−1; NO3-N: 131.4 mg L−1 | TP: 32 mg L−1; NO2-N: 2.36 mg L−1; NO3-N: 18.1 mg L−1; | [36] | |
| Tetraselmis suecica | 25% of treated municipal | 24 °C;150 rpm mixing; Continuous illumination | TN: 3.77 mg L−1 | nr | [50] |
COD (chemical oxygen demand), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate) and nr (not reported).
The possible utilization of a combined biogas slurry and municipal wastewater to grow Chlorella zofingiensis was studied [31]. They found that putting 8% of a pig biogas slurry in municipal wastewater (TN: 76.34 mg L−1; TP: 16.56 mg L−1) had a considerable impact on C. zofingiensis growth, removing 90% of total phosphorus and 93% of total nitrogen and increasing the lipid content by 8% compared to the BG11 medium [31].
Thus, the presented research has demonstrated that different species of microalgae can efficiently clean domestic wastewater, and that it would be much more beneficial if other nutrients such as glycerol and sodium acetate were introduced. Some of this research found that wastewater from the kitchen or laundry has an impact on microalgae growth and biochemical composition and suggested that kitchen wastewater can be diluted with water or anaerobically digested to improve bioremediation. This last procedure can remove organic debris and generate biogas under oxygen-free conditions, but the digestate includes a high concentration of ammonium.

Table 4.
Growth parameters and metabolites production of microalgae culture in domestic wastewater.
Table 4.
Growth parameters and metabolites production of microalgae culture in domestic wastewater.
| Algae Used | Wastewater Type | Growth Rate or Volumetric Productivity | Final Biomass Concentration | Production of Target Metabolites | References |
|---|---|---|---|---|---|
| Acutodesmus obliquus | Municipal and poultry litter | 140.36 mg L−1 d−1 | 1.9 g L−1 | Lipids: 38.49 mg L−1 d−1; Carbohydrates: 49.55 mg L−1 d−1 | [42] |
| Botryococcus Braunii | Secondary treated sewage | nr | 0.34 g L−1 | Hydrocarbon content: 53% | [13] |
| Botryococcus sp. | Domestic | 200 mg L−1 d−1 | 3.32 g L−1 | Carbohydrates: 1.12 g L−1; Lipids: 0.736 g L−1; | [14] |
| Chaetoceros sp. | Municipal | 2.79 g L−1 d−1 | 90.6 × 105 cells mL−1 | Lipids: 0.05 g L−1 d−1; Carbohydrates: 1.9 mg g−1 | [35] |
| Chlorella minutissima | Municipal | 0.196 d−1 | 191.66 mg L−1 d−1 | Lipids: 36.66 mg L−1 d−1 | [23] |
| Chlorella pyrenoidosa | Sewage treatment plant | nr | 4.5 g L−1 | nr | [16] |
| Domestic | nr | 1.71 g L−1 | nr | [20] | |
| Raw domestic | nr | 11.33 mg L−1; | nr | [21] | |
| Chlorella pyrenoidosa (FACHB-9) | Municipal | nr | 0.54 g L−1–0.67 g L−1 | Lipids: 30.61% | [18] |
| Municipal | nr | 0.6167 g L−1 | Lipids: 0.1083 g L−1; | [43] | |
| Septic tank effluents (toilet) | 0.54 d−1 | 1.68 g L−1 | Chlorophyll a: 4.3%; Lipids: 11.9%; Proteins: 57.2%; Carbohydrates: 19.3% | [33] | |
| Chlorella Sorokiniana MCC33 | Municipal | 0.269 d−1 | 208.35 mg L−1 d−1 | Lipids: 48.33 mg L−1 d−1 | [23] |
| Chlorella sp. | Kitchen | nr | 0.45 g L−1 | nr | [12] |
| Sewage | nr | 0.6 g L−1 | nr | [12] | |
| Primary effluent | 0.11 d−1 | nr | nr | [44] | |
| Raw centrate municipal | 0.677 d−1 | 0.92 g L−1 | FAMEs: 11.04%; | [45] | |
| Chlorella sp. IM-01 | Municipal | nr | nr | Carbohydrates: 61–94 µg mg−1 | [46] |
| Chlorella variabilisTH03 | Domestic | 0.41 d−1 | 1.67–1.85 g L−1 | nr | [37] |
| Chlorella vulgaris | Municipal | 0.32 d−1 | 0.89 g L−1 | Lipids: 0.16 g L−1 | [39] |
| Chlorella vulgaris (FC-16) | Municipal + glycerol | nr | 0.056 g L−1 d−1 | Lipids: 15.11% | [34] |
| Chlorella vulgaris Wu-G22 | Domestic (unsterilized) + Glycerol (50 mM) | nr | 1.65 g L−1 | Lipids: 15.7%; Carbohydrates: 7.3%; Proteins: 70% | [40] |
| Chlorella zofingiensis | Municipal + effluent from anaerobic digestion of piggery waste (92% + 8%) | 0.63 g L−1 d−1 | 2.51 g L−1 (indoor), 1.7 g L−1 (outdoor) | Lipids: 25.46% (indoor) 21.6% (outdoor); Carbohydrates: 21.2% (indoor) 26.9% (outdoor); | [31] |
| Desmodesmus sp. | Municipal (Ultrasound pretreatment) | nr | 75 g L−1 | Proteins: 97%; Carbohydrates: 89%; Lipids: 73%; | [52] |
| Municipal (Ozone pretreatment) | nr | 25 g L−1 | Carbohydrates: 85%; Lipids: 48%; Proteins: 25%; | ||
| Untreated Municipal | nr | nr | Lipids: 3.8%; Proteins: 8.23%; Carbohydrates: 37% | ||
| Dunaliella salina | 75% Municipal + 25% saline water | nr | 169.5 mg L−1 | nr | [38] |
| Haematococcus pluvialis | Raw primary effluent | 0.34 d−1 | nr | Astaxanthin: 3.26 mg L−1 | [47] |
| Domestic secondary effluent | 27.8 mg L−1 d−1 | 207 mg L−1 | Lipids: 43% | [48] | |
| Isochrysis sp. | 50% Municipal | 0.022 g L−1 d−1 | 110.5 × 105 cells mL−1 | Lipids: 0.02 g L−1 d−1; Carbohydrates: 2.6 mg g−1 | [35] |
| 80% Municipal | 1.27 g L−1 d−1 | nr | Lipids: 1.11 g L−1 d−1 | ||
| Micractinium reisseri | Municipal (Influent) | 1.15 d−1 | 0.22 g L−1 | Lipids: 23% | [49] |
| Municipal (Secondary effluent) | 1.04 d−1 | 0.19 g L−1 | Lipids: 30% | ||
| Municipal (Tertiary effluent) | 1.01 d−1 | 0.14 g L−1 | Lipids: 40% | ||
| Micractinium sp. | Primary effluent | 0.11 d−1 | nr | nr | [44] |
| Nannochloropsis gaditana | Municipal | 0.167 g L−1 d−1 | 2.33 g L−1 | Carbohydrates: 17.7% | [53] |
| Treatment plant | 0.15 mg L−1 d−1 | 72 mg L−1 | nr | [54] | |
| Nannochloropsis oceanica | Municipal sewage | 21.78 mg L−1 d−1 | nr | Lipids: 26.91% | [32] |
| Nannochloropsis oculata | Municipal + glycerol | nr | 0.044 g L−1 d−1 | Lipids: 8.91% | [34] |
| 75% of treated municipal | 0.5430 d−1 | 1.285 g L−1 | Carbohydrates: 2.39% | [50] | |
| Municipal | nr | 406 mg L−1 | nr | [51] | |
| Phaeodactylum tricornutum | Municipal and seawater (1:1) | 1.01 d−1 | 0.97 g L−1 | Lipids: 54.76 mg L−1 d−1 | [28] |
| Scenedesmus abundans | Raw domestic | nr | 7.23 mg L−1 | nr | [21] |
| Scenedesmus obliquus | Municipal | nr | 0.48 mg L−1 | Lipids: 9.02 mg L−1 d−1 | [27] |
| Municipal + 1 g L−1 of sodium acetate | nr | 0.2 mg L−1 | Lipids: 22.08 mg L−1 d−1 | ||
| Scenedesmus sp. | 15% Fresh leachate from transfer station | 0.17 d−1 | 133 mg L−1 d−1 | nr | [41] |
| Domestic | nr | 0.95 g L−1 | Lipids: 30.5% | [25] | |
| Primary urban | nr | 22.2 g m−2 d−1 | nr | [26] | |
| Domestic | nr | 0.84 g L−1 | Lipid productivity: 8.6 mg L−1 d−1 | [36] | |
| Tetraselmis suecica | 25% of treated municipal | 0.4778 d−1 | 0.76 g L−1 | Carbohydrates: 4.24% | [50] |
nr (not reported).
2.2. Agricultural Wastewater
2.2.1. Physicochemical Properties
A potential alternative growing medium for microalgae has been presented as the wastewater produced by agricultural activities. The properties of this type of wastewater are determined by the effluent’s source. There are four primary sources of agricultural wastewater (Figure 3), which are livestock, aquaculture, digested agricultural waste and drainage agricultural wastewater [55]. Organic carbons, volatile fatty acids, nutrients and metal ions are among the substances found in these sources [55]. Table 5 summarizes the physicochemical properties of agricultural wastewater used as a growth medium for microalgae that have been found in the literature. As described in this table, raw poultry litter extract had a pH of 7.45, chemical oxygen demand of 482.2 mg L−1, 13.25 mg L−1 of NO3-N and 108 mg L−1 of NH4-N [42]. This composition is significantly different from anaerobically digested poultry litter wastewater, which is characterized by a high concentration of ammonium (2000 mg L−1 to 3000 mg L−1 of NH4-N) and total nitrogen (2900 mg L−1 to 3200 mg L−1 of TN) [56]. Swine wastewater contained a high concentration of chemical oxygen demand (419.88 mg L−1 to 85,600 mg L−1), total nitrogen (163.40 mg L−1 to 5685 mg L−1) and total phosphate (26.2 mg L−1 to 284 mg L−1), and the pH was reported at 6.83–8.1 [30,57,58,59,60,61,62]. The mass cultivation of fish necessitates a large volume of freshwater and produces a large amount of aquaculture wastewater [5]. Bioremediation of aquaculture wastewater by microalgae was reported in the literature [60,63,64,65]. The pH was at 7.82–8.5, COD was at 30.30 mg L−1 to 367.39 mg L−1, TN was at 4.12–60 mg L−1 and the TP was at 0.16 mg L−1 to 6.8 mg L−1 for aquaculture wastewater physicochemical composition [60,63,64,65]. In addition, Table 5 showed that agricultural wastewater contains a high content of suspended particles (32.15 mg L−1 to 32,951.5 mg L−1) and different metal ions such as Zn, Cu, Mg, Ca and K, with concentrations depending on the wastewater source.
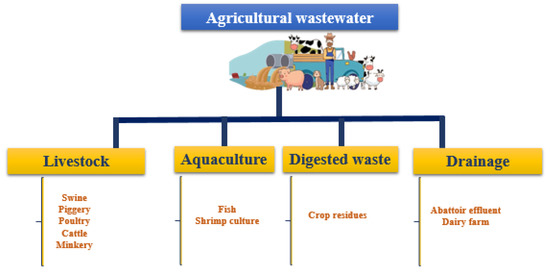
Figure 3.
Agricultural wastewater types.

Table 5.
Agricultural wastewater composition.
Table 5.
Agricultural wastewater composition.
| Type | pH | TSS (mg L−1) | TOC (mg L−1) | COD (mg L−1) | NO3-N (mg L−1) | NO2-N (mg L−1) | NH4-N (mg L−1) | NH3-N (mg L−1) | PO4-P (mg L−1) | TN (mg L−1) | TP (mg L−1) | References |
| Raw Poultry Litter Extract | 7.45 | 34.40 | - | 482.2 | 13.25 | - | - | 108.0 | 21.05 | - | - | [42] |
| Anaerobically Digested Poultry Litter | 9–10 | - | 500–1000 | 400–900 | - | - | 2000–3000 | - | - | 2900–3200 | 20–25 | [56] |
| Pretreated Piggery | 7.5–9.3 | - | 1409–3935 | 430–11,100 | 2–352 | 10 | 70–644 | 1388 | 129 | 981–1356.75 | 20–168 | [66,67,68,69,70] |
| Swine | 6.83–8.1 | 2375–7120 | 20,075 | 419.88–85,600 | 1.19–334 | - | 260–5351 | 578.27 | 36.7–6608 | 163.40–5685 | 26.2–284 | [30,57,58,60,61,62] |
| Nejayote | 9.80 | 9060 | - | 9153.30 | - | - | - | - | - | 120.69 | 41.16 | [62] |
| Cattle | 6.11 | - | - | 674 | 3041 | - | 22,358 | - | 760 | - | - | [71] |
| Dairy Farm | 6.05–8.18 | 32.15–65.65 | - - | 119.21–2593 | 1.45–5.44 | - | 0.75–181.50 | - | 4.33–7.01 | 283 | 115.90 | [72,73] |
| Swine Lagoon | 5.5 | - | - | 2386 | 4.33 | - | 336.2 | - | - | 348.2 | 26.62 | [74] |
| Aerated Swine Lagoon | 8.9 | - | - | 2328 | 103 | - | 22.3 | - | - | 177.9 | 19.48 | [74] |
| Minkery | 8.84 | - | - | 1200 | 10 | 10 | 3250 | - | - | - | 1400 | [75] |
| Paddy-Soaked | 6 | 7255 | 2900 | 2250 | - | - | - | 265.30 | 211.50 | - | - | [76] |
| Shrimp Culture | 8.18 | 32,951.5 | - | 73.5–367.39 | 12.9–73.67 | 7.58 | 8.07–109.91 | - | 15.59 | 21.9 | <0.4 | [63,64] |
| Fish Farm | 7.82–8.5 | - | - | 30.30–112 | 3.93–12 | 0.08 | 5.6 | - | - | 4.12–60 | 0.16–6.8 | [60,65] |
| Anaerobically Digested Abattoir Effluent | 7.1–7.4 | - | - | 302–514 | 0–8 | 0–7.5 | - | 200–210 | 70–80 | - | - | [77] |
| Type | Chloride (mg L−1) | Na (mg L−1) | F (mg L−1) | Fe (mg L−1) | Cu (mg L−1) | Zn (mg L−1) | Mg (mg L−1) | Ca (mg L−1) | K (mg L−1) | Pb (mg L−1) | Turbidity NTU | References |
| Anaerobically Digested Poultry Litter | - | - | - | 0.085 | 0.085 | - | - | 0.044 | 1314.24 | - | - | [56] |
| Pretreated Piggery | 52,524 | 139.55 | - | 1.62 | 0.026 | 0.14 | 54.75–81 | 28.55–105 | 400.75 | 0.0025 | - | [66,67,70] |
| Swine | 154.7 | 66.24–583.8 | - | 0.52–0.728 | 0.0015–0.94 | 0.087–1.7 | 8.3–37.49 | 3.89–63.88 | 229.9–666.7 | - | - | [58,59,61,62] |
| Dairy Farm | - | 121–165 | - | 0.02–0.03 | 0.004–0.007 | 0.001–0.003 | - | - | - | - | - | [72,73] |
TSS (total suspended solids), TOC (total organic carbon), COD (chemical oxygen demand), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), NH3-N (ammonia-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate), Na (sodium), F (fluorine), Fe (iron), Cu (copper), Zn (zinc), Mg (magnesium), Ca (calcium), K (potassium), Pb (lead), NTU (nephelometric turbidity units) and nr (not reported).
2.2.2. Pretreatment
The high suspended particles concentration in agricultural wastewater, similar to domestic wastewater, necessitates the use of a first treatment procedure prior to the cultivation stage. The same pretreatment methods (filtration, autoclaving and centrifugation) as for domestic wastewater were used for agricultural wastewater pretreatment, with other pretreatments such as flocculation, acid precipitation, digestion and stripping, as described in Table 6. Previous research has used the filtering approach to pretreat wastewater, utilizing filters with varying pore sizes depending on the wastewater source and the materials that need to be removed. Khatoon et al. [78] filtered aquaculture wastewater using a 22 µm pore size filter, followed by a 1.2 µm pore size filter. For swine wastewater pretreatment, a recent study used an 8 µm pore size filter after the centrifugation technique [30]. The centrifugation method was used by the majority of these studies, with different durations and forces, for instance, 8000 rpm for 10 min [79], 7000 rpm (8656× g) for 5 min [30], 10,000× g for 10 min at 4 °C [52], 3000× g for 5 min [64] and 3000× g for 10 min [65,69]. However, once more, these methods can only be used at lab-scale.
An air-stripping method was used in the study of Kim et al. [66] for decreasing the concentration of free ammonia, that affect the growth of microalgae, from piggery wastewater. The results showed that the concentration was reduced from 644 mg L−1 to 14.1 mg L−1 [66]. For chemical oxygen demand reduction, acid precipitation was used by Teràn Hilares et al. and Musetsho et al. [42,80]. Teràn Hilares et al. [80] discovered that by adjusting the pH of the poultry slaughterhouse from six to four using H2SO4, almost 80% of the COD was eliminated as sludge [80].

Table 6.
Pretreatment methods of agricultural wastewater before microalgae cultivation.
Table 6.
Pretreatment methods of agricultural wastewater before microalgae cultivation.
| Wastewater | Pretreatment Method | Microalgae | References |
|---|---|---|---|
| Swine | Settled for 24 h, then centrifuged at 8000 rpm for 10 min | Chlorella sp. HL | [79] |
| Swine | Centrifuged at 7000 rpm (8656× g) for 5 min, filtered with filter of pore diameter 8 µm | Scenedesmus sp. | [30] |
| Cattle | Filtered using Whatman filter paper 42 | Chlorella thermophila (MF179624) | [71] |
| Poultry litter extract | Acid pretreatment using 5 M of HCl or H2SO4 | Acutodesmus obliquus | [42] |
| Swine | Natural precipitation method for 1 day; and added with 5% sodium hypochlorite for 1 day. | Chlorella vulgaris MBFJNU-1 | [57] |
| Aquaculture | Filtered using a filter pump (pore size: 22 μm), then filtered with GF/C Whatman glass microfiber filters (pore size: 1.2 μm). | Tetraselmis sp. | [78] |
| Fishery | Sterilized in an autoclave at 121 °C and 150 kPa for 30 min. | Thalassiosira pseudonana and Isochrysis galbana | [81] |
| Poultry slaughterhouse | Acid precipitation (H2SO4) at pH 4 | Chlorella vulgaris | [80] |
| Swine | Sedimentation for 2 days, and anaerobically digested at 55 °C for 10 days | Chlorella pyrenoidosa | [59] |
| Swine | Filtered with a sieve of mesh number 140 (100 μm) | Chlorella sorokiniana AK-1 | [58] |
| Anaerobic digested swine | Sterilized in an autoclave at 121 °C and 150 kPa for 30 min. | Chlorella zofingiensis | [60] |
| Fishery | Sterilized in an autoclave at 121 °C and 150 kPa for 30 min. | Chlorella zofingiensis | [60] |
| Piggery | Centrifuged at 10,000× g for 10 min at 4 °C and pre-autoclaved at 121 °C for 20 min | Chlorella sp. GD | [59] |
| Minkery | Filtered through a filter cloth then filtered using 1.5 µm glass microfiber filters, autoclaved at 121 °C and 15 psi for 20 min. | Haematococcus pluvialis | [75] |
| Aquaculture | Centrifuged at 3000× g for 5 min. | Chlorella Sorokiniana MB-1-M12 | [64] |
| Piggery | Sedimented and sterilized by passing through a 0.45 μm filter | Chlorella pyrenoidosa | [62] |
| Piggery | Flocculated and filtered through 0.2 µm cellulose acetate membranes followed by stripping with air using an acrylic column (ID 5 cm × H 100 cm) | Acutodesmus obliquus | [66] |
| Aquaculture | Centrifuged at 3000× g for 10 min | Chlorella sp. GD | [65] |
| Aquaculture | Filtered using 0.45 mm Whatman GF/C filter papers followed by autoclaving | Chlorella sp. | [82] |
| Piggery | Autoclaved for 30 min at 121 °C then centrifuged (3000× g for 10 min) | Chlorella sp. GD | [69] |
2.2.3. Microalgae Treatment of Agricultural Wastewater
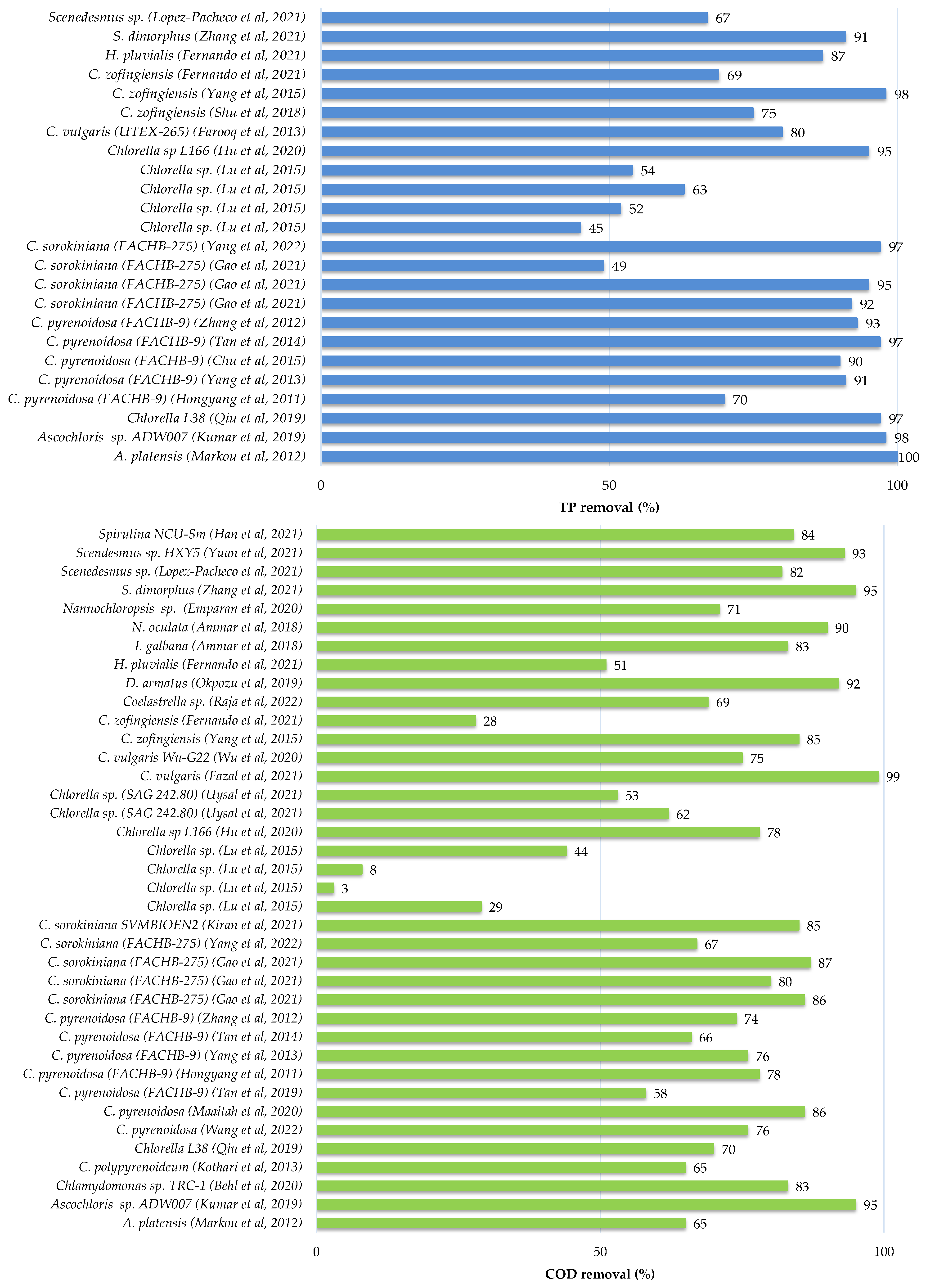
As shown in Table 5, nitrogen, phosphate, and some metal ions are present in agricultural effluent. Wastewaters are dangers to environmental safety if left untreated or cleaned incompetently, resulting in eutrophication of nearby water rivers and greenhouse gas emissions. Lately, considerable research has been conducted on the culture of microalgae in agricultural wastewater for their excellent nutrient removal, energy resource and the other products extracted from microalgal biomass (Table 7 and Table 8). The Figure 4 illustrates the removal efficiencies of total nitrate, total phosphate and chemical oxygen demand from agricultural wastewater by different species of microalgae.
