Abstract
This in vitro study compared the shear bond strength (SBS) and antibacterial efficacy of an orthodontic adhesive containing either cinnamon or titanium dioxide (TiO2) nanoparticles (NPs). A total sample of 120 freshly extracted teeth was randomly divided into three groups, according to the type of NPs incorporated into adhesive for metallic orthodontic brackets’ bonding: group 1—conventional orthodontic adhesive (TXT) as a control; group 2—conventional orthodontic adhesive mixed with TiO2 NPs (TXT + TNP); and group 3—conventional orthodontic adhesive mixed with cinnamon NPs (TXT + CNP). The SBS and adhesive remnant index (ARI) scores were evaluated and compared between the groups. The antibacterial efficacy against Streptococcus mutans for all the groups was assessed via a disc agar diffusion test. Data comparisons among groups were performed by ANOVA followed by Bonferroni post hoc test. Antibacterial efficacy comparison between the experimental groups was performed via an independent t-test. The significance level for all the tests was set at p ≤ 0.05. The highest mean SBS values (10.11 ± 1.88 MPa) were in the TXT control group followed by TXT + TNP (9.40 ± 1.78 MPa), and the lowest SBS was in the TXT + CNP (8.99 ± 1.77 MPa) group. The mean SBS among the experimental groups was non-significant (p = 0.241). Antibacterial effects significantly increased (p ≤ 0.05) in both experimental groups. However, TXT + TNP revealed a significantly higher antibacterial effect (p = 0.021) than TXT + CNP. In conclusion, incorporating cinnamon or TiO2 NPs into an orthodontic adhesive improves its antibacterial effects without compromising the bond strength for clinical purposes.
1. Introduction
The main objective of orthodontic treatment is to correct the malocclusion in the best possible manner without compromising the dental health and supporting oral tissues. Nevertheless, maintaining good oral hygiene with fixed orthodontic appliances is a challenge for both patients and orthodontists, since many fixed appliance components act as plaque-retentive areas [1,2].
Unfortunately, white spot lesions (WSLs) are considered one of the most common and worst problems that usually start by the end of the first month of orthodontic treatment and were reported in more than 40% of patients [3,4]. It occurs as a result of decreased oral pH and lactic acid production by cariogenic bacteria such as facultatively anaerobic, Gram-positive Streptococcus mutans [5,6,7,8]. This may be contributed to various factors, among which are treatment duration and appliance complexity, which induce accumulation of dental plaque on tooth surfaces and increased oral bacterial count [9,10].
In the literature, there are numerous recommendations to decrease or eliminate WSLs, including application of fluoride, selective etching technique, and oral hygiene instructions, which are primarily reliant on patients’ cooperation [11]. Indeed, several antibacterial components have been added to orthodontic adhesives to aid in minimizing or restraining the incidence of WSLs during orthodontic treatment, such as fluoride and chlorhexidine [7,12,13,14]. Yet, these agents have some drawbacks, such as the short-term release of antimicrobial agents, reduced mechanical properties and possible tooth discoloration [11].
Application of nanotechnology is an unlimited phase toward producing materials with superior chemical, optical and mechanical features, and numerous innovative antimicrobial nanoparticles (NPs) have been established for dental applications [15]. Presently, antimicrobial NPs have been widely used in several fields of dentistry. The large surface area and high charge density of NPs help them interact with bacterial cells more effectively and, consequently, increase the antimicrobial efficacy [15,16].
Hence, researchers have investigated other approaches that mentioned decreasing WSLs, among which are incorporation of NPs having anti-caries capacity into the orthodontic adhesives or orthodontic appliance surfaces without conceding the value of the bonded interface [17,18,19]. Lately, various sorts of NPs have been coated on either brackets or added to cements and orthodontic adhesives, such as gold, silver, copper, silicon dioxide, hydroxyapatite, zinc oxide and titanium dioxide (TiO2) [20,21,22,23,24,25,26,27,28,29,30].
It was suggested that TiO2 nanoparticles, unlike other NPs, have excellent optical properties consisting of a high reflective index, chemical stability and no color change of the resin composites around brackets. Incorporation of TiO2 NPs into dental composites also augmented mechanical properties, such as modulus of elasticity, microhardness and flexural strength, and also provided shear bond strength (SBS) values similar or even higher levels than those not containing NPs [27,30]. Moreover, numerous studies have reported that resins embodying TiO2 NPs display compelling antimicrobial properties without conceding the bond strength and may have good potential for preventing enamel demineralization and WSLs [6,8,31,32,33,34].
Stimulatingly, a recent meta-analysis confirmed that the incorporation of silver NPs into orthodontic adhesive enhanced its antibacterial activity [35]. However, another recent one concluded that incorporation of 1 wt% copper and 5 wt% TiO2 NPs were found to be less effective against bacterial growth as compared to non-modified ones and experienced no reduction in adhesion strength [36]. Currently, few reports have tried to incorporate adjunctive natural elements such as cinnamon NPs into orthodontic adhesives with possible promising antimicrobial effects [37].
However, based on the current orthodontic literature, it appears that these effects were not compared with those of other NPs. Therefore, this in vitro study aimed to investigate and compare shear bond strength (SBS) and antibacterial efficacy of orthodontic adhesives containing cinnamon and TiO2 nanoparticles. The null hypothesis of this study was that the cinnamon NPs contained in conventional orthodontic adhesive would demonstrate SBS and antibacterial efficacy similar to that of TiO2 NPs contained in conventional orthodontic adhesive.
