Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical
2.2. Preparation of A. agallocha and A. malaccensis Extract
2.3. Preparation of Overall Phenolic and Flavonoid Content
2.4. Determination of DPPH Radical Scavenging Activity
2.5. Reducing Power Assay
2.6. In Silico Biological Activity Prediction and ADMET Analysis
2.7. Molecular Docking
2.8. Molecular Dynamics Simulations
2.9. Cell Culture
2.10. In Vitro Cytotoxicity of Aquilaria agallocha and Aquilaria malaccensis
2.11. Evaluation of ROS Generation
2.12. Measurement of Nitrite Levels
2.13. Wound-Healing Assay
2.14. Gene Expression Analysis
2.15. Measurement of Antioxidant Enzyme Activity
2.16. Measurement of SOD Activity
2.17. Measurement of GPx Activity
2.18. Measurement of CAT Activity
2.19. Measurement of GST Activity
3. Results and Discussion
3.1. TPC, TFC, and Antioxidant Activities
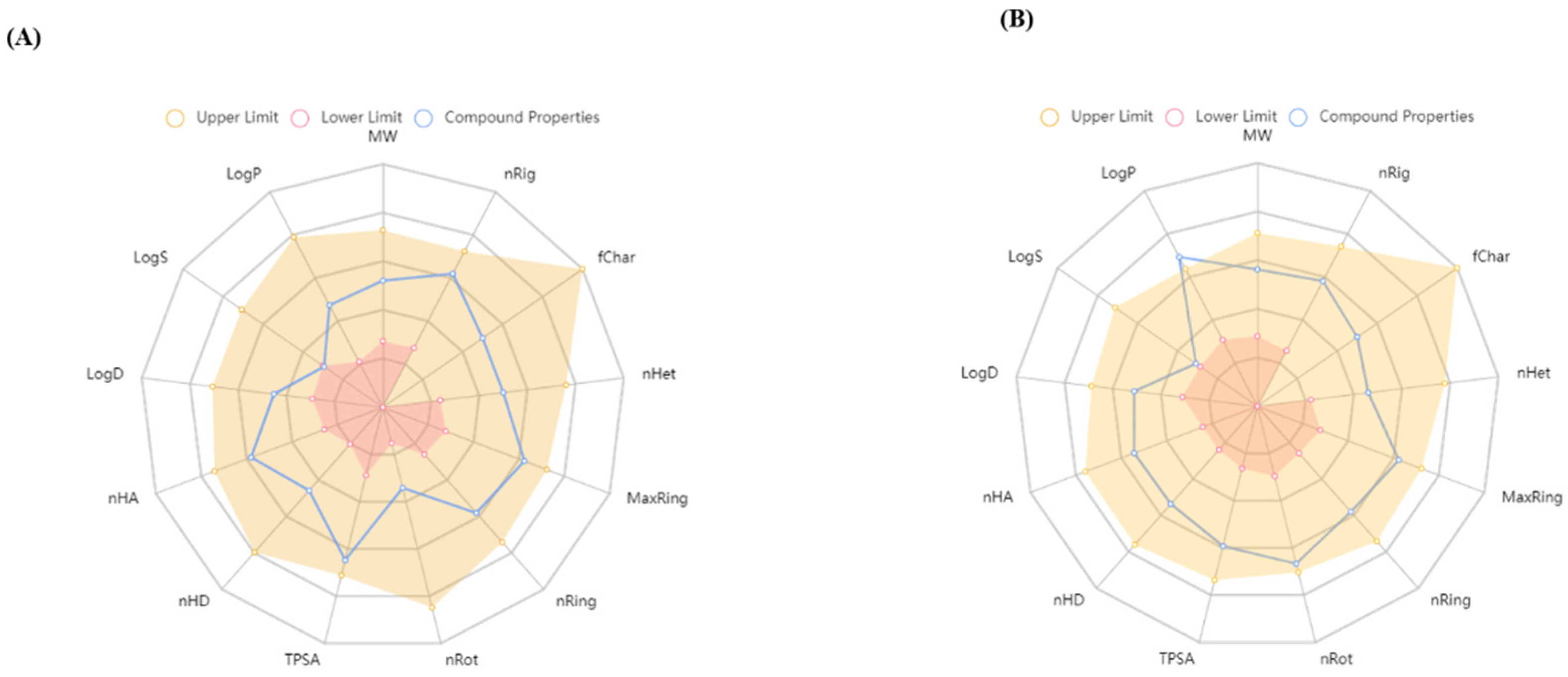
3.1.1. Biological Activity Prediction and ADMET Analysis
3.1.2. Molecular Docking Interaction
3.1.3. Analysis of Molecular Dynamics
3.2. Evaluation of Cytotoxicity
3.2.1. In Vitro ROS Induced by A. agallocha and A. malaccensis on Cancer Cells
3.2.2. A. agallocha and A. malaccensis Extract Increased NO Production and Inhibited ROS Generation Induced by LPS
3.2.3. Cancer Cells Migration Is Inhibited by A. agallocha and A. malaccensis
3.2.4. By Controlling Apoptotic Gene Expression, A. agallocha and A. malaccensis-Induced Apoptosis
3.2.5. A. agallocha and A. malaccensis Extract Inhibited the Increased Levels of Inflammation-Related Cytokines
3.3. Antioxidant Enzymatic Activity
Enhancing Antioxidative Gene Expression on Hacat Cells
4. Conclusions and Future Perspective