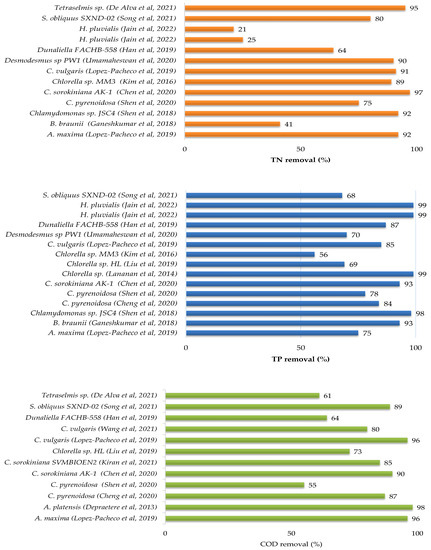
Figure 4.
Nutrients and COD removals from agricultural wastewater by microalgae species [56,58,59,61,62,63,68,70,72,74,75,79,80,82,83,84,85,86,87].
Swine wastewater treatment by microalgae was investigated in a recent study [79]. They evaluated the potential of seven strains which are Chlorella sp. HQ, Scenedesmus sp. LX1, Chlorella vulgaris (FACHB-8), C. pyrenoidosa (FACHB-5), S. obliquus (FACHB-417), Selenastrum capricomutum (FACHB-271) and Chlorella sp. HL, to remove pollutants from a ten-fold dilution of swine wastewater [79] and they reported that Chlorella sp. HL had the highest potential for growth in swine wastewater and nutrient removal among the other species. This strain showed the greatest specific growth rate (µ) and cell density, both of which were 0.51 d−1 and 2.43 × 107 cells mL−1 after nine days of culture, with the TP and COD removal rates of 69.13% and 72.95%, respectively. In addition, [79] suggested that Chlorella sp. HL can be a suitable candidate for energy production due to the higher heating value of 18.25 MJ kg−1 of Chlorella sp. HL biomass. The author of [57] studied the aptitude of Chlorella vulgaris MBFJNU-1 strain to reduce the pollutants of swine wastewater using a column photobioreactor in outdoor environments. At the same time, they evaluated the carbon dioxide fixation by this species. Their results suggested that the cultivation of Chlorella vulgaris MBFJNU-1 at 3% carbon dioxide, provided the maximum of biomass productivity by about 36 mg L−1 d−1 and the best removal efficiency rate of TN, TP and COD by about 16.84 mg L−1 d−1, 1.40 mg L−1 d−1 and 8.47 mg L−1 d−1, respectively [57]. However, these two studies did not assess the biochemical composition of the studied species grown in swine wastewater, which is a critical parameter to be able to use these microalgae for both wastewater treatment and the generation of high-value products at the same time [57,79].
Other research examined the microalgae immobilization method as an innovative technique to remove contaminants from swine wastewater [58,61]. The author of [58] assessed the ability of three species of Chlorella sorokiniana genera to grow and remove pollutants of a 2-fold diluted swine wastewater. They reported that Chlorella sorokiniana AK-1 had the highest biomass and protein production rate compared to other species (5.45 g L−1 and 0.27 g L−1 d−1, respectively) [58]. In the same study, they immobilized Chlorella sorokiniana AK-1 cells using sponge, activated carbon, clay and alginate beads as solid carriers in a 2-fold diluted swine wastewater, and found that the sponge immobilization method improved the biomass concentration and protein productivity (8.08 g L−1 and 0.272 g L−1 d−1, respectively) compared to the control and other solid carriers [58]. In addition, using the sponge as the solid carrier enhanced the removal efficiency of TN, TP and COD from 88.6% to 94.1%, 99.3% to 99.5% and from 84.1% to 91.6%, respectively, compared to the control [58]. A fixed-bed biofilm reactor, as another immobilization technique of algal cells, was used [61] to improve the productivity of the biofilm and the removal efficiencies of total nitrate, total phosphate and metal ion (Cu(II)) from swine wastewater. In this research, they used Chlamydomonas sp. JSC4 cells as an attached culture and assessed the effect of different parameters such as the volume of the swine wastewater and the concentration of Cu(II) on the productivity of the biofilm and removal efficiencies [61]. They reported that the swine wastewater had the best productivity levels (49.70 g−1 m−2 d−1) at an initial composition of 600 mg L−1 of total nitrate and 0.23 mg L−1 of Cu(II), but when the concentration of Cu(II) was increased to 15 mg L−1, the productivity of the biofilm was decreased to 37.73 g m−2 d−1 with a TN removal of 85.79%, TP removal of 96.56% and Cu(II) removal of 93.70% [61].
The microalga Desmodesmus sp. PW1 was used to treat piggery wastewater [83]. The results showed that this isolated strain from piggery wastewater had two important potentials, the first was the ability to reduce the pollutants in piggery wastewater, and the second was to self-flocculate in it and overcome the harvesting problem [80]. It can remove about 90% of total nitrogen and about 70% of total phosphorus from piggery wastewater with an initial composition of 296.7 mg L−1 of TN and 28.6 mg L−1 of TP under 25 °C and 4000 lx [83]. In addition, as this strain can self-flocculate more than 90% in 2.5 h of sedimentation, it could be a promising candidate for biomass and biodiesel production (1.76 g L−1 and 7.2%, respectively) [83].
To improve the efficiency of piggery wastewater treatment, [84] used the digestion technique before microalgae treatment, and they used a marine microalgal specie rather than the genus Chlorella and Scenedesmus, which have been widely used in the literature [84]. In the same investigation, they used the CO2 produced by the digester to feed the microalgae culture bioreactor during its operation [84]. Under these conditions (CO2 from digester (94.7%) and pH 7.5), Tetraselmis suecica had the highest production of biomass, lipids and carbohydrates, which were 59.8, 25 and 6.5 mg L−1 d−1, respectively, with TN and TP removals of 96 and 72%, respectively [84].
Microalgae have also demonstrated their ability to remediate aquaculture effluents [64,78]. Chen et al. [64] reported that Chlorella sorokiniana MB-1-M12 was the best candidate to treat shrimp culture wastewater because of its biomass and lutein productivities (1.9 mg L−1 d−1 and 5.55 mg L−1 d−1, respectively) in 75% of shrimp culture effluent. Another recent study used the immobilization method to treat aquaculture wastewater [78]. In this research, they immobilized Tetraselmis sp., a marine microalga, using alginate beads in aquaculture wastewater [78]. Tetraselmis sp. beads removed about 0.08 mg L−1, 0.10 mg L−1 and 0.17 mg L−1 from the initial concentration of the total ammonium (7.7 mg L−1), nitrite (3.1 mg L−1) and phosphorus (2 mg L−1) after two days, which corresponds to the following removal efficiencies of 98.9%, 97.7% and 91.1%, respectively [78].
Different wastewaters can also be combined, such as piggery farm wastewater and winery industry wastewater, to be treated by Chlorella sp. MM3 strain from the soil [70]. At the ratio 20:80 of piggery and winery wastewaters, Chlorella sp. MM3 removed 89.36% for the TN removal efficiency and 56.56% for the TP removal efficiency, and achieved a high lipid yield of about 51% [70]. Recently, 2.5% of cattle wastewater was diluted with domestic wastewater as a substitute for freshwater to be treated by Chlorella thermophila (MF179624), isolated from sewage wastewater [71]. They reported that Chlorella thermophila grew well (2.17 g L−1 of biomass) in 2.5% of cattle wastewater, compared to domestic wastewater (1.22 g L−1 of biomass) and bold basal medium (1.24 g L−1 of biomass), and gave interesting compounds such as lipids (18.27%), carbohydrates (29.39%) and proteins (44.91%) [71]. Chlorella thermophila had the highest removal rates of ammonium (53.74 mg L−1 d−1), nitrate (6.96 mg L−1 d−1) and more than 99% of phosphorus [71].

Table 7.
Treatment of agricultural wastewater by the cultivation of microalgae.
Table 7.
Treatment of agricultural wastewater by the cultivation of microalgae.
| Algae Used | Wastewater Type | Conditions of Culture | Nutrient Concentration | Average Nutrient Removal Rate | References |
|---|---|---|---|---|---|
| Acutodesmus obliquus | Piggery | 25 °C for 138 h | COD: 11,100 mg L−1; TN: 981 mg L−1; TP: 81 mg L−1 | TN: 175 mg g−1 d−1; TP: 1.5 mg g−1 d−1 COD: 1923 mg g−1 d−1) | [66] |
| Arthrospira maxima | 10% Nejayote | 21 °C; 1.5 L m−2 min−1 of aeration; 12:12 light: dark | COD: 9153.3 mg L−1; TN: 120.69 mg L−1; TP: 41.16 mg L−1 | COD: 96%; TN: 92%; TP: 75% | [62] |
| Arthrospira platensis | Dairy farm | 30 °C; 160 μE m−2 s−1 (from initial today 6) and 300 μE m−2 s−1 (from day 7); 12:12 light: dark | COD: 131.691 mg L−1; NO3-N: 3.452 mg L−1; NH4-N: 2.998 mg L−1; PO4-P: 5.672 mg L−1 | COD: 98.4%; NO3-N: 99.6%; NH4-N: ~100%; PO4-P: 98.8% | [72] |
| Botryococcus braunii | Aerated swine | 25 °C; 10 μE m−2 s−1 | TN: 177.9 mg L−1; TP: 19.48 mg L−1 | TN: 40.8%; TP: 93.3% | [74] |
| Chlamydomonas sp. JSC4 | Swine | 26 °C; 150 μE m−2 s−1; 16:8 light: dark; 5% CO2 at an aeration rate of 0.2 L min−1 | TN: 600 mg L−1; TP: 26.2 mg L−1 | TN: 92%; TP: 98% | [61] |
| Chlorella pyrenoidosa | Paddy-soaked | 33 °C to 37 °C; 3826–4240 μE m−2 s−1 | PO4-P: 211.50 mg L−1; NH4-N: 265.30 mg L−1 | NH3-N: 75.89%; PO4-P: 73.71% | [76] |
| Five times diluted anaerobically Digested swine | 25 °C; 80 μE m−2 s−1; 12:12 light: dark | NH4-N: 134.17 mg L−1; NO3-N: 14.49 mg L−1; TP: 6.65 mg L−1; COD: 116.10 mg L−1 | NH4-N: 94.1%; NO3-N: 85.2%; TP: 84.0%; COD: 86.8% | [59] | |
| Piggery | 25 °C to 27 °C; 63 μE m−2 s−1 | TN: 980 mg L−1; TP: 158 mg L−1; COD: 1000 mg L−1 | TN: 74.6%; TP: 77.7%; COD: 55.4% | [68] | |
| Chlorella sorokiniana AK-1 | 50% Swine | 27 °C, 150 μE m−2 s−1 | TN: 510 mg L−1; TP: 76.1 mg L−1; COD: 506.8 mg L−1 | : 97.0%; TP: 92.8%; COD: 90.1% | [58] |
| Chlorella sorokiniana MB-1-M12 | Shrimp culture | 26 °C; 150 μE m−2 s−1 | TN: 21.9 ppm; TP < 0.4 ppm; COD: 73.5 ppm | nr | [64] |
| Chlorella sorokinianaSVMBIOEN2 | Dairy farm | 25 °C; 100 μE m−2 s−1; 12:12 light: dark | COD: 2000 mg L−1 | COD: 85%; | [85] |
| Chlorella sp. | Aquaculture | 28 °C | NH4-N: 0.91 mg L−1; PO4-P: 2.6 mg L−1 | NH4-N: 98.7%; PO4-P: 92.2%; | [82] |
| Aquaculture | 25 °C; 3350 Lm | TP: 6.75 mg L−1 | TP: 99.15% | [86] | |
| Chlorella sp. GD | Aquaculture | 26 °C; 300 μE m−2 s−1; aerated with boiler flue gas (approximately 8% CO2) | pH: 8.5; COD: 112 mg L−1; TN: 60 mg L−1; TP: 6.8 mg L−1 | nr | [65] |
| Piggery | 26 °C; 300 μE m−2 s−1; 2% CO2 aeration rate of 0.2 vvm | pH: 8.5; COD: 430 mg L−1;TN: 550 mg L−1; TP: 20 mg L−1 | nr | [69] | |
| Chlorella sp. HL | Swine wastewater | 25 °C; 60 μE m−2 s−1; 16:8 light: dark | TP: 74.61 mg L−1; COD: 12,431.9 mg L−1 | TP: 69.13%; COD: 72.95% | [79] |
| Chlorella sp. MM3 | Mixed piggery and winery (20:80 ratio) | 23 °C; | TN: 284 mg L−1; TP: 11 mg L−1 | TN: 89.36%; TP: 56.56% | [70] |
| Chlorella thermophila MF179624 | 2.5% Cattle | 25 °C; 100 μE m−2 s−1; aeration 0.5 vvm | pH: 6.11, NH4-N: 22,358 mg L−1; NO3-N: 3041 mg L−1; PO4-P: 760 mg L−1 | NH4-N: 53.74 mg L−1 d−1; NO3-N: 6.96 mg L−1 d−1; PO4-P: more than 99% | [71] |
| Chlorella vulgaris | 10% swine | 21 °C; 12:12 light: dark; 1.5 L m−2 min−1 of aeration | COD: 10.933 mg L−1; TN: 163.40 mg L−1; TP: 147.0 mg L−1 | COD: 96%; TN: 91%; TP: 85% | [62] |
| Poultry slaughterhouse | First stage: acid precipitation of wastewater; Second stage: batch conditions: 25–27 °C; continuous illumination 440 μE m−2 s−1; Continuous process: 25 °C; continuous illumination 440 μE m−2 s−1 | COD: 2185–7313 mg L−1 | COD (first step): 80%; COD (second step): more than 83% | [80] | |
| Chlorella vulgaris MBFJNU-1 | Swine | Outdoor: (sunlight) | pH: 5.5–6.0; COD: 492.4–500.7 mg L−1; TN: 472.5–547.8 mg L−1;TP: 31.8–42.6 mg L−1 | COD: 8.47 mg L−1 d−1; TN: 16.84 mg L−1 d−1; TP: 1.40 mg L−1 d−1 | [57] |
| Desmodesmus sp. PW1 | Piggery | 25 °C; 4000 lx. | TN: 296.7 mg L−1; TP: 28.6 mg L−1 | TN:90%; TP:70% | [83] |
| Dunaliella FACHB-558 | Anaerobically digested poultry litter | 25 °C; 200 μE m−2 s−1; 12:12 light: dark | TN: 100–120 mg L−1; TP: 15–20 mg L−1; TOC: 400–500 mg L−1 | TN:63.8%; TP: 87.2%; TOC: 64.1% | [56] |
| Haematococcus pluvialis | 1% Minkery | Green stage: 20 °C; 50 μE m−2 s−1 continuous light; Red stage: 20 °C; 200 μE m−2 s−1 continuous light | NH4-N: 32.5 mg L−1; NO3-N: 0.1 mg L−1; NO2-N: 0.1 mg L−1; TP: 14.0 mg L−1 | TN: 24.8%, TP: 99.7% | [75] |
| 1.5% Minkery | Green stage: 20 °C; 50 μE m−2 s−1 continuous light; Red stage: 20 °C; 200 μE m−2 s−1 continuous light | NH4-N: 48.75 mg L−1; NO3-N: 0.15 mg L−1; NO2-N: 0.15 mg L−1 TP: 21.0 mg L−1 | TN: 20.7%, TP: 99.8% | ||
| Scenedesmus obliquus SXND-02 | Chicken farm + (7 g L−1) sodium acetate | 25 °C; 120 μE m−2 s−1; 12:12 light: dark; | nr | TN: 80%; TP: 68%; COD: 89% | [87] |
| Scenedesmus sp. MUR 272 | Anaerobically digested abattoir | Micro-ponds: 20 cm depth; pH 6.5; CO2 addition on demand; batch mode; 7.3–39.8 °C; 427.6–815.8 W m−2 | NH4-N: 45 mg L−1; PO4-P: 6.3 mg L−1 | NH4-N: 86%; PO4-P: 89% | [77] |
| Anaerobically digested piggery | 175 ± 25 μE m−2 s−1; 12:12 light: dark | nr | TN: up to 99%; TP: up to 73% | [84] | |
| Tetraselmis sp. | Synthetic mariculture | 23 °C; 1500 μE m−2 s−1; 12:12 light: dark | NO3-N: 45 mg L−1; NO2-N: 10 mg L−1; Orthophosphates: 17 mg L−1; NH4-N: 30 mg L−1; COD: 270 mg L−1; | TN:95.5%; Orthophosphates: 94.4%; COD: 61.4% | [63] |
| Aquaculture | 24 °C; 50 μE m−2 s−1 | NH4-N: 7.7 mg L−1; NO2-N: 3.1 mg L−1; PO4-P: 2 mg L−1; | NH4-N: 0.08 mg L−1; NO2-N: 0.1 mg L−1; PO4-P: 0.17 mg L−1 | [78] |
COD (chemical oxygen demand), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate) and nr (not reported).

Table 8.
Growth parameters and metabolites production of microalgae culture in agricultural wastewater.
Table 8.
Growth parameters and metabolites production of microalgae culture in agricultural wastewater.
| Algae Used | Wastewater Type | Growth Rate or Volumetric Productivity | Final Biomass Concentration | Production of Target Metabolites | References |
|---|---|---|---|---|---|
| Acutodesmus obliquus | Piggery | 1850 mg-cell L−1 d−1 | nr | nr | [66] |
| Arthrospira maxima | 10% Nejayote | 0.27 d−1 | 32 × 104 cell mL−1 | nr | [62] |
| Arthrospira platensis | Dairy farm | 0.50 g L−1 d−1 | 4.98 g L−1 | Lipids: 30.23% | [72] |
| Botryococcus Braunii | Aerated swine | nr | 0,94 mg L−1 | Hydrocarbon: 23.8% | [74] |
| Chlamydomonas sp. JSC4 | Swine | 49.70 g m−2 d−1 | 37.73 mg L−1 | Carbohydrates: 3920% Lipids: 17.67% Protein: 33.94% | [61] |
| Chlorella pyrenoidosa | Paddy-soaked | 0.42 d−1 | 1.56 g L−1 | Chlorophyll: 15.57 mg L−1 Lipids: 27.47% Carbohydrates: 23.77% Proteins: 46.12% | [76] |
| Five times diluted anaerobically Digested swine | 4.21 g m−2 d−1 | 42.20 g m−2 | Proteins: 57.30% Extracellular polysaccharides: 14.87% Crude fibre: 3.08% Crude ash: 5.57% | [59] | |
| Piggery | nr | nr | Lipids: 6.3 mg L−1 d−1 | [68] | |
| Chlorella sorokiniana AK-1 | 50% Swine | nr | 5.45 g L−1 | Proteins: 0.27 g L −1 d−1 | [58] |
| Chlorella sorokiniana MB-1-M12 | Shrimp culture | nr | 1.9 g L−1 d−1 | Lutein: 5.19 mg g−1 | [64] |
| Chlorella sorokiniana SVMBIOEN2 | Dairy farm | nr | 2.33 g L−1 | Carbohydrates: 10.2 mg g−1 Proteins: 14 mg g−1 | [85] |
| Chlorella sp | Aquaculture | nr | 213 cell mL−1 d−1 | nr | [82] |
| Aquaculture | nr | nr | nr | [86] | |
| Chlorella sp. GD | Aquaculture | 0.487 d−1 | 17.4 g L−1 | Lipids: 21.3% | [65] |
| Piggery | 0.839 d−1 | 0.681 g L−1 d−1 | Lipids: 21% | [69] | |
| Chlorella sp. HL | Swine | 0.51 d−1 | 2.43 × 107 cells mL−1 | nr | [79] |
| Chlorella sp. MM3 | Mixed piggery and winery (20:80 ratio) | nr | 4.4 × 106 cells mL−1 | Lipids: 51% | [70] |
| Chlorella thermophila MF179624 | 2.5% Cattle | nr | 2.17 g L−1 | Lipids: 18.27% Carbohydrates: 29.39% Proteins: 44.91% | [71] |
| Chlorella vulgaris | 10% swine | 0.57 d−1 | 128 × 106 cells mL−1 | nr | [62] |
| Poultry slaughterhouse | nr | 1.2 g L−1 | nr | [80] | |
| Chlorella vulgaris MBFJNU-1 | Swine | 0.11 d−1 | 36 mg L−1 d−1 | nr | [57] |
| Desmodesmus sp. PW1 | Piggery | nr | 1.76 g L−1 | 7.2% | [83] |
| Dunaliella FACHB-558 | Anaerobically digested poultry litter | nr | 678 mg L−1 | β-carotene: 4.02 mg L−1 | [56] |
| Haematococcus pluvialis | 1% Minkery | 0.399 mg L−1 d−1 | 681 mg L−1 | Astaxanthin: 39.72 mg L−1 | [75] |
| 1.5% Minkery | 0.451 mg L−1 d−1 | 906.33 mg L−1 | Astaxanthin: 16.64 mg L−1 | ||
| Scenedesmus obliquus SXND-02 | Chicken farm + (7 g L−1) sodium acetate | nr | 2.18 g L−1 | Lipids: 50.22% | [87] |
| Scenedesmus sp. (MUR 272) | Anaerobically digested abattoir | nr | 19.24 g m−2 d−1 | nr | [77] |
| Anaerobically digested piggery | nr | 59.8 mg L−1 d−1 | Lipids: 25 mg L−1 d−1 Carbohydrates: 6.5 mg L−1 d−1 | [84] | |
| Tetraselmis sp. | Synthetic mariculture | 0.067 d−1 | 1.19 g L−1 | Lipids: 62.16 mg g−1 | [63] |
nr (not reported).