2. Materials and Methods
2.1. Sample Size Calculation and Specimen Preparation
Based on a previous study [31], a sample size of 30 specimens per group was required to demonstrate a statistically significant difference between the groups. However, in the current study, 40 specimens were included to compensate for any specimen loss during the study process. Accordingly, 120 human premolars free from enamel cracks, hypomineralization, hypoplasia, caries, filling and endodontic treatment that were freshly extracted for orthodontic therapy were included in this study. The collected teeth were cleaned, residues on the teeth were removed and rinsed, and then the teeth were stored in isotonic saline solution at room temperature before use. Each tooth was independently embedded vertically in auto-polymerizing acrylic resin blocks (Acrostone Dental and Medical Supplies, Cairo, Egypt) to ensure that the facial surface was parallel to the applied force during the test [8,22]. The acrylic blocks were numbered and randomly allocated into three groups by an independent individual to mask the identity of the tested groups (40 teeth for each group) according to the utilized adhesive system as follows:
Group 1: Conventional orthodontic composite (TXT, Control);
Group 2: TiO2 NPs mixed with conventional orthodontic composite (TXT + TNP);
Group 3: Cinnamon NPs mixed with conventional orthodontic composite (TXT + CNP).
2.2. Nanocomposite Preparation
In the present study, 1% (w/w) of cinnamon and TiO2 nanocomposites were used. For the creation of composite containing 1% TiO2 NPs, 40 mg of TiO2 NP powder (anatase dry nano-powder, particle size: 21 ± 5 nm, P25; Plasma Chem GmbH, Berlin, Germany) was mixed with 3960 mg of Transbond XT composite (3M Unitek, Monrovia, CA, USA) to obtain a final 4000 mg of composite containing TiO2 NPs with 1% concentration. The TiO2 NPs and Transbond XT adhesive were mixed with a high-speed mixer (SpeedMixer™, FlackTek Inc., Landrum, SC, USA) after being weighed by a digital scale at 3500 rpm in a dark environment for 5 min to ensure proper distribution of the NPs [8,30,31,34].
An equivalent procedure was undertaken with cinnamon NPs that were prepared by a mechanical attrition method. The natural cinnamon product sample was prepared by cleaning 100 g of cinnamon crust, thoroughly washing it with water and drying it in the shade in the presence of air. After that, the crust was sliced into small pieces and crushed. The cinnamon NPs were determined to be 20–25 nm in size using scanning electron microscopy (JEM 1400 Flash; Jeol, Tokyo, Japan). The cinnamon-incorporating experimental adhesive was prepared in accordance with previous studies [37,38]. After mixing, the adhesive was examined under scanning electron microscopy to verify homogeneity of the mix. Subsequently, nano-composites were loaded into plastic syringes and kept in a dark and cold environment until use [6,8,24,27].
2.3. Bonding Procedures
Teeth were cleaned and polished with prophylactic rubber cups for 10 s using fluoride-free pumice followed by rinsing with water spray and drying for 30 s [7]. The enamel was etched for 20 s with 37% phosphoric acid gel (Scotchbond™, Monrovia, CA, USA), then rinsed with water for 40 s and dried with oil- and moisture-free air to get the chalky white texture of the enamel surface. Metallic premolar brackets (3M Unitek, Monrovia, CA, USA) with a 0.022-inch slot and an average base surface area of 11.55 mm2 were bonded on the buccal surface of teeth according to the manufacturers’ instructions by one investigator who was blinded to group allocation (A.A.E) [31,33].
A thin coat of primer (3M Unitek, Monrovia, CA, USA) was applied on etched enamel, spread on the surface by gentle air spray from a 15 cm distance and then cured for 10 s. In the first group, Transbond XT composite was applied on the bracket base. Brackets were then bonded at the center of the buccal surface of each tooth mesiodistally and occlusogingivally, and each tooth was light-cured (Woodpecker Guilin, Guangxi, China) with a light intensity of 1000 mW/cm2 for a total of 40 s from mesial, distal, occlusal, and gingival directions [7]. Similar to the previous group, the bonding process was carried out in the second and third groups using Transbond XT with TiO2 and cinnamon NPs, respectively [31,33,34].
To avoid or reduce bracket deformation during the debonding process, a 0.017 × 0.025-inch stainless steel wire was ligated into each bracket slot and then the blocks with teeth were stored in distilled water at 37 °C for 24 h [6,32,33]. Next, all samples were thermocycled to simulate the oral environment and clinical thermal stress conditions before testing in a thermocycling machine (SD Mechatronik, Feldkirchen-Westerham, Germany). The specimens were subjected to 2000 cycles in a 5 °C to 55 °C temperature bath of distilled water with 20 s dwell time and 10 s transfer time [20,21,22].
2.4. Shear Bond Strength Testing
All acrylic blocks were mounted vertically with buccal surfaces parallel to the debonding blade. Then, an occluso-gingival shear load was applied to brackets using an Instron universal testing machine (Lloyd Instruments, Fareham Hampshire, UK) at a crosshead speed of 1 mm/min [8,32,33]. The chisel edge mounted on the crosshead of the machine contacted the bracket base to generate a shear force at the bracket–tooth interface. The maximum force required to debond the brackets was measured in Newtons, and the SBS was calculated, in Megapascals (MPa), by dividing force values by bracket base area (MPa = N/mm2) [14,22].
2.5. Adhesive Remnant Index (ARI) Scoring
After debonding, the outer enamel surface was inspected using a light stereomicroscope (Nikon SM2-10, Tokyo, Japan) at ×10 magnification for determination of the amount of residual adhesive and scored for each tooth via the commonly used adhesive remnant index (ARI) scale as 0—No adhesive left on the enamel surface, 1—Less than half of the adhesive left on the enamel surface, 2—More than half of the adhesive left on the enamel surface, and 3—All the adhesive left on the enamel surface [32,37].
2.6. Determination of the Antibacterial Efficacy of the Nanoparticles
Composite discs (6 × 3 mm2) were prepared using plastic molds. Molds were covered by matrix strips on each side and light-cured for 20 s from each side, then sterilized by exposure to ultraviolet light for 15 min [8,37]
The disc agar diffusion test was used to evaluate antibacterial effects of composite discs containing cinnamon and TiO2 NPs against Streptococcus mutans by assessing the disc release of nanoparticles [24,30]. The bacterial strain used was isolated from carious dentine (Streptococcus mutans Clarke 25175™, ATCC, Manassas, VA, USA). Bacterial suspension was prepared in a brain–heart infusion broth and incubated for 1–2 h in 37 °C to get the logarithmic phase of bacterial growth. Then, suspension was cultured on agar plates using sterile swaps. Discs from each group were subsequently placed on the surface of plates with 2 cm distance from each other. The plates were incubated for 24 h at 37 °C and the bacterial growth was evaluated by measuring the diameter of bacterial growth inhibitory zone in mm around the discs [8,24,37].
