Bioinformatics-Based Characterization of ATP-Binding Cassette Subfamily B Member 1 (ABCB1) Gene Expression in Non-Small-Cell Lung Cancer (NSCLC)
Abstract
:1. Introduction
2. Methods
2.1. TNMplot Database
2.2. UALCAN Database
2.3. cBioPortal Database
2.4. PrognoScan Database
2.5. MEXPRESS Database
3. Results
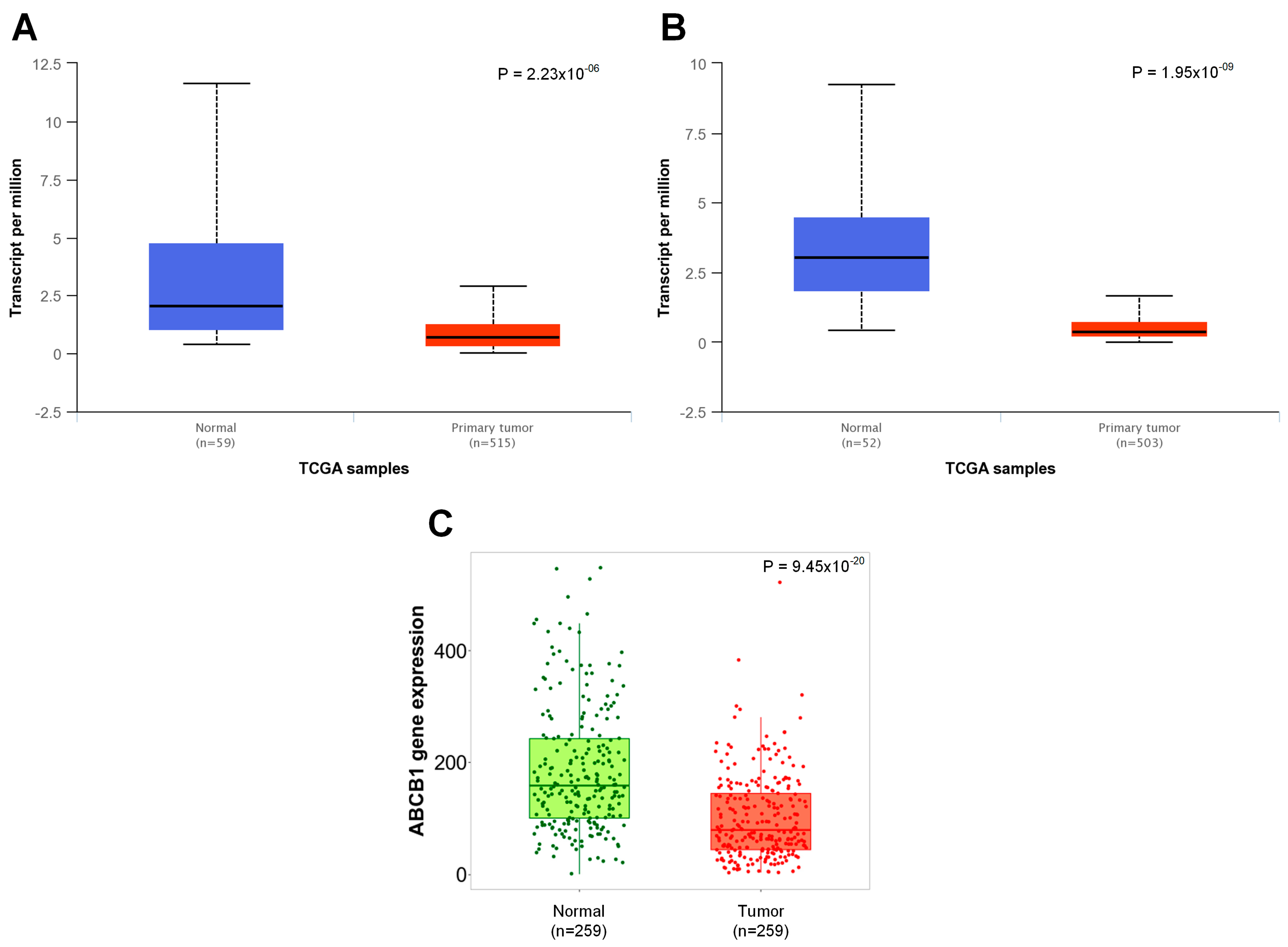
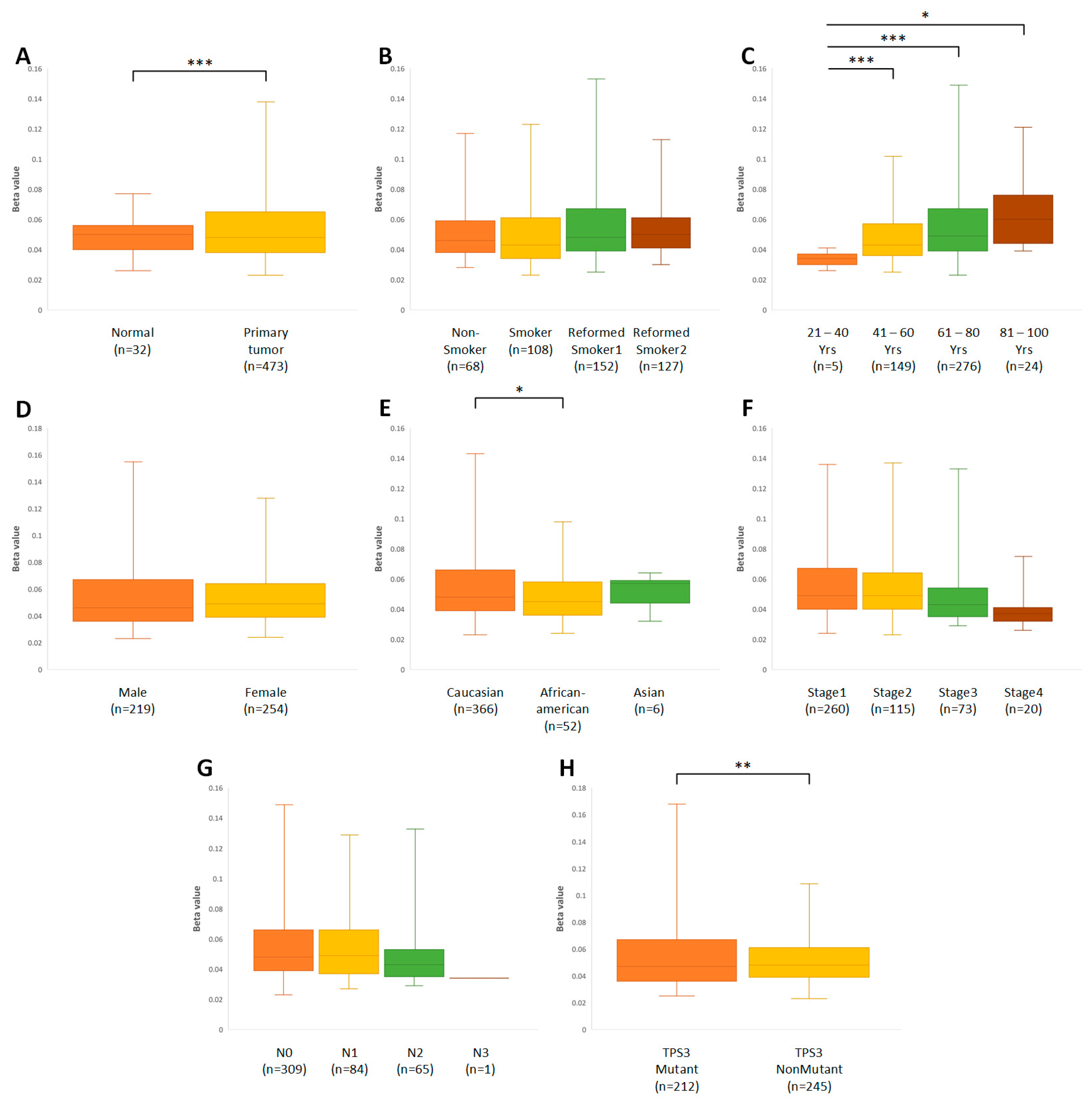
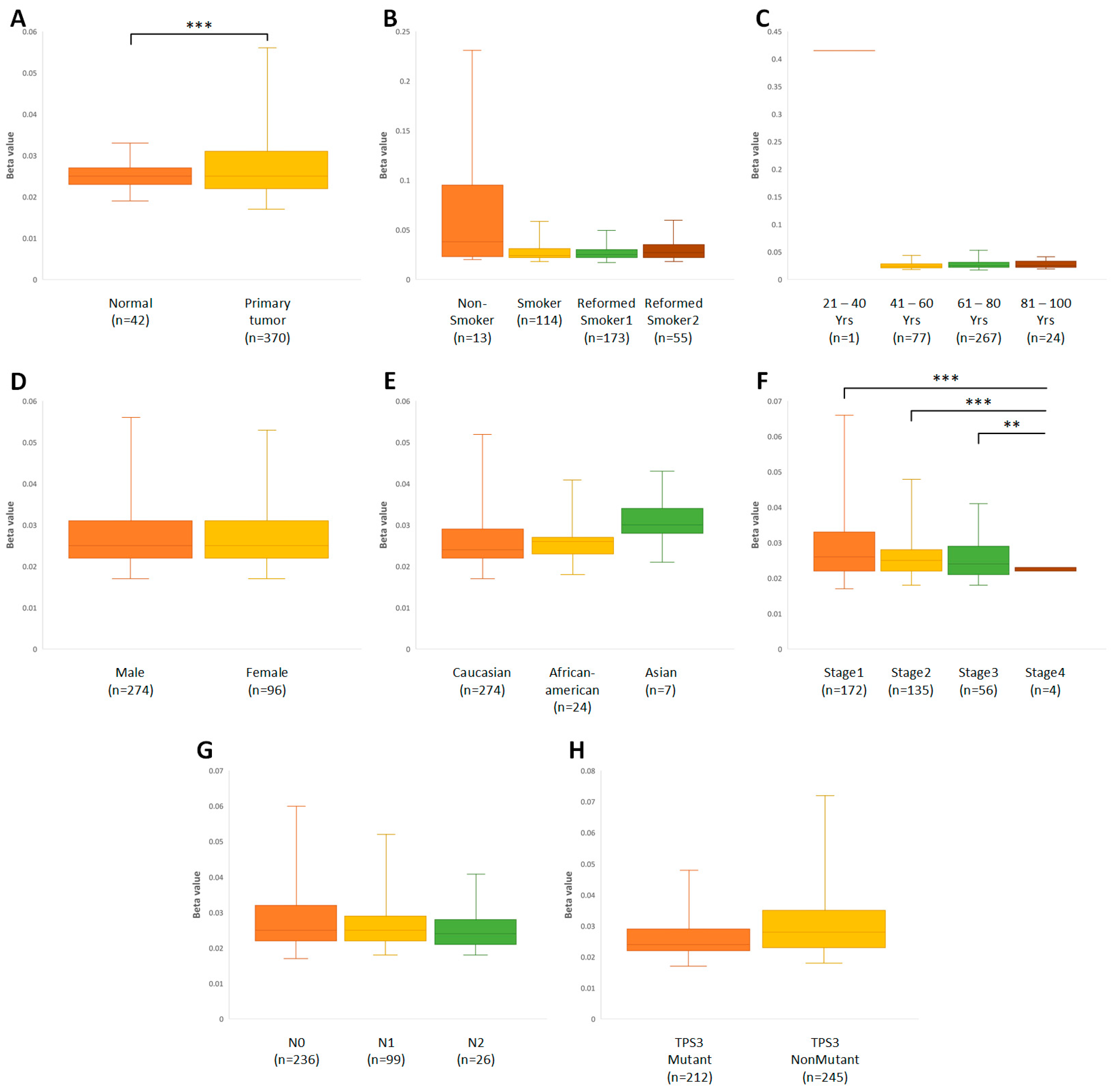
3.1. Physiologically Low Levels of ABCB1 Expression in Lung Tissue Are Significantly Downregulated in Cancer Cells
3.2. Time Elapsed since Smoking Cessation/Quitting Affects P-gp Gene Expression in Ex-Smokers with Squamous-Cell Lung Cancer
