Green Production of a High-Value Branched-Chain Diester: Optimization Based on Operating Conditions and Economic and Sustainability Criteria
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
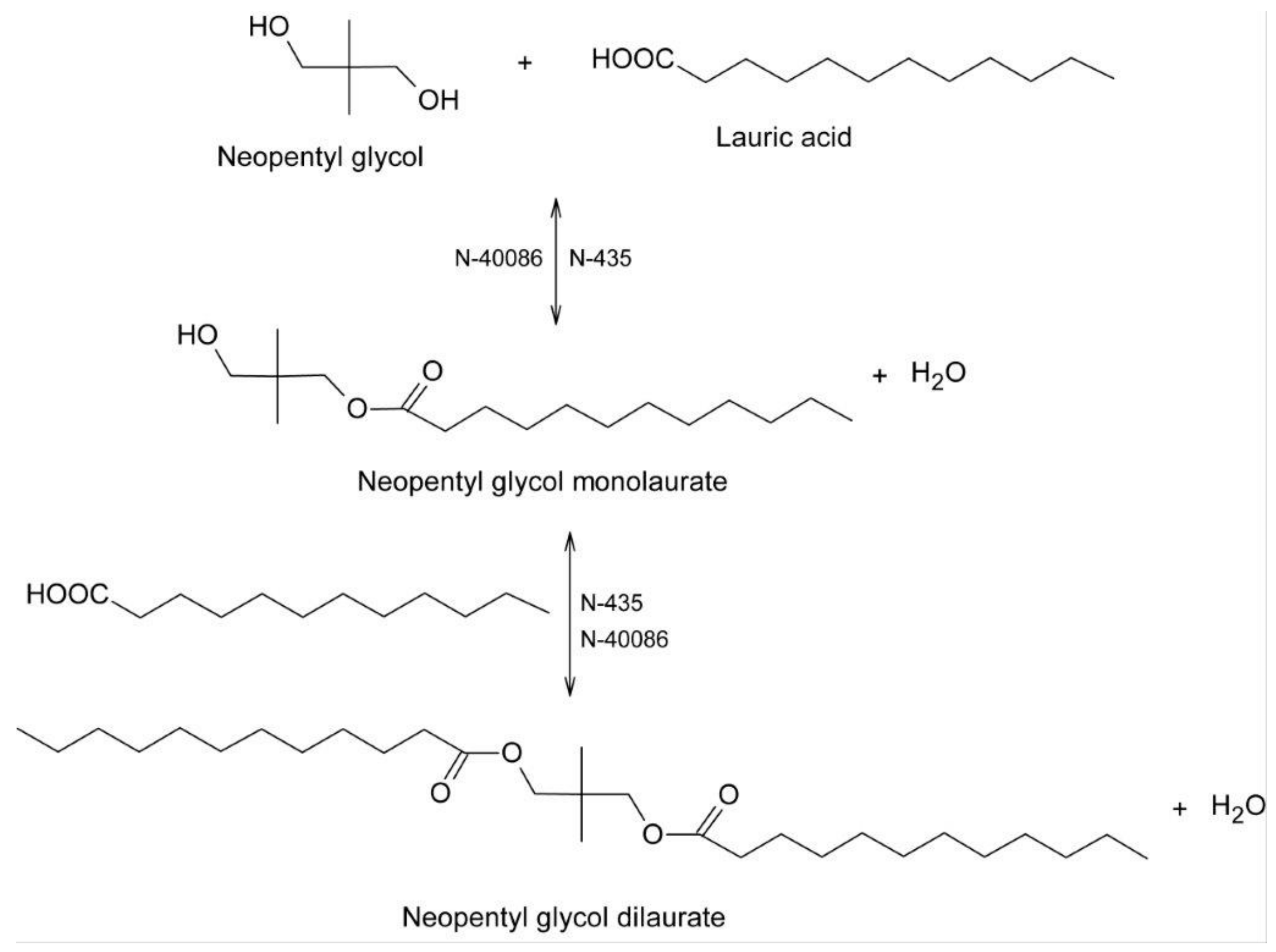
2.2. Biocatalytic Synthesis
2.3. Recovery and Reuse of the Biocatalysts
2.4. Energy Consumption
2.5. Gas Chromatography Analysis (GC)
3. Results and Discussion
3.1. Influence of the Amount of Biocatalyst
3.2. Influence of the Temperature
- It may cause enzyme denaturation;
- If not, it usually involves an improvement in reaction rate and a lower reaction media viscosity;
- Even if a biocatalyst’s activity is incremented by temperature, the process energy consumption may not be cost-effective.