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Woldesellassie, M.; Eyasu, M.; Kelbessa, U. In vivo anti-inflammatory activities of leaf extracts of Ocimum lamiifolium in mice model. J. Ethnopharmacol. 2011, 134, 32–36. [Google Scholar] [CrossRef]
- Rasheed, H. Studies of Oxygenation-1 (cox-1) and Oxygenation (cox-2) Inhibitors with Analgesic, Anti-Inflammatory and Anti-platelet Activities. Ph. D. Thesis, University of Karachi Pakistan, Karachi, Pakistan, 2003. [Google Scholar]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Miller, K.D.; Nogueira, L.; Mariotto, A.B.; Rowland, J.H.; Yabroff, K.R.; Alfano, C.M.; Jemal, A.; Kramer, J.L.; Siegel, R.L. Cancer treatment and survivorship statistics, 2019. CA Cancer J. Clin. 2019, 69, 363–385. [Google Scholar] [CrossRef]
- Ettinger, D.S.; Wood, D.E.; Aggarwal, C.; Aisner, D.L.; Akerley, W.; Bauman, J.R.; Bharat, A.; Bruno, D.S.; Chang, J.Y.; Chirieac, L.R. NCCN guidelines insights: Non–small cell lung cancer, version 1.2020: Featured updates to the NCCN guidelines. JNCCN J. Natl. Compr. Cancer Netw. 2019, 17, 1464–1472. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef] [PubMed]
- Dixit, S.; Ali, H. Anticancer activity of medicinal plant extract-a review. J. Chem. Chem. Sci. 2010, 1, 79–85. [Google Scholar]
- Azab, A.; Nassar, A.; Azab, A.N. Anti-inflammatory activity of natural products. Molecules 2016, 21, 1321. [Google Scholar] [CrossRef] [PubMed]
- International Expert Meeting on the Treatment of SbTCM; The Integration of Traditional Chinese Medicine with Western Medicine. SARS: Clinical trials on treatment using a combination of traditional Chinese medicine and Western medicine. In Proceedings of the Report of the WHO International Expert Meeting to Review and Analyse Clinical Reports on Combination Treatment for SARS, Beijing, China, 8–10 October 2003.
