How Does the Biocompatibility of Molybdenum Compare to the Gold Standard Titanium?—An In Vivo Rat Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Animal Model
2.1.2. Implants
Molybdenum Implants
Selective Laser Melting
Titanium Implants
2.2. Methods
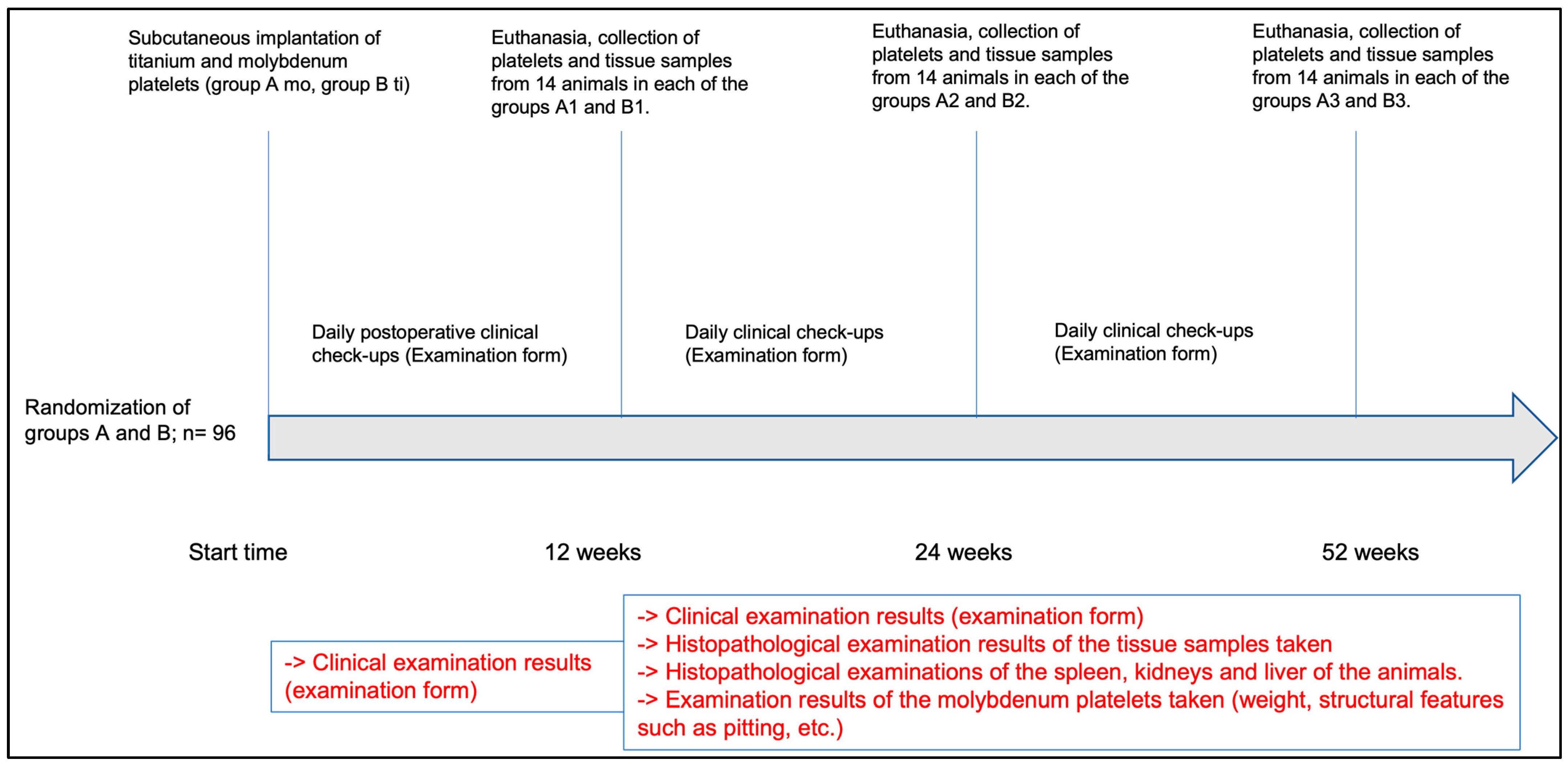
2.2.1. Study Design
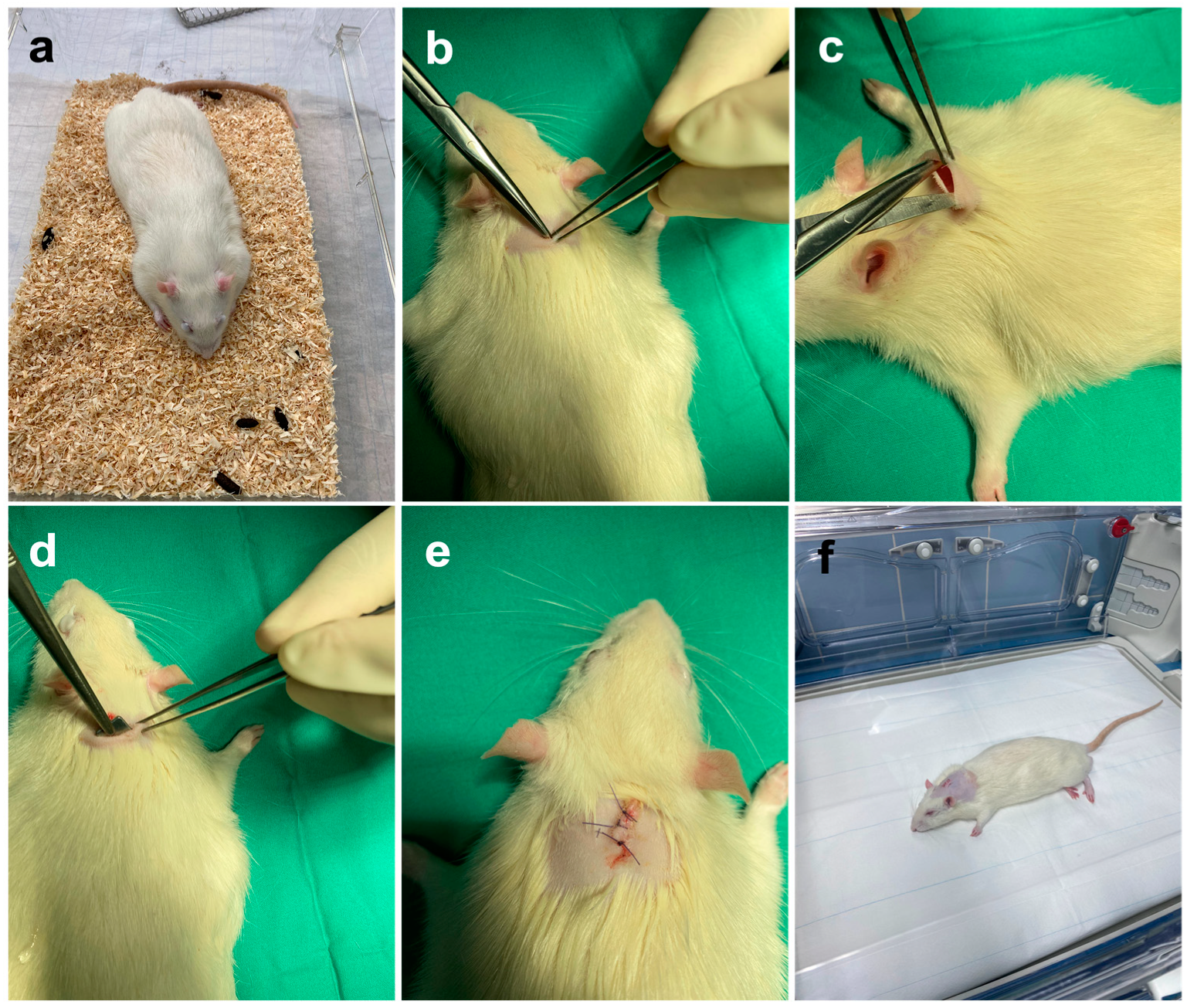
2.2.2. Surgical Implantation of the Molybdenum and Titanium Platelets
2.2.3. Postoperative Management
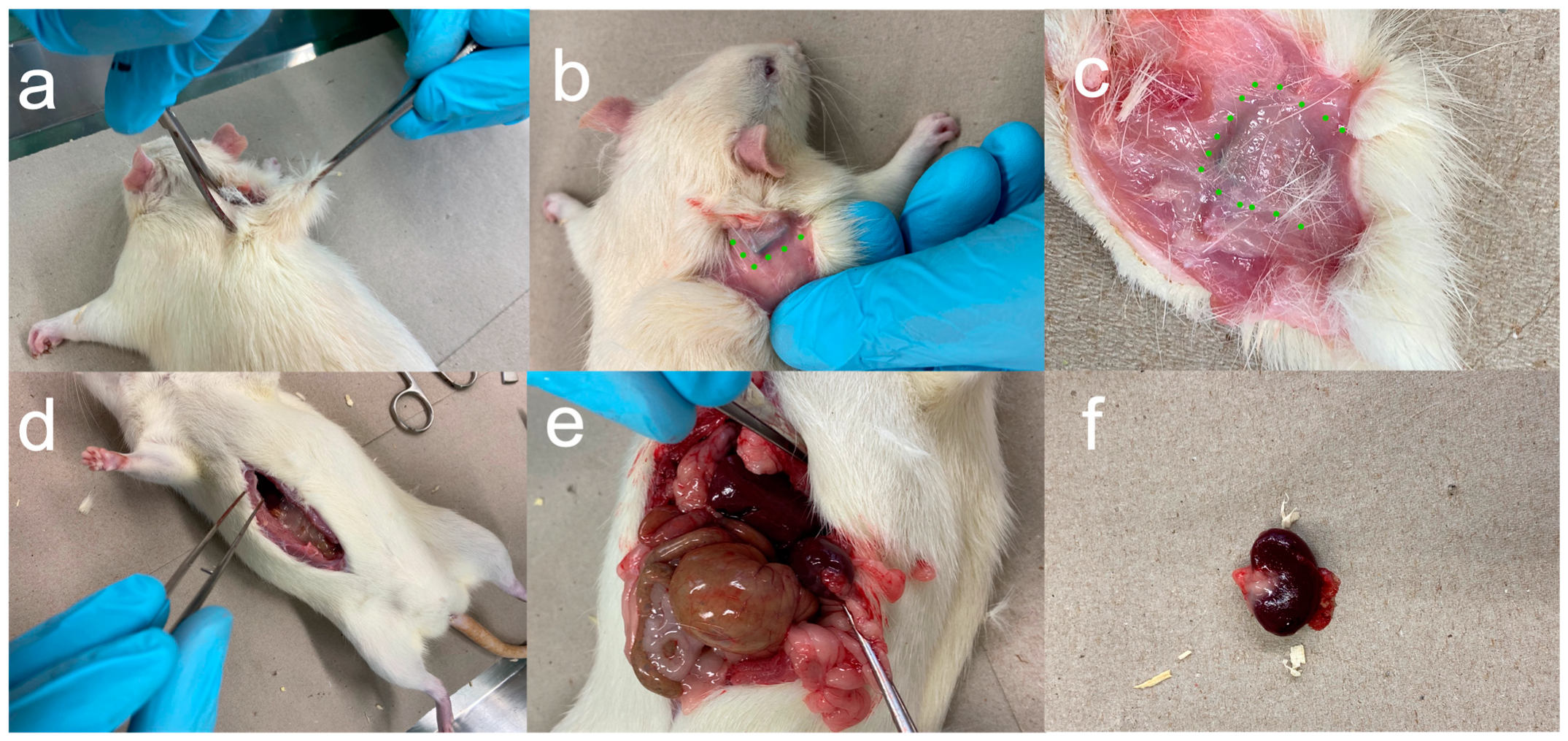
2.2.4. Euthanasia and Removal of Implants and Organs
2.2.5. Histopathological Processing and Examination
2.2.6. Analysis of the Metallographic Cross Sections
2.2.7. Statistics
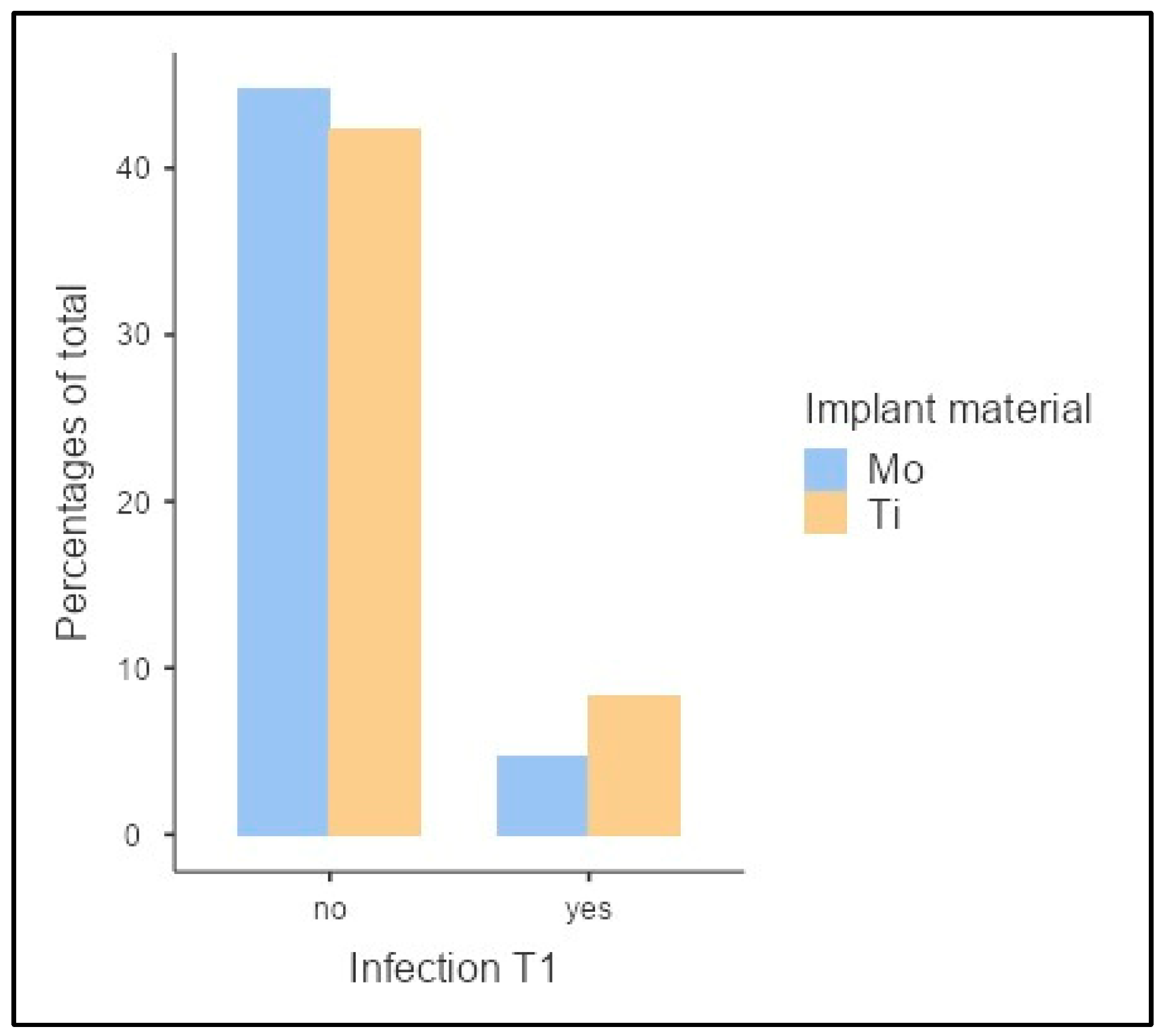
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haerle, F.; Champy, M. Atlas of Craniomaxillofacial Osteosynthesis: Microplates, Miniplates, and Screws; Thieme: New York, NY, USA, 2011. [Google Scholar]
- Yan, H.; Abel, T.J.; Alotaibi, N.M.; Anderson, M.; Niazi, T.N.; Weil, A.G.; Fallah, A.; Phillips, J.H.; Forrest, C.R.; Kulkarni, A.V.; et al. A systematic review and meta-analysis of endoscopic versus open treatment of craniosynostosis. Part 1: The sagittal suture. J. Neurosurg. Pediatr. 2018, 22, 352–360. [Google Scholar] [CrossRef] [PubMed]
- Yan, H.; Abel, T.J.; Alotaibi, N.M.; Anderson, M.; Niazi, T.N.; Weil, A.G.; Fallah, A.; Phillips, J.H.; Forrest, C.R.; Kulkarni, A.V.; et al. A systematic review of endoscopic versus open treatment of craniosynostosis. Part 2: The nonsagittal single sutures. J. Neurosurg. Pediatr. 