Studying the Tribological Properties of Coffee Oil-Loaded Water-Based Green Lubricant
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Physicochemical Properties of Extracted Coffee Oil and Investigated Lubricating Samples
2.3. Analysis of Fatty Acid Composition
2.4. Extraction and Analysis of Coffee Oil
2.5. Preparation of Lubricating Samples
2.6. Fourier-Transform Infrared (FTIR) Spectroscopy
2.7. Rust Prevention Study
2.8. Tribological Properties
3. Results and Discussions
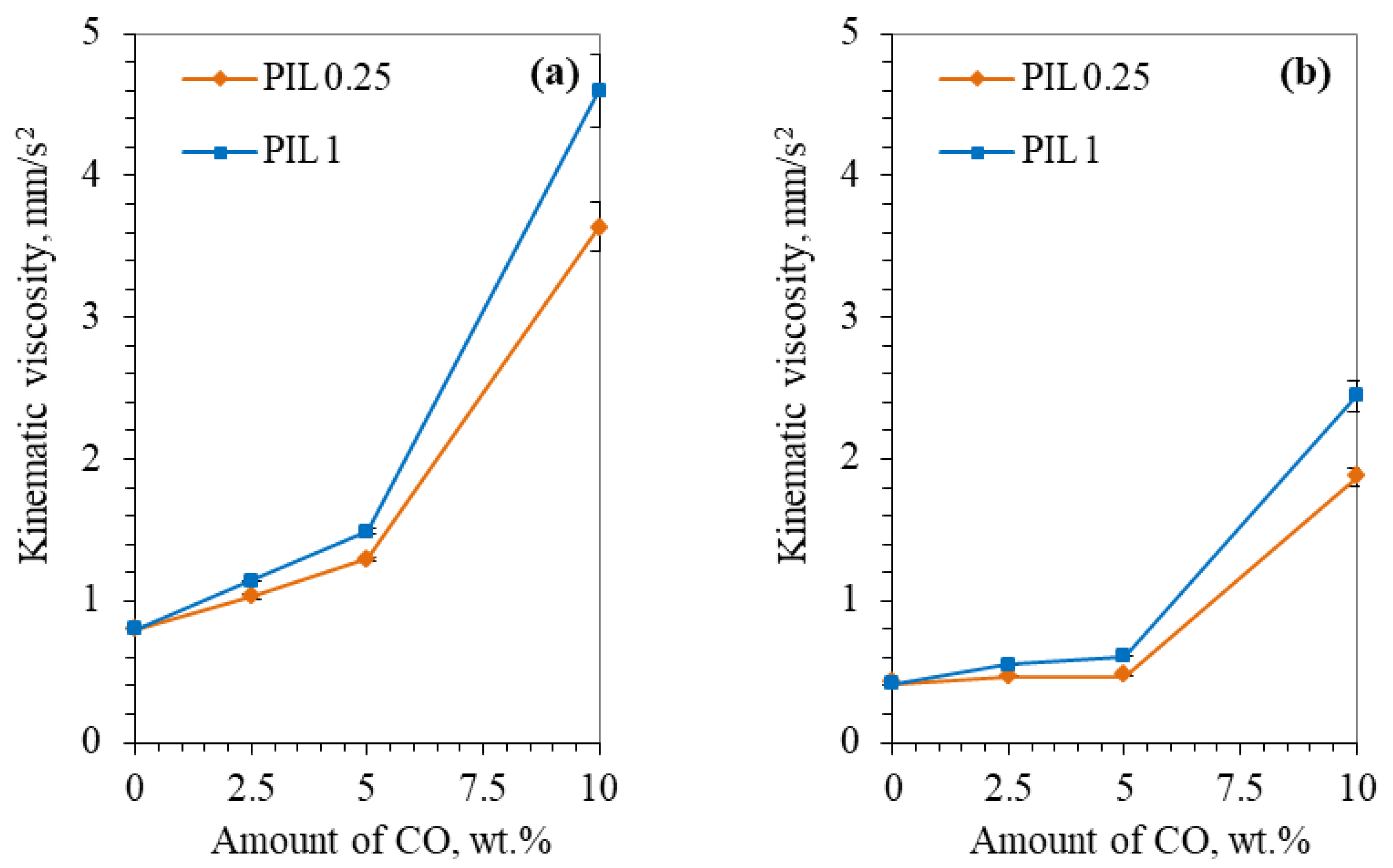
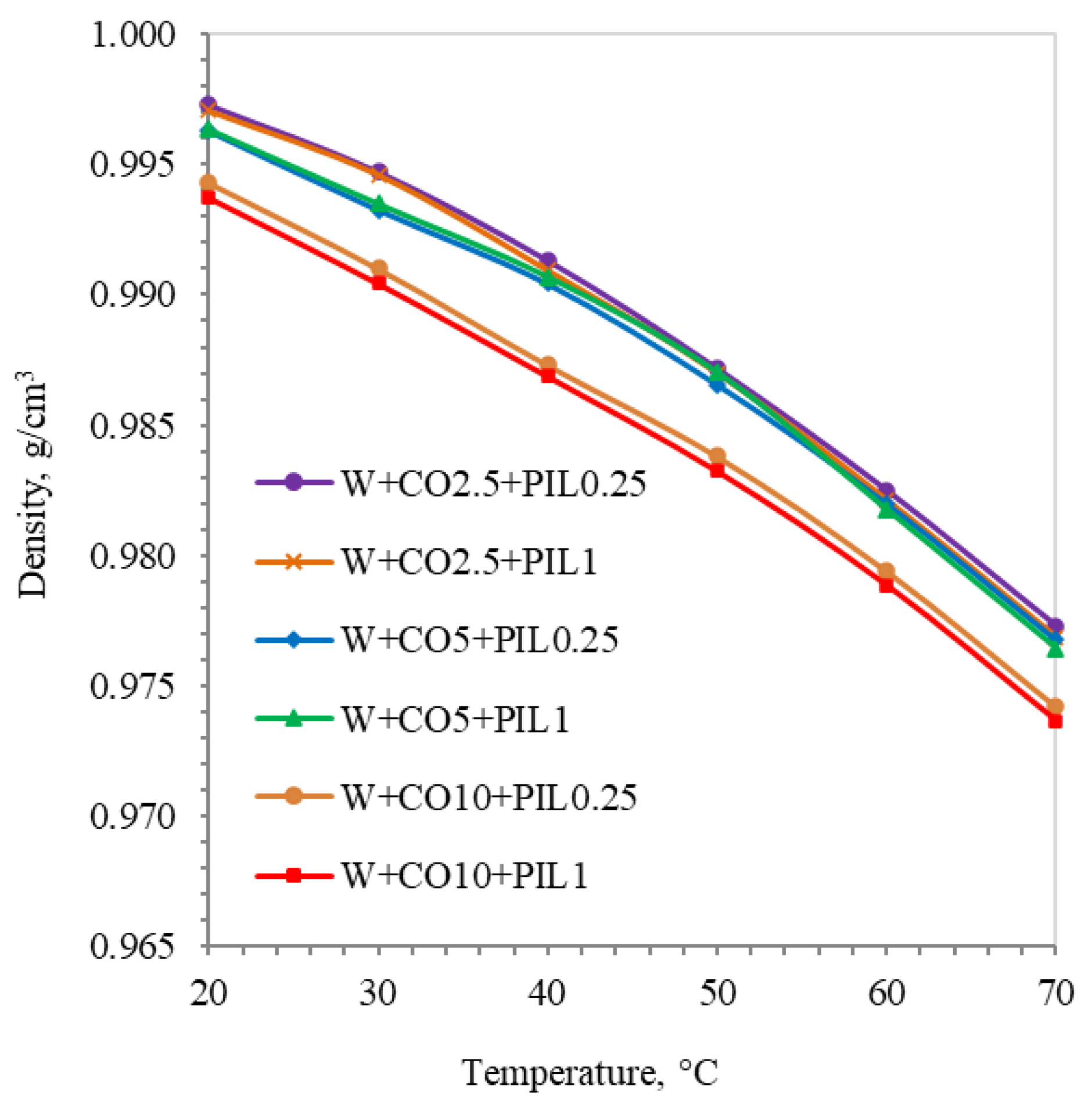