- Millaty, I.; Wijayanti, N.; Hidayati, L.; Nuringtyas, T. Identification of anticancer compounds in leaves extracts of agarwood (Aquilaria malaccensis (Lamk.)). In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2020; p. 012036. [Google Scholar]
- Tilburt, J.C.; Kaptchuk, T.J. Herbal medicine research and global health: An ethical analysis. Bull. World Health Organ. 2008, 86, 594–599. [Google Scholar] [CrossRef]
- World Health Organization. Programme on Traditional, M. In WHO Traditional Medicine Strategy 2002–2005; World Health Organizatio: Geneva, Switzerland, 2002. [Google Scholar]
- Tan, C.S.; Isa, N.M.; Ismail, I.; Zainal, Z. Agarwood induction: Current developments and future perspectives. Front. Plant Sci. 2019, 10, 122. [Google Scholar] [CrossRef]
- Wang, S.; Yu, Z.; Wang, C.; Wu, C.; Guo, P.; Wei, J. Chemical constituents and pharmacological activity of agarwood and Aquilaria plants. Molecules 2018, 23, 342. [Google Scholar] [CrossRef]
- Lee, S.Y.; Mohamed, R. The origin and domestication of Aquilaria, an important agarwood-producing genus. In Agarwood: Science Behind the Fragrance; Springer: Berlin/Heidelberg, Germany, 2016; pp. 1–20. [Google Scholar]
- Persoon, G.A.; van Beek, H.H. Growing ‘the wood of the gods’: Agarwood production in southeast Asia. In Smallholder Tree Growing for Rural Development and Environmental Services; Spinger: Berlin/Heidelberg, Germany, 2008; pp. 245–262. [Google Scholar]
- Eissa, M.A.; Hashim, Y.Z.-Y.; Salleh, H.M.; Abd-Azziz, S.S.; Isa, M.L.M.; Abd Warif, N.M.; Nor, Y.A.; El-Kersh, D.M.; Sani, S.A. Aquilaria species as potential anti-inflammatory agents—A review on in vitro and in vivo studies. Indian J. Nat. Prod. Resour. 2020, 11, 141–154. [Google Scholar]
- Zulkifle, N. Antidiabetic Activity of Aquilaria malaccensis (Agarwood) Leaves Extracts; University Malaysia Pahang Gambang: Pahang, Malaysia, 2018. [Google Scholar]
- Chitre, T.; Bhutada, P.; Nandakumar, K.; Somani, R.; Miniyar, P.; Mundhada, Y.; Gore, S.; Jain, K.J.P.O. Analgesic and anti-inflammatory activity of heartwood of Aquilaria agallocha in laboratory animals. Pharmacologyonline 2007, 1, 288–298. [Google Scholar]
- Yadav, D.K.; Mudgal, V.; Agrawal, J.; Maurya, A.K.; Bawankule, D.U.; Chanotiya, C.S.; Khan, F.; Thul, S.T. Molecular docking and ADME studies of natural compounds of Agarwood oil for topical anti-inflammatory activity. Curr. Comput.-Aided Drug Des. 2013, 9, 360–370. [Google Scholar] [CrossRef]
- Jin, Y.; Huynh, D.T.N.; Myung, C.-S.; Heo, K.-S. Ginsenoside Rh1 Prevents Migration and Invasion through Mitochondrial ROS-Mediated Inhibition of STAT3/NF-κB Signaling in MDA-MB-231 Cells. Int. J. Mol. Sci. 2021, 22, 10458. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Karakhanova, S.; Hartwig, W.; D’Haese, J.G.; Philippov, P.P.; Werner, J.; Bazhin, A.V. Mitochondria and mitochondrial ROS in cancer: Novel targets for anticancer therapy. J. Cell. Physiol. 2016, 231, 2570–2581. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Qi, H.; Liu, Y.; Duan, C.; Liu, X.; Xia, T.; Chen, D.; Piao, H.-L.; Liu, H.-X. The double-edged roles of ROS in cancer prevention and therapy. Theranostics 2021, 11, 4839. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Mazumdar, M.; Mukherjee, S.; Bhattacharjee, P.; Adhikary, A.; Manna, A.; Chakraborty, S.; Khan, P.; Sen, A.; Das, T. Restoration of p53/miR-34a regulatory axis decreases survival advantage and ensures Bax-dependent apoptosis of non-small cell lung carcinoma cells. FEBS Lett. 2014, 588, 549–559. [Google Scholar] [CrossRef]