2018, 22, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Gareb, B.; Roossien, C.C.; van Bakelen, N.B.; Verkerke, G.J.; Vissink, A.; Bos, R.R.; van Minnen, B. Comparison of the mechanical properties of biodegradable and titanium osteosynthesis systems used in oral and maxillofacial surgery. Sci. Rep. 2020, 10, 18143. [Google Scholar] [CrossRef] [PubMed]
- Barber, C.C.; Burnham, M.; Ojameruaye, O.; McKee, M.D. A systematic review of the use of titanium versus stainless steel implants for fracture fixation. OTA Int. 2021, 4, e138. [Google Scholar] [CrossRef]
- Comino-Garayoa, R.; Cortés-Bretón Brinkmann, J.; Peláez, J.; López-Suárez, C.; Martínez-González, J.M.; Suárez, M.J. Allergies to Titanium Dental Implants: What Do We Really Know about Them? A Scoping Review. Biology 2020, 9, 404. [Google Scholar] [CrossRef]
- Case, C.; Langkamer, V.; James, C.; Palmer; Kemp, A.; Heap, P.; Solomon, L. Widespread dissemination of metal debris from implants. J. Bone Jt. Surg. 1994, 76-B, 701–712. [Google Scholar] [CrossRef]
- Buijs, G.J.; Stegenga, B.; Bos, R. Efficacy and safety of biodegradable osteofixation devices in oral and maxillofacial surgery: A systematic review. J. Dent. Res. 2006, 85, 980–989. [Google Scholar] [CrossRef]
- Gareb, B.; Van Bakelen, N.; Buijs, G.; Jansma, J.; De Visscher, J.; Hoppenreijs, T.J.; Bergsma, J.; van Minnen, B.; Stegenga, B.; Bos, R. Comparison of the long-term clinical performance of a biodegradable and a titanium fixation system in maxillofacial surgery: A multicenter randomized controlled trial. PLoS ONE 2017, 12, e0177152. [Google Scholar] [CrossRef]