3.3. Biocatalyst Comparisons under Best Reaction Conditions
3.4. Biocatalyst Selection Based on Economic Evaluations and Sustainability Indicators
- Direct operating costs have been calculated for the production of 1 kg of NPGDL;
- Unit prices for both substrates have been obtained from suppliers who provide them in bulk, with a minimum purchase of 1 kg;
- The immobilized lipases have been donated by Novozymes, España S.A., and their prices were specified in personal communication;
- The energy costs have been calculated by measuring the energy consumption of the thermostatic bath and overhead mixer (Section 2.4) and considering an energy price of 0.2288 EUR/kW h (the average value of the second half-year of 2022 in Spain);
- Five consecutive uses of the biocatalysts have been considered in the calculations.
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sheldon, R.A.; Woodley, J.M. Role of biocatalysis in sustainable chemistry. Chem. Rev. 2018, 118, 801–838. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green chemistry: Principles and practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Faber, K. Biotransformations in Organic Chemistry: A Textbook, 6th ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 31–261. [Google Scholar]
- Thum, O.; Oxenbøll, K.M. Biocatalysis: A Sustainable Process for Production of Cosmetic Ingredients. Int. J. Appl. Sci. 2008, 134, 44–47. [Google Scholar]
- Badgujar, K.C.; Bhanage, B.M. The green metric evaluation and synthesis of diesel-blend compounds from biomass derived levulinic acid in supercritical carbon dioxide. Biomass Bioenergy 2016, 84, 12–21. [Google Scholar] [CrossRef]
- Box. Norma-Ecocert.pdf. Available online: https://ecocert.app.box.com/v/Norma-Ecocert (accessed on 29 March 2023).
- Cosmética Natural: Primer Macroanálisis Europeo Neutral y Objetivo, STANPA Asoc. Nac. Perfum. Cosmética. Available online: https://www.stanpa.com/notas-prensa/cosmetica-natural-primer-macroanalisis-europeo-neutral-y-objetivo/ (accessed on 4 April 2023).
- Montiel, M.C.; Serrano, M.; Máximo, M.F.; Gómez, M.; Ortega-Requena, S.; Bastida, J. Synthesis of cetyl ricinoleate catalyzed by immobilized Lipozyme® CalB lipase in a solvent-free system. Catal. Today 2015, 255, 49–53. [Google Scholar] [CrossRef]
- Chang, S.W.; Shaw, J.F.; Yang, K.H.; Shih, I.L.; Hsieh, C.H.; Shieh, C.J. Optimal lipase-catalyzed formation of hexyl laurate. Green Chem. 2005, 7, 547–551. [Google Scholar] [CrossRef]
- Verma, M.L.; Chauhan, G.S.; Kanwar, S.S. Enzymatic synthesis of isopropyl myristate using immobilized lipase from Bacillus cereus MTCC 8372. Acta Microbiol. Immunol. Hung. 2008, 55, 327–342. [Google Scholar] [CrossRef]
- Kuo, C.H.; Chen, H.H.; Chen, J.H.; Liu, Y.C.; Shieh, C.J. High yield of wax ester synthesized from cetyl alcohol and octanoic acid by Lipozyme RM IM and Novozym 435. Int. J. Mol. Sci. 2012, 13, 11694–11704. [Google Scholar] [CrossRef]
- Products & solutions—Evonik Personal Care—The Soul & Science of Beauty. Available online: https://personal-care.evonik.com/product/personal-care/en/products-solutions/ (accessed on 4 April 2023).