- Lu, H.-F.; Chie, Y.-J.; Yang, M.-S.; Lee, C.-S.; Fu, J.-J.; Yang, J.-S.; Tan, T.-W.; Wu, S.-H.; Ma, Y.-S.; Ip, S.-W. Apigenin induces caspase-dependent apoptosis in human lung cancer A549 cells through Bax-and Bcl-2-triggered mitochondrial pathway. Int. J. Oncol. 2010, 36, 1477–1484. [Google Scholar]
- Wang, J.-P.; Hsieh, C.-H.; Liu, C.-Y.; Lin, K.-H.; Wu, P.-T.; Chen, K.-M.; Fang, K. Reactive oxygen species-driven mitochondrial injury induces apoptosis by teroxirone in human non-small cell lung cancer cells. Oncol. Lett. 2017, 14, 3503–3509. [Google Scholar] [CrossRef]
- Piao, M.J.; Yoo, E.S.; Koh, Y.S.; Kang, H.K.; Kim, J.; Kim, Y.J.; Kang, H.H.; Hyun, J.W. Antioxidant effects of the ethanol extract from flower of Camellia japonica via scavenging of reactive oxygen species and induction of antioxidant enzymes. Int. J. Mol. Sci. 2011, 12, 2618–2630. [Google Scholar] [CrossRef]
- Vertuani, S.; Angusti, A.; Manfredini, S. The antioxidants and pro-antioxidants network: An overview. Curr. Pharm. Des. 2004, 10, 1677–1694. [Google Scholar] [CrossRef] [PubMed]
- Juráňová, J.; Franková, J.; Ulrichová, J. The role of keratinocytes in inflammation. J. Appl. Biomed. 2017, 15, 169–179. [Google Scholar] [CrossRef]
- Hu, T.-Y.; Ju, J.-M.; Mo, L.-H.; Ma, L.; Hu, W.-H.; You, R.-R.; Chen, X.-Q.; Chen, Y.-Y.; Liu, Z.-Q.; Qiu, S.-Q. Anti-inflammation action of xanthones from Swertia chirayita by regulating COX-2/NF-κB/MAPKs/Akt signaling pathways in RAW 264.7 macrophage cells. Phytomedicine 2019, 55, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.M.-D.; Fu, L.; Cheng, C.C.; Gao, R.; Lin, M.Y.; Su, H.L.; Belinda, N.E.; Nguyen, T.H.; Lin, W.-H.; Lee, P.C. Inhibition of LPS-induced oxidative damages and potential anti-inflammatory effects of Phyllanthus emblica extract via down-regulating NF-κB, COX-2, and iNOS in RAW 264.7 cells. Antioxidants 2019, 8, 270. [Google Scholar] [CrossRef]
- Ali, M.B.; Hahn, E.J.; Paek, K.-Y. CO2-induced total phenolics in suspension cultures of Panax ginseng CA Mayer roots: Role of antioxidants and enzymes. Plant Physiol. Biochem. 2005, 43, 449–457. [Google Scholar] [CrossRef]
- Tristantini, D.; Amalia, R. Quercetin concentration and total flavonoid content of anti-atherosclerotic herbs using aluminum chloride colorimetric assay. In Proceedings of the AIP Conference Proceedings; AIP Publishing LLC: College Park, MD, USA, 2019; p. 030012. [Google Scholar]
- Su, Y.; Li, L. Structural characterization and antioxidant activity of polysaccharide from four auriculariales. Carbohydr. Polym. 2020, 229, 115407. [Google Scholar] [CrossRef]
- Bhalodia, N.R.; Nariya, P.B.; Acharya, R.; Shukla, V. In vitro antioxidant activity of hydro alcoholic extract from the fruit pulp of Cassia fistula Linn. Ayu 2013, 34, 209. [Google Scholar] [CrossRef]
- Singh, D.; Gawande, D.Y.; Singh, T.; Poroikov, V.; Goel, R.K. Revealing pharmacodynamics of medicinal plants using in silico approach: A case study with wet lab validation. Comput. Biol. Med. 2014, 47, 1–6. [Google Scholar] [CrossRef]