- Kanno, T.; Sukegawa, S.; Furuki, Y.; Nariai, Y.; Sekine, J. Overview of innovative advances in bioresorbable plate systems for oral and maxillofacial surgery. Jpn. Dent. Sci. Rev. 2018, 54, 127–138. [Google Scholar] [CrossRef]
- Erdmann, N.; Bondarenko, A.; Hewicker-Trautwein, M.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Meyer-Lindenberg, A. Evaluation of the soft tissue biocompatibility of MgCa0.8 and surgical steel 316L in vivo: A comparative study in rabbits. Biomed. Eng. Online 2010, 9, 63. [Google Scholar] [CrossRef]
- Waizy, H.; Diekmann, J.; Weizbauer, A.; Reifenrath, J.; Bartsch, I.; Neubert, V.; Schavan, R.; Windhagen, H. In vivo study of a biodegradable orthopedic screw (MgYREZr-alloy) in a rabbit model for up to 12 months. J. Biomater. Appl. 2014, 28, 667–675. [Google Scholar] [CrossRef] [PubMed]
- Erdmann, N.; Angrisani, N.; Reifenrath, J.; Lucas, A.; Thorey, F.; Bormann, D.; Meyer-Lindenberg, A. Biomechanical testing and degradation analysis of MgCa0. 8 alloy screws: A comparative in vivo study in rabbits. Acta Biomater. 2011, 7, 1421–1428. [Google Scholar] [CrossRef] [PubMed]
- May, H.; Alper Kati, Y.; Gumussuyu, G.; Yunus Emre, T.; Unal, M.; Kose, O. Bioabsorbable magnesium screw versus conventional titanium screw fixation for medial malleolar fractures. J. Orthop. Traumatol. 2020, 21, 9. [Google Scholar] [CrossRef] [PubMed]
- Biber, R.; Pauser, J.; Brem, M.; Bail, H.J. Bioabsorbable metal screws in traumatology: A promising innovation. Trauma Case Rep. 2017, 8, 11–15. [Google Scholar] [CrossRef]