2.7. Energy-Dispersive X-ray (EDX) Spectroscopy and Scanning Electron Microscope (SEM) Analysis
In order to conform the homogeneity of the mix and confirm the elemental composition of the nanocomposites, energy-dispersive X-ray (EDX) spectroscopy was used in combination with a scanning electron microscope (SEM) (Evo 10, Carl Zeiss, Baden-Württemberg, Germany) operating under controlled atmospheric conditions at 15 kV, 20 µm distance and ×4000. A thin, electrically conductive gold layer was applied to the surface prior to SEM imaging to avoid electrostatic charge generation [6,8,24,27].
2.8. Statistical Analysis
Data were analyzed using statistical analysis software for Windows (IBM SPSS, version 22, IBM Corp., Armonk, NY, USA). Numerical data were summarized using mean, standard deviation, confidence intervals and range. Data were explored for normality by checking the data’s distribution using the Shapiro-Wilk test. Comparisons among groups with respect to normally distributed numeric variables (SBS) was performed by an analysis of variance (ANOVA) test, followed by Bonferroni post hoc test. Comparison between experimental groups regarding the antibacterial effect was performed via an independent t-test. Qualitative data of ARI were expressed as frequencies and percentages and compared using a chi-squared test. All p-values are two-sided with level of significance predetermined at p ≤ 0.05 level.
3. Results
3.1. Shear Bond Strength (SBS)
Figure 1 presents the mean SBS of the study groups. The mean SBS values were different in all the groups. The highest mean SBS was recorded in the TXT group (10.11 ± 1.88 MPa), followed by TXT + TNP (9.40 ± 1.78 MPa), and the lowest SBS was recorded in TXT + CNP (8.99 ± 1.77 MPa).
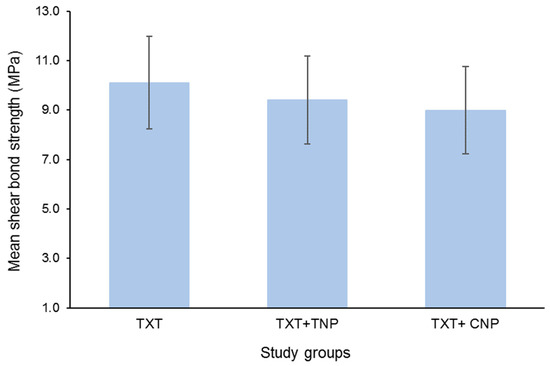
Figure 1.
Mean shear bond strength of the study groups. Bars indicate standard deviation (SD).
There was a statistically significant difference in SBS between the groups (p = 0.022). Pair-wise comparison revealed a significant difference in SBS between the conventional and cinnamon groups (p = 0.019). However, the mean SBS was non-significant between the conventional and TiO2 groups (p = 0.241) and between the nanoparticle groups (p = 0.944) (Table 1).

Table 1.
Mean comparison of SBS between the groups.
3.2. Adhesive Remnant Index
Table 2 demonstrated mean ARI scores that showed statistically non-significant differences (p = 0.96) among the three groups. In the TXT control group, 62.5% of samples recorded ARI 1, while 22.5% recorded ARI 2 and 10% recorded ARI 3, whereas ARI 0 was recorded in only 10% of the samples. In TXT + TNP group, 60% of the samples recorded ARI 1, while 25% recorded ARI 2 and 7.5% recorded ARI 3, whereas ARI 0 was noted in 7.5% of samples. In the TXT + CNP group, 55% of samples recorded ARI 1, while 32.5% recorded ARI 2 and 7.5% recorded ARI 3, and ARI 0 was noted in 5% of samples.

Table 2.
Descriptive statistics of adhesive remnant index (ARI) scores and mean comparison of ARI scores among the groups.
3.3. Assessment of Antimicrobial Efficacy
The results of antimicrobial test (Table 3) indicated that adhesive discs containing NPs had a potent effect against Streptococcus mutans as shown by the incidence of clear zone surrounding the discs in the agar plate. Conventional adhesive (TXT) disks had no antimicrobial effect, with no clear zone around disks. Upon comparing antibacterial effect between nanocomposite discs, TXT + TNP discs recorded a greater clear zone (6.38 ± 0.77 mm) in comparison to TXT + CNP (6 ± 0.65 mm). The difference in clear zone (0.38 ± 0.16 mm) between the nanocomposite groups was statistically significant (p = 0.021).

Table 3.
Mean comparison of bacterial growth inhibition diameter (clear zone) between the experimental groups.
3.4. EDX Spectroscopy and SEM Analysis Outcome
The TiO2 and cinnamon nanoparticles demonstrated irregular shape on the SEM micrograph (Figure 2a,b). Compared to cinnamon NPs, TiO2 was denser and congregated. EDX spectroscopy analysis verified the occurrence of titanium and oxygen in the TiO2 nanoparticles (Figure 3a). Contrarily, elements in cinnamon NPs were oxygen and Mg, P, K, Ca, Ti, Mn and Fe. Leading peaks were related to Mg and P elements (Figure 3b).
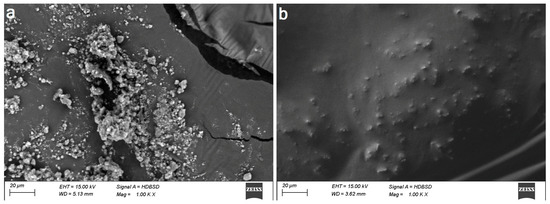
Figure 2.