- Carson, J.; Gallagher, K. Emollient Esters and Oils. In Conditioning Agents Hair Skin, 1st ed.; Schueller, R., Romanowski, P., Eds.; CRC Press: Boca Raton, FL, USA, 2020; pp. 111–137. [Google Scholar]
- Willing, A. Lubricants based on renewable resources—An environmentally compatible alternative to mineral oil products. Chemosphere 2001, 43, 89–98. [Google Scholar] [CrossRef]
- Mendes, A.A.; Soares, C.M.; Tardioli, P.W. Recent advances and future prospects for biolubricant base stocks production using lipases as environmentally friendly catalysts: A mini-review. World J. Microbiol. Biotechnol. 2023, 39, 1–18. [Google Scholar] [CrossRef]
- Knothe, G. Analyzing biodiesel: Standards and other methods. J. Am. Oil. Chem. Soc. 2006, 83, 823–833. [Google Scholar] [CrossRef]
- Dunn, R.O.; Ngo, H.L.; Haas, M.J. Branched-chain fatty acid methyl esters as cold flow improvers for biodiesel. J. Am. Oil. Chem. Soc. 2015, 92, 853–869. [Google Scholar] [CrossRef]
- Montiel, M.C.; Máximo, F.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Murcia, M.D.; Bastida, J. Biocatalytic solutions to cyclomethicones problem in cosmetics. Eng. Life Sci. 2019, 19, 370–388. [Google Scholar] [CrossRef]
- Commission Regulation (EU) 2018/35 of 10 January 2018 Amending Annex XVII to Regulation (EC) No 1907/2006 of the European Parliament and of the Council Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH) as Regards Octamethylcyclotetrasiloxane (‘D4’) and Decamethylcyclopentasiloxane (‘D5’). Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/PDF/?uri=CELEX:32018R0035&from=EN (accessed on 29 March 2023).
- CosIng-Cosmetics-GROWTH-EuropeanCommission. Available online: https://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=77342 (accessed on 29 March 2023).
- Aguieiras, E.C.G.; Cavalcanti, E.D.C.; da Silva, P.R.; Soares, V.F.; Fernandez-Lafuente, R.; Assunção, C.L.B.; da Silva, J.A.C.; Freire, D.M.G. Enzymatic synthesis of neopentyl glycol-bases biolubricants using biodiesel from soybean and castor bean as raw materials. Renew. Energy 2020, 148, 689–696. [Google Scholar] [CrossRef]
- Gryglewicz, S.; Muszynski, M.; Nowicki, J. Enzymatic synthesis of rapeseed oil-based lubricants. Ind. Crops. Prod. 2013, 45, 25–29. [Google Scholar] [CrossRef]
- Fernandes, K.V.; Cavalcanti, E.D.C.; Cipolatti, E.P.; Aguieiras, E.C.G.; Pinto, M.C.C.; Tavares, F.A.; da Silva, P.R.; Fernandez-Lafuente, R.; Arana-Peña, S.; Pinto, J.C.; et al. Enzymatic synthesis of biolubricants from by-product of soybean oil processing catalyzed by different biocatalysts of Candida rugosa lipase. Catal. Today 2021, 362, 122–129. [Google Scholar] [CrossRef]
- Åkerman, C.O.; Hagström, A.E.V.; Mollaahmad, M.A.; Karlsson, S.; Hatti-Kaul, R. Biolubricant synthesis using immobilised lipase: Process optimisation of trimethylolpropane oleate production. Process Biochem. 2011, 46, 2225–2231. [Google Scholar] [CrossRef]
- Pleiss, J.; Fischer, M.; Schmid, R.D. Anatomy of lipase binding sites: The scissile fatty acid binding site. Chem. Phys. Lipids 1998, 93, 67–80. [Google Scholar] [CrossRef]
- Naik, S.; Basu, A.; Saikia, R.; Madan, B.; Paul, P.; Chaterjee, R.; Brask, J.; Svendsen, A. Lipases for use in industrial biocatalysis: Specificity of selected structural groups of lipases. J. Mol. Catal. B Enzym. 2010, 65, 18–23. [Google Scholar] [CrossRef]
- Hessel, V.; Escribà-Gelonch, M.; Bricout, J.; Tran, N.N.; Anastasopoulou, A.; Ferlin, F.; Valentini, F.; Lanari, D.; Vaccaro, L. Quantitative sustainability assessment of flow chemistry—From simple metrics to holistic assessment. ACS Sustain. Chem. Eng. 2021, 9, 9508–9540. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E factor 25 years on: The rise of green chemistry and sustainability. Green Chem. 2017, 19, 18–43. [Google Scholar] [CrossRef]
- Sheldon, R.A. Metrics of green chemistry and sustainability: Past, present, and future. ACS Sustain. Chem. Eng. 2018, 6, 32–48. [Google Scholar] [CrossRef]
- Lima-Ramos, J.; Tufvesson, P.; Woodley, J.M. Application of environmental and economic metrics to guide the development of biocatalytic processes. Green Process Synth. 2014, 3, 195–213. [Google Scholar] [CrossRef]
- ASTM D974-02e1; Standard Test Method for Acid and Base Number by Color-Indicator Titration. ASTM International: West Conshohocken, PA, USA, 2002.