- Waizy, H.; Seitz, J.-M.; Reifenrath, J.; Weizbauer, A.; Bach, F.-W.; Meyer-Lindenberg, A.; Denkena, B.; Windhagen, H. Biodegradable magnesium implants for orthopedic applications. J. Mater. Sci. 2013, 48, 39–50. [Google Scholar] [CrossRef]
- Plaass, C.; Ettinger, S.; Sonnow, L.; Koenneker, S.; Noll, Y.; Weizbauer, A.; Reifenrath, J.; Claassen, L.; Daniilidis, K.; Stukenborg-Colsman, C.; et al. Early results using a biodegradable magnesium screw for modified chevron osteotomies. J. Orthop. Res. 2016, 34, 2207–2214. [Google Scholar] [CrossRef]
- Zheng, Y.F.; Gu, X.N.; Witte, F. Biodegradable metals. Mater. Sci. Eng. R Rep. 2014, 77, 1–34. [Google Scholar] [CrossRef]
- Eppley, B.L.; Morales, L.; Wood, R.; Pensler, J.; Goldstein, J.; Havlik, R.J.; Habal, M.; Losken, A.; Williams, J.K.; Burstein, F.; et al. Resorbable PLLA-PGA plate and screw fixation in pediatric craniofacial surgery: Clinical experience in 1883 patients. Plast. Reconstr. Surg. 2004, 114, 850–856; discussion 857. [Google Scholar] [CrossRef]
- Bos, R.R.; Boering, G.; Rozema, F.R.; Leenslag, J.W. Resorbable poly(L-lactide) plates and screws for the fixation of zygomatic fractures. J. Oral Maxillofac. Surg. 1987, 45, 751–753. [Google Scholar] [CrossRef]
- Wood, R.J.; Petronio, J.A.; Graupman, P.C.; Shell, C.D.; Gear, A.J.L. New Resorbable Plate and Screw System in Pediatric Craniofacial Surgery. J. Craniofacial Surg. 2012, 23, 845–849. [Google Scholar] [CrossRef]
