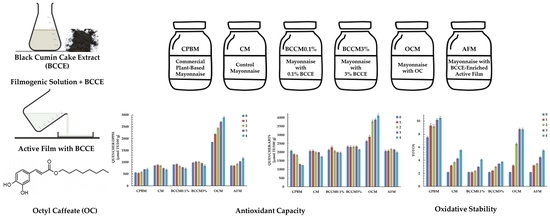
Effect of Black Cumin Cake Extract, Octyl Caffeate, and Active Packaging on Antioxidant Properties of Egg-Free Mayonnaise during Storage
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Preparation of Black Cumin Cake Extracts
2.3. Preparation of Active Film
2.4. Octyl Caffeate Synthesis
2.5. Preparation of Mayonnaises
2.6. Determination of Antioxidant Capacity
2.6.1. QUENCHER-DPPH Assay
2.6.2. QUENCHER-ABTS Assay
2.7. Determination of Oxidative Status
2.8. Statistical Analysis
3. Results and Discussion
3.1. Antioxidant Properties of Mayonnaises
3.2. Oxidative Status of Mayonnaises
3.3. Principal Component Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Mattia, C.; Balestra, F.; Sacchetti, G.; Neri, L.; Mastrocola, D.; Pittia, P. Physical and structural properties of extra-virgin olive oil based mayonnaise. LWT Food Sci. Technol. 2015, 62, 764–770. [Google Scholar] [CrossRef]
- Alizadeh, L.; Abdolmaleki, K.; Nayebzadeh, K.; Shahin, R. Effects of tocopherol, rosemary essential oil and Ferulago angulata extract on oxidative stability of mayonnaise during its shelf life: A comparative study. Food Chem. 2019, 285, 46–52. [Google Scholar] [CrossRef]
- Raikos, V.; McDonagh, A.; Ranawana, V.; Duthie, G. Processed beetroot (Beta vulgaris L.) as a natural antioxidant in mayonnaise: Effects on physical stability, texture and sensory attributes. Food Sci. Hum. Wellness 2016, 5, 191–198. [Google Scholar] [CrossRef] [Green Version]
- Mazaheri Tehrani, M. Ohmically extracted Zenyan essential oils as natural antioxidant in mayonnaise. Int. Food Res. J. 2013, 20, 3189–3195. [Google Scholar]
- Soltan, O.I.A.; Gazwi, H.S.S.; Ragab, A.E.; Aljohani, A.S.M.; El-Ashmawy, I.M.; Batiha, G.E.-S.; Hafiz, A.A.; Abdel-Hameed, S.M. Assessment of bioactive phytochemicals and utilization of Rosa canina fruit extract as a novel natural antioxidant for mayonnaise. Molecules 2023, 28, 3350. [Google Scholar] [CrossRef]
- Kishk, Y.F.M.; Elsheshetawy, H.E. Effect of ginger powder on the mayonnaise oxidative stability, rheological measurements, and sensory characteristics. Ann. Agric. Sci. 2013, 58, 213–220. [Google Scholar] [CrossRef] [Green Version]
- Lazăr, S.; Constantin, O.E.; Horincar, G.; Andronoiu, D.G.; Stănciuc, N.; Muresan, C.; Râpeanu, G. Beetroot by-product as a functional ingredient for obtaining value-added mayonnaise. Processes 2022, 10, 227. [Google Scholar] [CrossRef]
- Rafiee, Z.; Barzegar, M.; Sahari, M.A.; Maherani, B. Nanoliposomes containing pistachio green hull’s phenolic compounds as natural bio-preservatives for mayonnaise. Eur. J. Lipid Sci. Technol. 2018, 120, 1800086. [Google Scholar] [CrossRef]
- Altunkaya, A.; Hedegaard, R.V.; Harholt, J.; Brimer, L.; Gökmen, V.; Skibsted, L.H. Oxidative stability and chemical safety of mayonnaise enriched with grape seed extract. Food Funct. 2013, 4, 1647. [Google Scholar] [CrossRef]
- Li, C.-Y.; Kim, H.-W.; Li, H.; Lee, D.-C.; Rhee, H.-I. Antioxidative effect of purple corn extracts during storage of mayonnaise. Food Chem. 2014, 152, 592–596. [Google Scholar] [CrossRef]
- Sørensen, A.M.; Villeneuve, P.; Jacobsen, C. Alkyl caffeates as antioxidants in O/W emulsions: Impact of emulsifier type and endogenous tocopherols. Eur. J. Lipid Sci. Technol. 2017, 119, 1600276. [Google Scholar] [CrossRef] [Green Version]
- Ghelichi, S.; Hajfathalian, M.; Yesiltas, B.; Sørensen, A.M.; García-Moreno, P.J.; Jacobsen, C. Oxidation and oxidative stability in emulsions. Comp. Rev. Food Sci. Food Safe 2023, 22, 1864–1901. [Google Scholar] [CrossRef] [PubMed]