Scanning electron micrographs (×1000) of the nano orthodontic composites. (a) TXT + TNP and (b) TXT + CNP.
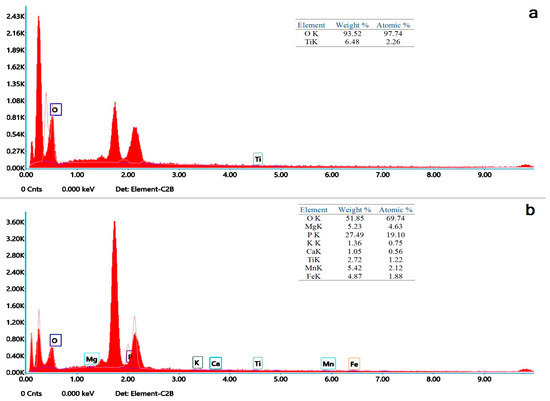
Figure 3.
Energy dispersive X-ray spectroscopic analysis of nanoparticles displaying elemental composition of nanocomposites. (a) TXT + TNP and (b) TXT + CNP.
4. Discussion
The effect of incorporating cinnamon and TiO2 NPs into orthodontic adhesives to prevent WSLs around orthodontic brackets is still poorly explained, the available literature is scarce, and to the best of the authors’ knowledge, there are no published data regarding comparisons of such alterations and consequences. In this in vitro study, the SBS and antibacterial efficacy of orthodontic adhesives containing cinnamon and TiO2 NPs were investigated and compared. The null hypothesis of this study was that the cinnamon NPs containing conventional orthodontic adhesive would demonstrate SBS and antibacterial efficacy similar to that of TiO2 NPs containing conventional orthodontic adhesive. The study outcomes demonstrated that TiO2 NPs had non-significantly higher SBS and significantly increased antibacterial activity compared to cinnamon NPs, thereby suggesting partial rejection of the null hypothesis.
The fixed orthodontic appliances make it challenging for the patient to practice good oral hygiene, decrease saliva’s capacity to self-clean, and create more areas where plaque might accumulate [5]. Mechanical plaque control techniques and strengthening enamel resistance through topical fluoride application are the main regimens for treating WSL. Fluoride products in various concentrations are frequently utilized as dental pastes, sealants, gels, mouthwashes, bonding materials, varnishes, and mouth rinses for the treatment of WSL [39,40,41]. Mouthwashes and toothpastes with fluoride are self-applied therapies [39,40], whereas dentists may perform some procedures with little clinical chair time, such as fluoride varnish or fluoride films. Most of these interventions also include advice on teeth brushing and oral hygiene instruction [40].
However, the last decade has seen a significant increase in the use of antibacterial NPs incorporated in orthodontic adhesives for preventing WSL [5,42]. When antibacterial NPs are transformed into nanometer size, they exhibit excellent antibacterial properties against both Gram-positive and Gram-negative bacteria, and these nanosized antibacterial agents are preferred to be added to dental materials due to the higher surface-to-volume ratio of NPs, which have intimate interactions with microbial membranes and provide a significantly larger surface area for antibacterial activity [5,6,42].
4.1. Shear Bond Strength
The mean SBS values were different in all tested groups, where the control TXT group recorded the highest SBS (10.11 ± 1.88 MPa), followed by TXT + TNP (9.40 ± 1.78 MPa), and the lowest SBS was found in the TXT + CNP group (8.99 ± 1.77 MPa). However, both experimental adhesives were not considerably different, and TXT + TNP was fairly similar to the control group. Furthermore, the TXT + CNP group displayed slightly lesser SBS than the control group.
The bond strength of orthodontic adhesives must be sufficient to withstand masticatory forces and stresses exerted by orthodontic arch wires. Brackets’ debonding may lead to extending treatment time and raising treatment expenses. It has been reported that a minimum SBS of 5.9–7.9 MPa offers acceptable clinical results for brackets’ bonding [6]. Nevertheless, it is imperative to note that excessive bond strength close to restorative materials is not necessarily desirable in orthodontic bonding since the enamel surface might be injured during brackets’ debonding [31]. Moreover, these values vary significantly from laboratory experiment values owing to the complex oral environment in which moisture contamination markedly reduces bonding strength. In the view of the current findings, both tested cinnamon and TiO2 nanocomposites revealed an acceptable SBS for clinical practice [7]. In line with the present outcomes, Yaseen et al. [37]. reported that an experimental adhesive with 3% cinnamon NPs had SBS with 7.20 ± 1.68 MPa compared to the control adhesive (8.50 ± 1.66 MPa).
The present concentration of 1% (w/w) NPs was selected in agreement with the results of previous reports which showed that adding 1% (w/w) to orthodontic adhesive resulted in enhanced antimicrobial effects without compromising its mechanical properties [6,8,34,37]. The SBS test has been commonly considered owing to its relative simplicity as compared to the tensile bond strength test in which it is difficult to align specimens in testing machines without creating a deleterious stress distribution. Moreover, evaluation of SBS at 24 h after bonding is commonly desired in most in vitro studies that offer a bonding substance with more homogeneity and consistency [43].
Consistent with the present results, Sodagar et al. [30] examined different percentages of TiO2 NPs and reported that Transbond adhesive with 1% had higher SBS (18.17 ± 4.65 MPa), which might be due to their smaller sample size. Likewise, Poosti et al. [8] found no significant difference in SBS of Transbond composite with (14.3 ± 1.2 MPa) and without 1% TiO2 NPs at 24 h. Yet, Felemban and Ebrahim [33] reported that adding ZrO2 − TiO2 NPs improved SBS (14.75 ± 0.25 MPa). However, their sample size was lower (n = 10), and adding ZrO2 could explain their higher SBS values.