- Eppley, B.L. Zygomaticomaxillary fracture repair with resorbable plates and screws. J. Craniofacial Surg. 2000, 11, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, J.E.; De Bruijn, W.; Rozema, F.; Bos, R.; Boering, G. Late degradation tissue response to poly(L-lactide) bone plates and screws. Biomaterials 1995, 16, 25–31. [Google Scholar] [CrossRef]
- Schwarz, G.; Mendel, R.R.; Ribbe, M.W. Molybdenum cofactors, enzymes and pathways. Nature 2009, 460, 839–847. [Google Scholar] [CrossRef] [PubMed]
- Bojinov, M.; Betova, I.; Raicheff, R. A model for the transpassivity of molybdenum in acidic sulphate solutions based on ac impedance measurements. Electrochim. Acta 1996, 41, 1173–1179. [Google Scholar] [CrossRef]
- Redlich, C.; Quadbeck, P.; Thieme, M.; Kieback, B. Molybdenum—A biodegradable implant material for structural applications? Acta Biomater. 2020, 104, 241–251. [Google Scholar] [CrossRef]
- Sikora-Jasinska, M.; Morath, L.M.; Kwesiga, M.P.; Plank, M.E.; Nelson, A.L.; Oliver, A.A.; Bocks, M.L.; Guillory, R.J.; Goldman, J. In-vivo evaluation of molybdenum as bioabsorbable stent candidate. Bioact. Mater. 2022, 14, 262–271. [Google Scholar] [CrossRef]
- Toschka, A.; Pöhle, G.; Quadbeck, P.; Suschek, C.V.; Strauß, A.; Redlich, C.; Rana, M. Molybdenum as a Potential Biocompatible and Resorbable Material for Osteosynthesis in Craniomaxillofacial Surgery—An In Vitro Study. Int. J. Mol. Sci. 2022, 23, 15710. [Google Scholar]
- Murray, F.J.; Sullivan, F.M.; Tiwary, A.K.; Carey, S. 90-Day subchronic toxicity study of sodium molybdate dihydrate in rats. Regul. Toxicol. Pharmacol. 2014, 70, 579–588. [Google Scholar] [CrossRef]