- Sun, S.; Tian, L. Novozym 40086 as a novel biocatalyst to improve benzyl cinnamate synthesis. RSC Adv. 2018, 8, 37184–37192. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Bastida, J.; Máximo, F.; Ortega-Requena, S.; Montiel, C. One-step solvent-free production of a spermaceti analogue using commercial immobilized lipases. ChemistrySelect 2018, 3, 748–752. [Google Scholar] [CrossRef]
- Boulifi, N.E.; Aracil, J.; Martínez, M. Lipase-catalyzed synthesis of isosorbide monoricinoleate: Process optimization by response surface methodology. Bioresour. Technol. 2010, 101, 8520–8525. [Google Scholar] [CrossRef]
- Badgujar, V.C.; Badgujar, K.C.; Yeole, P.M.; Bhanage, B.M. Immobilization of Rhizomucor miehei lipase on a polymeric film for synthesis of important fatty acid esters: Kinetics and application studies. Bioprocess Biosyst. Eng. 2017, 40, 1463–1478. [Google Scholar] [CrossRef]
- Murcia, M.D.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Máximo, F.; Bastida, J.; Montiel, M.C. Optimization of a sustainable biocatalytic process for the synthesis of ethylhexyl fatty acids esters. Catal. Today 2020, 346, 98–105. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; Montiel, M.C.; Ortega-Requena, S.; Máximo, F.; Bastida, J. Development and economic evaluation of an eco-friendly biocatalytic synthesis of emollient esters. Bioprocess Biosyst. Eng. 2020, 43, 495–505. [Google Scholar] [CrossRef]
- Kim, J.W.; Kim, B.H.; Kim, Y.; Lee, M.-W.; Im, D.J.; Kim, I.-H. Lipase mediated synthesis of neopentyl glycol diester using a combination of reduced and standard pressure. J. Am. Oil Chem. Soc. 2021, 98, 1001–1007. [Google Scholar] [CrossRef]
- Novozymes A/S, Enzymes for Biocatalysis. Available online: http://www.novozymes.com/en (accessed on 4 April 2023).