- Keramat, M.; Ehsandoost, E.; Golmakani, M.-T. Recent trends in improving the oxidative stability of oil-based food products by inhibiting oxidation at the interfacial region. Foods 2023, 12, 1191. [Google Scholar] [CrossRef] [PubMed]
- Alemán, M.; Bou, R.; Guardiola, F.; Durand, E.; Villeneuve, P.; Jacobsen, C.; Sørensen, A.-D.M. Antioxidative effect of lipophilized caffeic acid in fish oil enriched mayonnaise and milk. Food Chem. 2015, 167, 236–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sasaki, K.; Alamed, J.; Weiss, J.; Villeneuve, P.; López Giraldo, L.J.; Lecomte, J.; Figueroa-Espinoza, M.-C.; Decker, E.A. Relationship between the physical properties of chlorogenic acid esters and their ability to inhibit lipid oxidation in oil-in-water emulsions. Food Chem. 2010, 118, 830–835. [Google Scholar] [CrossRef]
- Sørensen, A.-D.M.; Durand, E.; Laguerre, M.; Bayrasy, C.; Lecomte, J.; Villeneuve, P.; Jacobsen, C. Antioxidant properties and efficacies of synthesized alkyl caffeates, ferulates, and coumarates. J. Agric. Food Chem. 2014, 62, 12553–12562. [Google Scholar] [CrossRef]
- Roman, M.J.; Decker, E.A.; Goddard, J.M. Performance of nonmigratory iron chelating active packaging materials in viscous model food systems: Iron chelating active packaging and food viscosity. J. Food Sci. 2015, 80, E1965–E1973. [Google Scholar] [CrossRef]
- Baghdadi, M.; Ahmadi, S.; Farhoodi, M.; Abedi, A.-S.; Omidi, N. The effect of high-density polyethylene active packages containing rosemary extract powder on oxidative stability of sunflower oil. J. Food Meas. Charact. 2019, 13, 2910–2920. [Google Scholar] [CrossRef]
- Kohannia, N.; Beigmohammadi, F.; Ramzani Ghara, A.; Nayebzadeh, K. Effect of polyethylene terephthalate incorporated with titanium dioxide and zinc oxide nanoparticles on shelf-life extension of mayonnaise sauce. J. Food. Process Preserv. 2021, 45, e15453. [Google Scholar] [CrossRef]
- Flórez, M.; Cazón, P.; Vázquez, M. Active packaging film of chitosan and Santalum album essential oil: Characterization and application as butter sachet to retard lipid oxidation. Food Packag. Shelf Life 2022, 34, 100938. [Google Scholar] [CrossRef]
- Ozdemir, N.; Kantekin-Erdogan, M.N.; Tat, T.; Tekin, A. Effect of black cumin oil on the oxidative stability and sensory characteristics of mayonnaise. J. Food Sci. Technol. 2018, 55, 1562–1568. [Google Scholar] [CrossRef]
- Tymczewska, A.; Furtado, B.U.; Nowaczyk, J.; Hrynkiewicz, K.; Szydłowska-Czerniak, A. Functional properties of gelatin/polyvinyl alcohol films containing black cumin cake extract and zinc oxide nanoparticles produced via casting technique. Int. J. Mol. Sci. 2022, 23, 2734. [Google Scholar] [CrossRef] [PubMed]
- Szydłowska-Czerniak, A.; Rabiej, D.; Krzemiński, M. Synthesis of novel octyl sinapate to enhance antioxidant capacity of rapeseed-linseed oil mixture: Octyl sinapate as a new lipophilic antioxidant. J. Sci. Food Agric. 2018, 98, 1625–1631. [Google Scholar] [CrossRef] [PubMed]
- Włodarczyk, K.; Zienkiewicz, A.; Szydłowska-Czerniak, A. Radical scavenging activity and physicochemical properties of aquafaba-based mayonnaises and their functional ingredients. Foods 2022, 11, 1129. [Google Scholar] [CrossRef] [PubMed]
- ISO 3960:2017; Animal and Vegetable Fats and Oils—Determination of Peroxide Value–Iodometric (Visual) Endpoint Determination. International Organization for Standardization: Geneva, Switzerland, 2017.
- ISO 6885:2016; Animal and Vegetable Fats and Oils—Determination of Anisidine Value. International Organization for Standardization: Geneva, Switzerland, 2016.