2.3. Industrial Wastewater
2.3.1. Physicochemical Properties
Industrial wastewater could come from a wide range of industries (Figure 5), such as the textile and food processing industries (sugar, starch, vegetable oil, shortening, potato, dairy, fruit juice and beverage, brewery and distillery industries) [88,89] and is one of the most significant pollution sources in the environment and has different properties. Different techniques have been used to treat this type of wastewater, among them is the treatment by microalgae [90]. Table 9 summarizes the properties of different industrial wastewaters which have been treated using microalgae, food-processing wastewater being the most investigated wastewater in the literature. This type of wastewater often has a high chemical oxygen demand and high total nitrate and total phosphate concentrations levels [5], for example, an olive oil mill wastewater contains 56,740–124,600 mg L−1 of COD, 130–190 mg L−1 of TN and 350 mg L−1 of TP, as well as soybean wastewater which has 5320–22,700 mg L−1 of COD, 267.1–950 mg L−1 of TN and 23.28–56.3 mg L−1 of TP [89,91,92,93,94,95,96]. Winery wastewater has a high COD content (119,300 mg L−1) and a lower TN (12.14 mg L−1) and TP content (3.46 mg L−1) [70]. On the other hand, textile wastewater is characterized by a high concentration of the total suspended and dissolved solids, chemical oxygen demand and a strong color [5,97]. As reported in Table 9, it contains 1050 mg L−1 of TSS, 1378.2–4458 mg L−1 of COD and 43.57 NTU, which is lower than a palm oil mill effluent (244 NTU) [98,99].
Figure 5.
Industrial wastewater types.
Chemical industry wastewater, such as that from pesticides and pharmaceutical processing industries, used in the literature as culture mediums for microalgae, contains high total organic carbon (TOC) and low total nitrate (TN) and total phosphate (TP) concentrations, with 7185.93 and 480.93 mg L−1 of TOC, 0.02 and 0.23 mg L−1 of TN and 1.01 and 0.52 mg L−1 of TP, for pharmaceutical and pesticide processing wastewaters, respectively [32].

Table 9.
Industrial wastewater composition.
Table 9.
Industrial wastewater composition.
| Type | pH | TSS (mg L−1) | TOC (mg L−1) | COD (mg L−1) | NO3-N (mg L−1) | NO2-N (mg L−1) | NH4-N (mg L−1) | NH3-N (mg L−1) | PO4-P (mg L−1) | TN (mg L−1) | TP (mg L−1) | References |
| Olive Oil Mill | 4–5.37 | 41,220–83,160 | - | 56,740–124,600 | 99.13 | - | 2.3 | 360 | 130–190 | 350 | [91,92] | |
| Textile | 10.5–10.9 | 1050 | - | 1378.2–4458 | 16.12 | - | 28.35 | - | 2.1–22.3 | 40 | - | [99,100] |
| Olive Oil Washing | 6.29 | - | 191.5 | 1362 | - | - | <4 | - | - | 7.49 | 1.63 | [17] |
| Starch | 2.13–7.3 | 1000–92,000 | 8770 | 792.28–7426 | 606 | 108 | 2.7–503 | - | 1.2–336 | 265.10–379.5 | 28.34–67.9 | [101,102,103,104,105] |
| Digested Starch | 7.3–7.5 | - | - | 702.4–102.5 | - | - | 217.6–334.7 | - | 19.3–32.9 | 240.3–382.7 | 22.7–40.2 | [106] |
| Alcohol | 3.2–4.5 | - | - | 45,638.06–65,000 | - | - | 214.56–279.72 | - | 19.71 | 618.68–725.34 | 47.16–64.38 | [104,105] |
| Reeling | 7.39 | - | - | 11 | - | - | - | - | - | 2.43 | 1.07 | [107] |
| Cooking Cocoon | 7.52 | - | - | 2925 | - | - | - | - | - | 267.5 | 23.1 | [107] |
| Frigon | >12 | - | - | 14,820 | - | - | - | - | - | 910.9 | 92.8 | [107] |
| Seafood | 7.92 | - | - | 1220.8 | 6.99 | 0.73 | 117.22 | - | - | 121.07 | 57.32 | [108] |
| Brewery | 5.5–6 | 300–320 | 1400–1500 | 2000–3000 | - | - | - | - | - | 30–45 | 12–16 | [109] |
| Rose Oil | 6.05–6.14 | - | - | 1200–2087 | 13.5 | - | 12.36 | - | <0.05 | - | - | [110] |
| Rolling Mill Industry | 6.05 | - | - | - | 11.5 | - | - | - | - | - | - | [111] |
| Vinegar | 5.6 | - | - | 740 | - | - | - | 17.7 | - | 20.5 | 7.4 | [112] |
| Dairy Industrial | 3.69–9.11 | 111–1510 | 143.3–722 | 342–7110 | 64 | 5.03 | 18.45–46.5 | 31.4–90.4 | 5.58–31.0 | 65.06–103.6 | 8–105.1 | [113,114,115,116,117,118] |
| Winery | 3.51–4.2 | 9780 | - | 119,300 | - | - | - | - | - | 12.14 | 3.46 | [70,119] |
| Soy Sauce | 6.548 | - | 992 | 3263.33 | - | - | 168.44 | - | - | 173.53 | 10.21 | [120] |
| Palm Oil Mill | 7–8 | 880–3900 | 1400 | 2898.72–4395.6 | - | - | 254 | - | 273 | 5.06–376 | 39.4–58 | [98,121,122,123] |
| Lactic Acid | 5.3 | - | - | 12,571 | - | - | - | 169 | - | 651 | 28.2 | [124] |
| Instant Coffee | 4.54 | 1000 | 7600 | 4940 | - | - | - | - | - | 75.63 | 7.81 | [125] |
| Dairy Products | 10.26 | 60 | 7420 | 190 | - | - | - | - | - | 18.04 | 2.63 | [125] |
| Pesticides Industry | 9.2 | - | 480.93 | - | - | - | - | - | - | 0.23 | 0.52 | [32] |
| Pharmaceutical Industry | 11.88 | - | 7185.93 | - | - | - | - | - | - | 0.02 | 1.01 | [32] |
| Petroleum Industry | 7.97 | - | 288.60 | - | - | - | - | - | - | 0.5 | 0.58 | [32] |
| Soybean | 4.2–6 | - | - | 5320–22,700 | - | - | 3–52.1 | 15.25 | 100 | 267.1–950 | 23.28–56.3 | [93,94,95,96] |
| Type | Chloride (mg L−1) | Na (mg L−1) | F (mg L−1) | Fe (mg L−1) | Cu (mg L−1) | Zn (mg L−1) | Mg (mg L−1) | Ca (mg L−1) | K (mg L−1) | Pb (mg L−1) | Turbidity NTU | References |
| Textile | 1019.46 | - | - | - | - | - | - | - | - | - | 43.57 | [99] |
| Olive Oil Washing | 172 | - | - | - | 1.6 | - | 62.4 | - | - | - | - | [17] |
| Starch | - | 315.21–719.40 | - | 0.94–32.86 | - | 0.86–1.24 | 3.711–181.16 | 98.40–126.36 | 112.34–174.47 | - | - | [102,104,105] |
| Digested Starch | - | 417.6–790.1 | - | 0.9–3.6 | 0.09–0.21 | - | 97.6–166.9 | 72.8–102.3 | - | - | - | [106] |
| Alcohol | - | 226.17–787.74 | - | 1.47–2.3 | - | 0.06–0.1 | 49.12–152.20 | 16.95–96.14 | 127.63–157.75 | - | - | [104,105] |
| Rolling Mill Industry | 172.3 | 60 | - | 3.51 | 8.5 | 0.75 | - | - | - | 2.48 | - | [111] |
| Vinegar Production | - | - | - | - | - | - | 13.9 | 39.5 | 69.9 | - | - | [112] |
| Dairy Industrial | 199–385 | 42 | 6 | - | - | - | 9.85 | 564 | - | - | - | [113,118] |
| Winery | - | 84.54 | - | 2.037 | 0.0059 | 0.6087 | 15.14 | 39.5 | 235.7 | 0.0004 | - | [70,119] |
| Palm Oil Mill | - | 2.99 | - | 1.67 | <0.01 | <0.01 | 3.8–672.22 | 0.8 | 3072.03–4393.89 | - | 244 | [98,121] |
| Instant Coffee | - | 0.059 | - | 9.762 | - | 0.032 | 28.10 | - | - | - | - | [125] |
| Dairy Products | - | 0.067 | - | - | - | 0.017 | 2.72 | - | - | - | - | [125] |
| Cassava Flour/Starch | - | 0.003 | - | - | 0.021 | 0.358 | 45.83 | - | - | - | - | [125] |
| Soybean Curd | 961 | 1387 | - | 5.16–41 | 0.55 | 6.91 | 35–173.5 | 51.47–366 | 1280 | - | - | [93,94,95] |
TSS (total suspended solids), TOC (total organic carbon), COD (chemical oxygen demand), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), NH3-N (ammonia-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate), Na (sodium), F (fluorine), Fe (iron), Cu (copper), Zn (zinc), Mg (magnesium), Ca (calcium), K (potassium), Pb (lead), NTU (nephelometric turbidity units) and nr (not reported).
2.3.2. Pretreatment
The industrial wastewater must be pretreated after characterization and before treatment with microalgae. Similarly to domestic and agricultural wastewaters, the procedures employed in the literature include sedimentation, filtration, acidification and centrifugation for the removal of suspended materials, as well as an autoclave or UV radiation sterilization for the removal of bacteria and other microorganisms found in industrial wastewater (Table 10). Filtration has also been used to remove germs depending on the pore size of the filter employed, for example, Hao et al. utilized the membrane filtration with a pore size of 0.22 µm to filter and sterilize industrial wastewater [8]. They reported that soy sauce wastewater composition had changed after sterilization by an autoclave at 121 °C for 30 min, with a change in the chemical oxygen demand from 3263.33 to 3463.33 mg L−1, total organic carbon from 992.00 to 1042.80 mg L−1, total nitrogen from 173.53 to 176.49 mg L−1, ammonium nitrogen from 168.44 to 158.85 mg L−1 and the total phosphorus from 10.21 to 5.57 mg L−1, and there was no change in the soy sauce wastewater color [120].

Table 10.
Pretreatment methods of industrial wastewater before microalgae cultivation.
Table 10.
Pretreatment methods of industrial wastewater before microalgae cultivation.
| Wastewater | Pretreatment Method | Microalgae | References |
|---|---|---|---|
| Tobacco | Filter-sterilized by a 0.22 μm membrane filter (Millipore, USA) | Chlorella pyrenoidosa (No.FACHB-863) | [8] |
| Palm oil mill (POME) | Filtered through a microfiber mesh and sterilized in an autoclave | Chlorella zofingiensis | [98] |
| Cassava processing | Autoclaved at 121 °C for 15 min, then filtered on filter paper | Arthrospira platensis | [101] |
| Palm oil mill (POME) | Filtered with filter cloth and re-filtered through glass microfibers filter (Whatman, Grade GF/C 1.2 mm) and autoclaved at 121 °C and at a pressure of 15 psi for 20–30 min | Nannochloropsis sp. | [121] |
| Soy sauce | Centrifuged (1644× g 5 min), then autoclaved at 121 °C for 30 min | Spirulina NCU-Sm | [120] |
| Potato | Acidified with sulfuric acid to pH 5, then autoclaved at 121 °C for 30 min | Scenedesmus sp. HXY5 | [126] |
| Olive oil washing | Centrifuged and filtered–sterilized through a glass wool pre-filter and cellulose nitrate membrane (0.45 μm) | Chlorella pyrenoidosa | [17] |
| Soybean processing | Centrifuged (5000 rpm), and filtered by a microporous filter membrane (0.45 μm), then sterilized by high-pressure steam at 121 °C | Chlorella sp. L166 | [96] |
| Vinegar production | Centrifuged for 10 min at 5000 rpm, and sterilized at 121 °C and 120 kPa for 20 min | Chlorella sp. | [112] |
| Soybean processing | Filtrated then autoclaved at 120 °C for 30 min | Chlorella L166 | [94] |
| Anaerobic palm oil mill | Centrifuged at 8000 rpm for 10 min | Scenedesmus sp. and Chlorella sp. | [122] |
| Starch processing plant | Filtered through 0.45 mm polyester filters and sterilized through UV-B radiation (UV doses 810 mJ cm−2 at a distance of 10 cm) | Chlorella pyrenoidosa (FACHB-9) | [102] |
| Cassava | Sterilized at 121 °C for 10 min | Desmodesmus aramatus | [103] |
| Anaerobically digested starch | Allowed to settle for 5–7 h in several tanks and filtered using polyester filters (1 μm) | Chlorella pyrenoidosa (FACHB-9) | [104] |
| Raw dairy | Filtered through a non-woven geotextile membrane (100 GSM grade) | Ascochloris sp. ADW007 | [113] |
| Seafood processing | Filtered using 0.45 μm pore size GF/C glass microfiber filters | Chlorella sp. | [108] |
| pesticides industry, pharmaceutical industry, petroleum industry | Filtered and sterilized using vacuum filtration unit and autoclaved at 121 °C for 15 min | Nannochloropsis oceanica | [32] |
| Anaerobic digested starch | Allowed to settle for several hours and filtered using a 0.45 µm polyester filter then sterilized | Chlorella pyrenoidosa (FACHB-9) | [105] |
| Anaerobic digested starch processing | Allowed to settle for 5–7 h in a settling tank and filtered with 270 mesh (53 lm) polyester filter bags | Chlorella pyrenoidosa (FACHB-9) | [127] |
| Raw dairy | Settled by gravity overnight and filtered through gauze | Chlorella sp. | [73] |
| Meat processing plant | Centrifuged at 8000 rpm for 10 min and sterilized at 121 °C for 30 min | Chlorella sp. | [128] |
| Pulp and paper industry | Addition of 1 mL of SuperFloc C-581 flocculant per 50 mL of effluent. Setteled for 20 min then filtred through a mesh filter with pore size of 5 µm and sterilized with a 500 mL bottle top polystyrene filter with pore size of 0.22 µm. | Nannochloropsis oculata | [129] |
| Raw dairy | Centrifuged (5000 rpm, 10 min) then autoclaved | Chlorella zofingiensis | [114] |
2.3.3. Microalgae Treatment of Industrial Wastewater
Despite the great variety of industrial effluent, (Table 11 and Table 12 and Figure 6 and Figure 7), microalgae have mostly been utilized to remediate wastewaters from the food industry, because they contain a lot of organic materials and are nontoxic for microalgae cultivation. For instance, in pilot scale conditions (open troughs with a semi-cylindrical barrel form, 29–42 °C, 28.944–196.015 W m−2 light intensity), Ascochloris sp. ADW007 demonstrated its capacity to reduce the pollutants found in raw industrial dairy wastewater (pH: 3.69, COD: 7110 mg L−1, TN: 137 mg L−1, TP: 105.1 mg L−1) as well as its ability to produce lipids for the generation of biodiesel [113]. In comparison to laboratory scale conditions, the strain produced the most biomass (0.207 g L−1 d−1) and lipids (34.98%) while also having the greatest wastewater treatment efficiency with hollow fiber filtration and activated carbon treatment as post-harvesting techniques (COD: 95.1%, TN: 79.7%, TP: 98.1%) [113]. Lactic acid wastewater was also used to cultivate Scenedesmus dimorphus for 10 days, allowing total nitrogen and total phosphate elimination rates of 96.31% and 90.78%, respectively, as well as a high lipid production of 1.54 g L−1 compared to a BG11 culture medium (1.04 g L−1 of lipids) [124]. Authors found that this treated wastewater might be used four times to produce lactic acid, potentially offsetting the expenses of lactic acid wastewater disposal [124]. Recently, a piece of research examined the potential of microalgae in producing electricity and industrial dairy wastewater treatment [115]. They isolated several marine microalgae and evaluated their electrogenic and redox peak activities. Of 18 microalgae isolates, Coelastrella sp. showed a better electrogenicity and redox peak and capability to remove nutrients from dairy wastewater, with the best removal efficiencies of NH4-N (90.38%), NO3-N (90.24%), P (66.75%), COD (69.44%) and TOC (83.51%) in a lab-scale raceway pond [115]. In addition, soy processing wastewaters treatment was also investigated using microalgae treatment [89,91,96]. Three different dilutions were used to cultivate two Chlorella species L166 and L38 in soybean effluent (0, 10 and 100 times) [94]. The results showed that Chlorella sp. L38, grown in a 10-times-diluted soybean effluent, had the highest removal efficiencies for TN (84.7%), TP (97.3%), and COD (70.5%), but that Chlorella L166 strain produced the highest levels of lipids by about 7.22 mg L−1 d−1 and polysaccharides by about 2.86 mg L−1 d−1 under the same conditions of culture [94].
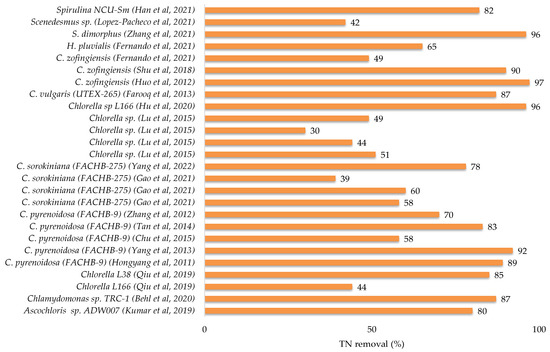
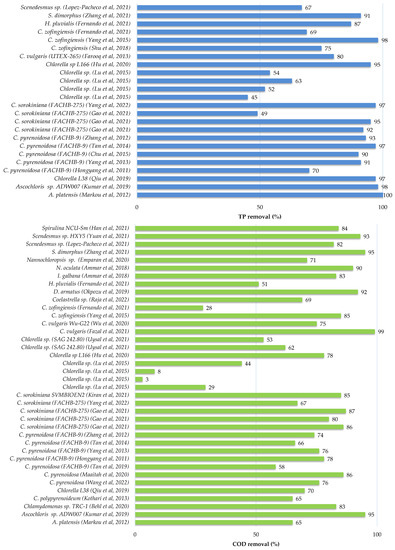
Figure 6.
Nutrients and COD removals from industrial wastewater by microalgae species [17,85,91,94,95,96,98,99,100,103,105,106,107,109,110,113,115,117,118,120,123,124,126,127,128,129,130,131,132,133,134,135,136,137].
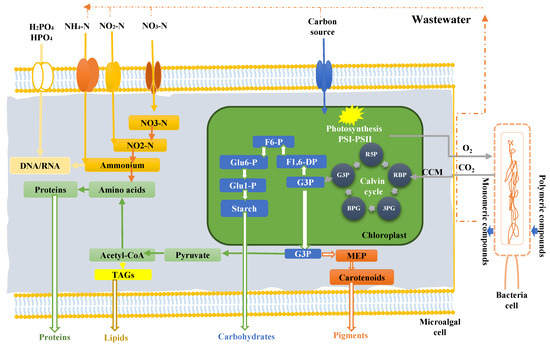
Figure 7.
Wastewater pollutants mechanisms of bioremediation by microalgae. NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), H2PO4 (dihydrogen phosphate), HPO4 (hydrogen phosphate), HMs (Heavy metals), CCM (carbon concentrating mechanism), RBP (ribulose biphosphate), R5P (ribulose 5 phosphate), G3P (glyceraldehyde 3 phosphate), BPG (bisphosphoglycerate), 3PG (3 phosphoglycerate), F1,6-DP (fructose 1,6-bisphosphate), F6-P (fructose 6 phosphate), Glu6-P (glucose 6 phosphate), Glu1-P (glucose 1 phosphate), MEP (methylerythritol phosphate) and TAGs (triacyl glycerols).
In another study, Chlorella sp. L166 was co-cultured with soybean effluent (COD: 5320 mg L−1, TN: 106.99 mg L−1, TP: 23.28 mg L−1) and CO2 from flue gas [96]. The concentration of chlorophyll a was 48.08 mg L−1, when the N/P ratio was 5:1 in the 20% soybean effluent and 5% CO2 (0.1 vvm) [96]. Furthermore, Chlorella sp. L166 obtained the maximum removal efficiencies of TN of 96.07, TP of 95.55%, COD of 78.20% and a CO2 fixation of 28.60% [96]. Therefore, it was discovered that Chlorella sp. L166 had the capacity to remove CO2 in flue gas while also treating a soybean processing effluent [96]. In a recent piece of work, Han et al. [120] employed a soy sauce processing effluent (NH4-N: 168.44 mg L−1,TN: 173.53 mg L−1, COD: 3263.33 mg L−1) as a culture medium to feed Spirulina and remove pollutants. The findings showed that Spirulina NCU-Sm grew well in raw soy sauce wastewater and produced a maximum biomass of 1.984 g L−1 with a removal efficiency 93.86% for NH4-N, 81.76% for TN and 84.08% for COD [120].
