The Effect of Circadian Photoreceptors Stimulation on the Stress Response of Subjects with High Anxiety: A Pilot Study
Abstract
:Featured Application
Abstract
1. Introduction
2. Materials and Methods
3. Results
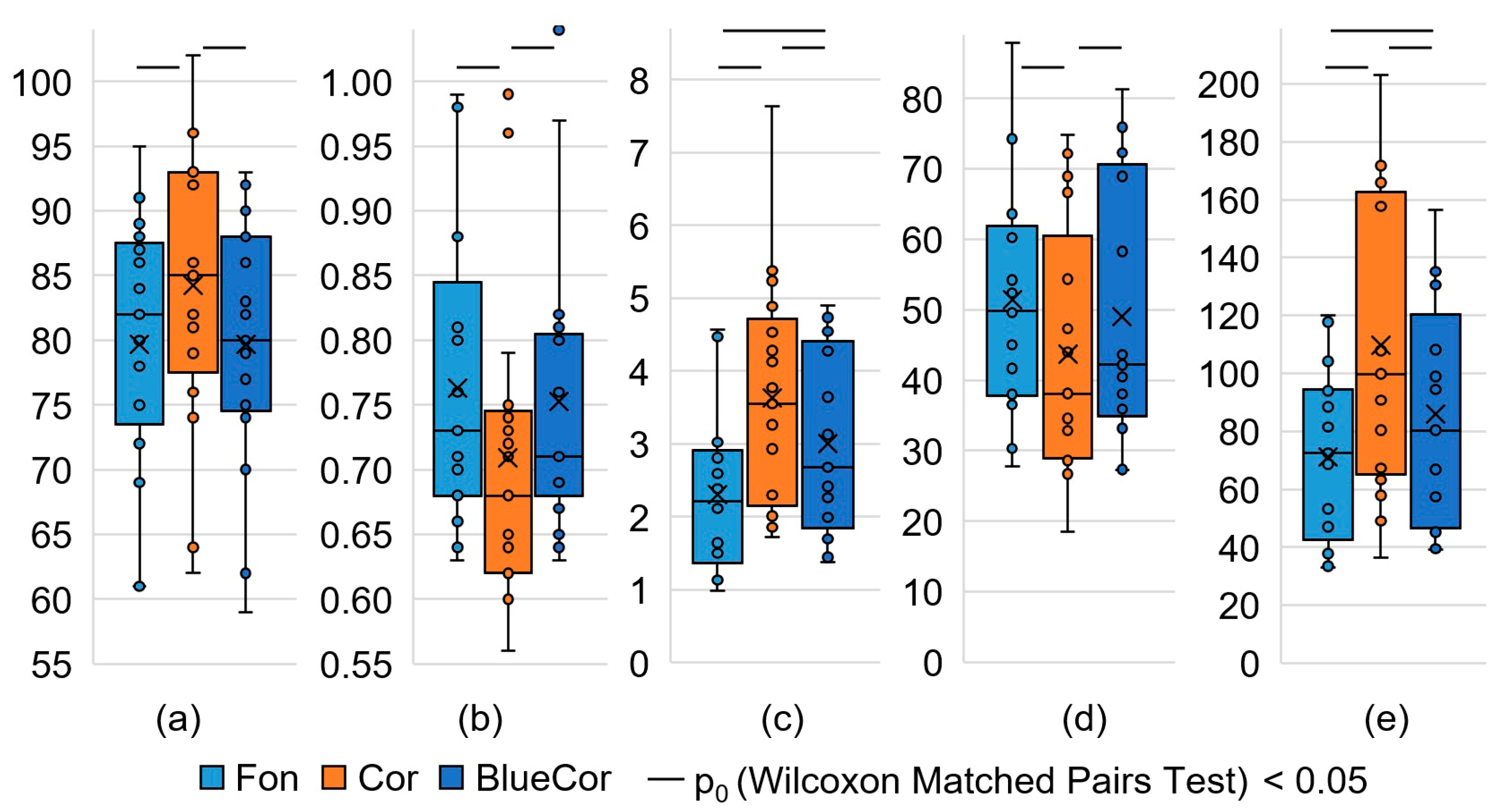
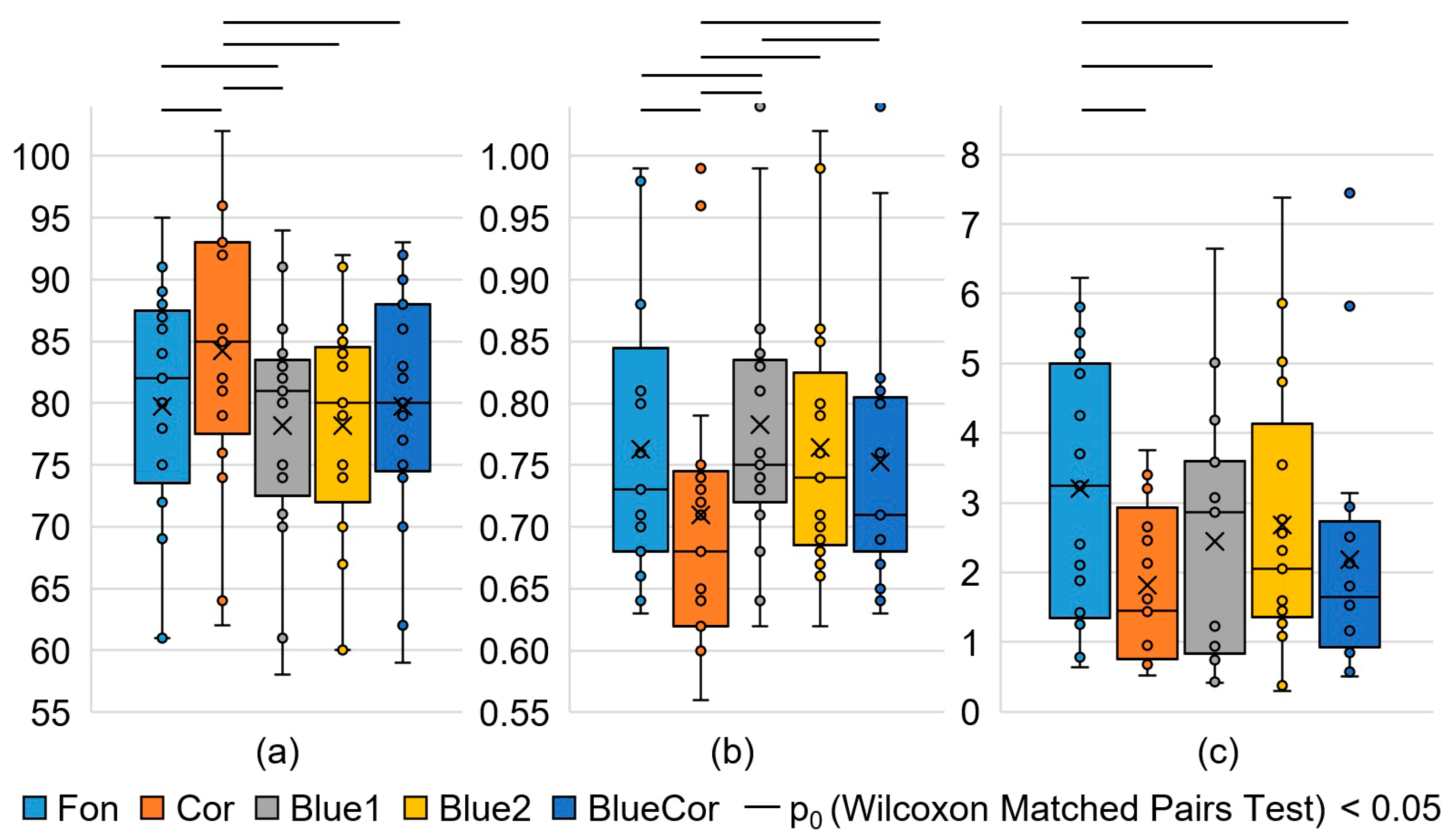
3.1. Analysis of HRV Parameters
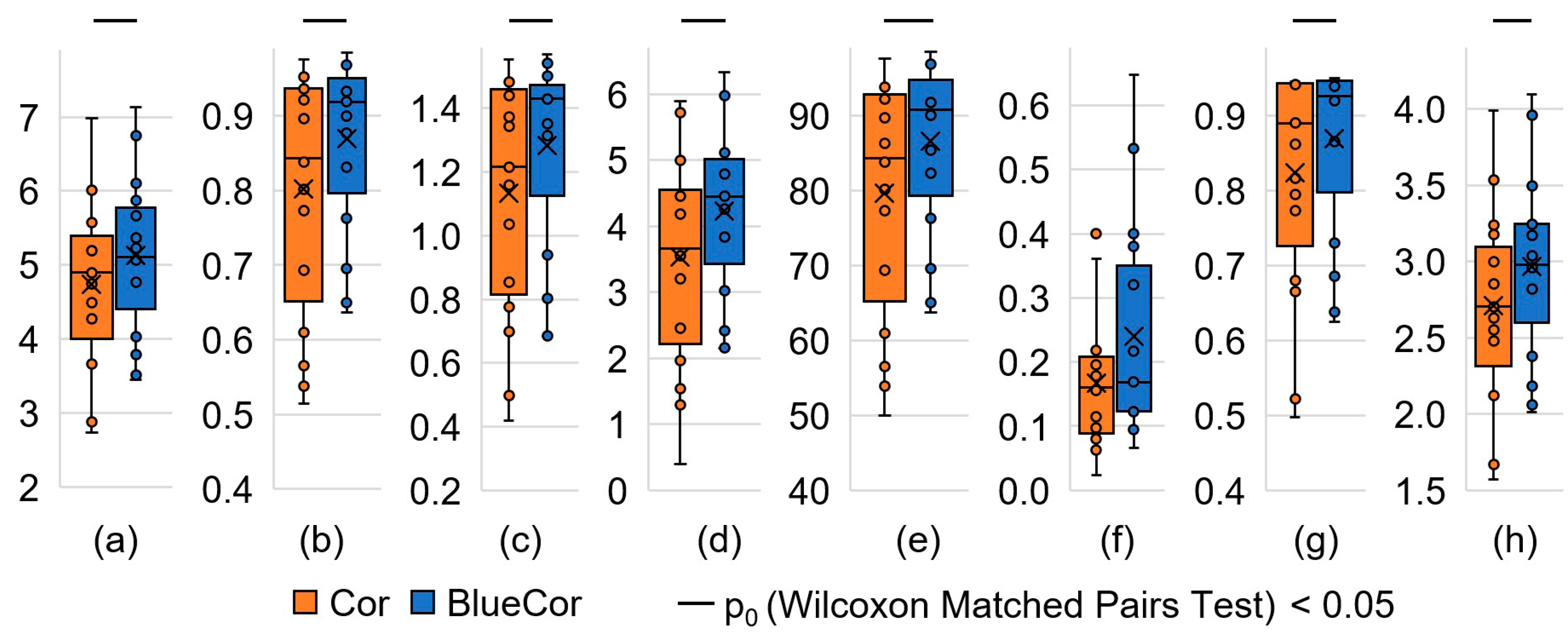
3.2. Analysis of the Cognitive Task Results
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Touitou, Y.; Coste, O.; Dispersyn, G.; Pain, L. Disruption of the Circadian System by Environmental Factors: Effects of Hypoxia, Magnetic Fields and General Anesthetics Agents☆. Adv. Drug Deliv. Rev. 2010, 62, 928–945. [Google Scholar] [CrossRef] [PubMed]
- Hattar, S.; Liao, H.-W.; Takao, M.; Berson, D.M.; Yau, K.-W. Melanopsin-Containing Retinal Ganglion Cells: Architecture, Projections, and Intrinsic Photosensitivity. Science 2002, 295, 1065–1070. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Kumbalasiri, T.; Carlson, S.M.; Wong, K.Y.; Krishna, V.; Provencio, I.; Berson, D.M. Induction of Photosensitivity by Heterologous Expression of Melanopsin. Nature 2005, 433, 745–749. [Google Scholar] [CrossRef] [PubMed]
- Bailes, H.J.; Lucas, R.J. Human Melanopsin Forms a Pigment Maximally Sensitive to Blue Light (λ max ≈ 479 Nm) Supporting Activation of G q/11 and G i/o Signalling Cascades. Proc. R. Soc. B 2013, 280, 20122987. [Google Scholar] [CrossRef]
- Richards, J.; Gumz, M.L. Advances in Understanding the Peripheral Circadian Clocks. FASEB J. 2012, 26, 3602–3613. [Google Scholar] [CrossRef]
- Agorastos, A.; Nicolaides, N.C.; Bozikas, V.P.; Chrousos, G.P.; Pervanidou, P. Multilevel Interactions of Stress and Circadian System: Implications for Traumatic Stress. Front. Psychiatry 2020, 10, 1003. [Google Scholar] [CrossRef]
- Leliavski, A.; Shostak, A.; Husse, J.; Oster, H. Impaired Glucocorticoid Production and Response to Stress in Arntl-Deficient Male Mice. Endocrinology 2014, 155, 133–142. [Google Scholar] [CrossRef]