3.1. Physicochemical Properties of Investigated Lubricating Samples
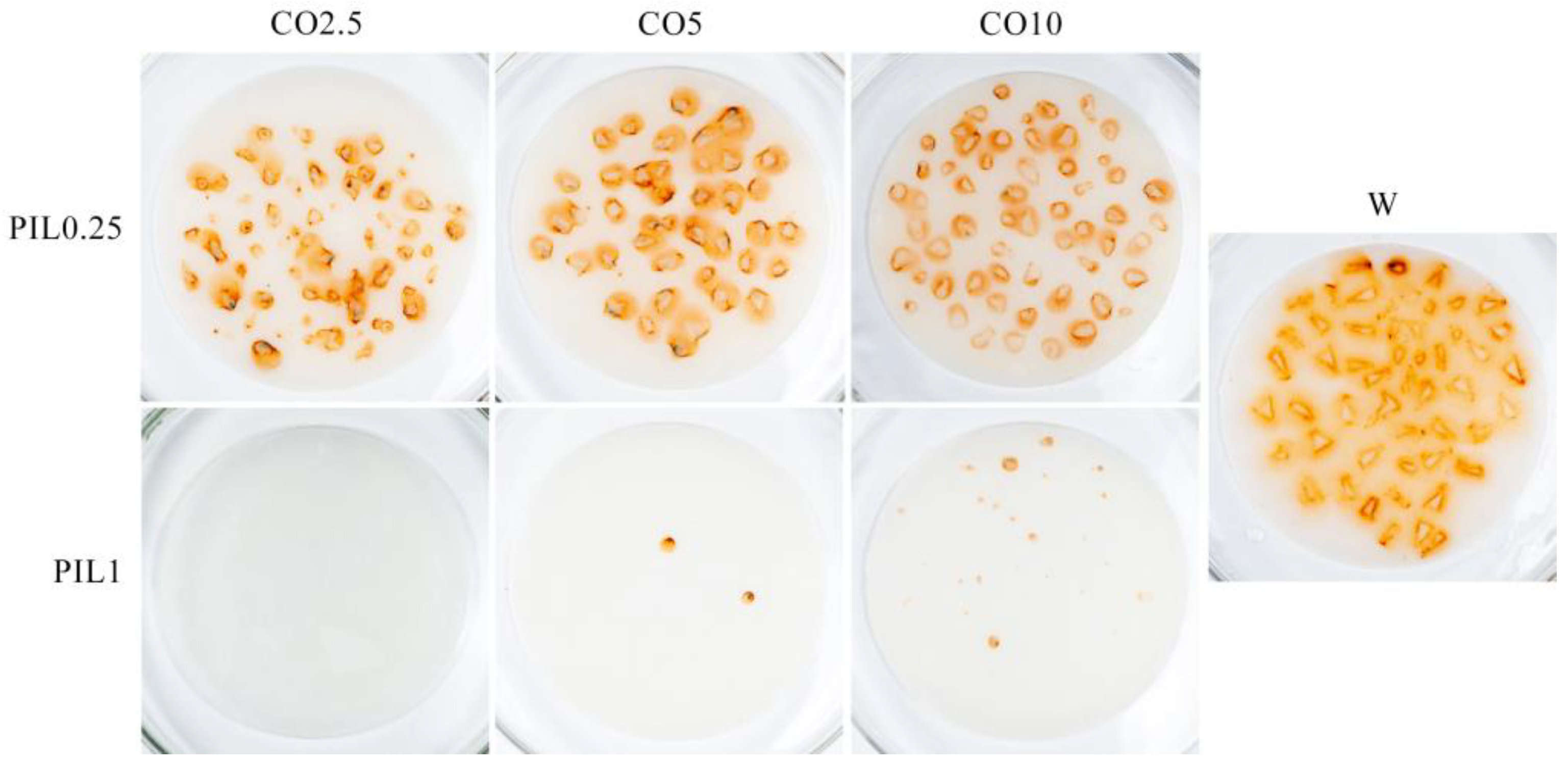
3.2. Corrosion Prevention Characteristics
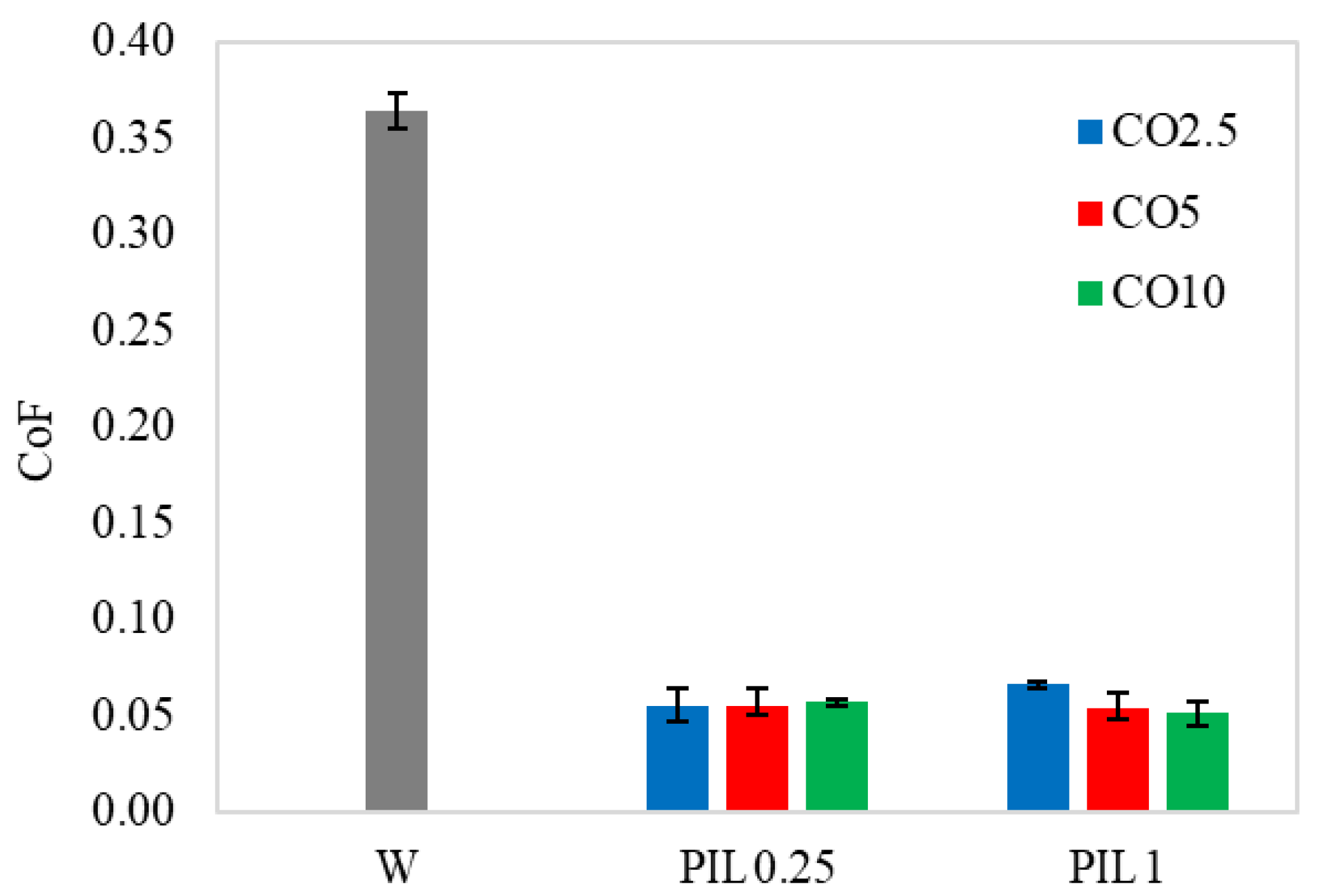
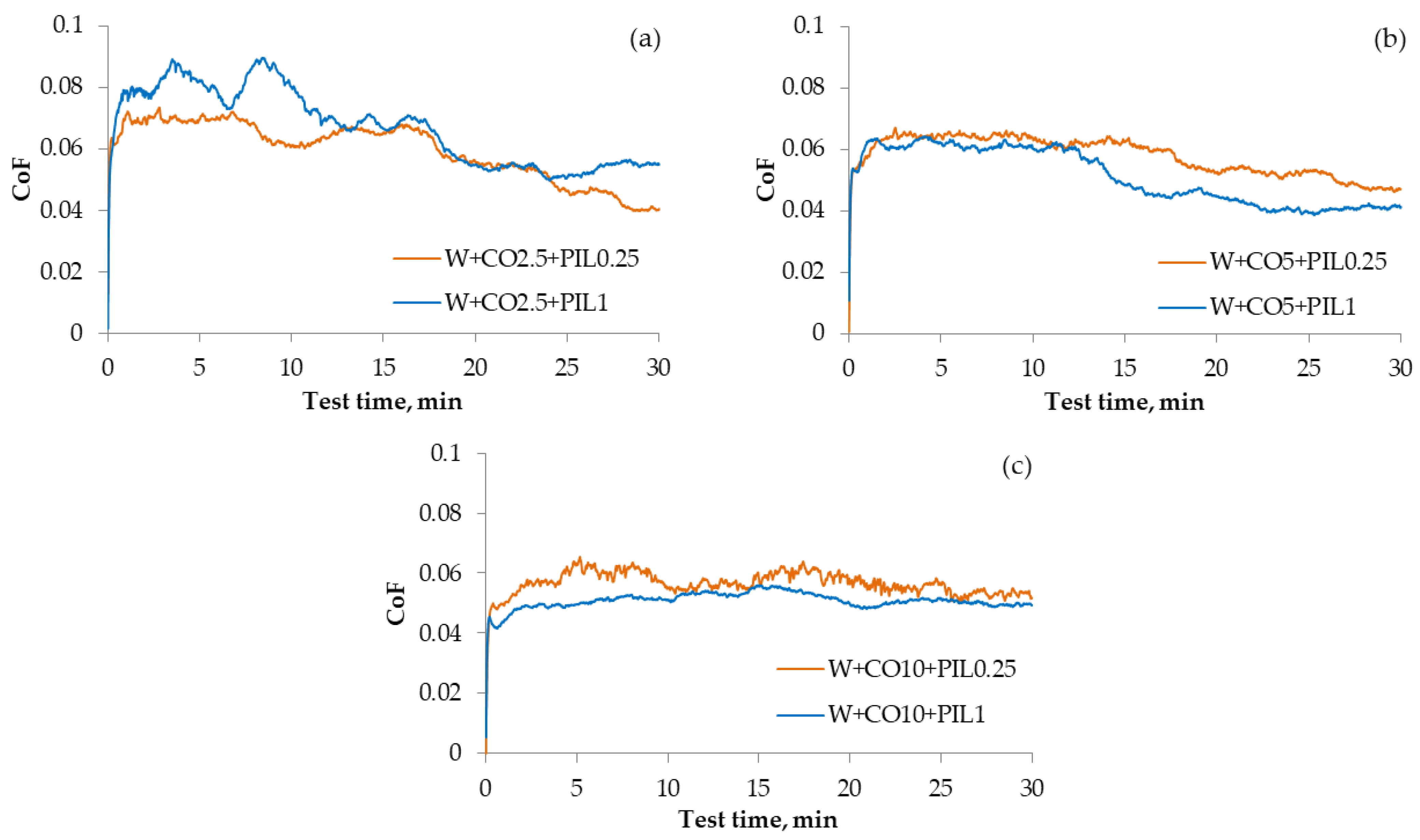
3.3. Friction and Wear Reduction Ability
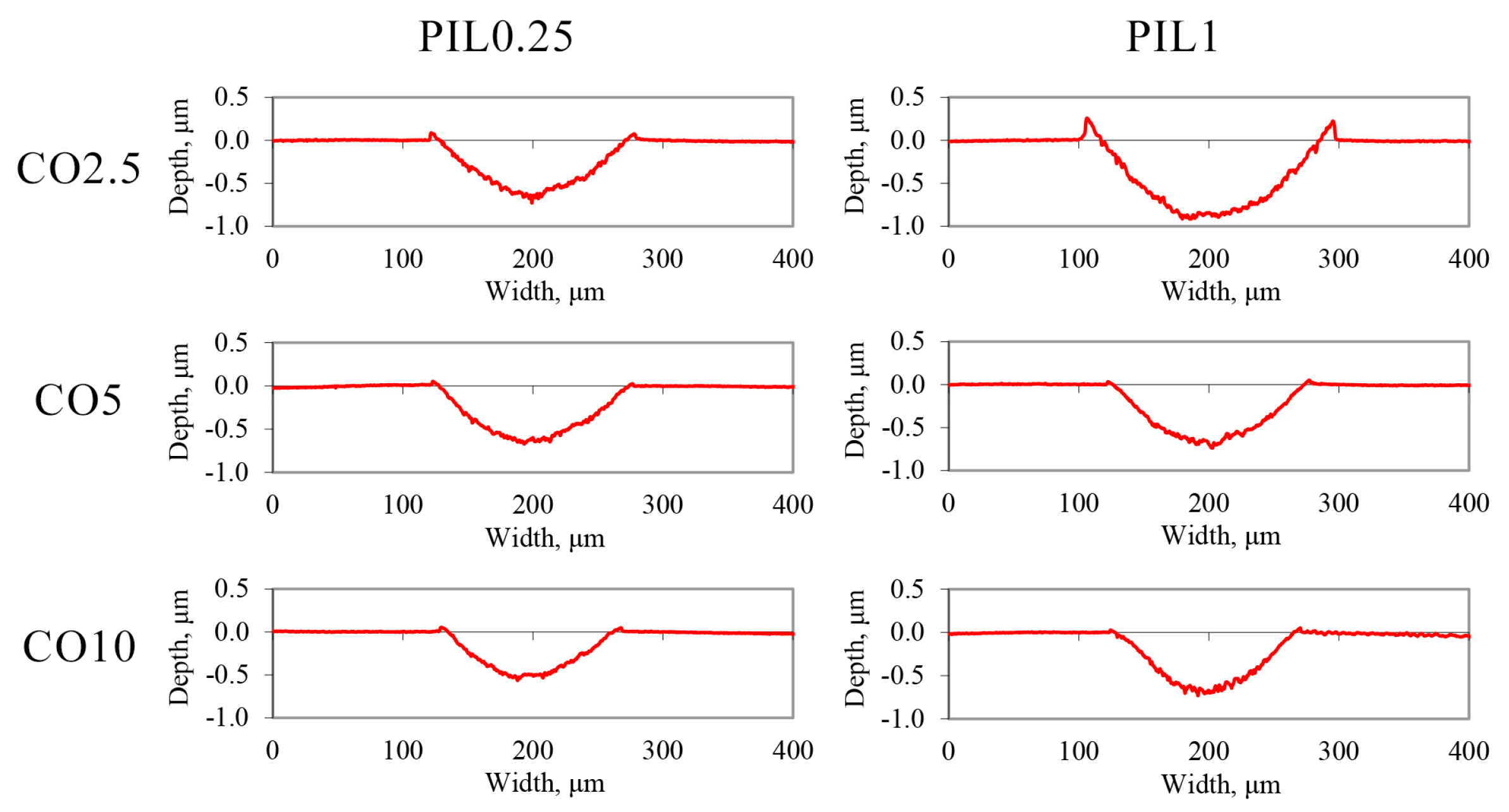
3.4. Analysis of the Worn Surfaces
4. Conclusions
- The extracted coffee oil comprises almost equal amounts of saturated and unsaturated fatty acids. Moreover, it has a high acid number which could result from the coffee preparation process. As an additive, coffee oil increases fluids’ viscosity.
- Protic ionic liquid facilitated the dispersion of coffee oil into the water. With the introduction of one wt.% of PIL, ten wt.% of coffee oil was dispersed. Moreover, PIL was found to be responsible for improved wettability and corrosion prevention ability.