In this study, TXT was used as the control adhesive as it is considered the gold standard light-cured orthodontic adhesive that is commonly exploited [20,30,33]. The sample included human maxillary premolars extracted for orthodontic purposes as these teeth are available and their results can be inferred directly to clinical practice, while the use of bovine teeth as an alternative is questionable in terms of the trace element contents of enamel [30]. Parallel findings were also obtained by Farzanegan et al. [32], and Assery et al. [6] reported that TXT composite containing 1% TiO2 NPs has no considerable effect on SBS with satisfactory clinical range (13.96 ± 1.97 MPa and 10.6 − 13.2 MPa, respectively). It is important to note that aging can deteriorate composite matrix by mechanisms such as swelling, depleting its free radicals by water sorption or thermal stresses, and hydrolytic degradation of the silane film over the fillers. Interestingly, Behnaz et al. [31] did not find any noteworthy differences between 1, 30, or 90 days of aging as mean SBS of 1% TiO2 did not significantly change over time, and they believed that TiO2 NPs might enhance TXT resin structure and reduce the deteriorating effect of aging.
The present outcome concurs with Pourhajibagher et al. [36], who advocated that minor reduction in SBS of orthodontic adhesives is anticipated after additions of 1% NPs as compared to that of non-modified adhesive. Conversely, the current findings differed from those of Behnaz et al. [31], who found that the mean SBS of TXT composite without TiO2 was about 20 units higher (147.44 ± 6.28 MPa) than that containing TiO2 (126.43 ± 5.93 Mpa), despite their recommendation for use in clinical situations. Furthermore, Reddy et al. [27] reported that SBS was relatively decreased by 30% (6.33 ± 1.51 MPa) for Transbond composite containing 1.0% TiO2 NPs. These discrepancies could be attributed to deficits in the in vitro models’ standardized protocols, smaller samples and altered methodological variants compared to the contemporary experimental settings. Additionally, saliva [44], blood [45], bleaching agents [46] or other contaminants have been demonstrated to have a significant influence on bond strength. Therefore, these variables should also be taken into careful consideration in combination with laser pretreatment in future clinical and laboratory tests.
4.2. Adhesive Remnant Index
The ARI is one of the most frequently used simple methods for evaluation and relating of the adhesion quality between composite and enamel surface, as well as between composite and bracket base via assessment of adhesive remnants following debonding. A high ARI score might indicate excessively strong adhesion between the adhesive and enamel surface, which could lead to enamel fracture at the interface. Thus, failure within the adhesive layer (low ARI score) during brackets’ deboning is desired to diminish the hazard of breakdown of enamel surface [6,32,35].
The present specimens presented that most failures had score 1 with less than 50% of the adhesive remaining on enamel surface after debonding, with no momentous difference among all groups (Table 2). Though, both cinnamon and TiO2 NPs adhesives had slightly lower ARI mean scores than the control group, the difference among groups was not substantial (Table 3). These findings are concomitant with those of Yaseen et al. [37] and Assery et al. [6]. Furthermore, in agreement with the existing findings, Behnaz et al. [31], Sodagar et al. [30], Farzanegan et al. [32] and Poosti et al. [8] reported that the ARI scores of TXT alone and TXT containing 1% TiO2 NPs were not considerably different after debonding.
However, it is noteworthy to remember that the ARI score depends on bracket base design, type of adhesive, position of the tooth within the arch, method of bracket debonding, and bracket base material and not simply on the bond strengths at the interfaces [31].
4.3. Antimicrobial Effect
The current results showed that Transbond adhesive disks containing either 1% cinnamon or TiO2 NPs had potent and marginally equivalent antimicrobial effect and significantly reduced the bacterial count (Table 3). These outcomes concur with Yaseen et al. [37], who showed that 3% cinnamon NPs have a potential antimicrobial effect against Streptococcus mutans. Considering that Streptococcus mutans is the main bacteria associated with initial carious lesions such as WSLs, the antimicrobial effect of the current cinnamon or TiO2 NPs was examined in accordance with the majority of earlier studies that utilized the diameter of growth inhibition zones [8,24,37]. The disc agar diffusion test was considered to evaluate the current nano-adhesives that could readily diffuse via agar to create the growth inhibition zone [25].
Cinnamon is one of the commonly used plant products in medical fields, and its advocated antimicrobial activity is linked to the presence of active extracts such as cinnamic aldehyde, cinnamate, cinnamic acid and numerous essential oils. These compounds are identified to be either bactericidal or bacteriostatic agents, depending upon concentration used [37,47,48]. Remarkably, Zainal-Abidin et al. [49] suggested that the minimal inhibitory concentration of cinnamon oil extract against main oral pathogens associated with caries and periodontal diseases was 0.21–0.63 mg/mL. Additionally, the least bactericidal concentration of cinnamaldehyde against Streptococcus mutans was recorded to be 0.2% [50].
As WSLs are frequently formed around orthodontic brackets, the evaluation of the antimicrobial effects due to the release of NPs in orthodontic adhesives is important. Hence, ideal antimicrobial NPs for adding to orthodontic adhesives must be able to diffuse into the environment. The current findings support those of Sodagar et al. [30], Assery et al. [6] and Poosti et al. [8], who observed that addition of 1% TiO2 to orthodontic composite demonstrated favorable antibacterial influence without affecting its bond strength. Moreover, Salehi et al. [28] found that N-doped TiO2-coated brackets could prevent the growth of Streptococcus mutans for at least three months and could effectively prevent enamel decalcification during orthodontic management.
The bactericidal mechanism of TiO2 is believed to be through its good anti-adhesive properties against Streptococcus mutans and the induction of oxidative damage in cell walls of microorganisms as a result of the production of free radicals when exposed to light irradiation, which are strong oxidants [6,33,36].
The NPs of different composition represent the most prevalent use of nanotechnology in the dental field, and many attempts have been made to apply antimicrobial NPs on an experimental scale to develop an orthodontic adhesive with antimicrobial activity to diminish WSLs around bonded orthodontic brackets [17]. Based on the present results, addition of cinnamon or TiO2 NPs to orthodontic adhesive considerably affected bacterial growth and could be considered as an effective technique to overcome WSLs.