Researchers have also evaluated the wastewater from oil-producing industries such as olive oil and palm oil to determine the potential of microalgae to treat this type of wastewater and produce a microalgal biomass as well as compounds with high added values such as lipids and carbohydrates [92,121,130]. During the 14 days of culture, Resdi et al. employed different ratios of palm oil effluent diluted with marine water (0, 20, 40, 60 and 80% dilutions) to cultivate Nannochloropsis sp. compared to palm oil effluent diluted with Walne’s medium [121]. They discovered that Nannochloropsis sp. thrived well in 60% palm oil mill effluent diluted with marine water, had the best specific growth rate of 0.39/d, the maximum cell density of 7.93 107 cell/mL, 61.60% lipids, and could remove 11.31 mg/mg/d of the chemical oxygen demand [121]. In contrast, Emparan et al. immobilized Nannochloropsis sp. in the form of beads using sodium alginate to assess the treatment of palm oil mill wastewater diluted 10 times in comparison with free cell culture [130]. Other authors immobilized Nannochloropsis sp. and found that it achieved the best biomass of 1.27 g L−1 and could remove 71% of the chemical oxygen demand (COD) from palm oil mill effluent, compared to culture with free-cells of Nannochloropsis sp. which showed 0.37 g L−1 for biomass production and 48% of the COD removal efficiency [130]. This sodium alginate immobilization technique did not interrupt the development of Nannochloropsis sp., but rather optimized it by facilitating the passage of nutrients from palm oil mill effluent through the membrane pores of beads, allowing the authors to conclude that this technique is viable for palm oil mill wastewater treatment [130]. Recently, [92] used raw and diluted olive mill wastewater after treatment by anaerobic digestion followed by ultrafiltration to cultivate Scenedesmus sp., which had the highest biomass productivity (0.15 g L−1 d−1) in 25% of the ultra-filtrated anaerobic liquid digestate of the olive mill with a nitrate removal efficiency of 98%. Additionally, Arthrospira platensis has been utilized to remediate cassava processing effluent [101]. The authors cultivated Arthrospira platensis for eight days in an SKM culture medium with modest changes (NaCl: 8 g L−1, NaHCO3: 5 g L−1, Ca(H2PO4)2·H2O: 0.01 g L−1, urea: 0.1 g L−1) and with a daily addition of a volume of cassava processing effluent as a source of nutrients (0–2 mL L−1 of culture) [101]. The findings demonstrated that when 1.5 mL d−1 L−1 of cassava wastewater was added to the culture, Arthrospira platensis could successfully remove nitrogen (88.4%) and phosphate (43.4%) [101]. Over 99% of ammonium, nitrites and nitrates may be reduced by adding up to 2.0 mL d−1 L−1 of cassava processing effluent [101]. The authors of [103] examined the reduction of the chemical oxygen demand of cassava wastewater (COD: 1570.00 mg L−1) and the biosynthesis of lipids by the microalga Desmodesmus armatus in both heterotrophic and mixotrophic growing modes. After 14 days of culture, they discovered that Desmodesmus armatus can biosynthesize 21.91% of lipids and remove 92% of the COD in the mixotrophic mode, as opposed to 74% of the COD and 20.86% of lipids in the heterotrophic mode [103]. Other food-processing wastewaters such as winery, potato and instant coffee wastewaters, have also shown their ability to be treated by microalgae [119,125,126]. The authors of [119] used a continuous flow photobioreactor with a membrane column to co-culture Chlorella vulgaris and Arthrospira platensis for 15 days, using winery wastewater (119.3 g L−1 COD) as the culture medium. This co-culture technique allowed the removal of 75% of the chemical demand from the industrial winery wastewater for a retention time of 2 days, producing a lipid yield of 278 mg g−1 and a biomass of 3.61 g L−1 [119]. In a lab test, five species of Scenedesmus and three species of Desmodesmus were examined to find the potential of microalgae for potato wastewater remediation with a high pigment content [126]. They found that Scenedesmus sp. HXY5 grew the fastest in potato wastewater, yielding the highest biomass of 2.64 g L−1 and a total pigment yield of 18.45 mg L−1, with a lutein yield of 11.46 mg L−1. It displayed an outstanding wastewater purification capability, with removal efficiencies of 59% for the total dissolved nitrogen (TDN), 32% for the total dissolved phosphate (TDP), and 93% for the COD [126]. Chlorella sorokiniana (IPRChs7104) was cultivated using wastewater from the instant coffee and cassava wastewater during 52 days of culture, under 28 °C, 130 μmol m−2 s−1 and a 12:12 h photoperiod [125]. The results showed a significant decrease in the chemical oxygen demand from 7200 mg L−1 to 435 mg L−1, and 4940 mg L−1 to 1240 mg L−1 for cassava and instant coffee wastewater, respectively [125].
The bioremediation of textile effluents was also assessed [99,100,131]. Chlorella vulgaris was cultivated in a raw and diluted (50%) textile wastewater and was compared to BG11 medium as the control during the 14 days of culture [131]. A maximum growth of 1.62 OD680 with a higher COD removal of 99.7% was obtained in the 50% textile wastewater, followed by 1.56 OD680 and 94.4% of the COD removal in the BG11 medium, and 0.89 OD680 with 76.3% of the COD removal in the raw textile wastewater [131]. They also demonstrated that C. vulgaris had the maximum of the fatty acid methyl esters (FAMEs) yield in the BG11 medium, followed by a diluted (50%) and raw textile wastewater of 31.26 mg g−1, 11.07 mg g−1 and 9.12 mg g−1, respectively [131]. In contrast, for Chlorella vulgaris Wu-G22 immobilized with sodium alginate (3%) and cultivated for 10 days in textile wastewater, 75% of chemical oxygen demand removal efficiency and maximum FAMEs accumulation were achieved compared to diluted effluent [100]. After the cultivation of Chlamydomonas sp. TRC-1 in textile wastewater for 7 days of culture, removal efficiencies of the COD, total nitrogen, nitrates and phosphates of 83.08%, 87.15%, 91.75% and 92.36%, respectively, were observed [99]. In addition, they demonstrated that the harvested biomass had a capacity for lipid accumulation (79.1%) and bioelectricity generation (current density of 3.6 A m−2, power of 4.13 × 10−4 W and power density of 1.83 W m−2) [99].

Table 11.
Treatment of industrial wastewater by the cultivation of microalgae.
Table 11.
Treatment of industrial wastewater by the cultivation of microalgae.
| Algae Used | Wastewater Type | Conditions of Culture | Nutrient Concentration | Average Nutrient Removal Rate | References |
|---|---|---|---|---|---|
| Arthrospira platensis | Cassava | 24 °C; 30 μE m−2 s−1 | pH: 3.9; PO4-P: 0.336 g L−1; NO3-N: 0.606 g L−1; NO2-N: 0.108 g L−1; NH4-N: 0.503 g L−1 | PO4-P: 43.4%; NO3-N: 88.4%; NO2-N: 100%; NH4-N: 99.9% | [101] |
| Arthrospira (Spirulina) platensis SAG 21.99 | 10% olive oil mill + 1 g L−1 NaNO3 + 5 g L−1 NaHCO3 | 30 °C; 10,000 lx; 20:4 light: dark | pH: 5.42; COD: 43.87 g L−1; TP: 0.23 g L−1; TN: 1.67 g L−1; Carbohydrates: 13.40 g L−1; Phenols: 3.12 g L−1 | COD: 65.53%; TP: 100%; Carbohydrates: 88.41%; Phénols: 100% | [91] |
| Ascochloris sp. ADW007 | Raw dairy | 29–42 °C; 28,944–196,015 W m−2; | pH: 3.69; COD: 7110 g L−1;TN: 137 mg L−1; TP: 105.1 mg L−1 | COD: 95.1%; TN: 79.7%; TP: 98.1% | [113] |
| Chlamydomonas sp. TRC-1 | Textile | 27 °C; 100 μE m−2 s−1; 16:8 light: dark; | COD: 1378.2 mg L−1;TDS: 8195 mg L−1; TSS:1050 mg L−1; TS: 9245 mg L−1; TN: 40 mg L−1; NO3-N: 16.129 mg L−1; PO4-P: 2.1 mg L−1; Hardness: 168.38 mg L−1; Cl: 1019.46 mg L−1; Turbidity: 43.57 NTU; Alkalinity: 1162.66 mg L−1 | COD: 83.08%; TDS: 82.11%; TSS: 87.40%; TS: 82.64%; TN: 87.15%; NO3-N: 91.75%; PO4-P: 92.36%; Cl: 41.43%; Turbidity: 72.45%; Alkalinity: 45.64% | [99] |
| Chlamydomonaspolypyrenoideum | Dairy industry | 28 ± 2 °C; 10 W m−2; 12:12 light: dark | NO3-N: 48.7 mg L−1; NO2-N: 3.05 mg L−1; PO4-P: 3.9 mg L−1; COD: 6000 mg L−1 | NO3-N: 90%; NO2-N: 74%; PO4-P: 70%; COD: 64.8% | [118] |
| Chlamydomonas reinhardtii CC124 | Olive mill | 28 °C; 70 μE m−2 s−1 (Acclimated for 3 weeks) | COD: 5065.35 mg L−1; TN: 7.01 mg L−1; TP: 42.94 mg L−1 | TN: 65 mg L−1 | [132] |
| Chlorella L166 | 10 times diluted Soybean | 22 °C; 6000 lx | TN: 0.95 g L−1; TP: 0.12 g L−1; COD: 22.70 g L−1 | TN: 43.9%; TP: 72.8–90.4%; COD: 37.1–61.1% | [94] |
| Chlorella L38 | 10 times diluted Soybean | 22 °C; 6000 lx | TN: 0.95 g L−1; TP: 0.12 g L−1; COD: 22.70 g L−1; | TN: 84.7%; TP: 97.3%; COD: 70.5%; | |
| Chlorella pyrenoidosa | Dairy | 27 °C; 120 μE m−2 s−1; 150 rpm | nr | COD: 76.17%; NH4-N: 98.10%; | [133] |
| 90% Olive oil washing + 10% urban | 25 °C; 126.2 μE m−2 s−1, 12:12 light: dark; pH 8 | PO4-P: 6.97 mg L−1; NO3-N: 40.8 mg L−1; COD: 1251 mg L−1 | PO4-P: 56.4%; NO3-N: 49%; COD: 86.3% | [17] | |
| Chlorella pyrenoidosa (FACHB-9) | A mixed acidified and secondary treated starch (1:1) | 32 °C, 127 μE m−2 s−1; 12:12 light: dark | pH: 2.7/7.2; TN: 362.6–302.4 mg L−1; NH4-N: 79.5–273.6 mg L−1; TP: 61.3–55.2 mg L−1 COD: 6196/892 mg L−1 | COD: 57.9% | [102] |
| Soybean | 27 °C; 40.5 μE m−2 s−1; 14:10 light: dark; | NH4-N: 52.1 mg L−1; TP: 56.3 mg L−1; TN: 267.1 mg L−1; COD: 13,215 mg L−1 | NH4-N:89.1%; TP:70.3%; TN:88.8%; COD: 77.8% | [95] | |
| Alcohol and anaerobically digested starch (1:15) | 30–32 °C; 12:12 light: dark | TN: 725.34–307.64 mg L−1; TP: 64.38–37.57 mg L−1; COD: 45,683.06–792.28 mg L−1 | COD: 405.18 mg L−1 d−1; TN: 49.15 mg L−1 d−1; TP: 6.72 mg L−1 d−1 | [104] | |
| Alcohol and anaerobically digested starch (AW/ADSW = 0.053:1, v/v) | 25 °C; 127 μE m−2 s−1; 12:12 light: dark | TN: 618.68–265.10 mg L−1;TP: 47.16–28.34 mg L−1; COD: 65,000–926.3 mg L−1; | COD: 75.78%; TN: 91.64%; TP: 90.74%; | [105] | |
| Anaerobic digested starch | 19.6–36.5 °C; 79,500 lx; 8.6:15.4 light: dark; | TN: 289.6 mg L−1; TP: 38.8 mg L−1 | TN: 57.9%; TP: 89.9% | [127] | |
| Anaerobic digested starch | 35–39 °C; 220 μE m−2 s−1 | TN: 240.3–382.7 mg L−1; TP: 22.7–40.2 mg L−1; COD: 702.4–1026.2 mg L−1 | TN: 83.06%; TP: 96.97%; COD: 65.99% | [106] | |
| Soybean | 28 °C in the dark | TN: 189.9 mg L−1; TP: 45.6 mg L−1; COD: 8087 mg L−1; | TN: 70%; TP: 92.7%; COD: 73.6% | [134] | |
| Chlorella pyrenoidosa (No. FACHB-863) | Tobacco | 25 °C, 80 μE m−2 s−1; 12:12 light: dark | pH: 5; TN: 151.91 mg L−1; NH4-N: 3.58 mg L−1; TP: 6.38 mg L−1; COD: 574.16 mg L−1 | TN: 94.58 mg L−1; NH4-N: 3.44 mg L−1; TP: 3.12 mg L−1; COD: 157.5 mg L−1 | [8] |
| Chlorella sorokiniana (FACHB-275) | Frigon + reeling | 25 °C; 50 μE m−2 s−1; 12:12 light: dark | COD: 5400.0 mg L−1; TN: 308.0 mg L−1; TP: 23.2 mg L−1 | COD: 86.1%; TN: 58.4%; TP: 91.9% | [107] |
| Frigon + distilled water | 25 °C; 50 μE m−2 s−1; 12:12 light: dark | COD: 5050.0 mg L−1; TN: 306.8 mg L−1; TP: 26.2 mg L−1 | COD: 80.0%; TN: 60.4%; TP: 94.9% | ||
| Cooking cocoon | 25 °C; 50 μE m−2 s−1; 12:12 light: dark | COD: 2925.0 mg L−1; TN: 267.5 mg L−1; TP: 23.1 mg L−1 | COD: 86.6%; TN: 38.9%; TP: 49.4% | ||
| Cooking cocoon | 25 °C; 150 μE m−2 s−1; 16:8 light: dark | nr | NH4-N: 92.61%; COD: 66.88%; TN: 78.50%; TP: 97.31% | [135] | |
| Chlorella sorokiniana (IPRChs7104) | Wastewater from the instant coffee | 28 °C; 130 μE m−2 s−1; 12:12 light: dark (2nd cycle, 52 d) | pH: 4.54; COD: 4940 mg L−1 | CODfin: 1240 mg L−1 | [124] |
| Dairy products | 28 °C; 130 μE m−2 s−1; 12:12 light: dark (2nd cycle, 52 d) | pH: 10.26; COD: 190 mg L−1 | CODfin: 158 mg L−1 | ||
| Cassava flour/starch | 28 °C; 130 μE m−2 s−1; 12:12 light: dark (2nd cycle, 52 d) | pH: 3.62; COD: 7200 mg L−1 | CODfin: 435 mg L−1 | ||
| Chlorella sorokiniana SVMBIOEN2 | Dairy | 25 °C; 100 μE m−2 s−1; 12:12 light: dark | COD: 2000 mg L−1 | COD: 85% | [85] |
| Chlorella sp. | Aerated Seafood processing | 25 °C; 135 μE m−2 s−1 | pH: 7.14; COD: 295.1 mg L−1; TN: 94.80 mg L−1; TP: 45.89 mg L−1 | TN: 4.98 mg L−1 d−1; TP: 1.91 mg L−1 d−1 | [108] |
| Meat processing (CUT + KILL) | 25 °C; 120 μE m−2 s−1 | COD: 2100 mg L−1; TN: 212.0 mg L−1; TP: 53.6 mg L−1 | COD: 29.52%; TN: 50.94%; TP: 44.95% | [128] | |
| Meat processing (DS + KILL) | 25 °C; 120 μE m−2 s−1 | COD: 2100 mg L−1; TN: 204.9 mg L−1; TP: 24.1 mg L−1 | COD: 3.21%; TN: 44.46%; TP: 52.11% | ||
| Meat processing (MPGP + KILL) | 25 °C; 120 μE m−2 s−1 | COD: 3020 mg L−1; TN: 197.6 mg L−1; TP: 44.7 mg L−1 | COD: 7.95%; TN: 30.06%; TP: 63.51% | ||
| Meat processing (REFINERY + KILL) | 25 °C; 120 μE m−2 s−1 | COD: 2340 mg L−1; TN: 251.0 mg L−1; TP: 31.3 mg L−1 | COD: 43.91%; TN: 49.48%; TP: 54.45% | ||
| Chlorella sp. L166 | Soybean processing | 25 °C; 6000 lx; 24:0 light: dark | pH: 6; COD: 5320 mg L−1; TN: 106.99 mg L−1; TP: 23.28 mg L−1 | COD: 78.20%; TN: 96.07%; TP: 95.55% | [96] |
| Chlorella sp. (SAG 242.80) | Rose oil processing effluent (Race way) | 17–19.5 °C; 256–329 μE m−2 s−1 | COD: 2343 mg L−1; NH4-N: 10.67 mg L−1; NO3-N: 2.40 mg L−1 | COD: 61.76%; NH4-N: 66.99%; NO3-N: 17.22% | [110] |
| Rose oil processing effluent (Tubular photobioreactor) | 28.5–31.5 °C; 256–329 μE m−2 s−1 | COD: 2342 mg L−1; NH4-N: 13.25 mg L−1; NO3-N: 1.85 mg L−1 | COD: 53.03%; NH4-N: 34.95%; NO3-N: 8.63% | ||
| Chlorella vulgaris | Industrial | 25 °C; 2500 lx; 12:12 light: dark | TN: 11.5 mg L−1; Cu: 8.50 mg L−1; Cd: 1.31 mg L−1; Ni: 0.16 mg L−1; Fe: 3.51 mg L−1; Pb: 2.48 mg L−1; | TN: 0.19 mg L−1; Cu: 7.63 mg L−1; Cd: 0.60 mg L−1; Ni: 0.14 mg L−1; Fe: 2.80 mg L−1; Pb: 1.72 mg L−1 | [111] |
| 50% Textile | 4000–5000 lx; | COD: 500–1200 mg L−1 | COD: 99.7%; NO3-N: 95.7%; PO4-P: 96.3% | [131] | |
| Chlorella vulgaris (UTEX-265) | Brewery | 25 °C; 100 μE m−2 s−1; 150 rpm; airflow rate of 100.0 cc/min; | pH: 5.5–6.0; COD: 2000–3000 mg L−1; TN: 30–45 mg L−1; TP: 12–16 mg L−1 | TN: 87%; TP: 80% | [109] |
| Brewery | 25 °C; 100 μE m−2 s−1 | pH: 5.5–6.0; COD: 2000–3000 mg L−1; TN: 30–45 mg L−1; TP: 12–16 mg L−1 | More than 70% of the nutrients | ||
| Chlorella vulgaris Wu-G22 | Textile | 30 °C; 4300 lx; pH 8.0 | pH: 10.5; COD: 4458 mg L−1; NH4-N: 8.35 mg L−1; TP: 22.3 mg L−1 | COD: 75%; NH4-N: 90% | [100] |
| Chlorella zofingiensis | 10% Dairy (pH regulation by CO2) | 6.2–20.8 °C; 310–1035 klx; 5–6% CO2 | TN: 11.8 mg L−1; PO4-P: 14.9 mg L−1;COD: 119.5 mg L−1 | TN: 79.6%; PO4-P: 42.0% | [136] |
| 10% Dairy (pH regulation by acetic acid) | 6.2–20.8 °C; 310–1035 klx; 5–6% CO2 | TN: 11.8 mg L−1; PO4-P: 14.9 mg L−1;COD: 119.5 mg L−1 | TN: 97.5%; PO4-P: 51.7% | ||
| Dairy | 25 °C; 200 μE m−2 s−1; 5–6% CO2 | TN: 75.5 mg L−1; TP: 48.0 mg L−1; COD: 1428 mg L−1 | TN: 90.3%; TP: ~75% | [117] | |
| Dairy | 25 °C; 200 μE m−2 s−1; 5% CO2 | TN: 136.5 mg L−1; TP: 85.0 mg L−1; COD: 1858 mg L−1 | TN: 93.64%; TP: 98.45%; COD: 85.05% | [114] | |
| 2.5% Palm oil mill effluent | Green stage: 28 °C; 3000 lx; 12:12 light: dark Red stage: 28 °C, 6000 lx continuous light | TN: 9.4 mg L−1; TP: 1.45 mg L−1; COD: 72.46 mg L−1 | TN: 49.3%, TP: 69.4%; COD: 28% | [98] | |
| Coelastrella sp. | Dairy | Under sunlight | pH: 8.65; P: 8.0 mg L−1; NH4-N: 39.3 mg L−1; NO3-N: 64.1 mg L−1; COD: 1970 mg L−1; TOC: 722 mg L−1 | P: 66.75%; NH4-N: 90.38%; NO3-N: 90.24%; COD: 69.44%; TOC: 83.51% | [115] |
| Desmodesmus armatus | Cassava | Heterotrophic: in the dark for 14 days | pH: 5.70; COD: 1570.00 mg L−1 | COD: 74% | [103] |
| Cassava | Mixotrophic: 4:20 light: dark for 14 days | pH: 5.70; COD: 1570.00 mg L−1 | COD: 92% | ||
| Haematococcus pluvialis | 7.5% Palm oil mill effluent | Green stage: 28 °C; 3000 lx; 12:12 light: dark Red stage: 28 °C; 6000 lx; 24:0 light: dark | TN: 28.2 mg L−1; TP: 4.4 mg L−1; COD: 217.40 mg L−1 | TN: 64.9%; TP: 86.7%; COD: 50.9% | [98] |
| Isochrysis galbana | Al-Ahdab oilfield produced water | 2000 lx; 25 °C; 18:6 light: dark | COD: 1300 mg L−1; NH4-N: 31 mg L−1 | COD: 83% | [137] |
| Nannochloropsis oculata | Al-Ahdab oilfield produced water | 25 °C; 2000 lx; 18:6 light: dark | COD: 1300 mg L−1; NH4-N: 31 mg L−1 | COD: 90% | [137] |
| Nannochloropsis sp. | Palm oil mill effluent | 23 ± 10.5 °C; 100 μE m−2 s−1 | COD: 4196.67 mg L−1 | COD: 11.31 mg mg−1 d−1 | [121] |
| 10% Palm oil mill effluent | Immobilized microalgae on sodium alginate beads | COD: 3250 mg L−1 | COD: 71% | [130] | |
| Nostoc commune | Industrial | 25 °C; 2500 lx; 12:12 light: dark | TN: 11.5 mg L−1; Cu: 8.50 mg L−1; Cd: 1.31 mg L−1; Ni: 0.16 mg L−1; Fe: 3.51 mg L−1; Pb: 2.48 mg L−1 | TN: 0.27 mg L−1; Cu: 7.77 mg L−1; Cd: 0.67 mg L−1; Ni: 0.14 mg L−1; Fe: 2.80 mg L−1; Pb: 1.66 mg L−1 | [111] |
| Oscillatoria limosa | Industrial | 25 °C; 2500 lx; 12:12 light: dark | TN: 11.5 mg L−1; Cu: 8.50 mg L−1; Cd: 1.31 mg L−1; Ni: 0.16 mg L−1; Fe: 3.51 mg L−1; Pb: 2.48 mg L−1 | TN: 0.15 mg L−1; Cu: 7.70 mg L−1; Cd: 0.61 mg L−1; Ni: 0.14 mg L−1; Fe: 2.7 mg L−1; TP: 1.68 mg L−1 | [111] |
| Scenedesmus dimorphus | Lactic acid | 25 °C; 2500 lx; 14:10 light: dark | COD: 12 571 mg L−1; TN: 651 mg L−1; NH3-N: 169 mg L−1; TP: 28.2 mg L−1 | COD: 95.06%; TN: 96.31%; NH3-N: 98.22; TP: 90.78% | [124] |
| Scenedesmus sp. | 25% of ultra-filtrated anaerobic liquid digestate of olive mill | 25 °C; 80.2 μE m−2 s−1; 24:0 light: dark | TN: 96 mg L−1 | TN: 98% | [92] |
| Industrial | nr | TN: 5.06 mg L−1; TP: 39.4 mg L−1; COD: 4395.6 mg L−1; | TN: 42%; TP: 67%; COD: 82% | [123] | |
| Scenedesmus sp. and chlorella sp. | Palm oil mill effluent | 25 °C; 14,000 lx; 24:0 light: dark | TN: 330 mg L−1; PO4-P: 273 mg L−1; COD: 2900 mg L−1; TOC: 1400 mg L−1 | TN: 86%; PO4-P: 85%; COD: 48%; TOC: 77% | [122] |
| Scendesmus sp. HXY5 | Potato | 25 °C; 60 μE m−2 s−1; 12:12 light: dark; | TDN: 127.98 mg L−1; TDP: 11.11 mg L−1; COD: 1504 mg L−1; | TN: 59%; TP: 32%; COD: 93% | [126] |
| Spirulina NCU-Sm | Soy sauce | 30 °C; 50 μE m−2 s−1 | NH4-N: 168.44 mg L−1; TN: 173.53 mg L−1; COD: 3263.33 mg L−1 | NH4-N: 93.86%; TN: 81.76%; COD: 84.08% | [120] |
TOC (total organic carbon), COD (chemical oxygen demand), TDS (total dissolved solid), TSS (total suspended solid), TS (total solid), NO3-N (nitrate-nitrogen), NO2-N (nitrite-nitrogen), NH4-N (ammonium-nitrogen), PO4-P (orthophosphate phosphate), TN (total nitrogen), TP (total phosphate), Cl (chloride), Cu (copper), Fe (iron), Cd (cadmium), Ni (nickel), Pb (lead).