- With the introduction of investigated additives, the lubricity of water could be significantly improved. The adsorption layer and viscosity were assigned responsibility for this.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lawal, S.A.; Choudhury, I.A.; Sadiq, I.O.; Oyewole, A. Vegetable-Oil Based Metalworking Fluids Research Developments for Machining Processes: Survey, Applications and Challenges. Manuf. Rev. 2014, 1. [Google Scholar] [CrossRef]
- Woma, T.Y.; Lawal, S.A.; Abdulrahman, A.S.; Olutoye, M.A.; Ojapah, M.M. Vegetable Oil Based Lubricants: Challenges and Prospects. Tribol. Online 2019, 14, 60–70. [Google Scholar] [CrossRef]
- Nune, M.M.R.; Chaganti, P.K. Development, Characterization, and Evaluation of Novel Eco-Friendly Metal Working Fluid. Measurement 2019, 137, 401–416. [Google Scholar] [CrossRef]
- Rahman, H.; Warneke, H.; Webbert, H.; Rodriguez, J.; Austin, E.; Tokunaga, K.; Rajak, D.K.; Menezes, P.L. Water-Based Lubricants: Development, Properties, and Performances. Lubricants 2021, 9, 73. [Google Scholar] [CrossRef]
- Gajrani, K.K.; Suvin, P.S.; Kailas, S.V.; Sankar, M.R. Hard Machining Performance of Indigenously Developed Green Cutting Fluid Using Flood Cooling and Minimum Quantity Cutting Fluid. J. Clean Prod 2019, 206, 108–123. [Google Scholar] [CrossRef]
- Del Sol, I.; Gámez, A.J.; Rivero, A.; Iglesias, P. Tribological Performance of Ionic Liquids as Additives of Water-Based Cutting Fluids. Wear 2019, 426–427, 845–852. [Google Scholar] [CrossRef]
- Zheng, D.; Zhao, Q.; Ju, C.; Wang, X. The Interaction of Two Anticorrosive Ionic Liquid Additives on the Friction Properties of Water Lubricants. Tribol. Int. 2020, 141, 105948. [Google Scholar] [CrossRef]
- Zheng, D.; Wang, X.; Liu, Z.; Ju, C.; Xu, Z.; Xu, J.; Yang, C. Synergy between Two Protic Ionic Liquids for Improving the Antiwear Property of Glycerol Aqueous Solution. Tribol. Int. 2020, 141, 105731. [Google Scholar] [CrossRef]
- Wu, Y.; He, Z.; Zeng, X.; Ren, T.; de Vries, E.; van der Heide, E. Tribological Properties and Tribochemistry Mechanism of Sulfur-Containing Triazine Derivatives in Water-Glycol. Tribol. Int. 2017, 109, 140–151. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, G.; Ding, T.; Xiang, X.; Li, F.; Ren, T.; Liu, S.; Zheng, L. Tribological Properties and Surface Interaction of Novel Water-Soluble Ionic Liquid in Water-Glycol. Tribol. Int. 2017, 116, 440–448. [Google Scholar] [CrossRef]
- Carrión, F.J.; Avilés, M.D.; Nakano, K.; Tadokoro, C.; Nagamine, T.; Bermúdez, M.D. Diprotic Ammonium Palmitate Ionic Liquid Crystal and Nanodiamonds in Aqueous Lubrication. Film Thickness and Influence of Sliding Speed. Wear 2019, 418–419, 241–252. [Google Scholar] [CrossRef]
- Noor El-Din, M.R.; Mishrif, M.R.; Kailas, S.V.; P.S, S.; Mannekote, J.K. Studying the Lubricity of New Eco-Friendly Cutting Oil Formulation in Metal Working Fluid. Ind. Lubr. Tribol. 2018, 70, 1569–1579. [Google Scholar] [CrossRef]
- Mueanmas, C.; Nikhom, R.; Petchkaew, A.; Iewkittayakorn, J.; Prasertsit, K. Extraction and Esterification of Waste Coffee Grounds Oil as Non-Edible Feedstock for Biodiesel Production. Renew. Energy 2019, 133, 1414–1425. [Google Scholar] [CrossRef]
- Uddin, M.N.; Techato, K.; Rasul, M.G.; Hassan, N.M.S.; Mofijur, M. Waste Coffee Oil: A Promising Source for Biodiesel Production. Energy Procedia 2019, 160, 677–682. [Google Scholar] [CrossRef]
- Grace, J.; Vysochanska, S.; Lodge, J.; Iglesias, P. Ionic Liquids as Additives of Coffee Bean Oil in Steel-Steel Contacts. Lubricants 2015, 3, 637–649. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Kupčinskas, A.; Kazancev, K.; Makarevičienė, V. Investigating the Tribological Properties of PILs Derived from Different Ammonium Cations and Long Chain Carboxylic Acid Anion. Tribol. Int. 2020, 141, 105905. [Google Scholar] [CrossRef]
- ISO 660:2020; Animal and Vegetable Fats and Oils—Determination of Acid Value and Acidity. International Organization for Standardization: Geneva, Switzerland, 2020.
- ISO 659:2009; Oilseeds—Determination of Oil Content. International Organization for Standardization: Geneva, Switzerland, 2009.