- ISO 3656:2011; Animal and Vegetable Fats and Oils—Determination of Ultraviolet Absorbance Expressed as Specific UV Extinction. International Organization for Standardization: Geneva, Switzerland, 2011.
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- Gökmen, V.; Serpen, A.; Fogliano, V. Direct measurement of the total antioxidant capacity of foods: The ‘QUENCHER’ approach. Trends Food Sci. Technol. 2009, 20, 278–288. [Google Scholar] [CrossRef]
- Speisky, H.; Shahidi, F.; Costa De Camargo, A.; Fuentes, J. Revisiting the oxidation of flavonoids: Loss, conservation or enhancement of their antioxidant properties. Antioxidants 2022, 11, 133. [Google Scholar] [CrossRef]
- Al-Maqtari, Q.A.; Ghaleb, A.D.S.; Mahdi, A.A.; Al-Ansi, W.; Noman, A.E.; Wei, M.; Al-Adeeb, A.; Yao, W. Stabilization of water-in-oil emulsion of Pulicaria jaubertii extract by ultrasonication: Fabrication, characterization, and storage stability. Food Chem. 2021, 350, 129249. [Google Scholar] [CrossRef]
- De Bruno, A.; Romeo, R.; Gattuso, A.; Piscopo, A.; Poiana, M. Functionalization of a vegan mayonnaise with high value ingredient derived from the agro-industrial sector. Foods 2021, 10, 2684. [Google Scholar] [CrossRef]
- Khalid, M.U.; Shabbir, M.A.; Mustafa, S.; Hina, S.; Quddoos, M.Y.; Mahmood, S.; Maryam, Y.; Faisal, F.; Rafique, A. Effect of apple peel as an antioxidant on the quality characteristics and oxidative stability of mayonnaise. Appl. Food Res. 2021, 1, 100023. [Google Scholar] [CrossRef]
- Kwon, H.; Ko, J.H.; Shin, H.-S. Evaluation of antioxidant activity and oxidative stability of spice-added mayonnaise. Food Sci. Biotechnol. 2015, 24, 1285–1292. [Google Scholar] [CrossRef]
- Lagunes-Galvez, L.; Cuvelier, M.E.; Odonnaud, C.; Berset, C. Oxidative stability of some mayonnaise formulations during storage and daylight irradiation. J. Food Lipids 2002, 9, 211–224. [Google Scholar] [CrossRef]
- Rabiej, D.; Szydłowska-Czerniak, A. Fluorescence and UV-VIS spectroscopy to determine the quality changes of rapeseed oil fortified with new antioxidant after storage under various conditions. Food Anal. Methods 2020, 13, 1973–1982. [Google Scholar] [CrossRef]



| Week | Type of Mayonnaise | |||||
|---|---|---|---|---|---|---|
| CPBM | CM | BCCM0.1% | BCCM3% | OCM | AFM | |
| QUENCHER-DPPH ± SD (μmol TE/100 g) | ||||||
| 0 | 557 ± 5 a,b,A | 850 ± 25 b,B | 890 ± 25 c,B | 978 ± 21 b,c,C | 1846 ± 50 a,D | 841 ± 21 a,B |
| 1 | 534 ± 4 a,A | 884 ± 41 b,B | 914 ± 41 c,B | 1014 ± 48 c,C | 2185 ± 92 b,D | 844 ± 36 a,B |