- Destici, E.; Jacobs, E.H.; Tamanini, F.; Loos, M.; van der Horst, G.T.J.; Oklejewicz, M. Altered Phase-Relationship between Peripheral Oscillators and Environmental Time in Cry1 or Cry2 Deficient Mouse Models for Early and Late Chronotypes. PLoS ONE 2013, 8, e83602. [Google Scholar] [CrossRef]
- Dallmann, R.; Touma, C.; Palme, R.; Albrecht, U.; Steinlechner, S. Impaired Daily Glucocorticoid Rhythm in Per1 Brd Mice. J. Comp. Physiol A 2006, 192, 769–775. [Google Scholar] [CrossRef]
- Wright, K.P.; Drake, A.L.; Frey, D.J.; Fleshner, M.; Desouza, C.A.; Gronfier, C.; Czeisler, C.A. Influence of Sleep Deprivation and Circadian Misalignment on Cortisol, Inflammatory Markers, and Cytokine Balance. Brain Behav. Immun. 2015, 47, 24–34. [Google Scholar] [CrossRef]
- Karatsoreos, I.N.; Bhagat, S.; Bloss, E.B.; Morrison, J.H.; McEwen, B.S. Disruption of Circadian Clocks Has Ramifications for Metabolism, Brain, and Behavior. Proc. Natl. Acad. Sci. USA 2011, 108, 1657–1662. [Google Scholar] [CrossRef] [PubMed]
- Hanifin, J.P.; Lockley, S.W.; Cecil, K.; West, K.; Jablonski, M.; Warfield, B.; James, M.; Ayers, M.; Byrne, B.; Gerner, E.; et al. Randomized Trial of Polychromatic Blue-Enriched Light for Circadian Phase Shifting, Melatonin Suppression, and Alerting Responses. Physiol. Behav. 2019, 198, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, A.V.; Khivintseva, E.V.; Pyatin, V.F.; Sergeeva, M.S.; Antipov, O.I. Melatonin—Known and Novel Areas of Clinical Application. Neurosci. Behav. Phys. 2019, 49, 60–63. [Google Scholar] [CrossRef]
- Zakharov, A.V.; Khivintseva, E.V.; Pytin, V.F.; Sergeeva, M.S.; Antipov, O.I. Melatonin—Known problems and perspectives of clinical usage. Z. Nevrol. Psikhiatr. S.S. Korsakova 2017, 117, 74. [Google Scholar] [CrossRef] [PubMed]
- Zakharov, A.; Khivintseva, E. Clinical Use of Melatonin in the Treatment of Sleep Disorders. In Melatonin—The Hormone of Darkness and its Therapeutic Potential and Perspectives; Vlachou, M., Ed.; IntechOpen: London, UK, 2020; ISBN 978-1-83962-908-2. [Google Scholar]