- Bompart, G.; Pécher, C.; Prévot, D.; Girolami, J.P. Mild renal failure induced by subchronic exposure to molybdenum: Urinary kallikrein excretion as a marker of distal tubular effect. Toxicol. Lett. 1990, 52, 293–300. [Google Scholar] [CrossRef]
- Krenn, V.; Morawietz, L.; Burmester, G.R.; Kinne, R.W.; Mueller-Ladner, U.; Muller, B.; Haupl, T. Synovitis score: Discrimination between chronic low-grade and high-grade synovitis. Histopathology 2006, 49, 358–364. [Google Scholar] [CrossRef]
- Goodman, S. A dirty dozen: Twelve p-value misconceptions. Semin. Hematol. 2008, 45, 135–140. [Google Scholar] [CrossRef] [PubMed]
- Greenland, S.; Senn, S.J.; Rothman, K.J.; Carlin, J.B.; Poole, C.; Goodman, S.N.; Altman, D.G. Statistical tests, P values, confidence intervals, and power: A guide to misinterpretations. Eur. J. Epidemiol. 2016, 31, 337–350. [Google Scholar] [CrossRef]
- Braun, J.; Kaserer, L.; Stajkovic, J.; Leitz, K.H.; Tabernig, B.; Singer, P.; Leibenguth, P.; Gspan, C.; Kestler, H.; Leichtfried, G. Molybdenum and tungsten manufactured by selective laser melting: Analysis of defect structure and solidification mechanisms. Int. J. Refract. Met. Hard Mater. 2019, 84, 104999. [Google Scholar] [CrossRef]
- Turnlund, J.R.; Weaver, C.M.; Kim, S.K.; Keyes, W.R.; Gizaw, Y.; Thompson, K.H.; Peiffer, G.L. Molybdenum absorption and utilization in humans from soy and kale intrinsically labeled with stable isotopes of molybdenum. Am. J. Clin. Nutr. 1999, 69, 1217–1223. [Google Scholar] [CrossRef] [PubMed]