3.3. Age, Gender, and Ethnicity Influence the Expression of the ABCB1 Gene
3.4. The Higher Stage of Cancer May Be Associated with a Decrease in the Amount of the ABCB1 Gene Transcript
3.5. Effect of ABCB1 Expression on Survival in Lung Cancer
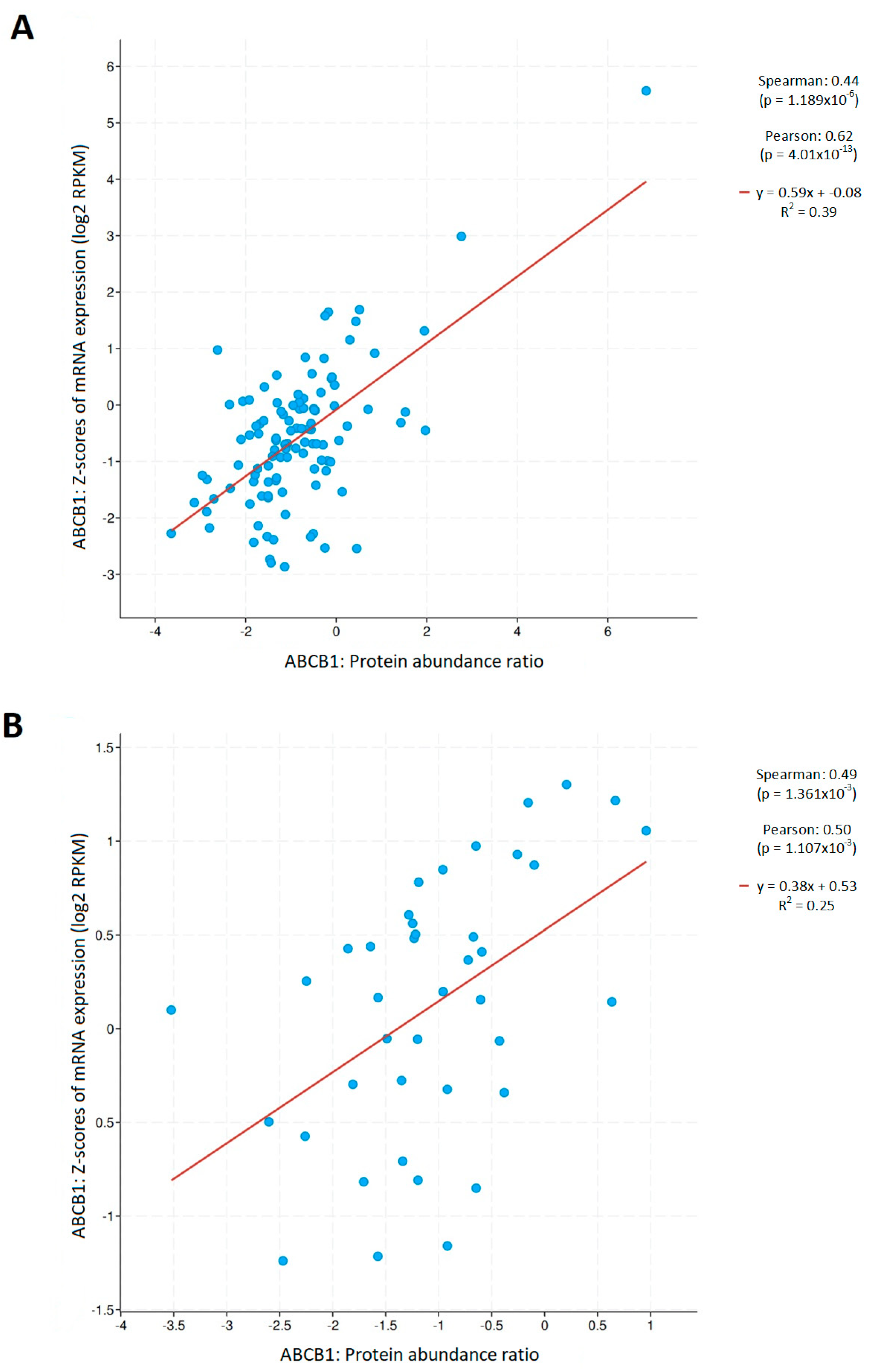
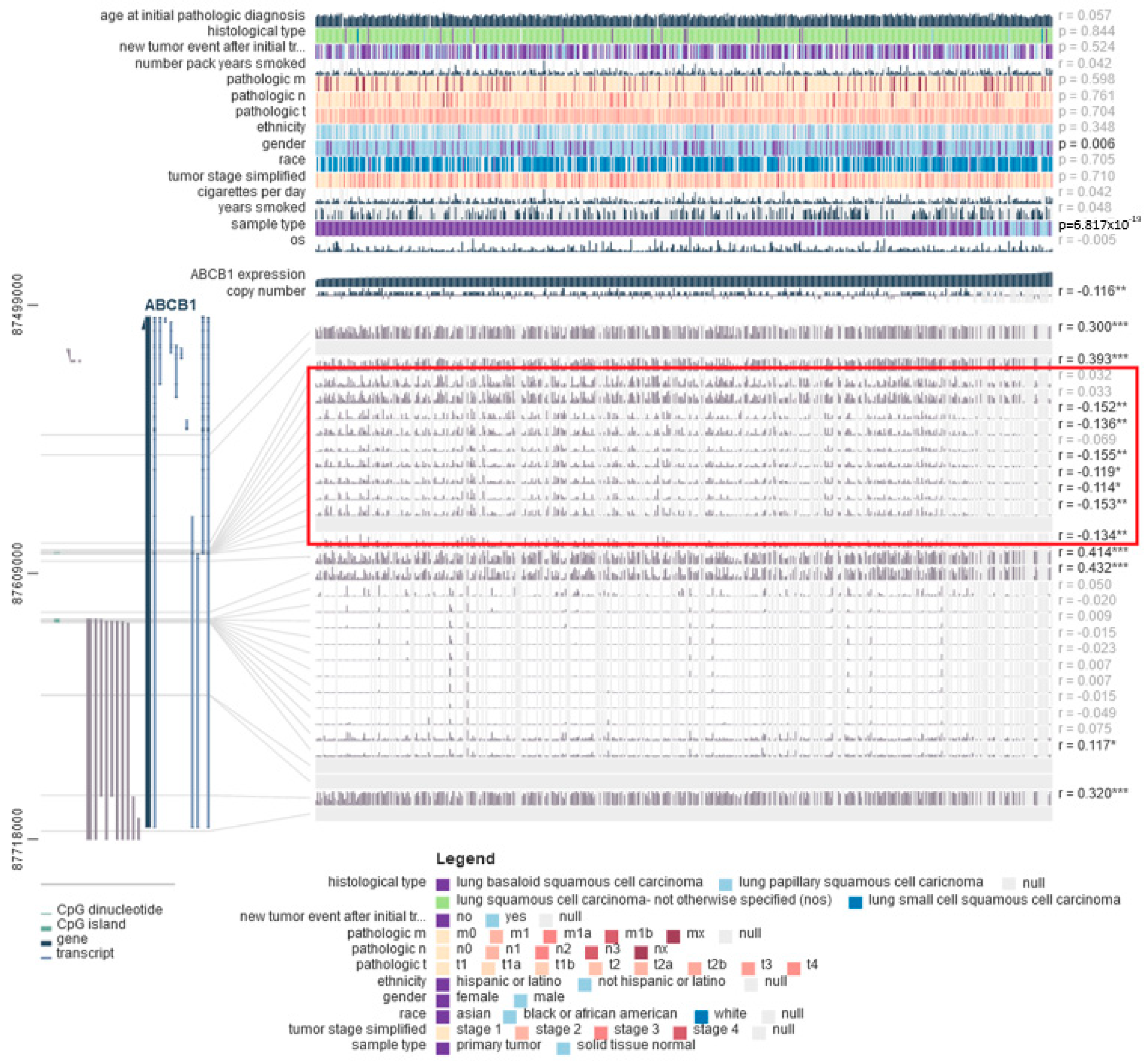
3.6. DNA Methylation Is Correlated with Decreased ABCB1 Gene Expression in Lung Cancer Cells
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wojciechowska, U.; Barańska, K.; Michałek, I.; Olasek, P.; Miklewska, M.; Didkowska, J.A. Cancer in Poland in 2020; Polish National Cancer Registry: Warsaw, Poland, 2022; Available online: http://onkologia.org.pl (accessed on 30 January 2023).