Table 12.
Growth parameters and metabolites production of microalgae culture in industrial wastewater.
Table 12.
Growth parameters and metabolites production of microalgae culture in industrial wastewater.
| Algae Used | Wastewater Type | Growth Rate or Volumetric Productivity | Final Biomass Concentration | Production of Target Metabolites | References |
|---|---|---|---|---|---|
| Arthrospira platensis | Cassava | 1.16 d−1 | 14,410 cells mL−1 | Exopolysaccharides: 0.41 g L−1 | [101] |
| Arthrospira (Spirulina) platensis SAG 21.99 | 10% olive oil mill + 1 g L−1 NaNO3 + 5 g L−1 NaHCO3 | 4.4 g L−1 h−1 | 1696 mg L−1 | Carbohydrates: 33.64%; Lipids: 16.91%; Proteins: 31.52%; Chlorophyll: 1.02% | [91] |
| Ascochloris sp. ADW007 | Raw dairy | 0.131 g L−1 d−1 | 2.23 g L−1 | Lipids: 34.67% | [113] |
| Raw dairy | 0.102 g L−1 d−1 | 1.73 g L−1 | Lipids: 24.99% | ||
| Raw dairy | 0.207 g L−1 d−1 | 1.44 g L−1 | Lipids: 34.98% | ||
| Chlamydomonas sp. TRC-1 | Textile | 0.28 g L−1 d−1 | 2.49 g L−1 | Lipids: 79.1%; Saturated fatty acid (SFA): 46.51%; Monounsaturated fatty acids (MUFAs): 20.7%; Polyunsaturated fatty acids (PUFAs): 11.3%; | [99] |
| Chlamydomonas polypyrenoideum | Dairy industry | nr | 3.8 g | Lipids: 1.6 g L−1 | [118] |
| Chlamydomonas reinhardtii CC124 | Olive mill | 22.2 mg L−1 h−1 | nr | Carbohydrates: 32.6%; Proteins: 53.87% | [132] |
| Chlorella L166 | 10 times diluted Soybean | nr | nr | Lipids: 7.22 mg L−1 d−1; Polysaccharides: 2.86 mg L−1 d−1 | [94] |
| Chlorella L38 | 10 times diluted Soybean | nr | nr | Lipids: 3.89 mg L−1 d−1; Polysaccharides: 1.38 mg L−1 d−1 | |
| Chlorella pyrenoidosa | Dairy | nr | nr | nr | [133] |
| 90% Olive oil washing +10% urban | 0.0203 h−1 | 1.73 × 10−3 g L−1 h−1 | Lipids: 51.5%; Proteins: 43.7% | [17] | |
| Chlorella pyrenoidosa (FACHB-9) | A mixed acidified and Secondary treated starch (1:1) | 1.27 d−1 | 3.3 g L−1 | Carbohydrates: 24.4%; Lipids: 18.7%; Proteins: 49.7%; Chlorophyll: 2.8% | [102] |
| Soybean processing | nr | 0.64 g L−1 d−1 | Lipids: 37% | [95] | |
| Alcohol and anaerobically digested starch (1:15) | 0.98 d−1 | 2.76 g L−1 | Lipids: 19.68% | [104] | |
| Alcohol and anaerobically digested starch (0.053:1, v/v) | 0.56 d−1 | 3.01 g L−1 | Lipids: 127.71 mg L−1 d−1 | [105] | |
| Anaerobic digested starch | 0.82 d−1 | 1.29 g L−1 | Lipids: 43.37 mg L−1 d−1; | [127] | |
| Anaerobic digested starch | 1.02 d−1 | 0.37 g L−1 d−1 | Lipids: 7.32%; | [106] | |
| Soybean | 0.058 d−1 | 6.2 g L−1 | Lipids: 0.53 mg L−1 d−1 | [134] | |
| Chlorella pyrenoidosa (No. FACHB-863) | Tobacco | nr | 540.24 mg L−1 | Lipids: 268.60 mg L−1 | [8] |
| Chlorella sorokiniana (FACHB-275) | Frigon + reeling | 0.36 d−1 | 19.3 mg L−1 | Lipids: 26.2%; Proteins: 49.5%; Carbohydrates: 21.2% | [107] |
| Frigon + distilled water | 0.35 d−1 | 17.7 mg L−1 | Lipids: 22.4%; Proteins: 47.3%; Carbohydrates: 20.2% | ||
| Cooking cocoon | 0.33 d−1 | 15.4 mg L−1 | Lipids: 22.1%; Proteins: 45.7%; Carbohydrates: 20.8% | ||
| Cooking cocoon | 0.496 d−1 | 3.43 mg L−1 | Lipids: 27.95%; Proteins: 53.25%; Carbohydrates: 14.25% Pigments: 2.66% | [135] | |
| Chlorella sorokiniana (IPRChs7104) | Wastewater from the instant coffee | nr | 0.88 g L−1 | nr | [125] |
| Dairy products | nr | 0.35 g L−1 | nr | ||
| Cassava flour/starch | nr | 1.23 g L−1 | nr | ||
| Chlorella sp. | Aerated Seafood | 0.156 d−1 | 77.7 mg L−1 d−1 | Lipids: 20.4 mg L−1 d−1 | [108] |
| Meat processing | nr | 1.538 g L−1 | Lipids: 17.54%; Proteins: 68.65% | [128] | |
| Meat processing | nr | 0.675 v | Lipids: 14.50%; Proteins: 60.87% | ||
| Meat processing | nr | 1.388 g L−1 | Lipids: 18.89%; Proteins: 61.20% | ||
| Meat processing | nr | 1.400 g L−1 | Lipids: 20.57%; Proteins: 64.76% | ||
| Brewery | nr | 1.22 g L−1 | Lipids:10% | [109] | |
| Brewery | nr | 2.74 g L−1 | Lipids: 50.23 mg L−1 d−1 | ||
| Dairy | 0.193 d−1 | 1.37 g L−1 | FAMEs: 87.09 mg L−1 | [138] | |
| Chlorella vulgaris | Dairy | 0.261 d−1 | 0.26 g L−1 | FAMEs: 27.18 mg L−1 | [138] |
| 50% Textile | nr | 1.62 OD680 | FAMEs: 11.07 mg g−1 | [131] | |
| Chlorella vulgaris (UTEX-265) | Brewery | nr | 1.5 g L−1 | Lipids: 18% | [109] |
| Brewery | nr | 3.2 g L−1 | Lipids: 108.0 g L−1 d | ||
| Chlorella zofingiensis | 10% Dairy (pH regulation by CO2) | nr | 10.9 × 106 cells mL−1 | Lipids: 17.9% | [136] |
| 10% Dairy (pH regulation by acetic acid) | nr | 9.05 × 106 cells mL−1 | Lipids: 31.8% | ||
| Dairy | nr | 3.8 g L−1 | Lipids: 27.7% | [117] | |
| Dairy | nr | 1.58–1.69 g L−1 | Lipids: 11.5–15.8% | [114] | |
| 2.5% Palm oil mill effluent | 0.2 d−1 | 0.48 g L−1 | Astaxanthin: 2.71 mg L−1 | [98] | |
| Coelastrella sp. | Dairy | nr | 4.61 g L−1 d−1 | nr | [115] |
| Desmodesmus armatus | Cassava | 0.14 d−1 | 52.14 mg L−1 d−1 | Lipids: 20.86% | [103] |
| Cassava | 0.15 d−1 | 70.83 mg L−1 d−1 | Lipids: 21.91% | ||
| Haematococcus pluvialis | 7.5% Palm oil mill effluent | 0.21 d−1 | 0.52 g L−1 | Astaxanthin: 22.43 mg L−1 | [98] |
| Isochrysis galbana | Al-Ahdab oilfield produced water | 0.169 d−1 | 0.899 g L−1 | Oil content: 82% | [137] |
| Nannochloropsis oceanica | Pesticides industry | 27.78 mg/L d−1 | nr | Lipids: 24.49% | [32] |
| Pharmaceutical industry | 5.59 mg L−1 d−1 | nr | Lipids: 25.22% | [32] | |
| Petroleum industry | 24.78 mg L−1 d−1 | nr | Lipids: 27.40% | [32] | |
| Nannochloropsis oculata | Al-Ahdab oilfield produced water | 0.179 d−1 | 1.0166 g L−1 | Oil content: 89% | [137] |
| Nannochloropsis sp. | Palm oil mill effluent | 0.39 d−1 | 7.93 × 107 cells/mL | Lipids: 61.60%, PUFAs: 59.13% | [121] |
| 10% Palm oil mill effluent | nr | 1.27 g L−1 | nr | [130] | |
| Scenedesmus dimorphus | Lactic acid | nr | 5.32 g L−1 | Lipids: 28.61% | [124] |
| Scenedesmus sp. | 25% of ultra-filtrated anaerobic liquid digestate of olive mill | 0.5 d−1 | 0.15 g L−1 d−1 | nr | [92] |
| Industrial | 0.15 d−1 | 0.69 g L−1 | nr | [123] | |
| Scenedesmus sp. and chlorella sp. | Palm oil mill effluent | stage 1: 0.1273 d−1 stage 2: 0.5858 d−1 | stage 1: 0.0204 g L−1 d−1 stage 2: 0.4403 g L−1 d−1 | Stage 1: Lipids: 17.19%; Carbohydrates: 2.06%; Proteins: 48.6% Stage 2: Lipids: 19.29%; Carbohydrates: 10.58%; Proteins: 57.36% | [122] |
| Scendesmus sp.HXY5 | Potato | 1.64 mg L−1 d−1 | 2.64 g L−1 | Total pigment yield: 18.45 mg L−1; Lutein yield: 11.46 mg L−1 | [126] |
| Spirulina NCU-Sm | Soy sauce | nr | 1.984 g L−1 | nr | [120] |
nr (not reported).
3. Microalgal Mechanisms for Wastewater Bioremediation
As shown in Table 1, Table 5 and Table 10, which summarize the wastewater composition utilized in the literature in the field of microalgae wastewater treatment, domestic, agricultural and industrial wastewaters contain carbon and various nitrogen sources such as nitrate, nitrite and ammonium, as well as phosphate from both inorganic and organic sources and heavy metals [139]. Microalgae can biodegrade these contaminants through a variety of methods, including absorption, fixation, bioabsorption and precipitation [5]. Microalgal–bacterial consortia have also been shown to improve the efficiency of wastewater treatment procedures [140,141]. Figure 7 illustrates the numerous microalgal techniques for biodegrading pollutants present in wastewater with the support of bacteria.
3.1. CO2 Fixation
The carbon concentrating mechanism (CCM) and photosynthetic carbon metabolic pathways are the mechanisms that microalgae utilize for CO2 fixation when they are grown in wastewater [142]. Microalgae may absorb photons from the sun and use CO2 or dissolved in wastewater as a carbon source for photosynthesis, CO2 can enter the cell through diffusion, and enters the cell through active transport [5,142]. Furthermore, they use heterotrophic or mixotrophic modes to absorb inorganic and organic carbon from wastewater for metabolic control and the production of biomolecules such as carbohydrates and lipids [5,143,144]. The biological reaction of carbon fixation and biomolecule production is shown in Equations (1) and (2) [5].
where Equation (1): CO2 fixation and carbohydrate generation; Equation (2): glucose utilization to pyruvate, acetyl-CoA, and fatty acid synthesis.
3.2. Nitrogen Sources Assimilation
Ammonium nitrogen (NH4-N), nitrate nitrogen (NO3-N), and nitrite nitrogen (NO2-N) enter microalgal cells via active transport [142]. Ammonium nitrogen (NH4-N) is a key component in microalgae growth [145]. For this reason, before being assimilated, nitrate and nitrite are converted to ammonium by enzymes nitrate reductase and nitrite reductase [142] as demonstrated by the equations below (3)–(5):
Here, Equation (3): ammonium-based nitrate and nitrite reduction; Equation (4): reaction of glutamine synthesis; Equation (5): proteins formation.
3.3. Phosphate Source Assimilation
Phosphorus restriction in a culture medium is an efficient technique to enhance microalgal metabolism since phosphorus is required for metabolic activities [144,146,147]. It is essential for energy transmission and nucleic acid synthesis [142]. Depending on the source of wastewater, phosphorus is present in the form of polyphosphate and orthophosphate [5]. These forms penetrate microalgal cells by active transport [142] and are consumed as and [139] are removed directly by incorporating them into organic substances (e.g., phospholipids) via the phosphorylation process [142].
Here, Equation (6): ATP, nucleic acid and phospholipids formation.
Furthermore, environmental conditions including the pH level, temperature and dissolved oxygen may influence P nutrient removal [142]. Phosphorus precipitation, for example, can be observed at a pH of 8.5 in the presence of carbonate and magnesium ions in wastewater as calcium or magnesium phosphates [5].
3.4. Heavy Metals Biodegradation
Heavy metals present in wastewater have a number of harmful consequences on the environment and people’s health, some of which can cause cancer [148]. Microalgae have demonstrated their capabilities despite the fact that heavy metals are more difficult to remove by physicochemical procedures than other wastewater elements [149]. This is due to the strong affinity interaction that exists between the microalgal cell wall and heavy metals [150]. There are five primary methods through which microalgae eliminate heavy metals from wastewater: intracellular bioaccumulation, ionic and covalent bond formation, exchange of cation and the complexes and chelates formation [139,148,149,151]. In addition, once inside the microalgae, heavy metals are biotransformed and compartmentalized in cell organelles [139,148,149,151]. These different mechanisms are illustrated in Figure 8.
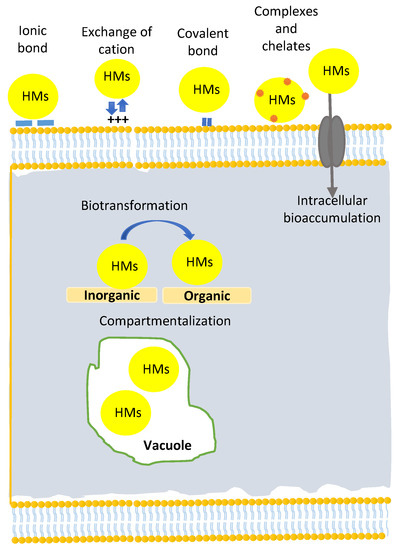
Figure 8.
Heavy metals (HMs) mechanisms of bioremediation by microalgae.
4. Economic Analysis of Microalgae Wastewater Treatment Technologies
4.1. Microalgae-Based Wastewater Treatment Technologies
Conventional wastewater treatment procedures consist of four main steps: preliminary, primary, secondary and tertiary treatments when physical, chemical and biological processes are used as the traditional methods [152]. Ion exchange, membrane filtration, coagulation or flocculation, bacteria-based remediation techniques and adsorption are only a few of the widely utilized techniques in traditional wastewater treatment (Figure 9).
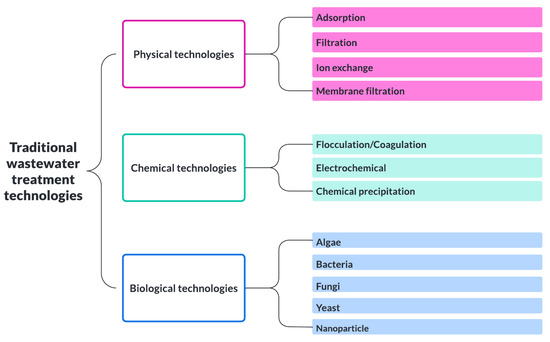
Figure 9.
Traditional wastewater treatment technologies.
Our literature review demonstrates that microalgae monoculture systems have shown to be beneficial as an innovative wastewater treatment technology. Recently, microalgae have been co-cultured with other microorganisms such as bacteria, fungi and yeast to enhance pollutant removal efficiencies and reduce the cost of the treatment process [153,154,155,156,157,158].
Microalgae-bacteria co-culture system was used by Liu et al. [155] to optimize ammonia nitrogen removal from actual rare earth element tailings (REEs) wastewater, using Chlorococcum robustum as microalga species. Liu et al. [155] found that this strategy of treatment achieved a 97.46% removal of ammonia nitrogen at a ratio of 1:3 of microalgae-bacteria consoritium. Walls et al. [158] used Scenedesmus sp. with a native wastewater yeast to treat municipal wastewater and achieved a 96% removal of nitrate and a 100% removal of total ammonia with a 93% removal of orthophosphate [158]. A filamentous fungi (Aspergillus oryzae) was cultured with Chlorella pyrenoidosa to valorize starch wastewater into high-value biomass [157]. Wang et al. [157] reported that this technology increased the removal efficiencies of major pollutants, with the chemical oxygen demand, total nitrogen, and total phosphate removal efficiencies reaching 92.08, 83.56 and 96.58%, respectively. Ray et al. [159] summarized these technologies and reported that microalgae such as Chlorella vulgaris, Chlorella variabilis, Scenedesmus obliquus and Scenedesmus capricornutum were co-cultured with Fungi species, among them Mucor circinelloides UMN-B34, Ganoderma lucidum, Aspergillus sp., Aspergillus niger, Pleurotus geesteranus and Pleurotus ostreatu to remove nutrients and cadmium by forming biopellets as well as harvesting a microalgal biomass. Furthermore, they indicated that Chlorella sorokiniana, Chlorella vulgaris, Scenedesmus obliquus, Selenastrum capricornutum and Spirulina platensis were used in microalgae-bacteria consortium with Proteobacteria, Pseudomonas sp., Acinetobacter sp., Rhodococcus sp., Mycobacterium sp., Burkholderia cepacia, Alcaligenes sp. and Azospirillum brasiliense for organic chemical pollutants and heavy metal removals from wastewater and greenhouse gas mitigation [159]. Chlorella vulgaris was cocultured with Yarrowia lipolytica for ammonia nitrogen and sulfate removals, but with Saccharomyces cerevisiae for in situ CO2 mitigation along with a reduction in the aeration costs of microalgae-yeast technology [159].
In addition to bacteria, fungi and yeast, nanoparticles such as iron oxide and ZnO were also used as a consortium with microalgae to remove pollutants from wastewater [160,161]. Vasistha et al. [160] used an integrated approach microalgae-ZnO nanoparticle association to improve the nutrient removal from sewage wastewater and biodiesel production, and demonstrated that the microalga Chlorosarcinopsis sp. MAS04-ZnO consortium achieved the maximum biomass of 3.43 g L−1 in primary treated wastewater with a 1.9-fold increase in cellular lipid compared to the BG11 medium.
4.2. Economic Assessment of Microalgae-Based Wastewater Treatment
The feasibility of wastewater treatment by microalgae toward a biorefinery system is influenced by a number of variables, including upstream and downstream processing, environmental effects and commercial analysis [5]. Assessment of the economic analysis can be examined by LCA and TEA, which are life cycle assessments and techno economic assessments, respectively [162]. Generally, LCA uses “cradle-to-grave” system boundaries that take into account every step of the treatment process to quantify the mass and energy exchanges between the treatment system and the overall environment. However, the TEA is often fixed to the treatment stage using “cradle-to-gate” system limitations, eliminating the waste treatment [163]. The five phases of TEA are generally the process design, mass and energy balance, cost projections, profitability and sensitivity analysis [163]. Therefore, a complete schematic design of the entire treatment process is essential to properly estimate the cost of the microalgae-based wastewater treatment process in a biorefinery system. The design process is the mean problem that limit both LCA and TEA analysis, thus, it is clear that there is huge research gap from lab-scale to pilot or large-scale, which limits the implementation of microalgae-based wastewater treatments in a biorefinery system.