- ISO 5508:1990; Animal and Vegetable Fats and Oils—Analysis by Gas Chromatography of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 1990.
- ISO 5509:2000; Animal and Vegetable Fats and Oils—Preparation of Methyl Esters of Fatty Acids. International Organization for Standardization: Geneva, Switzerland, 2000.
- Lourith, N.; Xivivadh, K.; Boonkong, P.; Kanlayavattanakul, M. Spent Coffee Waste: A Sustainable Source of Cleansing Agent for a High-Performance Makeup Remover. Sustain. Chem. Pharm. 2022, 29, 100826. [Google Scholar] [CrossRef]
- Phimsen, S.; Kiatkittipong, W.; Yamada, H.; Tagawa, T.; Kiatkittipong, K.; Laosiripojana, N.; Assabumrungrat, S. Oil Extracted from Spent Coffee Grounds for Bio-Hydrotreated Diesel Production. Energy Convers Manag. 2016, 126, 1028–1036. [Google Scholar] [CrossRef]
- Craig, A.P.; Franca, A.S.; Oliveira, L.S.; Irudayaraj, J.; Ileleji, K. Fourier Transform Infrared Spectroscopy and near Infrared Spectroscopy for the Quantification of Defects in Roasted Coffees. Talanta 2015, 134, 379–386. [Google Scholar] [CrossRef]
- Reis, N.; Franca, A.S.; Oliveira, L.S. Discrimination between Roasted Coffee, Roasted Corn and Coffee Husks by Diffuse Reflectance Infrared Fourier Transform Spectroscopy. LWT-Food Sci. Technol. 2013, 50, 715–722. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D.J. Spectrometric Identification of Organic Compounds; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 7. [Google Scholar]
- Esteban, B.; Riba, J.R.; Baquero, G.; Rius, A.; Puig, R. Temperature Dependence of Density and Viscosity of Vegetable Oils. Biomass Bioenergy 2012, 42, 164–171. [Google Scholar] [CrossRef]
- Rodenbush, C.M.; Hsieh, F.H.; Viswanath, D.S. Density and Viscosity of Vegetable Oils. J. Am. Oil Chem. Soc. 1999, 76, 1415–1419. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M. Investigation of Mixture of Vegetable Oil and Synthetic Esters as Environmentally Friendly Base Stock for Low-Temperature Lubrication Applications. Tribol. Ind. 2018, 40, 401–409. [Google Scholar] [CrossRef]
- Kreivaitis, R.; Gumbytė, M.; Treinytė, J. Investigation of Tribological Properties of Environmentally Friendly Ionic Liquids as a Potential Lubricity Improving Additives for Water-Based Lubricants. Ind. Lubr. Tribol. 2022, 74, 294–301. [Google Scholar] [CrossRef]
- Furey, M.J.; Kajdas, C.; Kempinski, R. Applications of the Concept of Tribopolymerisation in Fuels, Lubricants, Metalworking, and “minimalist” Lubrication. Lubr. Sci. 2002, 15, 73–82. [Google Scholar] [CrossRef]
- Blau, P.J. Friction Science and Technology From Concepts to Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2019; ISBN 9780367386665. [Google Scholar]
- Lin, W.; Klein, J. Control of Surface Forces through Hydrated Boundary Layers. Curr. Opin. Colloid Interface Sci. 2019, 44, 94–106. [Google Scholar] [CrossRef]
- Stachowiak, G.W.; Batchelor, A.W. Engineering Tribology, 4th ed.; Butterworth-Heinemann: Oxford, UK, 2013. [Google Scholar]





| Properties | This Study | Uddin et al. [14] | Lourith et al. [21] | Phimsen et al. [22] |
|---|---|---|---|---|
| Acid value, mg KOH/g | 61.94 ± 0.23 | 15.42 | 35.62 ± 0.30 | 12.20 |
| Acidity, % | 31.14 ± 0.42 | 6.14 | ||
| Oil yield, % (dry wt.) | 11 ± 0.25 | 12.70 ± 0.18 | 10–13 |
| Fatty Acid Composition | This Study | Uddin et al. [14] | Lourith et al. [21] | Phimsen et al. [22] |
|---|---|---|---|---|
| Saturated fatty acids (%): | 51.83 | 37.61 | 43.03 | 86.01 |