| 2 | 578 ± 15 b,A | 845 ± 34 b,B,C | 829 ± 32 b,B | 1006 ± 38 b,c,D | 2435 ± 101 c,E | 924 ± 35 b,C,D |
| 3 | 692 ± 27 c,A | 735 ± 33 a,A | 776 ± 19 a,b,A | 948 ± 26 b,B | 2708 ± 133 d,C | 1031 ± 50 c,B |
| 4 | 704 ± 17 c,A | 679 ± 30 a,A | 727 ± 34 a,A | 845 ± 28 a,B | 2887 ± 139 d,D | 1164 ± 38 d,C |
| QUENCHER-ABTS ± SD (μmol TE/100 g) | ||||||
| 0 | 2070 ± 94 c,A | 2068 ± 39 b,A | 2126 ± 40 b,B | 2326 ± 52 b,C | 2644 ± 41 a,D | 2065 ± 38 a,b,A |
| 1 | 1872 ± 84 b,A | 2079 ± 57 b,B | 2286 ± 61 b,C | 2302 ± 46 b,C | 2895 ± 149 b,D | 2075 ± 34 a,b,B |
| 2 | 1821 ± 57 b,A | 1990 ± 54 b,B | 2064 ± 95 a,B,C | 2311 ± 71 b,D | 3794 ± 80 c,E | 2175 ± 43 b,C |
| 3 | 1301 ± 50 a,A | 1988 ± 68 b,B | 1990 ± 41 a,B | 2334 ± 96 b,D | 3886 ± 131 c,d,E | 2138 ± 59 b,C |
| 4 | 1232 ± 42 a,A | 1735 ± 81 a,B | 1974 ± 64 a,C | 2139 ± 97 a,C | 4128 ± 198 d,D | 1989 ± 95 a,C |
| Week | Type of Mayonnaise | |||||
|---|---|---|---|---|---|---|
| CPBM | CM | BCCM0.1% | BCCM3% | OCM | AFM | |
| PV (meq O2/kg of oil) | ||||||
| 0 | 2.67 ± 0.05 a,C | 0.50 ± 0.02 a,A | 0.51 ± 0.03 a,A | 0.50 ± 0.03 a,A | 0.63 ± 0.03 a,B | 0.50 ± 0.03 a,A |
| 1 | 3.47 ± 0.03 b,D | 1.01 ± 0.04 b,C | 0.55 ± 0.01 b,B | 0.50 ± 0.01 a,A | 0.97 ± 0.01 b,C | 1.00 ± 0.01 b,C |
| 2 | 3.50 ± 0.01 b,F | 1.24 ± 0.01 c,E | 0.60 ± 0.01 c,A | 0.80 ± 0.01 b,B | 1.05 ± 0.05 c,C | 1.09 ± 0.01 c,D |
| 3 | 3.99 ± 0.02 c,E | 1.48 ± 0.02 d,C | 0.81 ± 0.02 d,A | 1.00 ± 0.01 c,B | 2.00 ± 0.01 d,D | 1.49 ± 0.03 d,C |
| 4 | 4.00 ± 0.01 c,E | 2.00 ± 0.01 e,D | 1.26 ± 0.02 e,B | 1.10 ± 0.02 d,A | 2.02 ± 0.02 d,D | 1.75 ± 0.01 e,C |
| AnV | ||||||
| 0 | 2.19 ± 0.11 a,B | 1.15 ± 0.06 a,A | 1.13 ± 0.04 a,A | 1.17 ± 0.07 a,A | 1.19 ± 0.05 a,A | 1.15 ± 0.03 a,A |
| 1 | 2.39 ± 0.08 a,b,D | 1.17 ± 0.07 a,A,B | 1.07 ± 0.07 a,A | 1.39 ± 0.07 b,C | 1.26 ± 0.06 a,B | 1.19 ± 0.05 a,b,B |
| 2 | 2.21 ± 0.10 a,C | 1.20 ± 0.05 a,b,A | 1.17 ± 0.06 a,A | 1.37 ± 0.06 b,B | 4.43 ± 0.14 b,D | 1.30 ± 0.07 b,A,B |
| 3 | 2.21 ± 0.11 a,C | 1.29 ± 0.03 b,A | 1.38 ± 0.04 b,B | 1.44 ± 0.07 b,B | 4.78 ± 0.08 c,D | 1.48 ± 0.06 c,B |
| 4 | 2.53 ± 0.12 b,C | 1.52 ± 0.07 c,A | 1.57 ± 0.07 c,A | 1.54 ± 0.07 c,A | 4.70 ± 0.23 c,D | 2.00 ± 0.10 d,B |
| TOTOX | ||||||
| 0 | 7.53 | 2.15 | 2.15 | 2.17 | 2.45 | 2.15 |
| 1 | 9.33 | 3.19 | 2.17 | 2.39 | 3.20 | 3.19 |
| 2 | 9.21 | 3.68 | 2.38 | 2.97 | 6.53 | 3.48 |
| 3 | 10.19 | 4.25 | 3.00 | 3.44 | 8.78 | 4.46 |
| 4 | 10.53 | 5.52 | 4.09 | 3.74 | 8.74 | 5.50 |
| CD | ||||||
| 0 | 2.30 ± 0.07 b,B | 2.20 ± 0.01 c,A | 2.19 ± 0.01 a,A | 2.20 ± 0.01 a,A | 2.23 ± 0.02 b,A | 2.20 ± 0.01 b,A |