- Schauer, A.; Redlich, C.; Scheibler, J.; Poehle, G.; Barthel, P.; Maennel, A.; Adams, V.; Weissgaerber, T.; Linke, A.; Quadbeck, P. Biocompatibility and Degradation Behavior of Molybdenum in an In Vivo Rat Model. Materials 2021, 14, 7776. [Google Scholar] [CrossRef] [PubMed]
- Petrova, M.; Bojinov, M.; Zanna, S.; Marcus, P. Mechanism of anodic oxidation of molybdenum in nearly-neutral electrolytes studied by electrochemical impedance spectroscopy and X-ray photoelectron spectroscopy. Electrochim. Acta 2011, 56, 7899–7906. [Google Scholar] [CrossRef]
- Jin, Z.; Hu, J.; Ma, D. Postoperative delirium: Perioperative assessment, risk reduction, and management. Br. J. Anaesth. 2020, 125, 492–504. [Google Scholar] [CrossRef]
- Johnson, J.W.; Chi, C.H.; Chen, C.K.; James, W.J. The Anodic Dissolution of Molybdenum. Corrosion 2013, 26, 338–342. [Google Scholar] [CrossRef]
- Pourbaix, M. Applications of electrochemistry in corrosion science and in practice. Corros. Sci. 1974, 14, 25–82. [Google Scholar] [CrossRef]

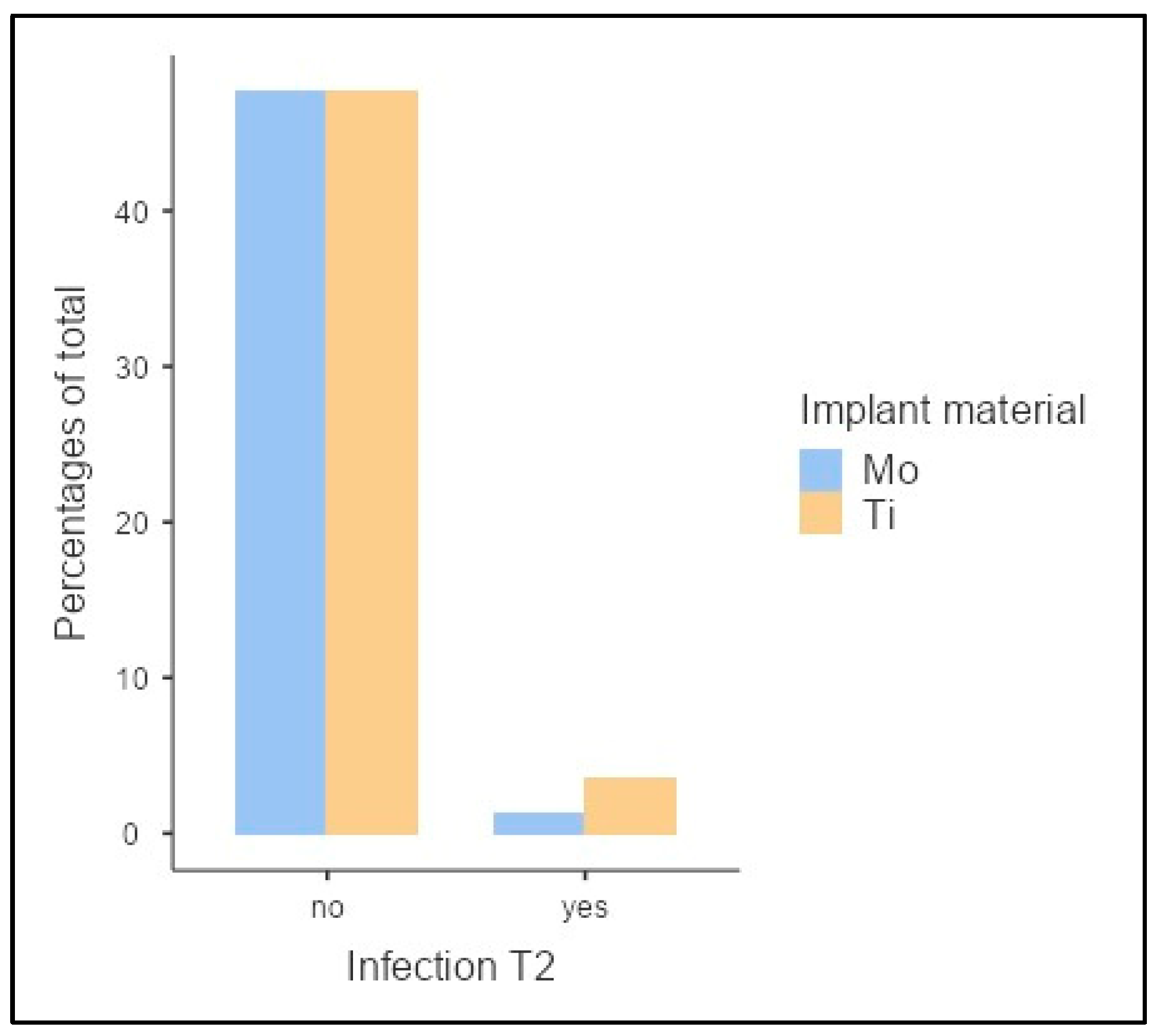
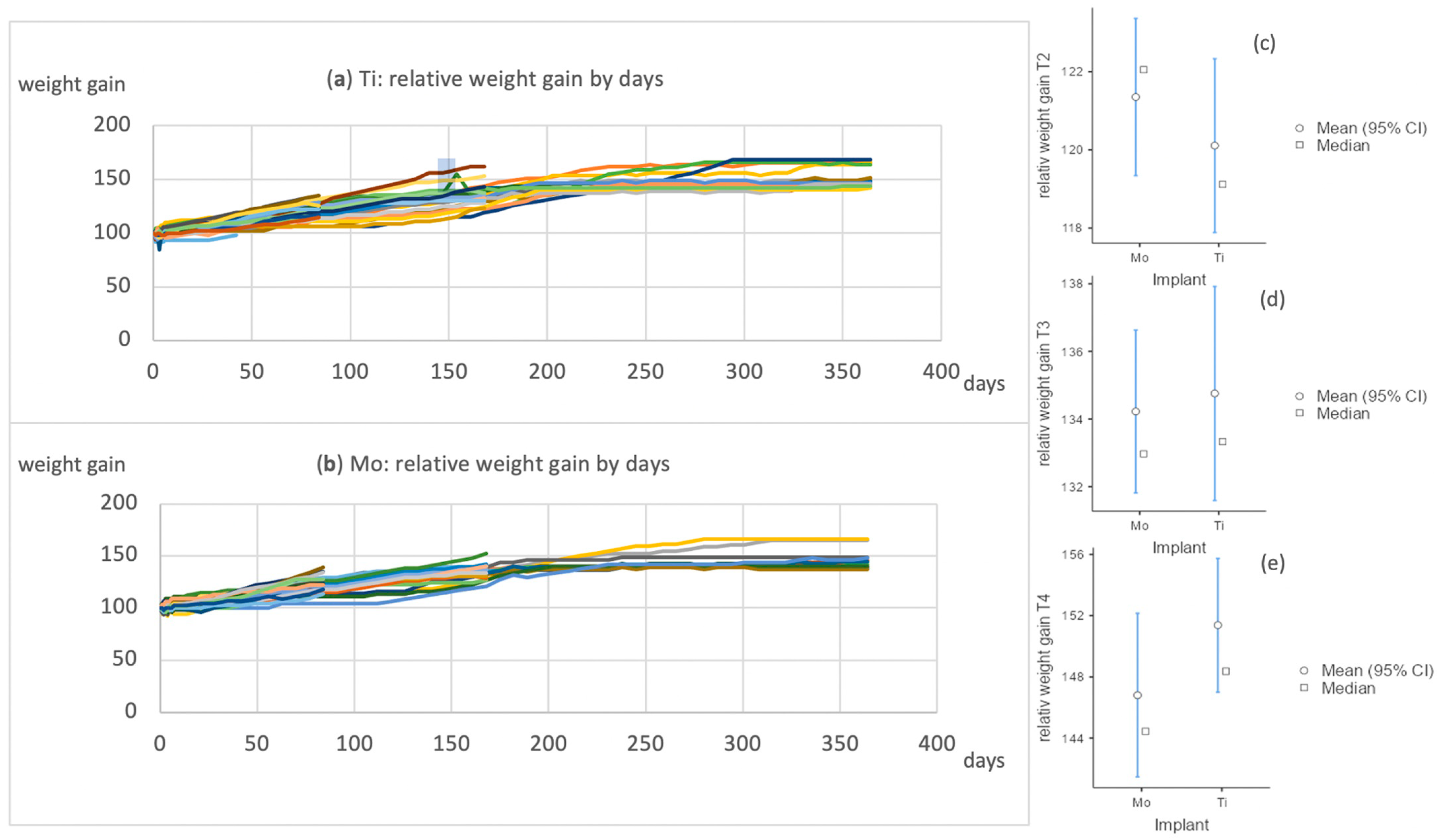

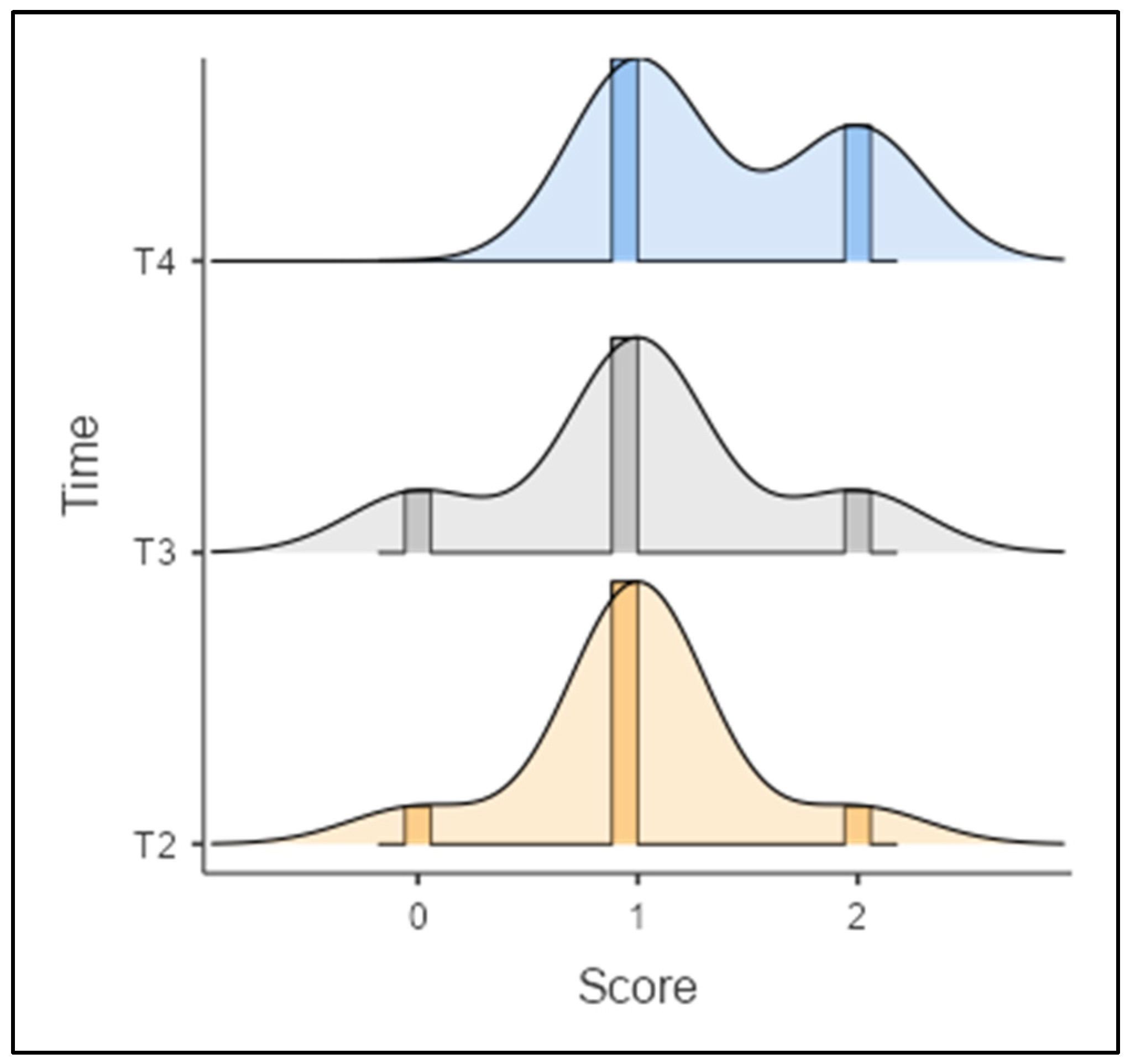

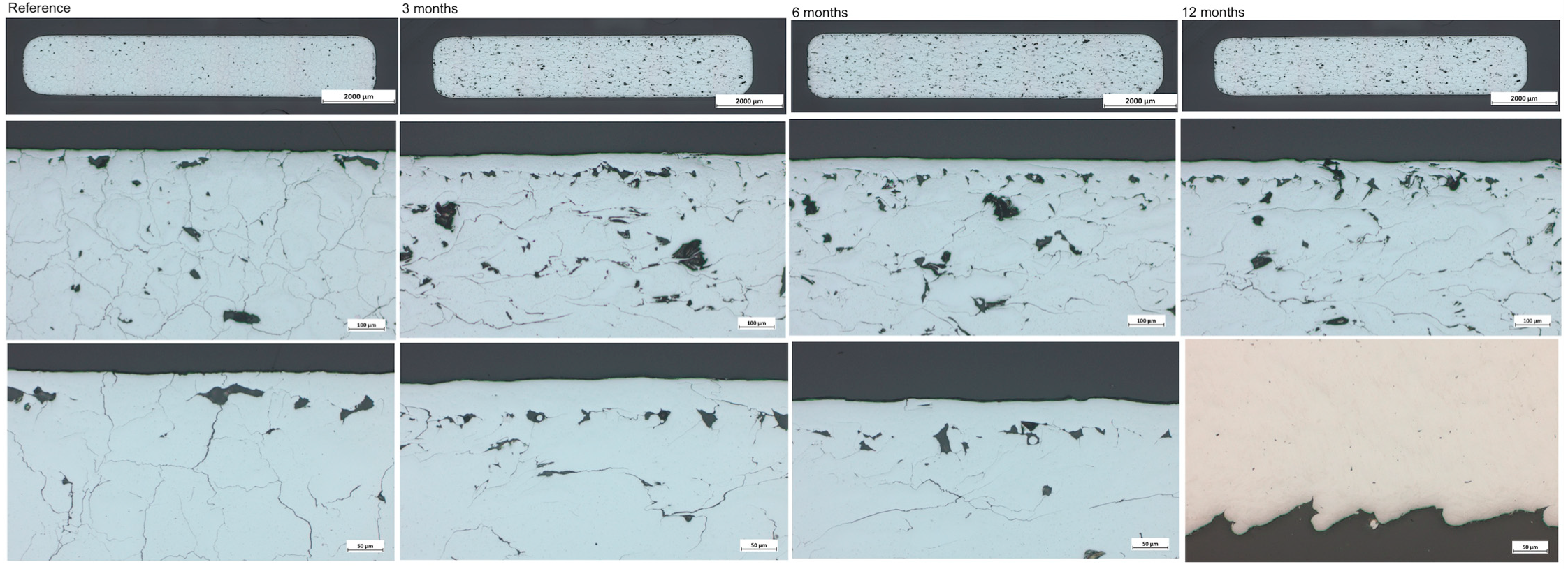
| Score | Total (n = 30) | Ti (n = 17) | Mo (n = 13) | ||||
|---|---|---|---|---|---|---|---|
| Synovial covering of the cell layer | n | % | n | % | n | % | |
| 0 | 2 | 6.57 | 0 | 0.0 | 2 | 15.4 | |
| 1 | 13 | 43.3 | 7 | 41.2 | 6 | 46.2 | |
| 2 | 9 | 30.0 | 7 | 41.2 | 2 | 15.4 | |
| 3 | 6 | 20.0 | 3 | 17.6 | 3 | 23.1 | |
| Cell density of the synovial stroma | n | % | n | % | n | % | |
| 0 | 8 | 26.7 | 4 | 23.5 | 4 | 30.8 | |
| 1 | 17 | 56.7 | 11 | 64.7 | 6 | 46.2 | |
| 2 | 4 | 13.3 | 1 | 5.9 | 3 | 23.1 | |