- European Respiratory Society. Respiratory Health and Disease in Europe: The European Lung White Book. (Chapter 19: Lung Cancer). Available online: https://www.erswhitebook.org (accessed on 14 August 2022).
- American Cancer Society. Cancer Statistics Center. Available online: http://cancerstatisticscenter.cancer.org (accessed on 30 January 2023).
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Adamek, M.; Biernat, W.; Chorostowska-Wynimko, J.; Didkowska, J.A.; Dziadziuszko, K.; Grodzki, T.; Jassem, J.; Kępka, L.; Kowalski, D.; Krawczyk, P.; et al. Lung Cancer in Poland. J. Thorac. Oncol. 2020, 15, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Clark, S.B.; Alsubait, S. Non-Small Cell Lung Cancer; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK562307/ (accessed on 30 January 2023).
- Jiang, W.; Cai, G.; Hu, P.C.; Wang, Y. Personalized medicine in non-small cell lung cancer: A review from a pharmacogenomics perspective. Acta Pharm. Sin. B. 2018, 8, 530–538. [Google Scholar] [CrossRef] [PubMed]
- Thandra, K.C.; Barsouk, A.; Saginala, K.; Aluru, J.S.; Barsouk, A. Epidemiology of lung cancer. Contemp. Oncol. 2021, 25, 45–52. [Google Scholar]
- Lung Cancer—Non-Small Cell: Statistics. Available online: https://www.cancer.net/cancer-types/lung-cancer-non-small-cell/statistics (accessed on 14 May 2023).