- Pyatin, V.F. Ustrojstvo Dlya Funkcional’nogo Upravleniya Cirkadiannymi Chasami Organizma Cheloveka. RU Patent 182615 U1, 15 January 2018. (In Russian). [Google Scholar]
- Hernando, D.; Roca, S.; Sancho, J.; Alesanco, Á.; Bailón, R. Validation of the Apple Watch for Heart Rate Variability Measurements during Relax and Mental Stress in Healthy Subjects. Sensors 2018, 18, 2619. [Google Scholar] [CrossRef]
- Baevskij, R.M. Problema Ocenki i Prognozirovaniya Funkcional’nogo Sostoyaniya Organizma i ee Razvitie v Kosmicheskoj Medicine. Uspekhi Fiziol. Nauk. 2006, 37, 42–57. (In Russian) [Google Scholar]
- Kim, H.-G.; Cheon, E.-J.; Bai, D.-S.; Lee, Y.H.; Koo, B.-H. Stress and Heart Rate Variability: A Meta-Analysis and Review of the Literature. Psychiatry Investig. 2018, 15, 235–245. [Google Scholar] [CrossRef]
- Dimitriev, D.A.; Saperova, E.V.; Dimitriev, A.D. State Anxiety and Nonlinear Dynamics of Heart Rate Variability in Students. PLoS ONE 2016, 11, e0146131. [Google Scholar] [CrossRef]
- Shook, N.; Pena, P.; Fazio, R.H.; Sollers, J.J.; Thayer, J.F. Friend or foe: Heart rate varability and the negativity bias in learning about novel objects, Proceedings of the 47th Annual Meeting of the Society-for-Psychophysiological-Research. Psychophysiology 2007, 44, S39. [Google Scholar]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A Meta-Analysis of Heart Rate Variability and Neuroimaging Studies: Implications for Heart Rate Variability as a Marker of Stress and Health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Bishop, S.J. Neurocognitive Mechanisms of Anxiety: An Integrative Account. Trends Cogn. Sci. 2007, 11, 307–316. [Google Scholar] [CrossRef]
- Blons, E.; Arsac, L.M.; Gilfriche, P.; McLeod, H.; Lespinet-Najib, V.; Grivel, E.; Deschodt-Arsac, V. Alterations in Heart-Brain Interactions under Mild Stress during a Cognitive Task Are Reflected in Entropy of Heart Rate Dynamics. Sci. Rep. 2019, 9, 18190. [Google Scholar] [CrossRef]
