Hydrothermal Preparation of Faceted Vesicles Made of Span 40 and Tween 40 and Their Characterization
Abstract
1. Introduction
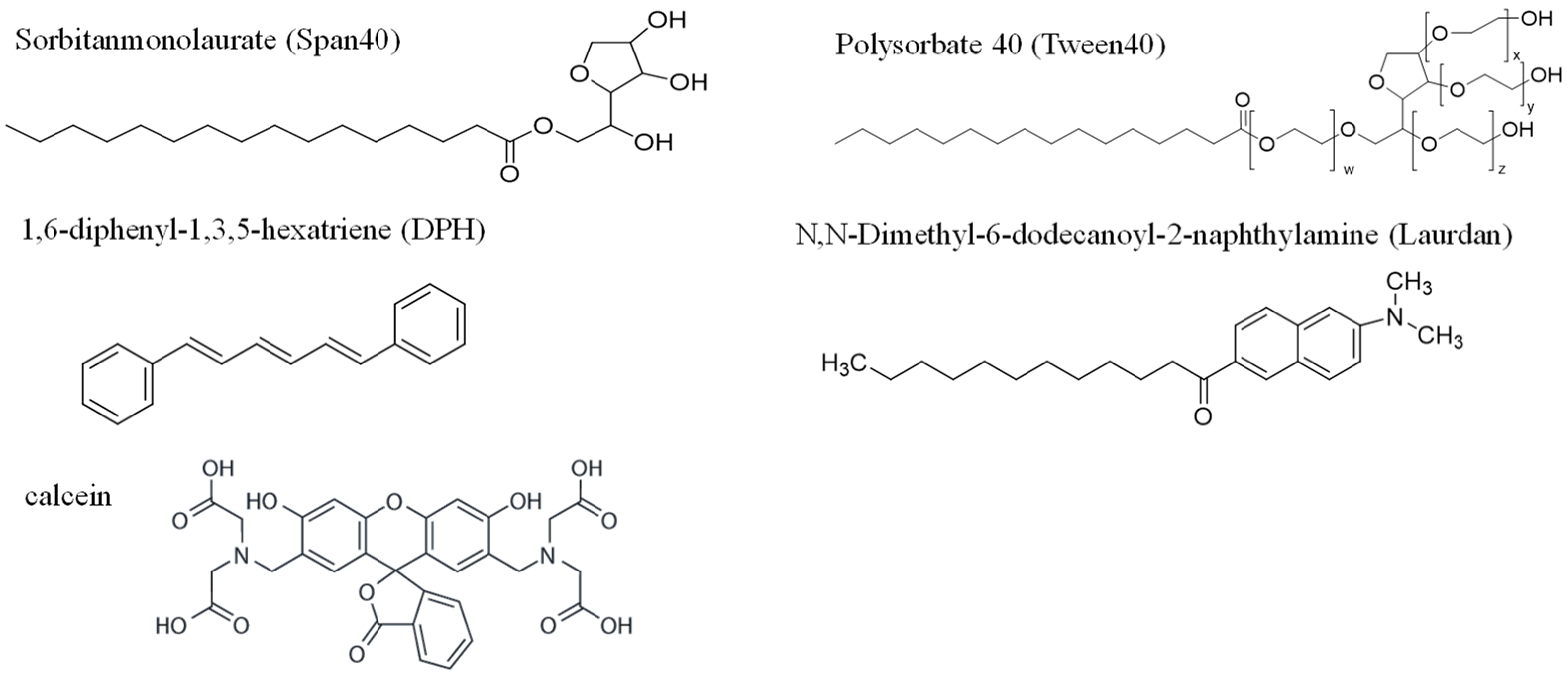
2. Materials and Methods
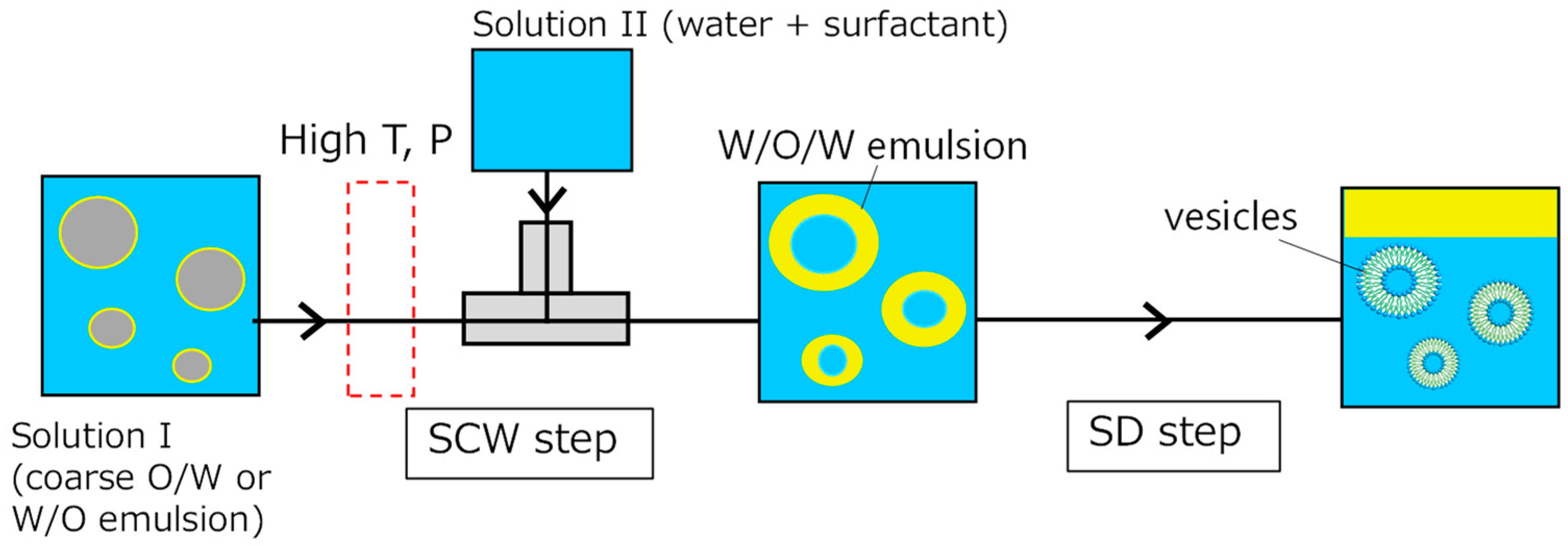
2.1. SCW/SD Method
2.2. Hydration Method
2.3. Membrane Characterization
2.4. Calcein Leakage
3. Results
3.1. Operational Conditions
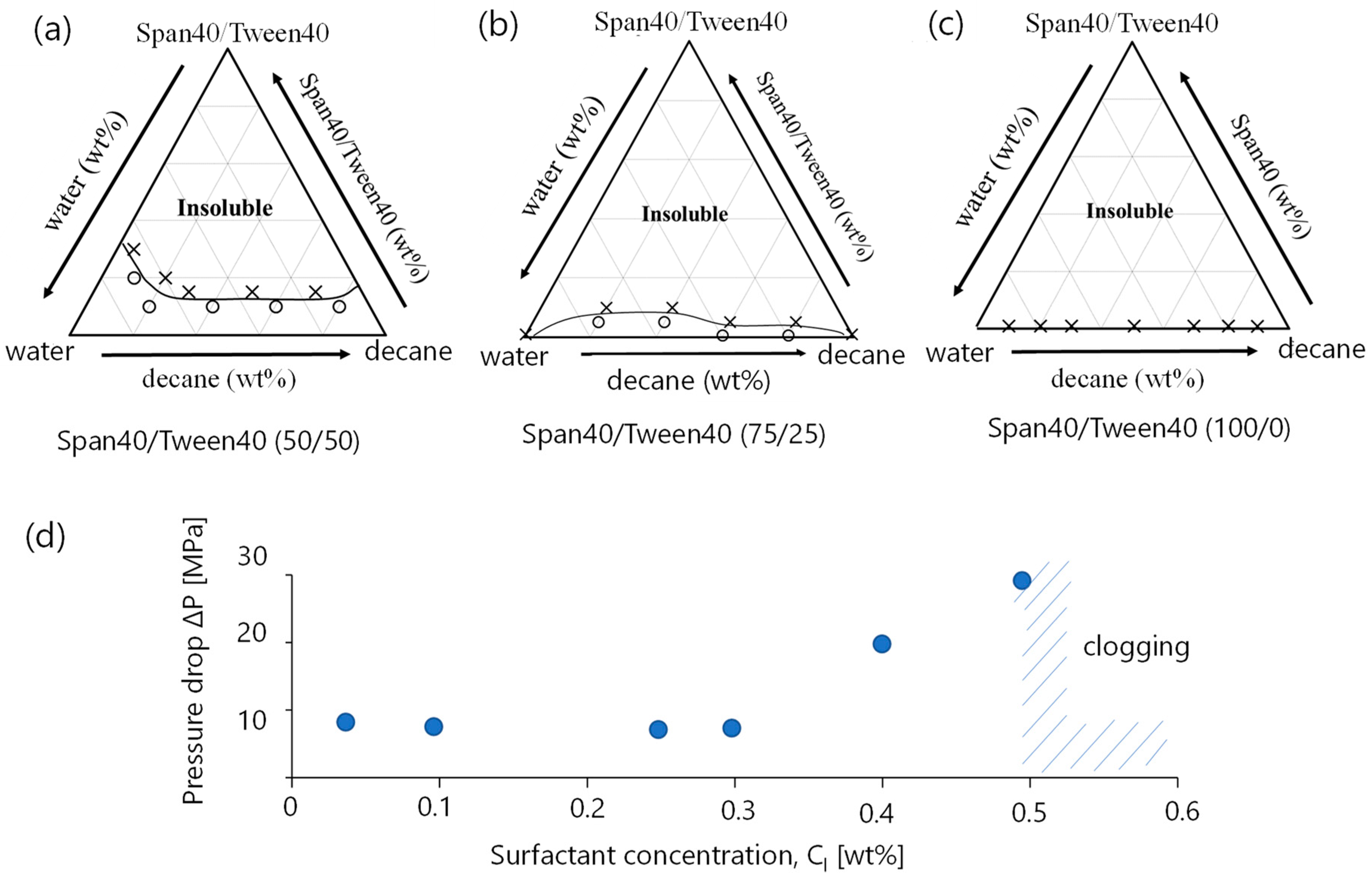
3.1.1. Phase Diagram
3.1.2. Pressure Drop
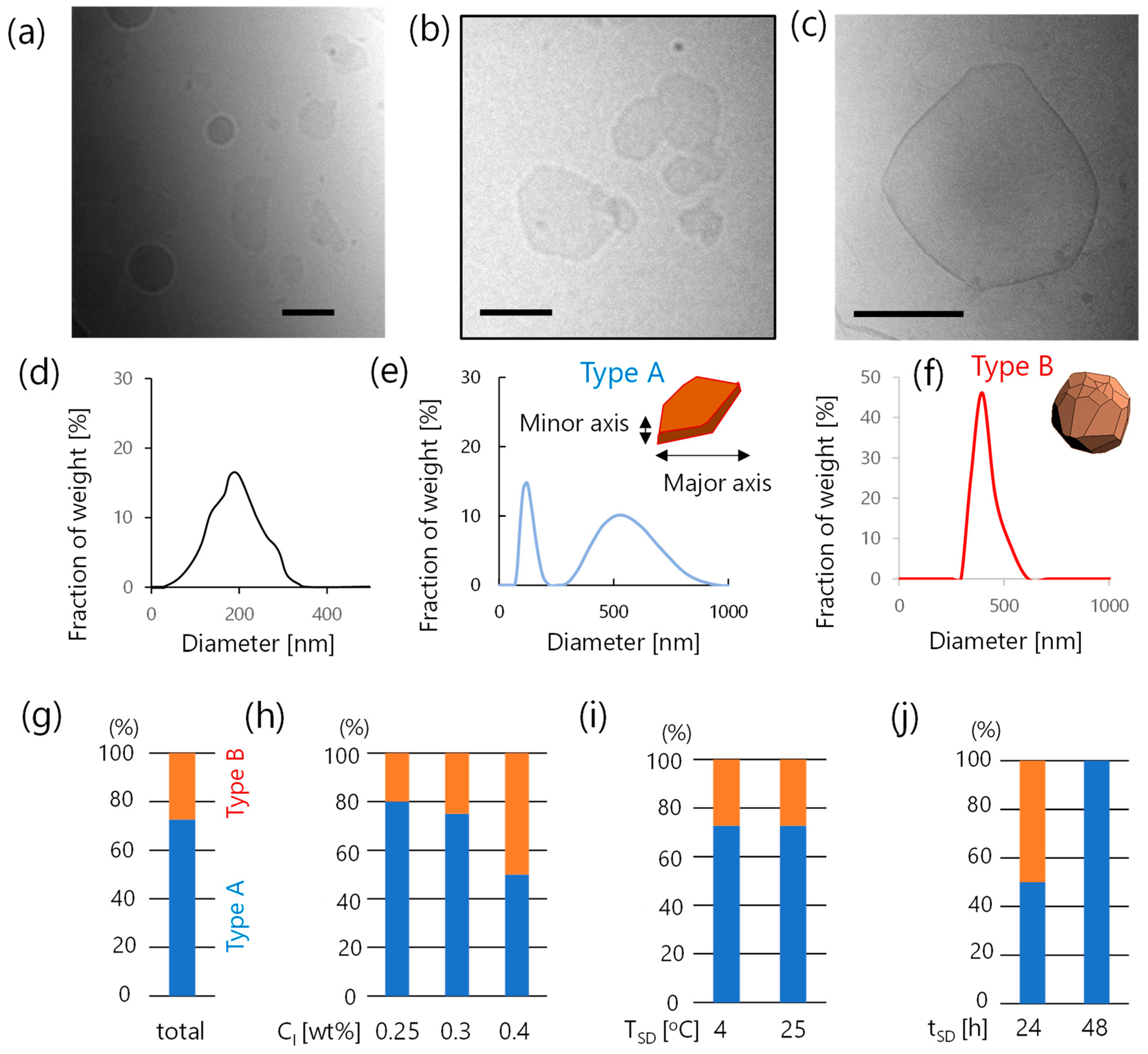
3.2. Formation Propensity of Vesicles
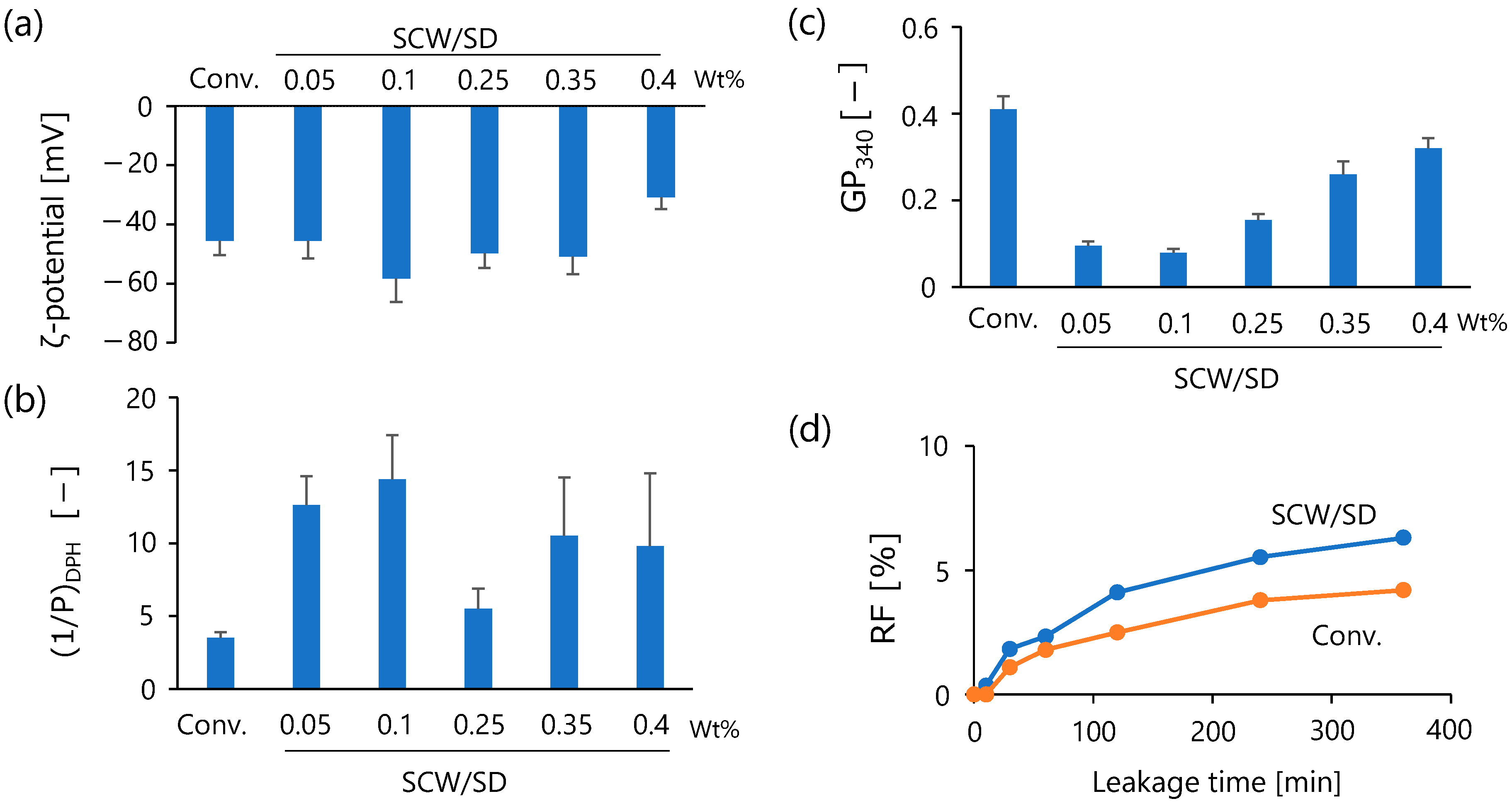
3.3. Characterization of Faceted Vesicles
3.3.1. Membrane Properties
3.3.2. Calcein Leakage Behavior