- Flores, R.; Patel, P.; Alpert, N.; Pyenson, B.; Taioli, E. Association of Stage Shift and Population Mortality Among Patients With Non-Small Cell Lung Cancer. JAMA Netw. Open 2021, 4, e2137508. [Google Scholar] [CrossRef]
- Nagl, L.; Pall, G.; Wolf, D.; Pircher, A.; Horvath, L. Molecular profiling in lung cancer. MEMO—Mag. Eur. Med. Oncol. 2022, 15, 201–205. [Google Scholar] [CrossRef]
- Hecht, S.S. Lung carcinogenesis by tobacco smoke. Int. J. Cancer 2012, 131, 2724–2732. [Google Scholar] [CrossRef] [PubMed]
- Pilotto Heming, C.; Muriithi, W.; Wanjiku Macharia, L.; Niemeyer Filho, P.; Moura-Neto, V.; Aran, V. P-glycoprotein and cancer: What do we currently know? Heliyon 2022, 8, e11171. [Google Scholar] [CrossRef]
- Lai, J.I.; Tseng, Y.J.; Chen, M.H.; Huang, C.F.; Chang, P.M. Clinical Perspective of FDA Approved Drugs with P-Glycoprotein Inhibition Activities for Potential Cancer Therapeutics. Front. Oncol. 2020, 10, 561936. [Google Scholar] [CrossRef]
- Wang, J.; Yang, D.H.; Yang, Y.; Wang, J.Q.; Cai, C.Y.; Lei, Z.N.; Teng, Q.X.; Wu, Z.X.; Zhao, L.; Chen, Z.S. Overexpression of ABCB1 Transporter Confers Resistance to mTOR Inhibitor WYE-354 in Cancer Cells. Int. J. Mol. Sci. 2020, 21, 1387. [Google Scholar] [CrossRef]
- Popęda, M.; Płuciennik, M.; Bednarek, A.K. Proteins in cancer resistance. Adv. Hyg. Exp. Med. 2014, 68, 616–632. [Google Scholar]
- Fellay, J.; Marzolini, C.; Meaden, E.R.; Back, D.J.; Buclin, T.; Chave, J.P.; Decosterd, L.A.; Furrer, H.; Opravil, M.; Pantaleo, G.; et al. Swiss HIV Cohort Study. Response to antiretroviral treatment in HIV-1-infected individuals with allelic variants of the multidrug resistance transporter 1: A pharmacogenetics study. Lancet 2002, 359, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Leschziner, G.D.; Andrew, T.; Pirmohamed, M.; Johnson, M.R. ABCB1 genotype and PGP expression, function and therapeutic drug response: A critical review and recommendations for future research. Pharm. J. 2007, 7, 154–179. [Google Scholar] [CrossRef]
- Seelig, A. P-Glycoprotein: One Mechanism, Many Tasks and the Consequences for Pharmacotherapy of Cancers. Front. Oncol. 2020, 10, 576559. [Google Scholar] [CrossRef]
- Mencalha, A.L.; Rodrigues, E.F.; Abdelhay, E.; Fernandez, T.S. Accurate monitoring of promoter gene methylation with high-resolution melting polymerase chain reaction using the ABCB1 gene as a model. Genet. Mol. Res. 2013, 12, 714–722. [Google Scholar] [CrossRef]
- Bartha, A.; Gyorffy, B. TNMplot.com: A Web Tool for the Comparison of Gene Expression in Normal, Tumor and Metastatic Tissues. Int. J. Mol. Sci. 2021, 22, 2622. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Bashel, B.; Balasubramanya, S.A.H.; Creighton, C.J.; Rodriguez, I.P.; Chakravarthi, B.V.S.K.; Varambally, S. UALCAN: A portal for facilitating tumor subgroup gene expression and survival analyses. Neoplasia 2017, 19, 649–658. [Google Scholar] [CrossRef]
- Chandrashekar, D.S.; Karthikeyan, S.K.; Korla, P.K.; Patel, H.; Shovon, A.R.; Athar, M.; Netto, G.J.; Qin, Z.S.; Kumar, S.; Manne, U.; et al. UALCAN: An update to the integrated cancer data analysis platform. Neoplasia 2022, 25, 18–27. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Jacobsen, A.; Byrne, C.J.; Heuer, M.L.; Larsson, E.; et al. The cBio cancer genomics portal: An open platform for exploring multidimensional cancer genomics data. Cancer Discov. 2012, 2, 401–404. [Google Scholar] [CrossRef]
- Gao, J.; Aksoy, B.A.; Dogrusoz, U.; Dresdner, G.; Gross, B.; Sumer, S.O.; Sun, Y.; Jacobsen, A.; Sinha, R.; Larsson, E.; et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci. Signal. 2013, 6, pl1. [Google Scholar] [CrossRef]