| Butyric acid (C4:0) | 0.04 ± 0.0009 | |||
| Caproic acid (C6:0) | 0.07 ± 0.01 | |||
| Caprylic acid (C8:0) | 0.18 ± 0.05 | 0.09 | ||
| Capric acid (C10:0) | 0.24 ± 0.07 | 0.01 | ||
| Undecylic acid (C11:0) | 0.04 ± 0.0002 | |||
| Lauric acid (C12:0) | 0.00 | 0.02 | ||
| Tridecanoic acid (C13:0) | 0.67 ± 0.15 | |||
| Myristic acid (C14:0) | 0.07 ± 0.13 | 3.82 | 0.18 | |
| Pentadecanoic acid (C15:0) | 0.03 ± 0.015 | |||
| Palmitic acid (C16:0) | 37.26 ± 1.78 | 19.00 | 35.39 | 65.07 |
| Margaric acid (C17:0) | 0.14 ± 0.045 | 0.11 | ||
| Stearic acid (C18:0) | 8.79 ± 0.26 | 6.73 | 7.64 | 13.00 |
| Arachidic acid (C20:0) | 3.26 ± 0.026 | 2.96 | 5.47 | |
| Heneicosylic acid (C21:0) | 0.1 ± 0.033 | |||
| Behenic acid (C22:0) | 0.8 ± 0.05 | 1.35 | ||
| Tricosylic acid (C23:0) | 0.12 ± 0.0013 | 5.11 | 0.19 | |
| Lignoceric acid (C24:0) | 0.02 ± 0.001 | 0.52 | ||
| Unsaturated fatty acids, %: | 48.17 | 62.27 | 45.63 | 13.99 |
| Monounsaturated fatty acids, %: | 10.19 | 33.56 | 9.78 | 8.98 |
| Myristoleic acid (C14:1 cis-9) | 0.14 ± 0.05 | 20.00 | ||
| Palmitoleic acid (C16:1 cis-9) | 0.04 ± 0.003 | |||
| Oleic acid (C18:1) | 9.26 ± 0.58 | 9.27 | 9.78 | 8.69 |
| Paullinic acid (C20:1) | 0.33 ± 0.006 | 0.29 | ||
| Erucic acid (C22:1) | 0.07 ± 0.002 | |||
| Nervonic acid (C24:1) | 0.35 ± 0.025 | 4.29 | ||
| Polyunsaturated fatty acids, %: | 37.98 | 28.71 | 35.85 | 5.01 |
| Linoleic acid (C18:2) | 36.59 ± 1.95 | 28.71 | 35.85 | 4.72 |
| Linolenic acid (C18:3) | 1.15 ± 0.045 | 0.04 | ||
| Eicosadienoic acid (C20:2) | 0.03 ± 0.0001 | |||
| Dihomo-gamma-linolenic acid (C20:3 8,11,14) | 0.03 ± 0.0002 | 0.15 | ||
| Arachidonic acid (C20:4) | 0.02 ± 0.005 | 0.10 | ||
| Eicosapentaenoic acid (C20:5) | 0.11 ± 0.0047 | |||
| Brassic acid (C22:2) | 0.02 ± 0.001 | |||
| Docosahexaenoic acid (C22:6) | 0.02 ± 0.0033 |
| Lubricating Sample | Coffee Oil wt.% | Protic Ionic Liquid wt.% | Water |
|---|---|---|---|
| W + CO2.5 + PIL0.25 | 2.5 | 0.25 | Balance |
| W + CO2.5 + PIL1 | 2.5 | 1 | |
| W + CO5 + PIL0.25 | 5 | 0.25 | |
| W + CO5 + PIL1 | 5 | 1 | |
| W + CO10 + PIL0.25 | 10 | 0.25 | |
| W + CO10 + PIL1 | 10 | 1 |
| Lubricating Sample | pH |
|---|---|
| W + CO2.5 + PIL0.25 | 8.62 ± 0.026 |
| W + CO2.5 + PIL1 | 8.78 ± 0.046 |
| W + CO5 + PIL0.25 | 8.26 ± 0.022 |
| W + CO5 + PIL1 | 8.53 ± 0.034 |
| W + CO10 + PIL0.25 | 8.02 ± 0.017 |
| W + CO10 + PIL1 | 8.52 ± 0.011 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kreivaitis, R.; Gumbytė, M.; Kupčinskas, A.; Treinytė, J.; Kazancev, K.; Sendžikienė, E. Studying the Tribological Properties of Coffee Oil-Loaded Water-Based Green Lubricant. Appl. Sci. 2023, 13, 6336. https://doi.org/10.3390/app13106336
Kreivaitis R, Gumbytė M, Kupčinskas A, Treinytė J, Kazancev K, Sendžikienė E. Studying the Tribological Properties of Coffee Oil-Loaded Water-Based Green Lubricant. Applied Sciences. 2023; 13(10):6336. https://doi.org/10.3390/app13106336
Chicago/Turabian StyleKreivaitis, Raimondas, Milda Gumbytė, Artūras Kupčinskas, Jolanta Treinytė, Kiril Kazancev, and Eglė Sendžikienė. 2023. "Studying the Tribological Properties of Coffee Oil-Loaded Water-Based Green Lubricant" Applied Sciences 13, no. 10: 6336. https://doi.org/10.3390/app13106336
APA StyleKreivaitis, R., Gumbytė, M., Kupčinskas, A., Treinytė, J., Kazancev, K., & Sendžikienė, E. (2023). Studying the Tribological Properties of Coffee Oil-Loaded Water-Based Green Lubricant. Applied Sciences, 13(10), 6336. https://doi.org/10.3390/app13106336