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guimarães, D.; Cavaco-Paulo, A.; Nogueira, E. Design of liposomes as drug delivery system for therapeutic applications. Int. J. Pharm. 2021, 601, 120571. [Google Scholar] [CrossRef] [PubMed]
- Walde, P.; Cosentino, K.; Engel, H.; Stano, P. Giant vesicles: Preparations and applications. ChemBioChem 2010, 11, 848–865. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.; Zhou, Q. Lipid Nanoparticles─From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Ge, X.; Wei, M.; He, S.; Yuan, W.-E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Chen, S.; Hanning, S.; Falconer, J.; Locke, M.; Wen, J. Recent advances in non-ionic surfactant vesicles (niosomes): Fabrication, characterization, pharmaceutical and cosmetic applications. Eur. J. Pharm. Biopharm. 2019, 144, 18–39. [Google Scholar] [CrossRef]
- Araste, F.; Aliabadi, A.; Abnous, K.; Taghdisi, S.M.; Ramezani, M.; Alibolandi, M. Self-assembled polymeric vesicles: Focus on polymersomes in cancer treatment. J. Control. Release 2021, 330, 502–528. [Google Scholar] [CrossRef]
- Kato, K.; Walde, P.; Koine, N.; Ichikawa, S.; Ishikawa, T.; Nagahama, R.; Ishihara, T.; Tsujii, T.; Shudou, M.; Omokawa, Y.; et al. Temperature-Sensitive Nonionic Vesicles Prepared from Span 80 (Sorbitan Monooleate). Langmuir 2008, 24, 10762–10770. [Google Scholar] [CrossRef]
- Hayashi, K.; Iwai, H.; Kamei, T.; Iwamoto, K.; Shimanouchi, T.; Fujita, S.; Nakamura, H.; Umakoshi, H. Tailor-made drug carrier: Comparison of