- Xiong, G.; Wu, Z.; Yi, J.; Fu, L.; Yang, Z.; Hsieh, C.; Yin, M.; Zeng, X.; Wu, C.; Lu, A. ADMETlab 2.0: An integrated online platform for accurate and comprehensive predictions of ADMET properties. Nucleic Acids Res. 2021, 49, W5–W14. [Google Scholar] [CrossRef]
- Souers, A.J.; Leverson, J.D.; Boghaert, E.R.; Ackler, S.L.; Catron, N.D.; Chen, J.; Dayton, B.D.; Ding, H.; Enschede, S.H.; Fairbrother, W.J. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat. Med. 2013, 19, 202–208. [Google Scholar] [CrossRef]
- Vainer, R.; Cohen, S.; Shahar, A.; Zarivach, R.; Arbely, E. Structural basis for p53 Lys120-acetylation-dependent DNA-binding mode. J. Mol. Biol. 2016, 428, 3013–3025. [Google Scholar] [CrossRef] [PubMed]
- Garner, T.P.; Reyna, D.E.; Priyadarshi, A.; Chen, H.-C.; Li, S.; Wu, Y.; Ganesan, Y.T.; Malashkevich, V.N.; Cheng, E.H.; Gavathiotis, E. An autoinhibited dimeric form of BAX regulates the BAX activation pathway. Mol. Cell 2016, 63, 485–497. [Google Scholar] [CrossRef] [PubMed]
- Maciag, J.J.; Mackenzie, S.H.; Tucker, M.B.; Schipper, J.L.; Swartz, P.; Clark, A.C. Tunable allosteric library of caspase-3 identifies coupling between conserved water molecules and conformational selection. Proc. Natl. Acad. Sci. USA 2016, 113, E6080–E6088. [Google Scholar] [CrossRef]
- Chao, Y.; Shiozaki, E.N.; Srinivasula, S.M.; Rigotti, D.J.; Fairman, R.; Shi, Y. Engineering a dimeric caspase-9: A re-evaluation of the induced proximity model for caspase activation. PLoS Biol. 2005, 3, e183. [Google Scholar] [CrossRef]
- Cosconati, S.; Forli, S.; Perryman, A.L.; Harris, R.; Goodsell, D.S.; Olson, A.J. Virtual screening with AutoDock: Theory and practice. Expert Opin. Drug Discov. 2010, 5, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar]
- Karpagam, V.; Sathishkumar, N.; Sathiyamoorthy, S.; Rasappan, P.; Shila, S.; Kim, Y.-J.; Yang, D.-C. Identification of BACE1 inhibitors from Panax ginseng saponins—An Insilco approach. Comput. Biol. Med. 2013, 43, 1037–1044. [Google Scholar] [CrossRef]
- Shrestha, S.; Natarajan, S.; Park, J.-H.; Lee, D.-Y.; Cho, J.-G.; Kim, G.-S.; Jeon, Y.-J.; Yeon, S.-W.; Yang, D.-C.; Baek, N.-I. Potential neuroprotective flavonoid-based inhibitors of CDK5/p25 from Rhus parviflora. Bioorg. Med. Chem. Lett. 2013, 23, 5150–5154. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Schüttelkopf, A.W.; Van Aalten, D.M.F. PRODRG: A tool for high-throughput crystallography of protein-ligand complexes. Acta Crystallogr. Sect. D Biol. Crystallogr. 2004, 60, 1355–1363. [Google Scholar] [CrossRef]
- Ljungh, A.; Moran, A.P.; Wadström, T. Interactions of bacterial adhesins with extracellular matrix and plasma proteins: Pathogenic implications and therapeutic possibilities. FEMS Immunol. Med. Microbiol. 1996, 16, 117–126. [Google Scholar] [CrossRef]
- Christen, M.; Hünenberger, P.H.; Bakowies, D.; Baron, R.; Bürgi, R.; Geerke, D.P.; Heinz, T.N.; Kastenholz, M.A.; Kräutler, V.; Oostenbrink, C.; et al. The GROMOS software for biomolecular simulation: GROMOS05. J. Comput. Chem. 2005, 26, 1719–1751. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, N.; Sathiyamoorthy, S.; Ramya, M.; Yang, D.U.; Lee, H.N.; Yang, D.C. Molecular docking studies of anti-apoptotic BCL-2, BCL-XL, and MCL-1 proteins with ginsenosides from Panax ginseng. J. Enzym. Inhib. Med. Chem. 2012, 27, 685–692. [Google Scholar] [CrossRef] [PubMed]