According to earlier research, regular open ponds or even sophisticated high-rate algal ponds (HRAP) are the most effective approach for growing microalgae in wastewater [164]. The environmental effect of HRAP systems for wastewater treatment has been evaluated through research using the life cycle assessment approach. They showed that, when compared to traditional systems (such as activated sludge systems), HRAPs might potentially aid to minimize environmental impacts and costs linked with wastewater treatment [165,166,167,168]. Kohlheb et al. [168] evaluated the sustainability of HRAP systems for municipal wastewater treatments by microalgae compared to sewage sludge systems. According to their findings, modern HRAP technology is more energy-efficient than an activated sludge-based sequencing batch reactor, requiring just 22% of their electricity demand [168]. Furthermore, HRAP is more cost-effective (0.18 € m−3) compared to activated sludge (0.26 € m−3) and more ecologically friendly, with reduced global warming (146.27.103 kg CO2 equivalent m−3) and eutrophication potentials (126.14 vs. 158.01.106 kg PO4 equivalent m−3), but the net environmental benefit of an activated sludge-based sequencing batch reactor was slightly larger than HRAPs because of the removal rate of nutrients [168].
Recently, Nasir et al. [169] compared co-pyrolyzing sewage sludge and wastewater-grown microalgae for biofuel production using LCA, global warming, energy recovery, and economic analysis in five scenarios while varying the mixture of sewage sludge and microalgae. They found that the 1:1 mixture produced the largest net profit (9% higher than sewage sludge). However, the environmental effect of the 1:1, 1:2 and 2:1 scenarios was lower than that of sewage sludge alone. For energy consumption, due to the high moisture content of the feedstock, they revealed that drying was the most energy-intensive operation, accounting for 69–88% of total used energy [169]. In another study, Arashiro [170] investigated the possible ecological effects of microalgae systems for wastewater treatment and bioproduct extraction. In this regard, a life cycle evaluation of two systems treating urban wastewater and food-processing wastewater with the recovery of bioproducts and bioenergy was performed. Furthermore, both solutions were compared to an existing method employing a typical growth medium for microalgae cultivation, in order to demonstrate the potential benefits of using wastewater against traditional cultivation methodologies [170]. They proved that the organization that treated food-processing wastewater with unialgal culture had lesser environmental consequences than the system that treated urban wastewater with mixed cultures [170]. When compared to a traditional system employing a standard growing medium, bioproduct recovery from microalgae wastewater treatment systems can minimize environmental impacts by up to five times [170].
Life cycle and technoeconomic analysis are being explored and provide a significant challenge for researchers, particularly for economic evaluation, with the goal of establishing an industrial-scale system for wastewater treatment by microalgae.
5. Challenges and Prospects
Domestic, agricultural and industrial wastewaters have recently received increased attention from microalgae researchers in order to assess the capability of microalgae to reduce contaminants found in these various forms of wastewater, and furthermore, project a microalgal biorefinery system. Microalgae have demonstrated their ability to remove these pollutants, but this ability can be increased if the difficulties encountered during culture are resolved. Figure 10 depicts a number of challenges (economic and biological) associated with the microalgae-based wastewater treatment process as well as ideas in order to deal with these issues.
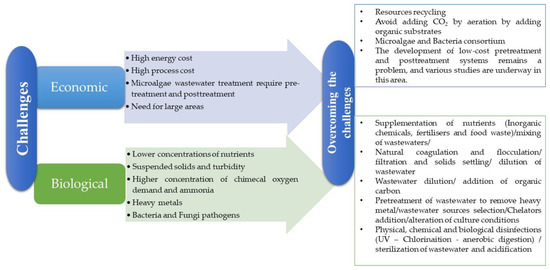
Figure 10.
Limitations in growing microalgae in wastewater and possible solutions.
Microalgae cell growth can be limited by low nutrient in wastewater, however this is one of the biological obstacles that can be solved by combining different wastewaters, adding inorganic compounds, fertilizers, and food waste [41,74,91,105]. Using agricultural waste (poultry litter) mixed with municipal wastewater to cultivate Acutodesmus obliquus result in a biomass 74.67% higher than using agricultural waste as culture medium [41]. The effect of alcohol wastewater as an anaerobic digested starch wastewater supplement on the removal of pollutant load by the microalgae Chlorella pyrenoidosa has been evaluated [105]. The strain was grown in various concentrations of anaerobic digested starch wastewater (ADS) and alcohol wastewater (A), but the proportion 1/0.053 of ADS/A wastewaters generated the highest results in terms of biomass (3.01 g/L), lipid production (127.70 mg/L/d), and pollutants removals (75.78%, 91.64 % and 90.74% of COD, TN and TP respectively) [105]. In contrast, researchers employed the dilutions approach to maximize the development of the microalgae in wastewater that had a high concentration of chemical oxygen demand and organic carbon addition to alleviate ammonia toxicity [162,171,172]. As reported, large amounts of ammonia can be toxic to cells by inhibiting development, photosynthesis and chlorophyll production, also destroying pH and C/N balances [145,171,173]. Temperature and pH both affect the presence of NH3-N, which predominates when pH is greater than 9.25 [145]. Two processes through which ammonia is toxic to the growth of microalgae can be observed: free NH3 diffusion in addition to NH4+ production in thylakoid under acidic conditions (reduces ATP to ADP conversion) and the promotion of NH3 ligation (harms the oxygen-evolution complex subunit) [145]. Additionally, in microalgae culture system with a temperature between 20 and 25 °C and a pH of 9.5, the photosynthesis is reduced up to 90% only because of 2 to 3 µM of NH3, hence the need to minimize ammonia toxicity [174]. Several different strategies exist to reduce toxicity of ammonia, including dilution, chemical stripping and addition of organic carbon [171]. Indeed, using glucose as carbon source for three species of microalgae (Chlorella sorokiniana, Coelastrum sp., and Desmodesmus communis) was found to minimize toxicity in all species at ammonia concentrations ranging from 50 to 1000 mg/L [171].
Others biological obstacles, such as high content of suspended solids, turbidity, heavy metals and bacteria can be overcome using appropriate pre-treatment methods for each obstacle. The previous Table 2, Table 6 and Table 9 describe a few pretreatment techniques that were employed in the literature prior to the cultivation of microalgae but most of them are not possible at large scale. One of the challenges could thus be to develop industrializable pre-treatments processes.
In the field of microalgae growth in wastewater for biomass generation and nutrient removal, the development and design of low-cost microalgae culturing systems is now a critical topic [175]. Closed photobioreactors often cost a lot of money and have a difficult design. The capital costs of a flat-panel photobioreactor, a tubular photobioreactor, and a bubble-column photobioreactor were reported to be 42.2, 26.2, and 15.5 $ m−2, respectively [175]. Ahmed et al. reported that the culture stage used the majority of the operational resources and photobioreactors (PBR) and require around ten times as much energy when utilizing pumping and/or aeration [150]. The conventional method for growing photoautotrophic microalgae uses CO2 as carbon source. However, procedures for purifying, storing, and distributing CO2 raise the cost of producing microalgae. Cultivation using carbon dioxide in dissolved form or using bacteria to produce CO2 were proposed as promising solution to overcome this challenge [7,175]. Bicarbonate could be used in the culture medium, that could result in productivity up to 50% greater than that of CO2 while decreasing the cost of culture by 55% [175]. The resources recycling approach is also a strategy to reduce the cost of cultivation system [176].
In addition, another barrier to large-scale wastewater microalgae-based treatment is the downstream processing of microalgae. Numerous studies are now being conducted to develop highly effective inexpensive harvesting approaches [4,157,177,178]. Ref. [4] harvested Chlorella sp. biomass using Aspergillus sp., a filamentous fungal in molasses wastewater, to simplify the harvesting technique and minimize the process’s production cost [4]. Coagulation (aluminum sulphate, ferricchloride, Tanfloc SG and Zetag 8185 coagulants) followed by dissolved air flotation (DAF) have shown high efficiency but the significant residual content of iron and aluminum in the final effluent necessitates prudence when using metal salts [177].
Although wastewater-grown microalgae have a significant potential, there have only been a few investigations of the value of biomass. Therefore, future studies should concentrate on finding the best wastewater sources, optimizing growing conditions, accumulation of product, and concomitantly extracting product and converting biomass. In order to provide suitable circumstances for microalgae development, it is also necessary to develop effective and affordable pretreatment techniques as well as integrated reactor systems.
6. Conclusions
In this review, it has been demonstrated in Section 2 that several species of microalgae, including Chlorella pyrenoidosa, Chlorella sorokiniana, Chlorella vulgaris, Chlorella zofingiensis, Nannochloropsis and Scenedesmus, can be used to remove pollutants from wastewater in a microalgal biorefinery system because of their capacity to accumulate high added value substances like lipids, carbohydrates, and carotenoids (astaxanthin and lutein). However, the cultivation of microalgae in wastewater requires efficient and less expensive pretreatment and downstream processes to be able to use the biomass produced as well as the treated wastewater (Section 2.1.2, Section 2.2.2 and Section 2.3.2). Indeed, this problem constitutes a major challenge on which scientists are working, in addition to the search for adequate sources of wastewater as well as technical and economic studies (Section 4).
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Gupta, S.; Pawar, S.B.; Pandey, R. Current practices and challenges in using microalgae for treatment of nutrient rich wastewater from agro-based industries. Sci. Total Environ. 2019, 687, 1107–1126. [Google Scholar] [CrossRef] [PubMed]
- Coyle, E.D.; Simmons, R.A. Understanding the Global Energy Crisis; Purdue studies in public policy; Purdue University Press: West Lafayette, IN, USA, 2014. [Google Scholar]
- Bi, Z.; He, B. Biodiesel from Microalgae. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–371. [Google Scholar]
- Yang, L.; Li, H.; Wang, Q. A novel one-step method for oil-rich biomass production and harvesting by co-cultivating microalgae with filamentous fungi in molasses wastewater. Bioresour. Technol. 2019, 275, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Goswami, R.K.; Mehariya, S.; Verma, P.; Lavecchia, R.; Zuorro, A. Microalgae-based biorefineries for sustainable resource recovery from wastewater. J. Water Process Eng. 2020, 40, 101747. [Google Scholar] [CrossRef]
- Roshan, A.; Kumar, M. Water end-use estimation can support the urban water crisis management: A critical review. J. Environ. Manag. 2020, 268, 110663. [Google Scholar] [CrossRef]
- Ferreira, A.; Gouveia, L. Microalgal Biorefineries. In Handbook of Microalgae-Based Processes and Products; Elsevier: Amsterdam, The Netherlands, 2020; pp. 771–798. [Google Scholar]
- Hao, T.-B.; Balamurugan, S.; Zhang, Z.-H.; Liu, S.-F.; Wang, X.; Li, D.-W.; Yang, W.-D.; Li, H.-Y. Effective bioremediation of tobacco wastewater by microalgae at acidic pH for synergistic biomass and lipid accumulation. J. Hazard. Mater. 2021, 426, 127820. [Google Scholar] [CrossRef]
- Mara, D. Domestic Wastewater Treatment in Developing Countries; Routledge: Oxfordshire, UK, 2013. [Google Scholar]
- Boutin, C.; Eme, C. Domestic Wastewater Characterization by Emission Source. In Proceedings of the 13th Congress on Small Xater and Wastewater Systems, Athens, Greece, 14–16 September 2016. [Google Scholar]
- Bhatia, S.K.; Mehariya, S.; Bhatia, R.K.; Kumar, M.; Pugazhendhi, A.; Awasthi, M.K.; Atabani, A.E.; Kumar, G.; Kim, W.; Seo, S.-O.; et al. Wastewater based microalgal biorefinery for bioenergy production: Progress and challenges. Sci. Total Environ. 2021, 751, 141599. [Google Scholar] [CrossRef]
- Kumar, P.K.; Krishna, S.V.; Naidu, S.S.; Verma, K.; Bhagawan, D.; Himabindu, V. Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions: Comparative study. Carbon Resour. Convers. 2019, 2, 126–133. [Google Scholar] [CrossRef]
- Sawayama, S.; Minowa, T.; Dote, Y.; Yokoyama, S. Growth of the hydrocarbon-rich microalga Botryococcus braunii in secondarily treated sewage. Appl. Microbiol. Biotechnol. 1992, 38, 135–138. [Google Scholar] [CrossRef]
- Ashokkumar, V.; Chen, W.-H.; Al-Muhtaseb, A.H.; Kumar, G.; Sathishkumar, P.; Pandian, S.; Ani, F.N.; Ngamcharussrivichai, C. Bioenergy production and metallic iron (Fe) conversion from Botryococcus sp. cultivated in domestic wastewater: Algal biorefinery concept. Energy Convers. Manag. 2019, 196, 1326–1334. [Google Scholar] [CrossRef]
- Carneiro, M.; Ranglová, K.; Lakatos, G.E.; Manoel, J.A.C.; Grivalský, T.; Kozhan, D.M.; Toribio, A.; Moreno, J.; Otero, A.; Varela, J.; et al. Growth and bioactivity of two chlorophyte (Chlorella and Scenedesmus) strains co-cultured outdoors in two different thin-layer units using municipal wastewater as a nutrient source. Algal Res. 2021, 56, 102299. [Google Scholar] [CrossRef]
- Kumari, P.; Varma, A.K.; Shankar, R.; Thakur, L.S.; Mondal, P. Phycoremediation of wastewater by Chlorella pyrenoidosa and utilization of its biomass for biogas production. J. Environ. Chem. Eng. 2021, 9, 104974. [Google Scholar] [CrossRef]
- Maaitah, M.; Hodaifa, G.; Malvis, A.; Sánchez, S. Kinetic growth and biochemical composition variability of Chlorella pyrenoidosa in olive oil washing wastewater cultures enriched with urban wastewater. J. Water Process Eng. 2020, 35, 101197. [Google Scholar] [CrossRef]
- Han, W.; Jin, W.; Ding, W.; Lu, S.; Song, K.; Chen, C.; Qin, C.; Chen, Y.; Tu, R.; Zhou, X. Effects of nutrient composition, lighting conditions, and metal ions on the growth and lipid yield of the high-lipid-yielding microalgae (Chlorella pyrenoidosa) cultivated in municipal wastewater. J. Environ. Chem. Eng. 2021, 9, 106491. [Google Scholar] [CrossRef]
- Wang, Q.; Jin, W.; Zhou, X.; Guo, S.; Gao, S.-H.; Chen, C.; Tu, R.; Han, S.-F.; Jiang, J.; Feng, X. Growth enhancement of biodiesel-promising microalga Chlorella pyrenoidosa in municipal wastewater by polyphosphate-accumulating organisms. J. Clean. Prod. 2019, 240, 118148. [Google Scholar] [CrossRef]
- Dahmani, S.; Zerrouki, D.; Ramanna, L.; Rawat, I.; Bux, F. Cultivation of Chlorella pyrenoidosa in outdoor open raceway pond using domestic wastewater as medium in arid desert region. Bioresour. Technol. 2016, 219, 749–752. [Google Scholar] [CrossRef] [PubMed]
- Lekshmi, B.; Joseph, R.S.; Jose, A.; Abinandan, S.; Shanthakumar, S. Studies on reduction of inorganic pollutants from wastewater by Chlorella pyrenoidosa and Scenedesmus abundans. Alex. Eng. J. 2015, 54, 1291–1296. [Google Scholar] [CrossRef]
- Kotoula, D.; Iliopoulou, A.; Irakleous-Palaiologou, E.; Gatidou, G.; Aloupi, M.; Antonopoulou, P.; Fountoulakis, M.S.; Stasinakis, A.S. Municipal wastewater treatment by combining in series microalgae Chlorella sorokiniana and macrophyte Lemna minor: Preliminary results. J. Clean. Prod. 2020, 271, 122704. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.; Kumar, A.; Bharti, R.K. An integrated approach for phycoremediation of municipal wastewater and production of sustainable transportation fuel using oleaginous Chlorella sp. J. Water Process Eng. 2021, 42, 102183. [Google Scholar] [CrossRef]
- Silambarasan, S.; Logeswari, P.; Sivaramakrishnan, R.; Incharoensakdi, A.; Cornejo, P.; Kamaraj, B.; Chi, N.T.L. Removal of Nutrients from Domestic Wastewater by Microalgae Coupled to Lipid Augmentation for Biodiesel Production and Influence of Deoiled Algal Biomass as Biofertilizer for Solanum Lycopersicum Cultivation. Chemosphere 2021, 268, 129323. [Google Scholar] [CrossRef]
- Thangam, K.R.; Santhiya, A.; Sri, S.A.; MubarakAli, D.; Karthikumar, S.; Kumar, R.S.; Thajuddin, N.; Soosai, M.R.; Varalakshmi, P.; Moorthy, I.G.; et al. Bio-refinery approaches based concomitant microalgal biofuel production and wastewater treatment. Sci. Total Environ. 2021, 785, 147267. [Google Scholar] [CrossRef]
- Morillas-España, A.; Sánchez-Zurano, A.; Lafarga, T.; Morales-Amaral, M.D.M.; Gómez-Serrano, C.; Acién-Fernández, F.G.; González-López, C.V. Improvement of wastewater treatment capacity using the microalga Scenedesmus sp. and membrane bioreactors. Algal Res. 2021, 60, 102516. [Google Scholar] [CrossRef]
- Liu, J.; Yin, J.; Ge, Y.; Han, H.; Liu, M.; Gao, F. Improved lipid productivity of Scenedesmus obliquus with high nutrient removal efficiency by mixotrophic cultivation in actual municipal wastewater. Chemosphere 2021, 285, 131475. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.-W.; Huang, L.; Ji, P.-Y.; Chen, C.-P.; Li, X.-S.; Gao, Y.-H.; Liang, J.-R. Using a mixture of wastewater and seawater as the growth medium for wastewater treatment and lipid production by the marine diatom Phaeodactylum tricornutum. Bioresour. Technol. 2019, 289, 121681. [Google Scholar] [CrossRef]
- Şirin, S.; Sillanpää, M. Cultivating and harvesting of marine alga Nannochloropsis oculata in local municipal wastewater for biodiesel. Bioresour. Technol. 2015, 191, 79–87. [Google Scholar] [CrossRef]
- Agustin, D.M.S.; Ledesma, M.T.O.; Ramírez, I.M.; Noguez, I.Y.; Pabello, V.M.L.; Velasquez-Orta, S.B. A non-sterile heterotrophic microalgal process for dual biomass production and carbon removal from swine wastewater. Renew. Energy 2021, 181, 592–603. [Google Scholar] [CrossRef]
- Zhou, W.; Wang, Z.; Xu, J.; Ma, L. Cultivation of microalgae Chlorella zofingiensis on municipal wastewater and biogas slurry towards bioenergy. J. Biosci. Bioeng. 2018, 126, 644–648. [Google Scholar] [CrossRef] [PubMed]
- Mitra, M.; Shah, F.; Bharadwaj, S.V.; Patidar, S.K.; Mishra, S. Cultivation of Nannochloropsis oceanica biomass rich in eicosapentaenoic acid utilizing wastewater as nutrient resource. Bioresour. Technol. 2016, 218, 1178–1186. [Google Scholar] [CrossRef] [PubMed]
- Tan, X.-B.; Wang, L.; Wan, X.-P.; Zhou, X.-N.; Yang, L.-B.; Zhang, W.-W.; Zhao, X.-C. Growth of Chlorella pyrenoidosa on different septic tank effluents from rural areas for lipids production and pollutants removal. Bioresour. Technol. 2021, 339, 125502. [Google Scholar] [CrossRef]