- Shook, N.J.; Fazio, R.H.; Vasey, M.W. Negativity Bias in Attitude Learning: A Possible Indicator of Vulnerability to Emotional Disorders? J. Behav. Ther. Exp. Psychiatry 2007, 38, 144–155. [Google Scholar] [CrossRef]
- Litscher, D.; Wang, L.; Gaischek, I.; Litscher, G. The Influence of New Colored Light Stimulation Methods on Heart Rate Variability, Temperature, and Well-Being: Results of a Pilot Study in Humans. Evid.-Based Complement. Altern. Med. 2013, 2013, 1–7. [Google Scholar] [CrossRef]
- Kuang, X.D.; Yu, X.B.; Cao, Y.; Li, D.S.; Zhu, H.Y. Interaction between the Circadian Clock and Chronic Stress. Biomed. Environ. Sci. 2018, 31, 686–699. [Google Scholar] [CrossRef]
- Helfrich-Förster, C. Interactions between Psychosocial Stress and the Circadian Endogenous Clock. PsyCh J. 2017, 6, 277–289. [Google Scholar] [CrossRef]
- Oster, H. The Interplay between Stress, Circadian Clocks, and Energy Metabolism. J. Endocrinol. 2020, 247, R13–R25. [Google Scholar] [CrossRef]
- Koch, C.E.; Leinweber, B.; Drengberg, B.C.; Blaum, C.; Oster, H. Interaction between Circadian Rhythms and Stress. Neurobiol. Stress 2017, 6, 57–67. [Google Scholar] [CrossRef]
- Landgraf, D.; Long, J.E.; Proulx, C.D.; Barandas, R.; Malinow, R.; Welsh, D.K. Genetic Disruption of Circadian Rhythms in the Suprachiasmatic Nucleus Causes Helplessness, Behavioral Despair, and Anxiety-like Behavior in Mice. Biol. Psychiatry 2016, 80, 827–835. [Google Scholar] [CrossRef]
- Portaluppi, F.; Tiseo, R.; Smolensky, M.H.; Hermida, R.C.; Ayala, D.E.; Fabbian, F. Circadian Rhythms and Cardiovascular Health. Sleep Med. Rev. 2012, 16, 151–166. [Google Scholar] [CrossRef]
- Vandewalle, G.; Gais, S.; Schabus, M.; Balteau, E.; Carrier, J.; Darsaud, A.; Sterpenich, V.; Albouy, G.; Dijk, D.J.; Maquet, P. Wavelength-Dependent Modulation of Brain Responses to a Working Memory Task by Daytime Light Exposure. Cereb. Cortex 2007, 17, 2788. [Google Scholar] [CrossRef] [PubMed]