| 3 | 1 | 3.3 | 1 | 5.9 | 0 | 0.0 | |
| Leukocytic infiltrate | n | % | n | % | n | % | |
| 0 | 3 | 10.0 | 1 | 5.9 | 2 | 15.4 | |
| 1 | 19 | 63.3 | 14 | 82.4 | 5 | 38.5 | |
| 2 | 8 | 26.7 | 2 | 11.8 | 6 | 46.2 | |
| Krenn score | n | % | n | % | n | % | |
| 0 | 3 | 10.0 | 1 | 5.9 | 2 | 15.4 | |
| 1 | 20 | 66.7 | 13 | 76.5 | 7 | 53.8 | |
| 2 | 7 | 23.3 | 3 | 17.6 | 4 | 30.8 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toschka, A.; Möllmann, H.; Hoppe, D.; Poehle, G.; van Meenen, L.; Seidl, M.; Karnatz, N.; Rana, M. How Does the Biocompatibility of Molybdenum Compare to the Gold Standard Titanium?—An In Vivo Rat Model. Appl. Sci. 2023, 13, 6312. https://doi.org/10.3390/app13106312
Toschka A, Möllmann H, Hoppe D, Poehle G, van Meenen L, Seidl M, Karnatz N, Rana M. How Does the Biocompatibility of Molybdenum Compare to the Gold Standard Titanium?—An In Vivo Rat Model. Applied Sciences. 2023; 13(10):6312. https://doi.org/10.3390/app13106312
Chicago/Turabian StyleToschka, André, Henriette Möllmann, Dominik Hoppe, Georg Poehle, Lutz van Meenen, Maximilian Seidl, Nadia Karnatz, and Majeed Rana. 2023. "How Does the Biocompatibility of Molybdenum Compare to the Gold Standard Titanium?—An In Vivo Rat Model" Applied Sciences 13, no. 10: 6312. https://doi.org/10.3390/app13106312
APA StyleToschka, A., Möllmann, H., Hoppe, D., Poehle, G., van Meenen, L., Seidl, M., Karnatz, N., & Rana, M. (2023). How Does the Biocompatibility of Molybdenum Compare to the Gold Standard Titanium?—An In Vivo Rat Model. Applied Sciences, 13(10), 6312. https://doi.org/10.3390/app13106312