- Mizuno, H.; Kitada, K.; Nakai, K.; Sarai, A. PrognoScan: A new database for meta-analysis of the prognostic value of genes. BMC Med. Genom. 2009, 2, 18. [Google Scholar] [CrossRef]
- Koch, A.; De Meyer, T.; Jeschke, J.; Van Criekinge, W. MEXPRESS: Visualizing expression, DNA methylation and clinical TCGA data. BMC Genom. 2015, 16, 636. [Google Scholar] [CrossRef]
- Koch, A.; Jeschke, J.; Van Criekinge, W.; van Engeland, M.; De Meyer, T. MEXPRESS update 2019. Nucleic Acids Res. 2019, 47, W561–W565. [Google Scholar] [CrossRef]
- Xiao, H.; Zheng, Y.; Ma, L.; Tian, L.; Sun, Q. Clinically-Relevant ABC Transporter for Anti-Cancer Drug Resistance. Front. Pharmacol. 2022, 12, 648407. [Google Scholar] [CrossRef]
- Waghray, D.; Zhang, Q. Inhibit or Evade Multidrug Resistance P-Glycoprotein in Cancer Treatment. J. Med. Chem. 2018, 61, 5108–5121. [Google Scholar] [CrossRef]
- Fojo, A.T.; Ueda, K.; Slamon, D.J.; Poplack, D.G.; Gottesman, M.M.; Pastan, I. Expression of a multidrug-resistance gene in human tumors and tissues. Proc. Natl. Acad. Sci. USA 1987, 84, 265–269. [Google Scholar] [CrossRef]
- Cordon-Cardo, C.; O’Brien, J.P.; Boccia, J.; Casals, D.; Bertino, J.R.; Melamed, M.R. Expression of the multidrug resistance gene product (P-glycoprotein) in human normal and tumor tissues. J. Histochem. Cytochem. 1990, 38, 1277–1287. [Google Scholar] [CrossRef]
- Lee, T.B.; Park, J.H.; Min, Y.D.; Kim, K.J.; Choi, C.H. Epigenetic mechanisms involved in differential MDR1 mRNA expression between gastric and colon cancer cell lines and rationales for clinical chemotherapy. BMC Gastroenterol. 2008, 8, 33. [Google Scholar] [CrossRef]
- Delou, J.M.A.; Vignal, G.M.; Índio-do-Brasil, V.; Accioly, M.T.S.; da Silva, T.S.L.; Piranda, D.N.; Sobral-Leite, M.; de Carvalho, M.A.; Capella, M.A.M.; Vianna-Jorge, R. Loss of constitutive ABCB1 expression in breast cancer associated with worse prognosis. Breast Cancer 2017, 9, 415–428. [Google Scholar] [CrossRef]
- Andersen, V.; Vogel, U.; Godiksen, S.; Frenzel, F.B.; Sæbø, M.; Hamfjord, J.; Kure, E.; Vogel, L.K. Low ABCB1 gene expression is an early event in colorectal carcinogenesis. PLoS ONE 2013, 8, e72119. [Google Scholar] [CrossRef]
- Reed, K.; Hembruff, S.L.; Laberge, M.L.; Villeneuve, D.J.; Côté, G.B.; Parissenti, A.M. Hypermethylation of the ABCB1 downstream gene promoter accompanies ABCB1 gene amplification and increased expression in docetaxel-resistant MCF-7 breast tumor cells. Epigenetics 2008, 3, 270–280. [Google Scholar] [CrossRef] [PubMed]
- Li, A.; Song, J.; Lai, Q.; Liu, B.; Wang, H.; Xu, Y.; Feng, X.; Sun, X.; Du, Z. Hypermethylation of ATP-binding cassette B1 (ABCB1) multidrug resistance 1 (MDR1) is associated with cisplatin resistance in the A549 lung adenocarcinoma cell line. Int. J. Exp. Pathol. 2016, 97, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Yabuki, N.; Sakata, K.; Yamasaki, T.; Terashima, H.; Mio, T.; Miyazaki, Y.; Fujii, T.; Kitada, K. Gene amplification and expression in lung cancer cells with acquired paclitaxel resistance. Cancer Genet. Cytogenet. 2007, 173, 1–9. [Google Scholar] [CrossRef]
- Takano, M.; Naka, R.; Sasaki, Y.; Nishimoto, S.; Yumoto, R. Effect of cigarette smoke extract on P-glycoprotein function in primary cultured and newly developed alveolar epithelial cells. Drug Metab. Pharmacokinet. 2016, 31, 417–424. [Google Scholar] [CrossRef]
- Erdő, F.; Krajcsi, P. Age-Related Functional and Expressional Changes in Efflux Pathways at the Blood-Brain Barrier. Front. Aging Neurosci. 2019, 11, 196. [Google Scholar] [CrossRef]
- Warrington, J.S.; Greenblatt, D.J.; von Moltke, L.L. The effect of age on P-glycoprotein expression and function in the Fischer-344 rat. J. Pharmacol. Exp. Ther. 2004, 309, 730–736. [Google Scholar] [CrossRef]