- Gupta, P.L.; Choi, H.J.; Lee, S.-M. Enhanced nutrient removal from municipal wastewater assisted by mixotrophic microalgal cultivation using glycerol. Environ. Sci. Pollut. Res. 2016, 23, 10114–10123. [Google Scholar] [CrossRef]
- Singh, P.K.; Bhattacharjya, R.; Saxena, A.; Mishra, B.; Tiwari, A. Utilization of wastewater as nutrient media and biomass valorization in marine Chrysophytes- Chaetoceros and Isochrysis. Energy Convers. Manag. X 2021, 10, 100062. [Google Scholar] [CrossRef]
- Baldev, E.; Ali, D.M.; Pugazhendhi, A.; Thajuddin, N. Wastewater as an economical and ecofriendly green medium for microalgal biofuel production. Fuel 2021, 294, 120484. [Google Scholar] [CrossRef]
- Van Do, T.C.; Nguyen, T.N.T.; Tran, D.T.; Le, T.G.; Nguyen, V.T. Semi-continuous removal of nutrients and biomass production from domestic wastewater in raceway reactors using Chlorella variabilis TH03-bacteria consortia. Environ. Technol. Innov. 2020, 20, 101172. [Google Scholar] [CrossRef]
- Liu, Y.; Yildiz, I. The effect of salinity concentration on algal biomass production and nutrient removal from municipal wastewater by Dunaliella salina. Int. J. Energy Res. 2018, 42, 2997–3006. [Google Scholar] [CrossRef]
- Leong, W.H.; Saman, N.A.M.; Kiatkittipong, W.; Assabumrungrat, S.; Najdanovic-Visak, V.; Wang, J.; Khoo, K.S.; Lam, M.K.; Mohamad, M.; Lim, J.W. Photoperiod-Induced Mixotrophic Metabolism in Chlorella Vulgaris for High Biomass and Lipid to Biodiesel Productions Using Municipal Wastewater Medium. Fuel 2022, 313, 123052. [Google Scholar] [CrossRef]
- Cabanelas, I.T.D.; Arbib, Z.; Chinalia, F.A.; Souza, C.O.; Perales, J.A.; Almeida, P.F.; Druzian, J.I.; Nascimento, I.A. From waste to energy: Microalgae production in wastewater and glycerol. Appl. Energy 2013, 109, 283–290. [Google Scholar] [CrossRef]
- Musetsho, P.; Renuka, N.; Guldhe, A.; Singh, P.; Pillay, K.; Rawat, I.; Bux, F. Valorization of Poultry Litter Using Acutodesmus Obliquus and Its Integrated Application for Lipids and Fertilizer Production. Sci. Total Environ. 2021, 796, 149018. [Google Scholar] [CrossRef]
- Feng, X.; Chen, Y.; Lv, J.; Han, S.; Tu, R.; Zhou, X.; Jin, W.; Ren, N. Enhanced lipid production by Chlorella pyrenoidosa through magnetic field pretreatment of wastewater and treatment of microalgae-wastewater culture solution: Magnetic field treatment modes and conditions. Bioresour. Technol. 2020, 306, 123102. [Google Scholar] [CrossRef]
- Wang, M.; Kuo-Dahab, W.C.; Dolan, S.; Park, C. Kinetics of nutrient removal and expression of extracellular polymeric substances of the microalgae, Chlorella sp. and Micractinium sp., in wastewater treatment. Bioresour. Technol. 2014, 154, 131–137. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Y.-F.; Chen, P.; Min, M.; Zhou, W.; Martinez, B.; Zhu, J.; Ruan, R. Characterization of a microalga Chlorella sp. well adapted to highly concentrated municipal wastewater for nutrient removal and biodiesel production. Bioresour. Technol. 2011, 102, 5138–5144. [Google Scholar] [CrossRef]
- Kiran, B.; Pathak, K.; Kumar, R.; Deshmukh, D. Cultivation of Chlorella sp. IM-01 in municipal wastewater for simultaneous nutrient removal and energy feedstock production. Ecol. Eng. 2014, 73, 326–330. [Google Scholar] [CrossRef]
- Sato, H.; Nagare, H.; Huynh, T.N.C.; Komatsu, H. Development of a new wastewater treatment process for resource recovery of carotenoids. Water Sci. Technol. 2015, 72, 1191–1197. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wu, Y.-H.; Yang, J.; Hu, H.-Y.; Yu, Y. Lipid-rich microalgal biomass production and nutrient removal by Haematococcus pluvialis in domestic secondary effluent. Ecol. Eng. 2013, 60, 155–159. [Google Scholar] [CrossRef]
- Abou-Shanab, R.A.I.; El-Dalatony, M.M.; El-Sheekh, M.M.; Ji, M.-K.; Salama, E.-S.; Kabra, A.N.; Jeon, B.-H. Cultivation of a new microalga, Micractinium reisseri, in municipal wastewater for nutrient removal, biomass, lipid, and fatty acid production. Biotechnol. Bioprocess Eng. 2014, 19, 510–518. [Google Scholar] [CrossRef]
- Reyimu, Z.; Özçimen, D. Batch cultivation of marine microalgae Nannochloropsis oculata and Tetraselmis suecica in treated municipal wastewater toward bioethanol production. J. Clean. Prod. 2017, 150, 40–46. [Google Scholar] [CrossRef]
- Parsy, A.; Sambusiti, C.; Baldoni-Andrey, P.; Elan, T.; Périé, F. Cultivation of Nannochloropsis oculata in saline oil & gas wastewater supplemented with anaerobic digestion effluent as nutrient source. Algal Res. 2020, 50, 101966. [Google Scholar] [CrossRef]
- Saleem, S.; Iftikhar, R.; Zafar, M.I.; Sohail, N.F. Growth kinetics of microalgae cultivated in different dilutions of fresh leachate for sustainable nutrient recovery and carbon fixation. Biochem. Eng. J. 2022, 178, 108299. [Google Scholar] [CrossRef]
- González-Balderas, R.; Velásquez-Orta, S.; Valdez-Vazquez, I.; Ledesma, M.O. Intensified recovery of lipids, proteins, and carbohydrates from wastewater-grown microalgae Desmodesmus sp. by using ultrasound or ozone. Ultrason. Sonochem. 2020, 62, 104852. [Google Scholar] [CrossRef]
- Onay, M. Bioethanol Production from Nannochloropsis Gaditana in Municipal Wastewater. Energy Procedia 2018, 153, 253–257. [Google Scholar] [CrossRef]
- Ledda, C.; Villegas, G.R.; Adani, F.; Fernández, F.A.; Grima, E.M. Utilization of centrate from wastewater treatment for the outdoor production of Nannochloropsis gaditana biomass at pilot-scale. Algal Res. 2015, 12, 17–25. [Google Scholar] [CrossRef]
- Mehta, N.; Shah, K.; Lin, Y.-I.; Sun, Y.; Pan, S.-Y. Advances in Circular Bioeconomy Technologies: From Agricultural Wastewater to Value-Added Resources. Environments 2021, 8, 20. [Google Scholar] [CrossRef]
- Han, T.; Lu, H.; Zhao, Y.; Xu, H.; Zhang, Y.; Li, B. Two-step strategy for obtaining Dunaliella sp. biomass and β-carotene from anaerobically digested poultry litter wastewater. Int. Biodeterior. Biodegrad. 2019, 143, 104714. [Google Scholar] [CrossRef]
- Zheng, M.; Dai, J.; Ji, X.; Li, D.; He, Y.; Wang, M.; Huang, J.; Chen, B. An integrated semi-continuous culture to treat original swine wastewater and fix carbon dioxide by an indigenous Chlorella vulgaris MBFJNU-1 in an outdoor photobioreactor. Bioresour. Technol. 2021, 340, 125703. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Kuo, E.-W.; Nagarajan, D.; Ho, S.-H.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. Cultivating Chlorella sorokiniana AK-1 with swine wastewater for simultaneous wastewater treatment and algal biomass production. Bioresour. Technol. 2020, 302, 122814. [Google Scholar] [CrossRef] [PubMed]
- Cheng, P.; Chu, R.; Zhang, X.; Song, L.; Chen, D.; Zhou, C.; Yan, X.; Cheng, J.J.; Ruan, R. Screening of the dominant Chlorella pyrenoidosa for biofilm attached culture and feed production while treating swine wastewater. Bioresour. Technol. 2020, 318, 124054. [Google Scholar] [CrossRef]
- Cheng, P.; Cheng, J.J.; Cobb, K.; Zhou, C.; Zhou, N.; Addy, M.; Chen, P.; Yan, X.; Ruan, R. Tribonema sp. and Chlorella zofingiensis co-culture to treat swine wastewater diluted with fishery wastewater to facilitate harvest. Bioresour. Technol. 2020, 297, 122516. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Yu, T.; Xie, Y.; Chen, J.; Ho, S.-H.; Wang, Y.; Huang, F. Attached culture of Chlamydomonas sp. JSC4 for biofilm production and TN/TP/Cu(II) removal. Biochem. Eng. J. 2018, 141, 1–9. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Carrillo-Nieves, D.; Salinas-Salazar, C.; Silva-Núñez, A.; Arévalo-Gallegos, A.; Barceló, D.; Afewerki, S.; Iqbal, H.M.; Parra-Saldívar, R. Combination of nejayote and swine wastewater as a medium for Arthrospira maxima and Chlorella vulgaris production and wastewater treatment. Sci. Total Environ. 2019, 676, 356–367. [Google Scholar] [CrossRef]
- de Alva, M.S.; Pabello, V.M.L. Phycoremediation by simulating marine aquaculture effluent using Tetraselmis sp. and the potential use of the resulting biomass. J. Water Process Eng. 2021, 41, 102071. [Google Scholar] [CrossRef]
- Chen, J.-H.; Kato, Y.; Matsuda, M.; Chen, C.-Y.; Nagarajan, D.; Hasunuma, T.; Kondo, A.; Dong, C.-D.; Lee, D.-J.; Chang, J.-S. A novel process for the mixotrophic production of lutein with Chlorella sorokiniana MB-1-M12 using aquaculture wastewater. Bioresour. Technol. 2019, 290, 121786. [Google Scholar] [CrossRef]
- Kuo, C.-M.; Jian, J.-F.; Lin, T.-H.; Chang, Y.-B.; Wan, X.-H.; Lai, J.-T.; Chang, J.-S.; Lin, C.-S. Simultaneous microalgal biomass production and CO2 fixation by cultivating Chlorella sp. GD with aquaculture wastewater and boiler flue gas. Bioresour. Technol. 2016, 221, 241–250. [Google Scholar] [CrossRef]
- Khatoon, H.; Penz, K.P.; Banerjee, S.; Rahman, M.R.; Minhaz, T.M.; Islam, Z.; Mukta, F.A.; Nayma, Z.; Sultana, R.; Amira, K.I. Immobilized Tetraselmis sp. for reducing nitrogenous and phosphorous compounds from aquaculture wastewater. Bioresour. Technol. 2021, 338, 125529. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-Y.; Hong, Y.; Zhao, G.-P.; Zhang, H.-K.; Zhai, Q.-Y.; Wang, Q. Microalgae-Based Swine Wastewater Treatment: Strain Screening, Conditions Optimization, Physiological Activity and Biomass Potential. Sci. Total Environ. 2022, 807, 151008. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Shao, S.; He, Y.; Luo, Q.; Zheng, M.; Zheng, M.; Chen, B.; Wang, M. Nutrients removal from piggery wastewater coupled to lipid production by a newly isolated self-flocculating microalga Desmodesmus sp. PW1. Bioresour. Technol. 2020, 302, 122806. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.-M.; Chen, T.-Y.; Lin, T.-H.; Kao, C.-Y.; Lai, J.-T.; Chang, J.-S.; Lin, C.-S. Cultivation of Chlorella sp. GD using piggery wastewater for biomass and lipid production. Bioresour. Technol. 2015, 194, 326–333. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-C.; Choi, W.J.; Chae, A.N.; Park, J.; Kim, H.J.; Song, K.G. Treating High-Strength Saline Piggery Wastewater Using the Heterotrophic Cultivation of Acutodesmus Obliquus. Biochem. Eng. J. 2016, 110, 51–58. [Google Scholar] [CrossRef]
- Hilares, R.T.; Bustos, K.A.G.; Vera, F.P.S.; Andrade, G.J.C.; Tanaka, D.A.P. Acid precipitation followed by microalgae (Chlorella vulgaris) cultivation as a new approach for poultry slaughterhouse wastewater treatment. Bioresour. Technol. 2021, 335, 125284. [Google Scholar] [CrossRef]
- Depraetere, O.; Foubert, I.; Muylaert, K. Decolorisation of Piggery Wastewater to Stimulate the Production of Arthrospira platensis. Bioresour. Technol. 2013, 148, 366–372. [Google Scholar] [CrossRef]
- Wang, H.; Xiong, H.; Hui, Z.; Zeng, X. Mixotrophic Cultivation of Chlorella Pyrenoidosa with Diluted Primary Piggery Wastewater to Produce Lipids. Bioresour. Technol. 2012, 104, 215–220. [Google Scholar] [CrossRef]
- Ganeshkumar, V.; Subashchandrabose, S.R.; Dharmarajan, R.; Venkateswarlu, K.; Naidu, R.; Megharaj, M. Use of Mixed Wastewaters from Piggery and Winery for Nutrient Removal and Lipid Production by Chlorella Sp. MM3. Bioresour. Technol. 2018, 256, 254–258. [Google Scholar] [CrossRef]
- Jain, R.; Mishra, S.; Mohanty, K. Cattle Wastewater as a Low-Cost Supplement Augmenting Microalgal Biomass under Batch and Fed-Batch Conditions. J. Environ. Manag. 2022, 304, 114213. [Google Scholar] [CrossRef]
- Hena, S.; Znad, H.; Heong, K.; Judd, S. Dairy farm wastewater treatment and lipid accumulation by Arthrospira platensis. Water Res. 2018, 128, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Lu, W.; Wang, Z.; Wang, X.; Yuan, Z. Cultivation of Chlorella Sp. Using Raw Dairy Wastewater for Nutrient Removal and Biodiesel Production: Characteristics Comparison of Indoor Bench-Scale and Outdoor Pilot-Scale Cultures. Bioresour. Technol. 2015, 192, 382–388. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ge, Y.; Cheng, H.; Wu, L.; Tian, G. Aerated swine lagoon wastewater: A promising alternative medium for Botryococcus braunii cultivation in open system. Bioresour. Technol. 2013, 139, 190–194. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Yildiz, I. Bioremediation of Minkery Wastewater and Astaxanthin Production by Haematococcus Pluvialis. Int. J. Glob. Warm. 2019, 19, 145–157. [Google Scholar] [CrossRef]
- Wang, H.; Qi, M.; Bo, Y.; Zhou, C.; Yan, X.; Wang, G.; Cheng, P. Treatment of Fishery Wastewater by Co-Culture of Thalassiosira Pseudonana with Isochrysis Galbana and Evaluation of Their Active Components. Algal Res. 2021, 60, 102498. [Google Scholar] [CrossRef]
- Shayesteh, H.; Vadiveloo, A.; Bahri, P.A.; Moheimani, N.R. Can CO2 Addition Improve the Tertiary Treatment of Anaerobically Digested Abattoir Effluent (ADAE) by Scenedesmus Sp. (Chlorophyta)? Algal Res. 2021, 58, 102379. [Google Scholar] [CrossRef]
- Nasir, N.M.; Bakar, N.S.A.; Lananan, F.; Abdul Hamid, S.H.; Lam, S.S.; Jusoh, A. Treatment of African Catfish, Clarias Gariepinus Wastewater Utilizing Phytoremediation of Microalgae, Chlorella Sp. with Aspergillus Niger Bio-Harvesting. Bioresour. Technol. 2015, 190, 492–498. [Google Scholar] [CrossRef]
- Umamaheswari, J.; Kavitha, M.S.; Shanthakumar, S. Outdoor Cultivation of Chlorella Pyrenoidosa in Paddy-Soaked Wastewater and a Feasibility Study on Biodiesel Production from Wet Algal Biomass through in-Situ Transesterification. Biomass Bioenergy 2020, 143, 105853. [Google Scholar] [CrossRef]
- Herold, C.; Ishika, T.; Nwoba, E.G.; Tait, S.; Ward, A.; Moheimani, N.R. Biomass Production of Marine Microalga Tetraselmis Suecica Using Biogas and Wastewater as Nutrients. Biomass Bioenergy 2021, 145, 105945. [Google Scholar] [CrossRef]
- Kiran, B.R.; Mohan, S.V. Photosynthetic transients in Chlorella sorokiniana during phycoremediation of dairy wastewater under distinct light intensities. Bioresour. Technol. 2021, 340, 125593. [Google Scholar] [CrossRef]
- Lananan, F.; Hamid, S.H.A.; Din, W.N.S.; Ali, N.; Khatoon, H.; Jusoh, A.; Endut, A. Symbiotic bioremediation of aquaculture wastewater in reducing ammonia and phosphorus utilizing Effective Microorganism (EM-1) and microalgae (Chlorella sp.). Int. Biodeterior. Biodegrad. 2014, 95, 127–134. [Google Scholar] [CrossRef]
- Song, Y.; Wang, X.; Cui, H.; Ji, C.; Xue, J.; Jia, X.; Ma, R.; Li, R. Enhancing growth and oil accumulation of a palmitoleic acid–rich Scenedesmus obliquus in mixotrophic cultivation with acetate and its potential for ammonium-containing wastewater purification and biodiesel production. J. Environ. Manag. 2021, 297, 113273. [Google Scholar] [CrossRef] [PubMed]
- Rosenwinkel, K.-H.; Austermann-Haun, U.; Meyer, H. Industrial Wastewater Sources and Treatment Strategies. In Environmental Biotechnology; Jördening, H.-J., Winter, J., Eds.; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2005; pp. 49–77. [Google Scholar]
- Li, S.; Zhao, S.; Yan, S.; Qiu, Y.; Song, C.; Li, Y.; Kitamura, Y. Food processing wastewater purification by microalgae cultivation associated with high value-added compounds production—A review. Chin. J. Chem. Eng. 2019, 27, 2845–2856. [Google Scholar] [CrossRef]
- Ahmad, A.; Banat, F.; Alsafar, H.; Hasan, S.W. Algae Biotechnology for Industrial Wastewater Treatment, Bioenergy Production, and High-Value Bioproducts. Sci. Total Environ. 2022, 806, 150585. [Google Scholar] [CrossRef]
- Markou, G.; Chatzipavlidis, I.; Georgakakis, D. Cultivation of Arthrospira (Spirulina) Platensis in Olive-Oil Mill Wastewater Treated with Sodium Hypochlorite. Bioresour. Technol. 2012, 112, 234–241. [Google Scholar] [CrossRef]
- Karray, R.; Elloumi, W.; Ben Ali, R.; Loukil, S.; Chamkha, M.; Karray, F.; Sayadi, S. A Novel Bioprocess Combining Anaerobic Co-Digestion Followed by Ultra-Filtration and Microalgae Culture for Optimal Olive Mill Wastewater Treatment. J. Environ. Manag. 2022, 303, 114188. [Google Scholar] [CrossRef]
- Yonezawa, N.; Matsuura, H.; Shiho, M.; Kaya, K.; Watanabe, M.M. Effects of Soybean Curd Wastewater on the Growth and Hydrocarbon Production of Botryococcus Braunii Strain BOT-22. Bioresour. Technol. 2012, 109, 304–307. [Google Scholar] [CrossRef]
- Qiu, Y.; Zu, Y.; Song, C.; Xie, M.; Qi, Y.; Kansha, Y.; Kitamura, Y. Soybean Processing Wastewater Purification via Chlorella L166 and L38 with Potential Value-Added Ingredients Production. Bioresour. Technol. Rep. 2019, 7, 100195. [Google Scholar] [CrossRef]
- Hongyang, S.; Yalei, Z.; Chunmin, Z.; Xuefei, Z.; Jinpeng, L. Cultivation of Chlorella Pyrenoidosa in Soybean Processing Wastewater. Bioresour. Technol. 2011, 102, 9884–9890. [Google Scholar] [CrossRef]
- Hu, X.; Song, C.; Mu, H.; Liu, Z.; Kitamura, Y. Optimization of Simultaneous Soybean Processing Wastewater Treatment and Flue Gas CO2 Fixation via Chlorella Sp. L166 Cultivation. J. Environ. Chem. Eng. 2020, 8, 103960. [Google Scholar] [CrossRef]
- Fazal, T.; Mushtaq, A.; Rehman, F.; Khan, A.U.; Rashid, N.; Farooq, W.; Rehman, M.S.U.; Xu, J. Bioremediation of textile wastewater and successive biodiesel production using microalgae. Renew. Sustain. Energy Rev. 2018, 82, 3107–3126. [Google Scholar] [CrossRef]
- Fernando, J.S.R.; Premaratne, M.; Dinalankara, D.M.S.D.; Perera, G.L.N.J.; Ariyadasa, T.U. Cultivation of microalgae in palm oil mill effluent (POME) for astaxanthin production and simultaneous phycoremediation. J. Environ. Chem. Eng. 2021, 9, 105375. [Google Scholar] [CrossRef]
- Behl, K.; SeshaCharan, P.; Joshi, M.; Sharma, M.; Mathur, A.; Kareya, M.S.; Jutur, P.P.; Bhatnagar, A.; Nigam, S. Multifaceted Applications of Isolated Microalgae Chlamydomonas Sp. TRC-1 in Wastewater Remediation, Lipid Production and Bioelectricity Generation. Bioresour. Technol. 2020, 304, 122993. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-Y.; Lay, C.-H.; Chiong, M.-C.; Chew, K.W.; Chen, C.-C.; Wu, S.-Y.; Zhou, D.; Kumar, G.; Show, P.L. Immobilized Chlorella species mixotrophic cultivation at various textile wastewater concentrations. J. Water Process Eng. 2020, 38, 101609. [Google Scholar] [CrossRef]
- Araujo, G.S.; Santiago, C.S.; Moreira, R.T.; Neto, M.P.D.; Fernandes, F.A. Nutrient removal by Arthrospira platensis cyanobacteria in cassava processing wastewater. J. Water Process Eng. 2020, 40, 101826. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhao, X.-C.; Yang, L.-B. Strategies for Enhanced Biomass and Lipid Production by Chlorella Pyrenoidosa Culture in Starch Processing Wastewater. J. Clean. Prod. 2019, 236, 117671. [Google Scholar] [CrossRef]
- Okpozu, O.O.; Ogbonna, I.O.; Ikwebe, J.; Ogbonna, J.C. Phycoremediation of Cassava Wastewater by Desmodesmus Armatus and the Concomitant Accumulation of Lipids for Biodiesel Production. Bioresour. Technol. Rep. 2019, 7, 100255. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhao, X.-C.; Zhang, Y.-L.; Zhou, Y.-Y.; Yang, L.-B.; Zhang, W.-W. Enhanced Lipid and Biomass Production Using Alcohol Wastewater as Carbon Source for Chlorella pyrenoidosa Cultivation in Anaerobically Digested Starch Wastewater in Outdoors. Bioresour. Technol. 2018, 247, 784–793. [Google Scholar] [CrossRef]
- Yang, L.; Tan, X.; Li, D.; Chu, H.; Zhou, X.; Zhang, Y.; Yu, H. Nutrients Removal and Lipids Production by Chlorella pyrenoidosa Cultivation Using Anaerobic Digested Starch Wastewater and Alcohol Wastewater. Bioresour. Technol. 2015, 181, 54–61. [Google Scholar] [CrossRef]