- Kuts, A.; Poluektov, M.; Zakharov, A.; Govzman, A.; Ponomareva, I.; Yakupov, E.; Tikhomirova, O.; Sviryaev, Y.; Yakovlev, A.; Polyakov, A.; et al. Clinical and neurophysiological characteristics of 89 patients with narcolepsy and cataplexy from the Russian Narcolepsy Network. J. Clin. Sleep Med. 2023, 19, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Kudielka, B.M.; Kirschbaum, C. Sex Differences in HPA Axis Responses to Stress: A Review. Biol. Psychol. 2005, 69, 113–132. [Google Scholar] [CrossRef] [PubMed]
- Bangasser, D.A.; Valentino, R.J. Sex Differences in Stress-Related Psychiatric Disorders: Neurobiological Perspectives. Front. Neuroendocrinol. 2014, 35, 303–319. [Google Scholar] [CrossRef]
- Joye, D.A.M.; Evans, J.A. Sex Differences in Daily Timekeeping and Circadian Clock Circuits. Semin. Cell Dev. Biol. 2022, 126, 45–55. [Google Scholar] [CrossRef]
| Parameter | Definition |
|---|---|
| SDNN, ms | Standard deviation of the NN-interval duration for the sample |
| RMSSD, ms | The square root of the sum of the squared differences in the durations of consecutive NN-intervals pairs |
| pNN50, % | Percentage of the adjacent NN-intervals pairs that differ in duration by more than 50 ms |
| HRVind, c.u. | Triangular index of heart rate variability |
| Heart rate, beats/min | Heart rate |
| Mode, ms | NN-interval duration mode |
| AMo, % | Amplitude of the NN-interval duration mode |
| DX, ms | Variation range of NN-interval duration |
| VLF, thousand ms2 | The power of very low-frequency (0.003–0.04 Hz) fluctuations in the duration of NN-interval |
| LF, thousand ms2 | The power of low-frequency (0.04–0.15 Hz) fluctuations in the duration of NN-interval |
| HF, ms2 | The power of high-frequency (0.15–0.40 Hz) fluctuations in the duration NN-interval |
| Total, thousand ms2 | The power of fluctuations in the duration of NN-interval in all ranges (0.003–0.40 Hz) |
| LF norm, % | The normalized value of the power of low-frequency (0.04–0.15 Hz) fluctuations in the NN-interval duration |