The in vitro design of the study did not completely simulate in vivo conditions, which is a major limitation of this study. Certain factors such as patients’ dietary intake and oral hygiene may significantly influence the study outcome in in vivo conditions. Furthermore, saliva, which is considered crucial for oral defense mechanisms, was not considered in this study. Nevertheless, further in vivo studies are required to assess their clinical applicability, efficacy and long-term antibacterial effects and mechanical properties. Moreover, identification of proper concentration, particle size and preparation form (addition to primer or adhesive) of NPs is still greatly recommended.
5. Conclusions
Based on the outcome of this study, the following conclusions are made:
- (a)
- The mean SBS values were different in all the groups. The highest mean SBS was recorded in the conventional group, followed by the TiO2 NPs group, and the lowest SBS was recorded in the cinnamon NP group.
- (b)
- The results of the antimicrobial test indicated that adhesive discs containing NPs had a potent effect against Streptococcus mutans. Conventional adhesive disks had no antimicrobial effect, with no clear zone around disks. Upon comparing antibacterial effects between nanocomposite discs, TiO2 NPs recorded a greater clear zone in comparison to the cinnamon NP group, and the difference in clear zone between the nanocomposite groups was statistically significant.
- (c)
- Overall, the current in vitro findings verified that incorporating cinnamon or TiO2 nanoparticles into an orthodontic adhesive improves its antibacterial effects without compromising or disturbing its SBS for clinical usage.
Author Contributions
Conceptualization, A.A.E.-A., M.M.A., K.F.A., A.A.S.M., K.S.E. and F.A.H.; Data curation, H.N.A.-K., M.M.A., M.M.H. and K.S.E.; Formal analysis, H.N.A.-K., K.F.A., A.A.S.M. and F.A.H.; Investigation, H.N.A.-K., R.E.M., K.F.A., M.M.H. and K.S.E.; Methodology, A.A.E.-A. and H.N.A.-K.; Project administration, K.S.E.; Resources, A.A.E.-A., R.E.M., M.M.A., A.A.S.M. and K.S.E.; Software, R.E.M.; Supervision, F.A.H.; Validation, M.M.H.; Visualization, M.M.A. and K.F.A.; Writing—original draft, A.A.E.-A., R.E.M., K.F.A. and M.M.H.; Writing—review and editing, A.A.S.M. and F.A.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bishara, S.E.; Ostby, A.W. White Spot Lesions: Formation, Prevention, and Treatment. Semin. Orthod. 2008, 14, 174–182. [Google Scholar] [CrossRef]
- Sardana, D.; Zhang, J.; Ekambaram, M.; Yang, Y.; Mcgrath, C.P.; Yiu, C.K.Y. Effectiveness of Professional Fluorides against Enamel White Spot Lesions During Fixed Orthodontic Treatment: A Systematic Review and Meta-Analysis. J. Dent. 2019, 82, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Richter, A.E.; Arruda, A.O.; Peters, M.C.; Sohn, W. Incidence of Caries Lesions among Patients Treated with Comprehensive Orthodontics. Am. J. Orthod. Dentofac. Orthop. 2011, 139, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Tufekci, E.; Dixon, J.S.; Gunsolley, J.C.; Lindauer, S.J. Prevalence of White Spot Lesions During Orthodontic Treatment with Fixed Appliances. Angle Orthod. 2011, 81, 206–210. [Google Scholar] [CrossRef]
- Asiry, M.A.; Alshahrani, I.; Alqahtani, N.D.; Durgesh, B. Efficacy of Yttrium (Iii) Fluoride Nanoparticles in Orthodontic Bonding. J. Nanosci. Nanotechnol. 2019, 19, 1105–1110. [Google Scholar] [CrossRef]
- Assery, M.; Ajwa, N.; Alshamrani, A.; Alanazi, B.; Durgesh, B.; Matinlinna, J. Titanium Dioxide Nanoparticles Reinforced Experimental Resin Composite for Orthodontic Bonding. Mater. Res. Express 2019, 6, 125098. [Google Scholar] [CrossRef]
- Durgesh, B.H.; Alkheraif, A.A.; Pavithra, D.; Hashem, M.I.; Alkhudhairy, F.; Elsharawy, M.; Divakar, D.D.; Vallittu, P.K.; Matinlinna, J.P. Evaluation of an Experimental Adhesive Resin for Orthodontic Bonding. Mech. Compos. Mater. 2017, 53, 389–398. [Google Scholar] [CrossRef]
- Poosti, M.; Ramazanzadeh, B.; Zebarjad, M.; Javadzadeh, P.; Naderinasab, M.; Shakeri, M.T. Shear Bond Strength and Antibacterial Effects of Orthodontic Composite Containing TiO2 Nanoparticles. Eur. J. Orthod. 2013, 35, 676–679. [Google Scholar] [CrossRef]
- Freitas, A.O.; Marquezan, M.; Nojima Mda, C.; Alviano, D.S.; Maia, L.C. The Influence of Orthodontic Fixed Appliances on the Oral Microbiota: A Systematic Review. Dent. Press J. Orthod. 2014, 19, 46–55. [Google Scholar] [CrossRef]
- Sundararaj, D.; Venkatachalapathy, S.; Tandon, A.; Pereira, A. Critical Evaluation of Incidence and Prevalence of White Spot Lesions During Fixed Orthodontic Appliance Treatment: A Meta-Analysis. J. Int. Soc. Prev. Community Dent. 2015, 5, 433–439. [Google Scholar]