- Tan, X.; Chu, H.; Zhang, Y.; Yang, L.; Zhao, F.; Zhou, X. Chlorella Pyrenoidosa Cultivation Using Anaerobic Digested Starch Processing Wastewater in an Airlift Circulation Photobioreactor. Bioresour. Technol. 2014, 170, 538–548. [Google Scholar] [CrossRef]
- Gao, K.; Liu, Q.; Gao, Z.; Xue, C.; Qian, P.; Dong, J.; Gao, Z.; Deng, X. A Dilution Strategy Used to Enhance Nutrient Removal and Biomass Production of Chlorella Sorokiniana in Frigon Wastewater. Algal Res. 2021, 58, 102438. [Google Scholar] [CrossRef]
- Gao, F.; Peng, Y.-Y.; Li, C.; Yang, G.-J.; Deng, Y.-B.; Xue, B.; Guo, Y.-M. Simultaneous Nutrient Removal and Biomass/Lipid Production by Chlorella Sp. in Seafood Processing Wastewater. Sci. Total Environ. 2018, 640–641, 943–953. [Google Scholar] [CrossRef] [PubMed]
- Farooq, W.; Lee, Y.-C.; Ryu, B.-G.; Kim, B.-H.; Kim, H.-S.; Choi, Y.-E.; Yang, J.-W. Two-Stage Cultivation of Two Chlorella Sp. Strains by Simultaneous Treatment of Brewery Wastewater and Maximizing Lipid Productivity. Bioresour. Technol. 2013, 132, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Uysal, Ö.; Ekinci, K. Treatment of Rose Oil Processing Effluent with Chlorella Sp. Using Photobioreactor and Raceway. J. Environ. Manag. 2021, 295, 113089. [Google Scholar] [CrossRef] [PubMed]
- Atoku, D.I.; Ojekunle, O.Z.; Taiwo, A.M.; Shittu, O.B. Evaluating the Efficiency of Nostoc commune, Oscillatoria limosa and Chlorella vulgaris in a Phycoremediation of Heavy Metals Contaminated Industrial Wastewater. Sci. Afr. 2021, 12, e00817. [Google Scholar] [CrossRef]
- Huo, S.; Kong, M.; Zhu, F.; Qian, J.; Huang, D.; Chen, P.; Ruan, R. Co-Culture of Chlorella and Wastewater-Borne Bacteria in Vinegar Production Wastewater: Enhancement of Nutrients Removal and Influence of Algal Biomass Generation. Algal Res. 2020, 45, 101744. [Google Scholar] [CrossRef]
- Kumar, A.K.; Sharma, S.; Shah, E.; Parikh, B.S.; Patel, A.; Dixit, G.; Gupta, S.; Divecha, J.M. Cultivation of Ascochloris Sp. ADW007-Enriched Microalga in Raw Dairy Wastewater for Enhanced Biomass and Lipid Productivity. Int. J. Environ. Sci. Technol. 2019, 16, 943–954. [Google Scholar] [CrossRef]
- Yang, K.; Qin, L.; Wang, Z.; Feng, W.; Feng, P.; Zhu, S.; Xu, J.; Yuan, Z. Water-saving analysis on an effective water reuse system in biodiesel feedstock production based on Chlorella zofingiensis fed-batch cultivation. Water Sci. Technol. 2015, 71, 1562–1568. [Google Scholar] [CrossRef]
- Raja, S.W.; Thanuja, K.G.; Karthikeyan, S.; Marimuthu, S. Exploring the Concurrent Use of Microalgae Coelastrella Sp. for Electricity Generation and Dairy Wastewater Treatment. Bioresour. Technol. Rep. 2022, 17, 100889. [Google Scholar] [CrossRef]
- Gani, P.; Sunar, N.M.; Matias-Peralta, H.M.; Abdul Latiff, A.A.; Joo, I.T.K.; Parjo, U.K.; Emparan, Q.; Er, C.M. Phycoremediation of Dairy Wastewater by Using Green Microlgae: Botryococcus sp. Appl. Mech. Mater. 2015, 773–774, 1318–1323. [Google Scholar] [CrossRef]
- Shu, Q.; Qin, L.; Yuan, Z.; Zhu, S.; Xu, J.; Xu, Z.; Feng, P.; Wang, Z. Comparison of Dairy Wastewater and Synthetic Medium for Biofuels Production by Microalgae Cultivation. Energy Sources Part A Recovery Util. Environ. Eff. 2018, 40, 751–758. [Google Scholar] [CrossRef]
- Kothari, R.; Prasad, R.; Kumar, V.; Singh, D. Production of biodiesel from microalgae Chlamydomonas polypyrenoideum grown on dairy industry wastewater. Bioresour. Technol. 2013, 144, 499–503. [Google Scholar] [CrossRef] [PubMed]
- Spennati, E.; Mirizadeh, S.; Casazza, A.A.; Solisio, C.; Converti, A. Chlorella Vulgaris and Arthrospira Platensis Growth in a Continuous Membrane Photobioreactor Using Industrial Winery Wastewater. Algal Res. 2021, 60, 102519. [Google Scholar] [CrossRef]
- Han, P.; Lu, Q.; Zhong, H.; Xie, J.; Leng, L.; Li, J.; Fan, L.; Li, J.; Chen, P.; Yan, Y.; et al. Recycling Nutrients from Soy Sauce Wastewater to Culture Value-Added Spirulina maxima. Algal Res. 2021, 53, 102157. [Google Scholar] [CrossRef]
- Resdi, R.; Lim, J.S.; Idris, A. Batch Kinetics of Nutrients Removal for Palm Oil Mill Effluent and Recovery of Lipid by Nannochloropsis Sp. J. Water Process Eng. 2021, 40, 101767. [Google Scholar] [CrossRef]
- Hariz, H.B.; Takriff, M.S.; Yasin, N.H.M.; Ba-Abbad, M.M.; Hakimi, N.I.N.M. Potential of the microalgae-based integrated wastewater treatment and CO2 fixation system to treat Palm Oil Mill Effluent (POME) by indigenous microalgae; Scenedesmus sp. and Chlorella sp. J. Water Process Eng. 2019, 32, 100907. [Google Scholar] [CrossRef]
- López-Pacheco, I.Y.; Castillo-Vacas, E.I.; Castañeda-Hernández, L.; Gradiz-Menjivar, A.; Rodas-Zuluaga, L.I.; Castillo-Zacarías, C.; Sosa-Hernández, J.E.; Barceló, D.; Iqbal, H.M.; Parra-Saldívar, R. CO2 biocapture by Scenedesmus sp. grown in industrial wastewater. Sci. Total Environ. 2021, 790, 148222. [Google Scholar] [CrossRef]
- Zhang, C.; Wu, D.-J.; Zhong, C.-Q. Cultivating Scenedesmus Dimorphus in Lactic Acid Wastewater for Cost-Effective Biodiesel Production. Sci. Total Environ. 2021, 792, 148428. [Google Scholar] [CrossRef]
- Melo, J.M.; Telles, T.S.; Ribeiro, M.R.; Junior, O.D.C.; Andrade, D.S. Chlorella sorokiniana as bioremediator of wastewater: Nutrient removal, biomass production, and potential profit. Bioresour. Technol. Rep. 2021, 17, 100933. [Google Scholar] [CrossRef]
- Yuan, S.; Ye, S.; Yang, S.; Luo, G. Purification of Potato Wastewater and Production of Byproducts Using Microalgae Scenedesmus and Desmodesmus. J. Water Process Eng. 2021, 43, 102237. [Google Scholar] [CrossRef]
- Chu, H.-Q.; Tan, X.-B.; Zhang, Y.-L.; Yang, L.-B.; Zhao, F.-C.; Guo, J. Continuous Cultivation of Chlorella pyrenoidosa Using Anaerobic Digested Starch Processing Wastewater in the Outdoors. Bioresour. Technol. 2015, 185, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Zhou, W.; Min, M.; Ma, X.; Chandra, C.; Doan, Y.T.T.; Ma, Y.; Zheng, H.; Cheng, S.; Griffith, R.; et al. Growing Chlorella Sp. on Meat Processing Wastewater for Nutrient Removal and Biomass Production. Bioresour. Technol. 2015, 198, 189–197. [Google Scholar] [CrossRef]
- Polishchuk, A.; Valev, D.; Tarvainen, M.; Mishra, S.; Kinnunen, V.; Antal, T.; Yang, B.; Rintala, J.; Tyystjärvi, E. Cultivation of Nannochloropsis for eicosapentaenoic acid production in wastewaters of pulp and paper industry. Bioresour. Technol. 2015, 193, 469–476. [Google Scholar] [CrossRef]
- Emparan, Q.; Jye, Y.S.; Danquah, M.K.; Harun, R. Cultivation of Nannochloropsis sp. microalgae in palm oil mill effluent (POME) media for phycoremediation and biomass production: Effect of microalgae cells with and without beads. J. Water Process Eng. 2020, 33, 101043. [Google Scholar] [CrossRef]
- Fazal, T.; Rehman, M.S.U.; Javed, F.; Akhtar, M.; Mushtaq, A.; Hafeez, A.; Din, A.A.; Iqbal, J.; Rashid, N.; Rehman, F. Integrating bioremediation of textile wastewater with biodiesel production using microalgae (Chlorella vulgaris). Chemosphere 2021, 281, 130758. [Google Scholar] [CrossRef]
- Li, J.; Zheng, X.; Liu, K.; Sun, S.; Li, X. Effect of Tetracycline on the Growth and Nutrient Removal Capacity of Chlamydomonas reinhardtii in Simulated Effluent from Wastewater Treatment Plants. Bioresour. Technol. 2016, 218, 1163–1169. [Google Scholar] [CrossRef]
- Wang, B.; Qin, L.; Yao, C.; Chen, H.; Feng, P.; Zhu, S.; Zhou, W.; Wang, Z. Enhancement of Co-Conversion of Endogenous Carbon and Nitrogen of Dairy Wastewater in Mesophilic Hydrolysis-Acidification Coupled Microalgae Culture System by Rhamnolipid. Biochem. Eng. J. 2022, 179, 108314. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, H.; Zhong, Y.; Zhang, C.; Shen, Z.; Sang, W.; Yan, G.; Zhou, X. The Effect of Bacterial Contamination on the Heterotrophic Cultivation of Chlorella pyrenoidosa in Wastewater from the Production of Soybean Products. Water Res. 2012, 46, 5509–5516. [Google Scholar] [CrossRef]
- Yang, M.; Xue, C.; Li, L.; Gao, Z.; Liu, Q.; Qian, P.; Dong, J.; Gao, K. Design and Performance of a Low-Cost Microalgae Culturing System for Growing Chlorella sorokiniana on Cooking Cocoon Wastewater. Algal Res. 2022, 62, 102607. [Google Scholar] [CrossRef]
- Huo, S.; Wang, Z.; Zhu, S.; Zhou, W.; Dong, R.; Yuan, Z. Cultivation of Chlorella zofingiensis in Bench-Scale Outdoor Ponds by Regulation of PH Using Dairy Wastewater in Winter, South China. Bioresour. Technol. 2012, 121, 76–82. [Google Scholar] [CrossRef]
- Ammar, S.H.; Khadim, H.J.; Mohamed, A.I. Cultivation of Nannochloropsis oculata and Isochrysis galbana Microalgae in Produced Water for Bioremediation and Biomass Production. Environ. Technol. Innov. 2018, 10, 132–142. [Google Scholar] [CrossRef]
- Choi, Y.-K.; Jang, H.M.; Kan, E. Microalgal Biomass and Lipid Production on Dairy Effluent Using a Novel Microalga, Chlorella Sp. Isolated from Dairy Wastewater. Biotechnol. Bioprocess Eng. 2018, 23, 333–340. [Google Scholar] [CrossRef]
- Koul, B.; Sharma, K.; Shah, M.P. Phycoremediation: A Sustainable Alternative in Wastewater Treatment (WWT) Regime. Environ. Technol. Innov. 2022, 25, 102040. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Lei, Z. Wastewater Treatment Using Microalgal-Bacterial Aggregate Process at Zero-Aeration Scenario: Most Recent Research Focuses and Perspectives. Bioresour. Technol. Rep. 2022, 17, 100943. [Google Scholar] [CrossRef]
- Qi, F.; Jia, Y.; Mu, R.; Ma, G.; Guo, Q.; Meng, Q.; Yu, G.; Xie, J. Convergent Community Structure of Algal–Bacterial Consortia and Its Effects on Advanced Wastewater Treatment and Biomass Production. Sci. Rep. 2021, 11, 21118. [Google Scholar] [CrossRef]
- Kong, W.; Shen, B.; Lyu, H.; Kong, J.; Ma, J.; Wang, Z.; Feng, S. Review on Carbon Dioxide Fixation Coupled with Nutrients Removal from Wastewater by Microalgae. J. Clean. Prod. 2021, 292, 125975. [Google Scholar] [CrossRef]
- Maheshwari, N.; Krishna, P.K.; Thakur, I.S.; Srivastava, S. Biological Fixation of Carbon Dioxide and Biodiesel Production Using Microalgae Isolated from Sewage Waste Water. Environ. Sci. Pollut. Res. 2020, 27, 27319–27329. [Google Scholar] [CrossRef]
- Silvello, M.A.D.C.; Gonçalves, I.S.; Azambuja, S.P.H.; Costa, S.S.; Silva, P.G.P.; Santos, L.O.; Goldbeck, R. Microalgae-based carbohydrates: A green innovative source of bioenergy. Bioresour. Technol. 2021, 344, 126304. [Google Scholar] [CrossRef]
- Chai, W.S.; Chew, C.H.; Munawaroh, H.S.H.; Ashokkumar, V.; Cheng, C.K.; Park, Y.-K.; Show, P.L. Microalgae and Ammonia: A Review on Inter-Relationship. Fuel 2021, 303, 121303. [Google Scholar] [CrossRef]
- Li, X.; Li, W.; Zhai, J.; Wei, H. Effect of Nitrogen Limitation on Biochemical Composition and Photosynthetic Performance for Fed-Batch Mixotrophic Cultivation of Microalga Spirulina platensis. Bioresour. Technol. 2018, 263, 555–561. [Google Scholar] [CrossRef]
- Ran, W.; Wang, H.; Liu, Y.; Qi, M.; Xiang, Q.; Yao, C.; Zhang, Y.; Lan, X. Storage of Starch and Lipids in Microalgae: Biosynthesis and Manipulation by Nutrients. Bioresour. Technol. 2019, 291, 121894. [Google Scholar] [CrossRef] [PubMed]
- Yan, C.; Qu, Z.; Wang, J.; Cao, L.; Han, Q. Microalgal Bioremediation of Heavy Metal Pollution in Water: Recent Advances, Challenges, and Prospects. Chemosphere 2022, 286, 131870. [Google Scholar] [CrossRef] [PubMed]
- Priya, A.; Jalil, A.; Vadivel, S.; Dutta, K.; Rajendran, S.; Fujii, M.; Soto-Moscoso, M. Heavy metal remediation from wastewater using microalgae: Recent advances and future trends. Chemosphere 2022, 305, 135375. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.F.; Mofijur, M.; Parisa, T.A.; Islam, N.; Kusumo, F.; Inayat, A.; Le, V.G.; Badruddin, I.A.; Khan, T.Y.; Ong, H.C. Progress and challenges of contaminate removal from wastewater using microalgae biomass. Chemosphere 2022, 286, 131656. [Google Scholar] [CrossRef] [PubMed]
- Leong, Y.K.; Chang, J.-S. Bioremediation of Heavy Metals Using Microalgae: Recent Advances and Mechanisms. Bioresour. Technol. 2020, 303, 122886. [Google Scholar] [CrossRef]
- Sathinathan, P.; Parab, H.; Yusoff, R.; Ibrahim, S.; Vello, V.; Ngoh, G. Photobioreactor design and parameters essential for algal cultivation using industrial wastewater: A review. Renew. Sustain. Energy Rev. 2023, 173, 113096. [Google Scholar] [CrossRef]
- Nagarajan, D.; Lee, D.-J.; Varjani, S.; Lam, S.S.; Allakhverdiev, S.I.; Chang, J.-S. Microalgae-Based Wastewater Treatment—Microalgae-Bacteria Consortia, Multi-Omics Approaches and Algal Stress Response. Sci. Total Environ. 2022, 845, 157110. [Google Scholar] [CrossRef]
- Aditya, L.; Mahlia, T.I.; Nguyen, L.N.; Vu, H.P.; Nghiem, L.D. Microalgae-bacteria consortium for wastewater treatment and biomass production. Sci. Total Environ. 2022, 838, 155871. [Google Scholar] [CrossRef]
- Liu, Z.; Cui, D.; Liu, Y.; Wang, H.; Yang, L.; Chen, H.; Qiu, G.; Xiong, Z.; Shao, P.; Luo, X. Enhanced Ammonia Nitrogen Removal from Actual Rare Earth Element Tailings (REEs) Wastewater by Microalgae-Bacteria Symbiosis System (MBS): Ratio Optimization of Microalgae to Bacteria and Mechanism Analysis. Bioresour. Technol. 2023, 367, 128304. [Google Scholar] [CrossRef]
- Leng, L.; Li, W.; Chen, J.; Leng, S.; Chen, J.; Wei, L.; Peng, H.; Li, J.; Zhou, W.; Huang, H. Co-Culture of Fungi-Microalgae Consortium for Wastewater Treatment: A Review. Bioresour. Technol. 2021, 330, 125008. [Google Scholar] [CrossRef]
- Wang, S.-K.; Yang, K.-X.; Zhu, Y.-R.; Zhu, X.-Y.; Nie, D.-F.; Jiao, N.; Angelidaki, I. One-Step Co-Cultivation and Flocculation of Microalgae with Filamentous Fungi to Valorize Starch Wastewater into High-Value Biomass. Bioresour. Technol. 2022, 361, 127625. [Google Scholar] [CrossRef] [PubMed]
- Walls, L.E.; Velasquez-Orta, S.B.; Romero-Frasca, E.; Leary, P.; Noguez, I.Y.; Ledesma, M.T.O. Non-sterile heterotrophic cultivation of native wastewater yeast and microalgae for integrated municipal wastewater treatment and bioethanol production. Biochem. Eng. J. 2019, 151, 107319. [Google Scholar] [CrossRef]
- Ray, A.; Nayak, M.; Ghosh, A. A Review on Co-Culturing of Microalgae: A Greener Strategy towards Sustainable Biofuels Production. Sci. Total Environ. 2022, 802, 149765. [Google Scholar] [CrossRef] [PubMed]
- Vasistha, S.; Khanra, A.; Rai, M.P. Influence of Microalgae-ZnO Nanoparticle Association on Sewage Wastewater towards Efficient Nutrient Removal and Improved Biodiesel Application: An Integrated Approach. J. Water Process Eng. 2021, 39, 101711. [Google Scholar] [CrossRef]
- Markeb, A.A.; Llimós-Turet, J.; Ferrer, I.; Blánquez, P.; Alonso, A.; Sánchez, A.; Moral-Vico, J.; Font, X. The use of magnetic iron oxide based nanoparticles to improve microalgae harvesting in real wastewater. Water Res. 2019, 159, 490–500. [Google Scholar] [CrossRef]
- Nishshanka, G.K.S.H.; Liyanaarachchi, V.C.; Premaratne, M.; Nimarshana, P.; Ariyadasa, T.U.; Kornaros, M. Wastewater-based microalgal biorefineries for the production of astaxanthin and co-products: Current status, challenges and future perspectives. Bioresour. Technol. 2021, 342, 126018. [Google Scholar] [CrossRef]
- Fu, R.; Kang, L.; Zhang, C.; Fei, Q. Application and progress of techno-economic analysis and life cycle assessment in biomanufacturing of fuels and chemicals. Green Chem. Eng. 2022, S2666952822000802. [Google Scholar] [CrossRef]
- Pooja, K.; Priyanka, V.; Rao, B.C.S.; Raghavender, V. Cost-Effective Treatment of Sewage Wastewater Using Microalgae Chlorella Vulgaris and Its Application as Bio-Fertilizer. Energy Nexus 2022, 7, 100122. [Google Scholar] [CrossRef]
- Leong, Y.K.; Huang, C.-Y.; Chang, J.-S. Pollution Prevention and Waste Phycoremediation by Algal-Based Wastewater Treatment Technologies: The Applications of High-Rate Algal Ponds (HRAPs) and Algal Turf Scrubber (ATS). J. Environ. Manag. 2021, 296, 113193. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Montero, N.; Ferrer, I.; Acién, F.G.; Gómez, C.; Garfí, M. Life Cycle Assessment of High Rate Algal Ponds for Wastewater Treatment and Resource Recovery. Sci. Total Environ. 2018, 622–623, 1118–1130. [Google Scholar] [CrossRef]
- Maga, D. Life Cycle Assessment of Biomethane Produced from Microalgae Grown in Municipal Waste Water. Biomass Conv. Bioref. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Kohlheb, N.; van Afferden, M.; Lara, E.; Arbib, Z.; Conthe, M.; Poitzsch, C.; Marquardt, T.; Becker, M.-Y. Assessing the Life-Cycle Sustainability of Algae and Bacteria-Based Wastewater Treatment Systems: High-Rate Algae Pond and Sequencing Batch Reactor. J. Environ. Manag. 2020, 264, 110459. [Google Scholar] [CrossRef] [PubMed]
- Nasir, A.S.M.; Mohamed, B.; Li, L.Y. Comparative life cycle assessment of co-pyrolysing sewage sludge and wastewater-grown microalgae for biofuel production. Resour. Conserv. Recycl. 2023, 190, 106780. [Google Scholar] [CrossRef]
- Arashiro, L.T.; Josa, I.; Ferrer, I.; Van Hulle, S.W.; Rousseau, D.P.; Garfí, M. Life Cycle Assessment of Microalgae Systems for Wastewater Treatment and Bioproducts Recovery: Natural Pigments, Biofertilizer and Biogas. Sci. Total Environ. 2022, 847, 157615. [Google Scholar] [CrossRef]
- Sutherland, D.L. Improving Microalgal Tolerance to High Ammonia with Simple Organic Carbon Addition for More Effective Wastewater Treatment. J. Water Process Eng. 2022, 47, 102667. [Google Scholar] [CrossRef]
- Li, J.; Wang, L.; Lu, Q.; Zhou, W. Toxicity Alleviation for Microalgae Cultivation by Cationic Starch Addition and Ammonia Stripping and Study on the Cost Assessment. RSC Adv. 2019, 9, 38235–38245. [Google Scholar] [CrossRef]
- Wang, J.; Zhou, W.; Chen, H.; Zhan, J.; He, C.; Wang, Q. Ammonium Nitrogen Tolerant Chlorella Strain Screening and Its Damaging Effects on Photosynthesis. Front. Microbiol. 2019, 9, 3250. [Google Scholar] [CrossRef]
- Azov, Y.; Goldman, J.C. Free Ammonia Inhibition of Algal Photosynthesis in Intensive Culturest. Appl. Environ. Microbiol. 1982, 43, 735–739. [Google Scholar] [CrossRef]
- Hanifzadeh, M.; Sarrafzadeh, M.-H.; Nabati, Z.; Tavakoli, O.; Feyzizarnagh, H. Technical, economic and energy assessment of an alternative strategy for mass production of biomass and lipid from microalgae. J. Environ. Chem. Eng. 2018, 6, 866–873. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, L.; Wu, D.; Wang, L.; Yang, Z.; Yan, W.; Jin, Y.; Chen, F.; Song, Y.; Cheng, X. Biochemical wastewater from landfill leachate pretreated by microalgae achieving algae’s self-reliant cultivation in full wastewater-recycling chain with desirable lipid productivity. Bioresour. Technol. 2021, 340, 125640. [Google Scholar] [CrossRef]
- Leite, L.D.S.; Hoffmann, M.T.; Daniel, L.A. Coagulation and dissolved air flotation as a harvesting method for microalgae cultivated in wastewater. J. Water Process Eng. 2019, 32, 100947. [Google Scholar] [CrossRef]
- Kurniawan, S.B.; Ahmad, A.; Imron, M.F.; Abdullah, S.R.S.; Othman, A.R.; Hasan, H.A. Potential of Microalgae Cultivation Using Nutrient-Rich Wastewater and Harvesting Performance by Biocoagulants/Bioflocculants: Mechanism, Multi-Conversion of Biomass into Valuable Products, and Future Challenges. J. Clean. Prod. 2022, 365, 132806. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).