| HF norm, % | The normalized value of the power of high-frequency (0.15–0.40 Hz) fluctuations in the NN-interval duration |
| LF/HF | The ratio of low-frequency (0.04–0.15 Hz) and high-frequency (0.15–0.40 Hz) fluctuations in the NN-interval duration |
| SIM, c.u. | Intensity index of influences of the sympathetic division of the autonomic nervous system |
| PAR, c.u. | Intensity index of influences of the parasympathetic division of the autonomic nervous system |
| IB, c.u. | Stress index of regulatory systems according to R.M. Baevsky |
| Parameter | Definition | Formula |
|---|---|---|
| A, symbols/s | Attention speed | Number of letters viewed/operating time |
| T1, c.u. | Work accuracy (option 1) | Total crossed out/should have been crossed out |
| T2, c.u. | Work accuracy (option 2) | Correctly crossed out/should have been crossed out |
| T3, c.u. | Work accuracy (option 3) | Correctly crossed out/(crossed out + skipped) |
| E, signs | Coefficient of mental productivity | Number of letters viewed × 2nd option for work accuracy |
| Au, symbols/s | Coefficient of mental performance | Attention speed × ((correctly crossed out − skipped)/should have been crossed out) |
| K, % | Concentration of attention | Correctly crossed out/should have been crossed out |
| Ku, c.u. | Stability of concentration | Lines viewed × (lines viewed/(letters omitted and erroneously crossed out + 1)) |
| V, symbols | The volume of visual information | 0.5936 × number of letters viewed |
| Q, symbols/s | The speed of processing visual information | (Volume of visual information − 2.807 × (skipped + erroneously crossed out))/operating time |
| Baseline Condition | The Letter Cancellation Test | Blue Sky Pro Session | The Letter Cancellation Test | |
|---|---|---|---|---|
| 5 min | 5 min | 20 min | 5 min | |
| 5 min | 5 min | |||
| HRV | HRV | HRV | HRV | HRV |
| Fon | Cor | Blue1 | Blue2 | BlueCor |