- Bak, M.-J.; Jun, M.; Jeong, W.-S. Antioxidant and hepatoprotective effects of the red ginseng essential oil in H2O2-treated HepG2 cells and CCl4-treated mice. Int. J. Mol. Sci. 2012, 13, 2314–2330. [Google Scholar] [CrossRef]
- Sikdar, S.; Lallemand, B.; Dubois, J. Induction of phase II enzymes glutathione-s-transferase and NADPH: Quinone oxydoreductase 1 with novel sulforaphane derivatives in human keratinocytes: Evaluation of the intracellular GSH level. Pharmacol. Pharm. 2014, 5, 937. [Google Scholar] [CrossRef]
- Saeed, N.; Khan, M.R.; Shabbir, M. Antioxidant activity, total phenolic and total flavonoid contents of whole plant extracts Torilis leptophylla L. BMC Complement. Altern. Med. 2012, 12, 221. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.B.; Shishodia, S. Molecular targets of dietary agents for prevention and therapy of cancer. Biochem. Pharmacol. 2006, 71, 1397–1421. [Google Scholar] [CrossRef]
- Okwu, D.; Ekeke, O. Phytochemical screening and mineral composition of chewing sticks in South Eastern Nigeria. Glob. J. Pure Appl. Sci. 2003, 9, 235–238. [Google Scholar] [CrossRef]
- Petti, S.; Scully, C. Polyphenols, oral health and disease: A review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Poroikov, V.V.; Filimonov, D.A.; Ihlenfeldt, W.D.; Gloriozova, T.A.; Lagunin, A.A.; Borodina, Y.V.; Stepanchikova, A.V.; Nicklaus, M.C. PASS biological activity spectrum predictions in the enhanced open NCI database browser. J. Chem. Inf. Comput. Sci. 2003, 43, 228–236. [Google Scholar] [CrossRef]
- Assaf, A.M.; Haddadin, R.N.; Aldouri, N.A.; Alabbassi, R.; Mashallah, S.; Mohammad, M.; Bustanji, Y.J. Anti-cancer, anti-inflammatory and anti-microbial activities of plant extracts used against hematological tumors in traditional medicine of Jordan. J. Ethnopharmacol. 2013, 145, 728–736. [Google Scholar] [CrossRef] [PubMed]
- Khlifi, D.; Sghaier, R.M.; Amouri, S.; Laouini, D.; Hamdi, M.; Bouajila, J. Composition and anti-oxidant, anti-cancer and anti-inflammatory activities of Artemisia herba-alba, Ruta chalpensis L. and Peganum harmala L. Food Chem. Toxicol. 2013, 55, 202–208. [Google Scholar] [CrossRef] [PubMed]
- Amaral, S.; Mira, L.; Nogueira, J.; da Silva, A.P.; Florêncio, M.H. Plant extracts with anti-inflammatory properties—A new approach for characterization of their bioactive compounds and establishment of structure–antioxidant activity relationships. Food Chem. Toxicol. 2009, 17, 1876–1883. [Google Scholar] [CrossRef]
- Zou, Z.; Chang, H.; Li, H.; Wang, S.J.A. Induction of reactive oxygen species: An emerging approach for cancer therapy. Apoptosis 2017, 22, 1321–1335. [Google Scholar] [CrossRef] [PubMed]
- Rao, P.C.; Begum, S.; Sahai, M.; Sriram, D.S. Coptisine-induced cell cycle arrest at G2/M phase and reactive oxygen species–dependent mitochondria-mediated apoptosis in non-small-cell lung cancer A549 cells. Tumor Biol. 2017, 39, 1010428317694565. [Google Scholar] [CrossRef]
- Zaidieh, T.; Smith, J.R.; Ball, K.E.; An, Q. ROS as a novel indicator to predict anticancer drug efficacy. BMC Cancer 2019, 19, 1224. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-mediated cellular signaling. Oxidative Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in cancer therapy: The bright side of the moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef]
- Nakamura, H.; Takada, K. Reactive oxygen species in cancer: Current findings and future directions. Cancer Sci. 2021, 112, 3945–3952. [Google Scholar] [CrossRef]