- Ragupathy, L.; Ziener, U.; Dyllick-Brenzinger, R.; von Vacano, B.; Landfester, K. Enzyme-catalyzed polymerizations at higher temperatures: Synthetic methods to produce polyamides and new poly(amide-co-ester)s. J. Mol. Catal. B. Enzym. 2012, 76, 94–105. [Google Scholar] [CrossRef]
- Aguieiras, E.C.G.; Veloso, C.O.; Bevilaqua, J.V.; Rosas, D.O.; da Silva, M.A.P.; Langone, M.A.P. Estolides synthesis catalyzed by immobilized lipases. Enzyme Res. 2011, 2011, 432746. [Google Scholar] [CrossRef]
- Smith, J.K.; Liu, Z.; Vedachalam, M.; Compton, D. Process of making D-panthenyl. triacetate. Patent US 2005/0002972 A1, 2005. [Google Scholar]
- Máximo, F.; Asensi, M.; Serrano-Arnaldos, M.; Ortega-Requena, S.; Montiel, C. Biocatalytic intensified process for the synthesis of neopentyl glycol dicaprylate/dicaprate. Sus. Chem. Pharm. 2022, 30, 100882. [Google Scholar] [CrossRef]
- Nieto, S.; Bernal, J.M.; Villa, R.; García-Verdugo, E.; Donaire, A.; Lozano, P. Sustainable setups for the biocatalytic production and scale-up of panthenyl monoacyl esters under solvent-free conditions. ACS Sustain. Chem. Eng. 2023, 11, 5737–5747. [Google Scholar] [CrossRef]
- Serrano-Arnaldos, M.; García-Martínez, J.J.; Ortega-Requena, S.; Bastida, J.; Máximo, F.; Montiel, M.C. Reaction strategies for the enzymatic synthesis of neopentyl glycol diheptanoate. Enzyme Microb. Technol. 2020, 132, 109400. [Google Scholar] [CrossRef]






| Cost | Cost (EUR/kg NPGDL) | ||
|---|---|---|---|
| Novozym® 40086 | Novozym® 435 | ||
| Lauric acid 1 | 2 EUR/kg | 1.41 | 1.30 |
| Neopentyl glycol 2 | 1.5 EUR/kg | 0.49 | 0.45 |
| Biocatalyst 3 | |||
| Novozym® 40086 | 600 EUR/kg | 10.69 | |
| Novozym® 435 | 1300 EUR/kg | 10.64 | |
| Thermostatic bath | |||
| Initial | 6.8 10−3 EUR/min | 3.23 (8 min) | 5.19 (14 min) |
| Maintenance | 2 10−4 EUR/min | 6.27 (8 h) | 13.10 (5 h) |
| Overhead stirrer | 10−4 EUR/min | 3.18 | 1.71 |
| Total direct cost | 25.28 | 32.39 | |
| Novozym® 40086 | Novozym® 435 | |
|---|---|---|
| Atom economy (AE) 1 (%) | 92.87 | 92.87 |
| Simple E-factor 2 | 0.017 | 0.018 |
| Complete E-factor 3 | 0.095 | 0.093 |
| Carbon mass efficiency (CME) 4 (%) | 91.35 | 91.46 |
| Process mass intensity (PMI) 5 | 1.05 | 1.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montiel, C.; Gimeno-Martos, S.; Ortega-Requena, S.; Serrano-Arnaldos, M.; Máximo, F.; Bastida, J. Green Production of a High-Value Branched-Chain Diester: Optimization Based on Operating Conditions and Economic and Sustainability Criteria. Appl. Sci. 2023, 13, 6177. https://doi.org/10.3390/app13106177
Montiel C, Gimeno-Martos S, Ortega-Requena S, Serrano-Arnaldos M, Máximo F, Bastida J. Green Production of a High-Value Branched-Chain Diester: Optimization Based on Operating Conditions and Economic and Sustainability Criteria. Applied Sciences. 2023; 13(10):6177. https://doi.org/10.3390/app13106177
Chicago/Turabian StyleMontiel, Claudia, Silvia Gimeno-Martos, Salvadora Ortega-Requena, Mar Serrano-Arnaldos, Fuensanta Máximo, and Josefa Bastida. 2023. "Green Production of a High-Value Branched-Chain Diester: Optimization Based on Operating Conditions and Economic and Sustainability Criteria" Applied Sciences 13, no. 10: 6177. https://doi.org/10.3390/app13106177
APA StyleMontiel, C., Gimeno-Martos, S., Ortega-Requena, S., Serrano-Arnaldos, M., Máximo, F., & Bastida, J. (2023). Green Production of a High-Value Branched-Chain Diester: Optimization Based on Operating Conditions and Economic and Sustainability Criteria. Applied Sciences, 13(10), 6177. https://doi.org/10.3390/app13106177