| Parameter | Condition 1. | Comparison 2 (p0) | ||||||
|---|---|---|---|---|---|---|---|---|
| Fon | Cor | Blue1 | Blue2 | BlueCor | Fon vs. Cor | Fon vs. BluCor | Cor vs. BluCor | |
| SDNN, ms | 61.6 ±2.9 | 55.1 ±3.7 | 61.7 ±4.6 | 62.4 ±3.4 | 56.9 ±3.3 | 0.049 | 0.136 | 0.379 |
| RMSSD, ms | 50 (38; 60) | 38 (29; 54) | 48 (36; 63) | 45 (38; 67) | 42 (36; 69) | 0.044 | 0.177 | 0.044 |
| NN50, ms | 86 (58; 127) | 49 (29; 117) | 92 (44; 124) | 81 (46; 145) | 57 (45; 155) | 0.080 | 0.276 | 0.055 |
| HRVind, c.u. | 11.9 ± 0.6 | 10.2 ± 0.7 | 11.1 ± 0.7 | 12.1 ± 0.7 | 10.5 ± 0.6 | 0.023 | 0.079 | 0.351 |
| Moda, ms | 730 (680; 810) | 680 (620; 740) | 750 (730; 830) | 740 (690; 800) | 710 (690; 800) | 0.002 | 0.394 | 0.002 |
| AMo, % | 7.8 ± 0.4 | 9.5 ± 0.6 | 8.8 ± 0.7 | 7.7 ± 0.5 | 9.0 ± 0.4 | 0.016 | 0.081 | 0.209 |
| DX, ms | 350 (330; 390) | 330 (260; 390) | 360 (310; 390) | 310 (300; 370) | 330 (310; 380) | 0.047 | 0.281 | 0.551 |
| Heart rate, bpm | 79.7 ± 2.4 | 84.2 ± 2.7 | 78.2 ± 2.3 | 78.2 ± 2.3 | 79.7 ± 2.4 | 0.002 | 0.778 | 0.001 |
| VLF, thousand ms2 | 2.0 (1.2; 3.1) | 1.5 (1.0; 2.8) | 2.9 (1.5; 3.4) | 2.7 (1.6; 5.2) | 1.6 (1.1; 2.3) | 0.523 | 0.266 | 0.868 |
| LF, thousand ms2 | 3.9 (2.4; 4.3) | 2.1 (1.7; 3.4) | 2.6 (1.6; 3.9) | 4.6 (2.1; 6.1) | 2.3 (2.2; 2.9) | 0.025 | 0.035 | 0.554 |
| HF, thousand ms2 | 3.2 (1.4; 4.8) | 1.4 (0.8; 2.7) | 2.9 (0.9; 3.6) | 2.1 (1.5; 3.5) | 1.6 (0.9; 2.5) | 0.006 | 0.044 | 0.332 |
| Total, thousand ms2 | 9.5 ± 1.1 | 6.5 ± 0.7 | 9.5 ± 1.5 | 10.0 ± 1.2 | 6.9 ± 0.8 | 0.007 | 0.019 | 0.554 |
| LF norm, % | 55.1 ± 3.8 | 57.7 ± 3.5 | 58.1 ± 3.7 | 60.8 ± 3.5 | 57.9 ± 4.5 | 0.523 | 0.831 | 0.868 |
| HF norm, % | 44.9 ± 3.8 | 42.3 ± 3.5 | 41.9 ± 3.7 | 39.2 ± 3.5 | 42.1 ± 4.5 | 0.523 | 0.831 | 0.868 |
| LF/HF | 1.02 (0.81; 2.04) | 1.30 (0.93; 1.99) | 1.18 (0.86; 2.11) | 1.53 (1.08; 2.57) | 1.53 (0.68; 3.03) | 0.796 | 0.758 | 0.943 |
| SIM, c.u. | 2.3 ± 0.3 | 3.6 ± 0.4 | 2.9 ± 0.5 | 2.4 ± 0.3 | 3.0 ± 0.3 | 0.006 | 0.006 | 0.049 |