- Chambers, C.; Stewart, S.; Su, B.; Sandy, J.; Ireland, A. Prevention and Treatment of Demineralisation During Fixed Appliance Therapy: A Review of Current Methods and Future Applications. Br. Dent. J. 2013, 215, 505–511. [Google Scholar] [CrossRef]
- Chung, S.H.; Cho, S.; Kim, K.; Lim, B.S.; Ahn, S.J. Antimicrobial and Physical Characteristics of Orthodontic Primers Containing Antimicrobial Agents. Angle Orthod. 2017, 87, 307–312. [Google Scholar] [CrossRef]
- Cohen, W.J.; Wiltshire, W.A.; Dawes, C.; Lavelle, C.L. Long-Term in Vitro Fluoride Release and Rerelease from Orthodontic Bonding Materials Containing Fluoride. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 571–576. [Google Scholar] [CrossRef]
- Hussein, F.A.; Hashem, M.I.; Chalisserry, E.P.; Anil, S. The Impact of Chlorhexidine Mouth Rinse on the Bond Strength of Polycarbonate Orthodontic Brackets. J. Contemp. Dent. Pract. 2014, 15, 688–692. [Google Scholar]
- Ozak, S.T.; Ozkan, P. Nanotechnology and Dentistry. Eur. J. Dent. 2013, 7, 145–151. [Google Scholar]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef]
- Borzabadi-Farahani, A.; Borzabadi, E.; Lynch, E. Nanoparticles in Orthodontics, a Review of Antimicrobial and Anti-Caries Applications. Acta Odontol. Scand. 2014, 72, 413–417. [Google Scholar] [CrossRef]
- Govindankutty, D. Applications of Nanotechnology in Orthodontics and Its Future Implications. Int. J. Appl. Dent. Sci. 2015, 1, 166–171. [Google Scholar]
- Varon-Shahar, E.; Sharon, E.; Zabrovsky, A.; Houri-Haddad, Y.; Beyth, N. Antibacterial Orthodontic Cements and Adhesives: A Possible Solution to Streptococcus mutans Outgrowth Adjacent to Orthodontic Appliances. Oral Health Prev. Dent. 2019, 17, 49–56. [Google Scholar]
- Akhavan, A.; Sodagar, A.; Mojtahedzadeh, F.; Sodagar, K. Investigating the Effect of Incorporating Nanosilver/Nanohydroxyapatite Particles on the Shear Bond Strength of Orthodontic Adhesives. Acta Odontol. Scand. 2013, 71, 1038–1042. [Google Scholar] [CrossRef]
- Argueta-Figueroa, L.; Scougall-Vilchis, R.J.; Morales-Luckie, R.A.; Olea-Mejía, O.F. An Evaluation of the Antibacterial Properties and Shear Bond Strength of Copper Nanoparticles as a Nanofiller in Orthodontic Adhesive. Aust. Orthod. J. 2015, 31, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Blöcher, S.; Frankenberger, R.; Hellak, A.; Schauseil, M.; Roggendorf, M.J.; Korbmacher-Steiner, H.M. Effect on Enamel Shear Bond Strength of Adding Microsilver and Nanosilver Particles to the Primer of an Orthodontic Adhesive. BMC Oral Health 2015, 15, 42. [Google Scholar] [CrossRef] [PubMed]
- Degrazia, F.W.; Leitune, V.C.; Garcia, I.M.; Arthur, R.A.; Samuel, S.M.; Collares, F.M. Effect of Silver Nanoparticles on the Physicochemical and Antimicrobial Properties of an Orthodontic Adhesive. J. Appl. Oral Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Eslamian, L.; Borzabadi-Farahani, A.; Karimi, S.; Saadat, S.; Badiee, M.R. Evaluation of the Shear Bond Strength and Antibacterial Activity of Orthodontic Adhesive Containing Silver Nanoparticle, an In-Vitro Study. Nanomaterials 2020, 10, 1466. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Sierra, J.F.; Ruiz, F.; Pena, D.C.; Martínez-Gutiérrez, F.; Martínez, A.E.; Guillén Ade, J.; Tapia-Pérez, H.; Castañón, G.M. The Antimicrobial Sensitivity of Streptococcus Mutans to Nanoparticles of Silver, Zinc Oxide, and Gold. Nanomedicine 2008, 4, 237–240. [Google Scholar] [CrossRef]
- Panchali, B.; Mushtaq, A.; Mazumder, J.; Rizvi, M.S.; Miglani, R. Nanoparticles and Their Applications in Orthodontics. Adv. Dent. Oral Health 2016, 2, 41–50. [Google Scholar]
- Reddy, A.K.; Kambalyal, P.B.; Patil, S.R.; Vankhre, M.; Khan, M.Y.; Kumar, T.R. Comparative Evaluation and Influence on Shear Bond Strength of Incorporating Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles in Orthodontic Adhesive. J. Orthod. Sci. 2016, 5, 127–131. [Google Scholar]
- Salehi, P.; Babanouri, N.; Roein-Peikar, M.; Zare, F. Long-Term Antimicrobial Assessment of Orthodontic Brackets Coated with Nitrogen-Doped Titanium Dioxide against Streptococcus Mutans. Prog. Orthod. 2018, 19, 35. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhavan, A.; Hashemi, E.; Arab, S.; Pourhajibagher, M.; Sodagar, K.; Kharrazifard, M.J.; Bahador, A. Evaluation of the Antibacterial Activity of a Conventional Orthodontic Composite Containing Silver/Hydroxyapatite Nanoparticles. Prog. Orthod. 2016, 17, 40. [Google Scholar] [CrossRef]
- Sodagar, A.; Akhoundi, M.S.A.; Bahador, A.; Jalali, Y.F.; Behzadi, Z.; Elhaminejad, F.; Mirhashemi, A.H. Effect of TiO2 Nanoparticles Incorporation on Antibacterial Properties and Shear Bond Strength of Dental Composite Used in Orthodontics. Dent. Press J. Orthod. 2017, 22, 67–74. [Google Scholar] [CrossRef]