- van Assema, D.M.E.; Lubberink, M.; Boellaard, R.; Schuit, R.C.; Windhorst, A.D.; Scheltens, P.; Lammertsma, A.A.; van Berckel, B.N. P-glycoprotein function at the blood-brain barrier: Effects of age and gender. Mol. Imaging Biol. 2012, 14, 771–776. [Google Scholar] [CrossRef]
- Zou, F.; Seike, M.; Noro, R.; Kunugi, S.; Kubota, K.; Gemma, A. Prognostic significance of ABCB1 in stage I lung adenocarcinoma. Oncol. Lett. 2017, 14, 313–321. [Google Scholar] [CrossRef]
- Chen, C.; Clark, D.; Ueda, K.; Pastan, I.; Gottesman, M.M.; Roninson, I.B. Genomic organization of the human multidrug resistance (MDR1) gene and origin of P-glycoproteins. J. Biol. Chem. 1990, 265, 506–514. [Google Scholar] [CrossRef] [PubMed]
- Genovese, I.; Ilari, A.; Assaraf, Y.G.; Fazi, F.; Colotti, G. Not only P-glycoprotein: Amplification of the ABCB1-containing chromosome region 7q21 confers multidrug resistance upon cancer cells by coordinated overexpression of an assortment of resistance-related proteins. Drug Resist. Updates 2017, 32, 23–46. [Google Scholar] [CrossRef]
- Trussardi-Regnier, A.; Lavenus, S.; Gorisse, M.C.; Dufer, J. Thalidomide alters nuclear architecture without ABCB1 gene modulation in drug-resistant myeloma cells. Int. J. Oncol. 2009, 35, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Spitzwieser, M.; Pirker, C.; Koblmüller, B.; Pfeiler, G.; Hacker, S.; Berger, W.; Heffeter, P.; Cichna-Markl, M. Promoter methylation patterns of ABCB1, ABCC1 and ABCG2 in human cancer cell lines, multidrug-resistant cell models and tumor, tumor-adjacent and tumor-distant tissues from breast cancer patients. Oncotarget 2016, 7, 73347–73369. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Geng, J.; Ma, K.; Yu, J.; Sun, J.; Shen, Z.; Bao, G.; Chen, Y.; Zhang, H.; He, Y.; et al. RASSF1A, APC, ESR1, ABCB1 and HOXC9, but not p16INK4A, DAPK1, PTEN and MT1G genes were frequently methylated in the stage I non-small cell lung cancer in China. J. Cancer Res. Clin. Oncol. 2009, 135, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Yegnasubramanian, S.; Kowalski, J.; Gonzalgo, M.L.; Zahurak, M.; Piantadosi, S.; Walsh, P.C.; Bova, G.S.; De Marzo, A.M.; Isaacs, W.B.; Nelson, W.G. Hypermethylation of CpG islands in primary and metastatic human prostate cancer. Cancer Res. 2004, 64, 1975–1986. [Google Scholar] [CrossRef]
- Gao, P.; Yang, X.; Xue, Y.W.; Zhang, X.F.; Wang, Y.; Liu, W.J.; Wu, X.J. Promoter methylation of glutathione S-transferase pi1 and multidrug resistance gene 1 in bronchioloalveolar carcinoma and its correlation with DNA methyltransferase 1 expression. Cancer 2009, 115, 3222–3232. [Google Scholar] [CrossRef]
- Enokida, H.; Shiina, H.; Igawa, M.; Ogishima, T.; Kawakami, T.; Bassett, W.W.; Anast, J.W.; Li, L.C.; Urakami, S.; Terashima, M.; et al. CpG hypermethylation of MDR1 gene contributes to the pathogenesis and progression of human prostate cancer. Cancer Res. 2004, 64, 5956–5962. [Google Scholar] [CrossRef]
- Zappe, K.; Cichna-Markl, M. Aberrant DNA Methylation of ABC Transporters in Cancer. Cells 2020, 9, 2281. [Google Scholar] [CrossRef]
- Sharma, G.; Mirza, S.; Parshad, R.; Srivastava, A.; Datta Gupta, S.; Pandya, P.; Ralhan, R. CpG hypomethylation of MDR1 gene in tumor and serum of invasive ductal breast carcinoma patients. Clin. Biochem. 2010, 43, 373–379. [Google Scholar] [CrossRef]
- Zawadzka, I.; Jeleń, A.; Pietrzak, J.; Żebrowska-Nawrocka, M.; Michalska, K.; Szmajda-Krygier, D.; Mirowski, M.; Łochowski, M.; Kozak, J.; Balcerczak, E. The impact of ABCB1 gene polymorphism and its expression on non-small-cell lung cancer development, progression and therapy—Preliminary report. Sci. Rep. 2020, 10, 6188. [Google Scholar] [CrossRef]





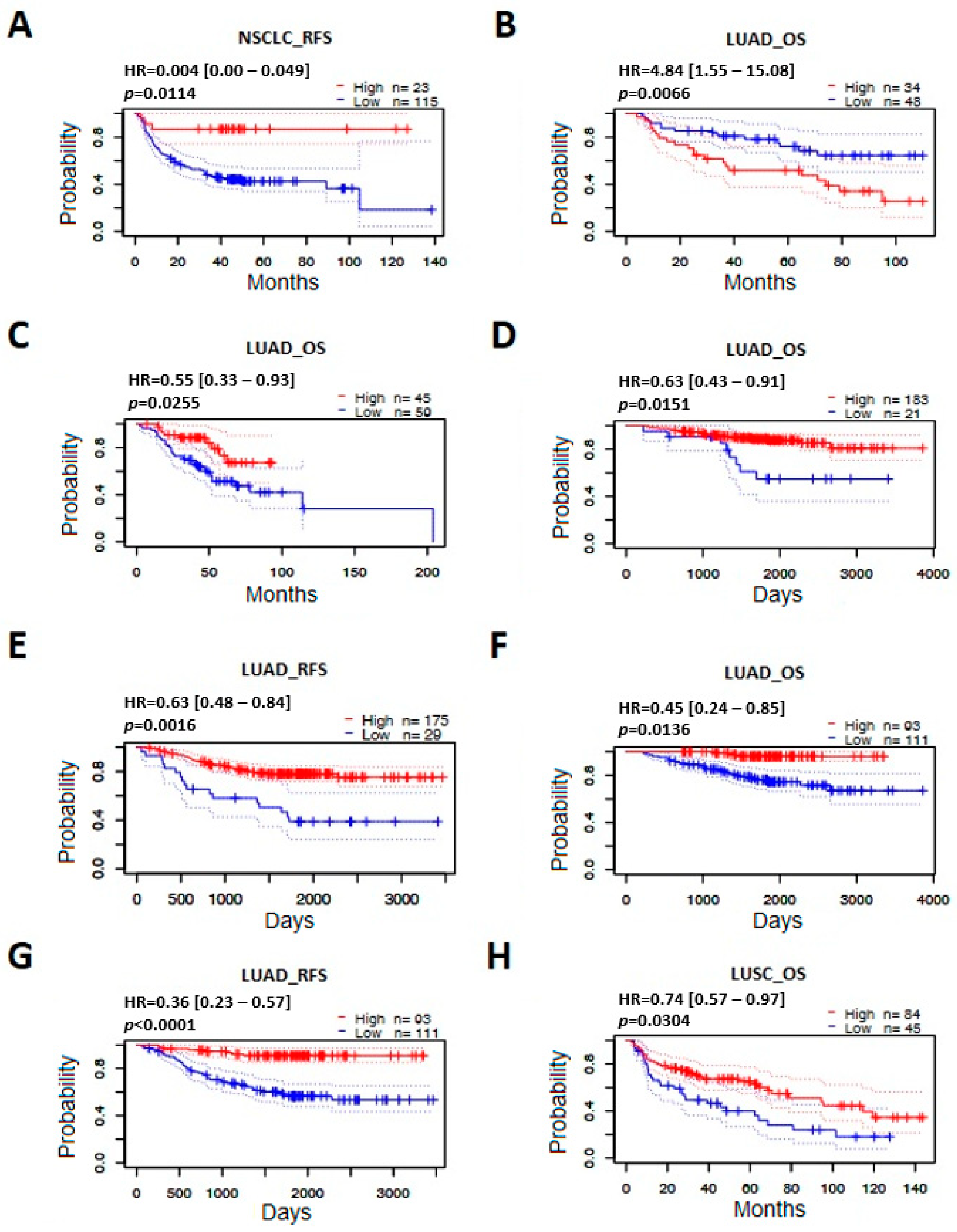

| Dataset | Lung Cancer Type | N | End Point | Cut Point | p (Cox) | HR [95% Cl] |