| PAR, c.u. | 14.6 ± 0.8 | 12.0 ± 0.9 | 13.7 ± 0.9 | 14.5 ± 0.9 | 13.1 ± 0.8 | 0.031 | 0.093 | 0.332 |
| IB, c.u. | 71 ± 7 | 110 ± 13 | 81 ± 10 | 73 ± 8 | 86 ± 9 | 0.006 | 0.049 | 0.028 |
| Parameter | Condition 1 | Comparison (Wilcoxon Matched Pairs Test) | |||
|---|---|---|---|---|---|
| Cor | BlueCor | W | Z | p0 | |
| A, symbols/s | 4.7 ± 0.3 | 5.1 ± 0.3 | 18 | 2.769 | 0.006 |
| T1, c.u. | 0.84 (0.69; 0.94) | 0.92 (0.83; 0.93) | 24 | 2.485 | 0.013 |
| T2, c.u. | 0.84 (0.69; 0.92) | 0.91 (0.82; 0.93) | 23 | 2.533 | 0.011 |
| T3, c.u. | 0.94 (0.91; 0.96) | 0.95 (0.91; 0.97) | 58 | 0.876 | 0.381 |
| E, symbols | 1216 (853; 1439) | 1430 (1314; 1446) | 21 | 2.627 | 0.009 |
| Au, symbols/s | 3.5 ± 0.4 | 4.2 ± 0.3 | 5 | 3.385 | 0.001 |
| K, % | 84 (69; 92) | 91 (82; 93) | 20 | 2.675 | 0.007 |
| Ku, c.u. | 160 (97; 200) | 169 (123; 320) | 42 | 1.633 | 0.102 |
| V, symbols | 890 (773; 943) | 925 (865; 946) | 22 | 2.379 | 0.017 |
| Q, symbols/s | 2.7 ± 0.2 | 3.0 ± 0.1 | 9 | 3.195 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergeeva, M.; Borisova, O.; Romanchuk, N.; Videnin, A.; Pyatin, V.; Shusharina, N.; Zakharov, A.V.; Kolsanov, A. The Effect of Circadian Photoreceptors Stimulation on the Stress Response of Subjects with High Anxiety: A Pilot Study. Appl. Sci. 2023, 13, 6679. https://doi.org/10.3390/app13116679
Sergeeva M, Borisova O, Romanchuk N, Videnin A, Pyatin V, Shusharina N, Zakharov AV, Kolsanov A. The Effect of Circadian Photoreceptors Stimulation on the Stress Response of Subjects with High Anxiety: A Pilot Study. Applied Sciences. 2023; 13(11):6679. https://doi.org/10.3390/app13116679
Chicago/Turabian StyleSergeeva, Mariya, Oksana Borisova, Natalia Romanchuk, Arseniy Videnin, Vasiliy Pyatin, Natalia Shusharina, Alexander V. Zakharov, and Alexander Kolsanov. 2023. "The Effect of Circadian Photoreceptors Stimulation on the Stress Response of Subjects with High Anxiety: A Pilot Study" Applied Sciences 13, no. 11: 6679. https://doi.org/10.3390/app13116679