- Snezhkina, A.V.; Kudryavtseva, A.V.; Kardymon, O.L.; Savvateeva, M.V.; Melnikova, N.V.; Krasnov, G.S.; Dmitriev, A.A. ROS generation and antioxidant defense systems in normal and malignant cells. Oxidative Med. Cell. Longev. 2019, 2019, 6175804. [Google Scholar] [CrossRef]
- Huynh, D.T.N.; Jin, Y.; Myung, C.-S.; Heo, K.-S. Ginsenoside Rh1 induces MCF-7 cell apoptosis and autophagic cell death through ROS-mediated Akt signaling. Cancers 2021, 13, 1892. [Google Scholar] [CrossRef] [PubMed]
- Zamzami, N.; Marchetti, P.; Castedo, M.; Decaudin, D.; Macho, A.; Hirsch, T.; Susin, S.A.; Petit, P.X.; Mignotte, B.; Kroemer, G. Sequential reduction of mitochondrial transmembrane potential and generation of reactive oxygen species in early programmed cell death. J. Exp. Med. 1995, 182, 367–377. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Chen, X.; Nguyen, T.; Chai, J.; Gao, Y.; Wu, J.; Li, J.; Abdel-Rahman, M.A.; Chen, X.; Xu, X. The Strong Anti-Tumor Effect of Smp24 in Lung Adenocarcinoma A549 Cells Depends on Its Induction of Mitochondrial Dysfunctions and ROS Accumulation. Toxins 2022, 14, 590. [Google Scholar] [CrossRef]
- Dai, C.-Q.; Luo, T.-T.; Luo, S.-C.; Wang, J.-Q.; Wang, S.-M.; Bai, Y.-H.; Yang, Y.-L.; Wang, Y.-Y. p53 and mitochondrial dysfunction: Novel insight of neurodegenerative diseases. J. Bioenerg. Biomembr. 2016, 48, 337–347. [Google Scholar] [CrossRef] [PubMed]
- Knott, A.B.; Bossy-Wetzel, E. Nitric oxide in health and disease of the nervous system. Antioxid. Redox Signal. 2009, 11, 541–553. [Google Scholar] [CrossRef]
- Korhonen, R.; Lahti, A.; Kankaanranta, H.; Moilanen, E. Nitric oxide production and signaling in inflammation. Curr. Drug Targets-Inflamm. Allergy 2005, 4, 471–479. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Liu, X.; Bao, Y.; Wang, X.; Zhai, J.; Zhan, X.; Zhang, H. Characterization and anti-inflammation of a polysaccharide produced by Chaetomium globosum CGMCC 6882 on LPS-induced RAW 264.7 cells. Carbohydr. Polym. 2021, 251, 117129. [Google Scholar] [CrossRef] [PubMed]
- Sahai, E. Mechanisms of cancer cell invasion. Curr. Opin. Genet. Dev. 2005, 15, 87–96. [Google Scholar] [CrossRef]
- Johnson, T.M.; Yu, Z.-X.; Ferrans, V.J.; Lowenstein, R.A.; Finkel, T. Reactive oxygen species are downstream mediators of p53-dependent apoptosis. Proc. Natl. Acad. Sci. USA 1996, 93, 11848–11852. [Google Scholar] [CrossRef]
- Liu, B.; Chen, Y.; Clair, D.K.S. ROS and p53: A versatile partnership. Free Radic. Biol. Med. 2008, 44, 1529–1535. [Google Scholar] [CrossRef]
- Aubrey, B.J.; Kelly, G.L.; Janic, A.; Herold, M.J.; Strasser, A. How does p53 induce apoptosis and how does this relate to p53-mediated tumour suppression? Cell Death Differ. 2018, 25, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, Y.; Yu, X.; Yu, T.; Zheng, X.; Chu, Q. Radix tetrastigma inhibits the non-small cell lung cancer via Bax/Bcl-2/Caspase-9/Caspase-3 pathway. Nutr. Cancer 2022, 74, 320–332. [Google Scholar] [CrossRef] [PubMed]
- Cao, J.; Naeem, M.; Noh, J.-K.; Lee, E.H.; Yoo, J.-W. Dexamethasone phosphate-loaded folate-conjugated polymeric nanoparticles for selective delivery to activated macrophages and suppression of inflammatory responses. Macromol. Res. 2015, 23, 485–492. [Google Scholar] [CrossRef]