|---|---|---|---|---|---|---|
| GSE3141/ 243951_at | NSCLC | 111 | OS | 0.47 | 0.9497 | 0.99 [0.71–1.38] |
| GSE3141/ 209994_s_at | NSCLC | 111 | OS | 0.85 | 0.2595 | 1.19 [0.88–1.61] |
| GSE3141/ 209993_at | NSCLC | 111 | OS | 0.14 | 0.3762 | 0.86 [0.60–1.21] |
| GSE14814/ 209994_s_at | NSCLC | 90 | OS | 0.61 | 0.5441 | 1.44 [0.44–4.73] |
| GSE14814/ 209994_s_at | NSCLC | 90 | DSS | 0.67 | 0.2909 | 1.98 [0.56–7.04] |
| GSE14814/ 209993_at | NSCLC | 90 | OS | 0.23 | 0.1610 | 2.10 [0.74–5.94] |
| GSE14814/ 209993_at | NSCLC | 90 | DSS | 0.70 | 0.1069 | 2.44 [0.82–7.24] |
| GSE4716-GPL3694/ 2144 | NSCLC | 50 | OS | 0.28 | 0.3411 | 1.51 [0.65–3.51] |
| GSE4716-GPL3696/ 4235 | NSCLC | 50 | OS | 0.14 | 0.5693 | 1.23 [0.60–2.52] |
| GSE8894/ 209993_at | NSCLC | 138 | RFS | 0.68 | 0.2389 | 0.56 [0.22–1.47] |
| GSE8894/ 243951_at | NSCLC | 138 | RFS | 0.83 | 0.0114 | 0.04 [0.00–0.49] |
| GSE8894/ 209994_s_at | NSCLC | 138 | RFS | 0.63 | 0.4021 | 0.85 [0.58–1.25] |
| jacob-00182-CANDF/ 209994_s_at | LUAD | 82 | OS | 0.78 | 0.6817 | 1.12 [0.66–1.89] |
| jacob-00182-CANDF/ 209993_at | LUAD | 82 | OS | 0.59 | 0.0066 | 4.84 [1.55–15.08] |
| jacob-00182-HLM/ 209994_s_at | LUAD | 79 | OS | 0.27 | 0.7065 | 0.94 [0.66–1.32] |
| jacob-00182-HLM/ 209993_at | LUAD | 79 | OS | 0.80 | 0.2640 | 0.57 [0.21–1.53] |
| jacob-00182-MSK/ 209993_at | LUAD | 104 | OS | 0.39 | 0.9669 | 0.97 [0.26–3.68] |
| jacob-00182-MSK/ 209994_s_at | LUAD | 104 | OS | 0.57 | 0.0255 | 0.55 [0.33–0.93] |
| GSE13213/ A_23_P82523 | LUAD | 117 | OS | 0.26 | 0.9598 | 0.99 [0.66–1.48] |
| GSE31210/ 209993_at | LUAD | 204 | OS | 0.10 | 0.0151 | 0.63 [0.43–0.91] |
| GSE31210/ 209993_at | LUAD | 204 | RFS | 0.14 | 0.0016 | 0.63 [0.48–0.84] |
| GSE31210/ 243951_at | LUAD | 204 | OS | 0.54 | 0.0136 | 0.45 [0.24–0.85] |
| GSE31210/ 209994_s_at | LUAD | 204 | OS | 0.17 | 0.9183 | 1.03 [0.55–1.95] |
| GSE31210/ 243951_at | LUAD | 204 | RFS | 0.54 | 0.0000 | 0.36 [0.23–0.57] |
| GSE31210/ 209994_s_at | LUAD | 204 | RFS | 0.21 | 0.5167 | 0.85 [0.52–1.39] |
| jacob-00182-UM/ 209993_at | LUAD | 178 | OS | 0.52 | 0.6339 | 0.85 [0.44–1.64] |
| jacob-00182-UM/ 209994_s_at | LUAD | 178 | OS | 0.83 | 0.6900 | 0.94 [0.68–1.29] |
| GSE4573/ 209993_at | LUSC | 129 | OS | 0.43 | 0.4648 | 0.80 [0.44–1.46] |
| GSE4573/ 209994_s_at | LUSC | 129 | OS | 0.34 | 0.0304 | 0.74 [0.57–0.97] |
| GSE17710/ 31702 | LUSC | 56 | RFS | 0.45 | 0.3395 | 1.17 [0.85–1.61] |
| GSE17710/ 42809 | LUSC | 56 | OS | 0.64 | 0.6609 | 1.17 [0.59–2.33] |
| GSE17710/ 20677 | LUSC | 56 | RFS | 0.55 | 0.4410 | 1.22 [0.73–2.05] |
| GSE17710/ 31702 | LUSC | 56 | OS | 0.48 | 0.3012 | 1.20 [0.85–1.68] |
| GSE17710/ 20677 | LUSC | 56 | OS | 0.88 | 0.5863 | 1.17 [0.67–2.05] |
| GSE17710/ 42809 | LUSC | 56 | RFS | 0.64 | 0.5124 | 1.23 [0.66–2.31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeleń, A.M.; Strehl, B.; Szmajda-Krygier, D.; Pązik, M.; Balcerczak, E. Bioinformatics-Based Characterization of ATP-Binding Cassette Subfamily B Member 1 (ABCB1) Gene Expression in Non-Small-Cell Lung Cancer (NSCLC). Appl. Sci. 2023, 13, 6576. https://doi.org/10.3390/app13116576
Jeleń AM, Strehl B, Szmajda-Krygier D, Pązik M, Balcerczak E. Bioinformatics-Based Characterization of ATP-Binding Cassette Subfamily B Member 1 (ABCB1) Gene Expression in Non-Small-Cell Lung Cancer (NSCLC). Applied Sciences. 2023; 13(11):6576. https://doi.org/10.3390/app13116576
Chicago/Turabian StyleJeleń, Agnieszka Maria, Bartłomiej Strehl, Dagmara Szmajda-Krygier, Milena Pązik, and Ewa Balcerczak. 2023. "Bioinformatics-Based Characterization of ATP-Binding Cassette Subfamily B Member 1 (ABCB1) Gene Expression in Non-Small-Cell Lung Cancer (NSCLC)" Applied Sciences 13, no. 11: 6576. https://doi.org/10.3390/app13116576