- Behnaz, M.; Dalaie, K.; Mirmohammadsadeghi, H.; Salehi, H.; Rakhshan, V.; Aslani, F. Shear Bond Strength and Adhesive Remnant Index of Orthodontic Brackets Bonded to Enamel Using Adhesive Systems Mixed with TiO2 Nanoparticles. Dent. Press J. Orthod. 2018, 23, 43.e1–43.e7. [Google Scholar] [CrossRef]
- Farzanegan, F.; Shafaee, H.; Darroudi, M.; Rangrazi, A. Effect of the Incorporation of Chitosan and TiO2 Nanoparticles on the Shear Bond Strength of an Orthodontic Adhesive: An in Vitro Study. J. Adv. Oral Res. 2021, 12, 261–266. [Google Scholar] [CrossRef]
- Felemban, N.H.; Ebrahim, M.I. The Influence of Adding Modified Zirconium Oxide-Titanium Dioxide Nano-Particles on Mechanical Properties of Orthodontic Adhesive: An in Vitro Study. BMC Oral Health 2017, 17, 43. [Google Scholar] [CrossRef]
- Heravi, F.; Ramezani, M.; Poosti, M.; Hosseini, M.; Shajiei, A.; Ahrari, F. In Vitro Cytotoxicity Assessment of an Orthodontic Composite Containing Titanium-Dioxide Nano-Particles. J. Dent. Res. Dent. Clin. Dent. Prospect. 2013, 7, 192–198. [Google Scholar]
- Alam, M.K.; Alsuwailem, R.; Alfawzan, A.A. Antibacterial Activity and Bond Strength of Silver Nanoparticles Modified Orthodontic Bracket Adhesive: A Systematic Review and Meta-Analysis of In-Vitro and In-Vivo Studies. Int. J. Adhes. Adhes. 2022, 113, 103040. [Google Scholar] [CrossRef]
- Pourhajibagher, M.; Sodagar, A.; Bahador, A. An in Vitro Evaluation of the Effects of Nanoparticles on Shear Bond Strength and Antimicrobial Properties of Orthodontic Adhesives: A Systematic Review and Meta-Analysis Study. Int. Orthod. 2020, 18, 203–213. [Google Scholar] [CrossRef]
- Yaseen, S.N.; Taqa, A.A.; Al-Khatib, A.R. The Effect of Incorporation Nano Cinnamon Powder on the Shear Bond of the Orthodontic Composite (an In Vitro Study). J. Oral Biol. Craniofac. Res. 2020, 10, 128–134. [Google Scholar] [CrossRef]
- Rajput, N. Methods of Preparation of Nanoparticles—A Review. Int. J. Adv. Eng. Technol. 2015, 7, 1806–1811. [Google Scholar]
- Amaechi, B.T.; Mcgarrell, B.; Luong, M.N.; Okoye, L.O.; Gakunga, P.T. Prevention of White Spot Lesions around Orthodontic Brackets Using Organoselenium-Containing Antimicrobial Enamel Surface Sealant. Heliyon 2021, 7, e06490. [Google Scholar] [CrossRef]
- Hu, H.; Feng, C.; Jiang, Z.; Wang, L.; Shrestha, S.; Su, X.; Shu, Y.; Ge, L.; Lai, W.; Hua, F.; et al. Effectiveness of Remineralising Agents in Prevention and Treatment of Orthodontically Induced White Spot Lesions: A Protocol for a Systematic Review Incorporating Network Meta-Analysis. Syst. Rev. 2019, 8, 339. [Google Scholar] [CrossRef]
- Khoroushi, M.; Kachuie, M. Prevention and Treatment of White Spot Lesions in Orthodontic Patients. Contemp. Clin. Dent. 2017, 8, 11–19. [Google Scholar] [PubMed]
- Yun, Z.; Qin, D.; Wei, F.; Xiaobing, L. Application of Antibacterial Nanoparticles in Orthodontic Materials. Nanotechnol. Rev. 2022, 11, 2433–2450. [Google Scholar] [CrossRef]
- Yamamoto, A.; Yoshida, T.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Miyazaki, M. Orthodontic Bracket Bonding: Enamel Bond Strength vs. Time. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 435.e1–435.e6. [Google Scholar] [CrossRef] [PubMed]
- Robaski, A.W.; Pamato, S.; Tomás-De Oliveira, M.; Pereira, J.R. Effect of Saliva Contamination on Cementation of Orthodontic Brackets Using Different Adhesive Systems. J. Clin. Exp. Dent. 2017, 9, e919–e924. [Google Scholar] [CrossRef] [PubMed]
- Cacciafesta, V.; Sfondrini, M.F.; Scribante, A.; De Angelis, M.; Klersy, C. Effects of Blood Contamination on the Shear Bond Strengths of Conventional and Hydrophilic Primers. Am. J. Orthod. Dentofac. Orthop. 2004, 126, 207–212. [Google Scholar] [CrossRef]
- Cacciafesta, V.; Sfondrini, M.F.; Stifanelli, P.; Scribante, A.; Klersy, C. The Effect of Bleaching on Shear Bond Strength of Brackets Bonded with a Resin-Modified Glass Ionomer. Am. J. Orthod. Dentofac. Orthop. 2006, 130, 83–87. [Google Scholar] [CrossRef]
- Abdalla, W. Antibacterial and Antifungal Effect of Cinnamon. Microbiol. Res. J. Int. 2018, 23, 1–8. [Google Scholar] [CrossRef]
- Rezvani, M.B.; Niakan, M.; Kamalinejad, M.; Ahmadi, F.S.; Hamze, F. The Synergistic Effect of Honey and Cinnamon against Streptococcus mutans Bacteria. Asian Pac. J. Trop. Biomed. 2017, 7, 314–320. [Google Scholar] [CrossRef]
- Zainal-Abidin, Z.; Mohd-Sais, S.; Abdul Majid, F.A.; Wan Mustapha, W.A.; Jantan, I. Antibacterial Activity of Cinnamon Oil on Oral Pathogens. Open Conf. Proc. J. 2013, 4, 12–16. [Google Scholar] [CrossRef]
- Choi, O.; Cho, S.K.; Kim, J.; Park, C.G.; Kim, J. In Vitro Antibacterial Activity and Major Bioactive Components of Cinnamomum verum Essential Oils against Cariogenic Bacteria, Streptococcus mutans and Streptococcus sobrinus. Asian Pac. J. Trop. Biomed. 2016, 6, 308–314. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).