- Yang, M.; Wang, Y.; Patel, G.; Xue, Q.; Njateng, G.S.S.; Cai, S.; Cheng, G.; Kai, G. In vitro and in vivo anti-inflammatory effects of different extracts from Epigynum auritum through down-regulation of NF-κB and MAPK signaling pathways. J. Ethnopharmacol. 2020, 261, 113105. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Hu, G.; Wang, C.; Xu, H.; Chen, X.; Qian, A. Cepharanthine, an alkaloid from Stephania cepharantha Hayata, inhibits the inflammatory response in the RAW264. 7 cell and mouse models. Inflammation 2014, 37, 235–246. [Google Scholar] [CrossRef]
- Alscher, R.G.; Erturk, N.; Heath, L.S. Role of superoxide dismutases (SODs) in controlling oxidative stress in plants. J. Exp. Bot. 2002, 53, 1331–1341. [Google Scholar] [CrossRef]











| Samples | Total Phenolic Contents (µg GAE/mg Extract) | Total Flavonoid Contents (µg RE/mg Extract) | In Vitro Antioxidant | |
|---|---|---|---|---|
| DPPH Scavenging (ug/GAE mg Extract) | Reducing Power (ug/GAE mg Extract) | |||
| A. agallocha | 37.203 ± 0.14 | 276.745 ± 0.09 | 2.394 ± 0.009 | 9.746 ± 0.06 |
| A. malaccensis | 30.026 ± 0.11 | 292.095 ± 0.08 | 2.271 ± 0.006 | 9.243 ± 0.03 |
| Predicted Biological Activity | Pi a (%) | Pa b (%) |
|---|---|---|
| TP53 expression enhancer | 0.907 | 0.002 |
| Hepato-protectant | 0.907 | 0.002 |
| Monophenol monooxygenase inhibitor | 0.907 | 0.002 |
| Free radical scavenger | 0.906 | 0.002 |
| Membrane integrity agonist | 0.850 | 0.024 |
| Antimutagenic | 0.816 | 0.004 |
| Caspase 3 stimulant | 0.811 | 0.005 |
| Anticarcinogenic | 0.803 | 0.005 |
| UDP-glucuronosyltransferase substrate | 0.794 | 0.007 |
| Chemo-preventive | 0.741 | 0.005 |
| Protein | Compound | Binding Energy (kcal/mol) | Hydrogen Bond Interactions | Hydrophobic Interactions | No. of Hydrogen Bonds |
|---|---|---|---|---|---|
| BCL-2 | Grewin | −7.5 | ASP108; ARG143 | PHE109; MET112; ALA146 | 2 |
| Gmelofuran | −7.4 | - | ARG104; LEU198; PHE195 | 0 | |
| BDA-366 | −6.3 | LEU134; ARG143 | ALA146; GLU133; TRY105 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nahar, J.; Boopathi, V.; Rupa, E.J.; Awais, M.; Valappil, A.K.; Morshed, M.N.; Murugesan, M.; Akter, R.; Yang, D.U.; Mathiyalagan, R.; et al. Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study. Appl. Sci. 2023, 13, 6321. https://doi.org/10.3390/app13106321
Nahar J, Boopathi V, Rupa EJ, Awais M, Valappil AK, Morshed MN, Murugesan M, Akter R, Yang DU, Mathiyalagan R, et al. Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study. Applied Sciences. 2023; 13(10):6321. https://doi.org/10.3390/app13106321
Chicago/Turabian StyleNahar, Jinnatun, Vinothini Boopathi, Esrat Jahan Rupa, Muhammad Awais, Anjali Kariyarath Valappil, Md Niaj Morshed, Mohanapriya Murugesan, Reshmi Akter, Dong Uk Yang, Ramya Mathiyalagan, and et al. 2023. "Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study" Applied Sciences 13, no. 10: 6321. https://doi.org/10.3390/app13106321
APA StyleNahar, J., Boopathi, V., Rupa, E. J., Awais, M., Valappil, A. K., Morshed, M. N., Murugesan, M., Akter, R., Yang, D. U., Mathiyalagan, R., Yang, D. C., & Jung, S.-K. (2023). Protective Effects of Aquilaria agallocha and Aquilaria malaccensis Edible Plant Extracts against Lung Cancer, Inflammation, and Oxidative Stress—In Silico and In Vitro Study. Applied Sciences, 13(10), 6321. https://doi.org/10.3390/app13106321