| 1 | 2.07 ± 0.02 a,A | 2.03 ± 0.03 b,A | 2.33 ± 0.09 a,b,D | 2.32 ± 0.07 b,c,C | 2.25 ± 0.02 b,A,B | 2.19 ± 0.03 b,B |
| 2 | 2.41 ± 0.05 c,A | 2.38 ± 0.02 d,A | 2.43 ± 0.10 b,c,A | 2.47 ± 0.02 d,e,A,B | 2.63 ± 0.05 c,C | 2.57 ± 0.05 d,B |
| 3 | 2.50 ± 0.05 d,C,D | 2.42 ± 0.03 d,C | 2.56 ± 0.08 c,D | 2.54 ± 0.03 e,C,D | 2.00 ± 0.04 a,A | 2.28 ± 0.04 c,B |
| 4 | 2.91 ± 0.02 e,C | 1.98 ± 0.03 a,A | 2.44 ± 0.06 b,c,B | 2.39 ± 0.09 c,d,B | 1.95 ± 0.02 a,A | 1.95 ± 0.04 a,A |
| CT | ||||||
| 0 | 1.96 ± 0.09 a,D | 0.53 ± 0.01 c,B | 0.56 ± 0.02 b,C | 0.51 ± 0.01 b,A | 0.55 ± 0.01 a,C | 0.53 ± 0.01 c,B |
| 1 | 2.16 ± 0.05 b,D | 0.55 ± 0.01 d,B | 0.45 ± 0.01 a,A | 0.46 ± 0.01 a,A | 0.71 ± 0.01 e,C | 0.58 ± 0.01 e,B |
| 2 | 2.28 ± 0.04 c,E | 0.50 ± 0.01 b,B | 0.45 ± 0.01 a,A | 0.46 ± 0.01 a,A,B | 0.66 ± 0.02 d,D | 0.54 ± 0.01 d,C |
| 3 | 2.41 ± 0.03 d,E | 0.49 ± 0.01 a,B | 0.45 ± 0.02 a,A | 0.46 ± 0.01 a,A,B | 0.64 ± 0.01 c,D | 0.51 ± 0.01 b,C |
| 4 | 2.61 ± 0.01 e,C | 0.48 ± 0.01 a,A,B | 0.46 ± 0.02 a,A | 0.48 ± 0.02 a,A,B | 0.58 ± 0.02 b,B | 0.48 ± 0.01 a,A,B |
| AV (mg KOH/g of oil) | ||||||
| 0 | 1.20 ± 0.01 a,C | 0.08 ± 0.00 a,A | 0.07 ± 0.00 a,A | 0.09 ± 0.00 a,A | 0.13 ± 0.01 a,B | 0.08 ± 0.00 a,A |
| 1 | 1.33 ± 0.01 b,F | 0.12 ± 0.01 c,D | 0.08 ± 0.00 b,A | 0.09 ± 0.00 a,B | 0.17 ± 0.00 b,E | 0.11 ± 0.00 b,C |
| 2 | 1.34 ± 0.02 b,D | 0.12 ± 0.00 c,B | 0.10 ± 0.00 c,A,B | 0.09 ± 0.00 a,A | 0.17 ± 0.01 b,C | 0.11 ± 0.00 b,A,B |
| 3 | 1.36 ± 0.01 b,E | 0.11 ± 0.00 b,C | 0.10 ± 0.00 c,B | 0.09 ± 0.00 a,A | 0.23 ± 0.01 c,D | 0.11 ± 0.00 b,C |
| 4 | 1.38 ± 0.05 b,C | 0.12 ± 0.00 c,A | 0.10 ± 0.00 c,A | 0.11 ± 0.01 b,A | 0.23 ± 0.00 c,B | 0.11 ± 0.00 b,A |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Włodarczyk, K.; Tymczewska, A.; Rabiej-Kozioł, D.; Szydłowska-Czerniak, A. Effect of Black Cumin Cake Extract, Octyl Caffeate, and Active Packaging on Antioxidant Properties of Egg-Free Mayonnaise during Storage. Appl. Sci. 2023, 13, 6245. https://doi.org/10.3390/app13106245
Włodarczyk K, Tymczewska A, Rabiej-Kozioł D, Szydłowska-Czerniak A. Effect of Black Cumin Cake Extract, Octyl Caffeate, and Active Packaging on Antioxidant Properties of Egg-Free Mayonnaise during Storage. Applied Sciences. 2023; 13(10):6245. https://doi.org/10.3390/app13106245
Chicago/Turabian StyleWłodarczyk, Katarzyna, Alicja Tymczewska, Dobrochna Rabiej-Kozioł, and Aleksandra Szydłowska-Czerniak. 2023. "Effect of Black Cumin Cake Extract, Octyl Caffeate, and Active Packaging on Antioxidant Properties of Egg-Free Mayonnaise during Storage" Applied Sciences 13, no. 10: 6245. https://doi.org/10.3390/app13106245