formation-dependent physicochemical properties within self-assembled aggregates for an optimal drug carrier. Colloids Surf. B Biointerfaces 2017, 152, 269–276. [Google Scholar] [CrossRef]
- Hayashi, K.; Shimanouchi, T.; Kato, K.; Miyazaki, T.; Nakamura, A.; Umakoshi, H. Span 80 vesicles have a more fluid, flexible and “wet” surface than phospholipid liposomes. Colloids Surf. B Biointerfaces 2011, 87, 28–35. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Hayashi, T.; Toramoto, K.; Fukuma, S.; Hayashi, K.; Yasuhara, K.; Kimura, Y. Microfluidic and hydrothermal preparation of vesicles using sorbitan monolaurate/polyoxyethylene (20) sorbitan monolaurate(Span20/Tween20). Colloids Surf. B Biointerfaces 2021, 205, 111836. [Google Scholar] [CrossRef]
- Zumbuehl, A. Artificial Phospholipids and Their Vesicles. Langmuir 2018, 35, 10223–10232. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Demé, B.; Gulik-Krzywicki, T.; Dedieu, J.-C.; Vautrin, C.; Désert, S.; Perez, E.; Zemb, T. Self-assembly of regular hollow icosahedra in salt-free catanionic solutions. Nature 2001, 411, 672–675. [Google Scholar] [CrossRef]
- Deng, Y.; Ling, J.; Li, M. Physical stimuli-responsive liposomes and polymersomes as drug delivery vehciles based on phase transitions in the membrane. Nanoscale 2018, 10, 6781–6800. [Google Scholar] [CrossRef]
- Rosa, M.; Infante, M.R.; Miguel, M.d.G.; Lindman, B. Spontaneous Formation of Vesicles and Dispersed Cubic and Hexagonal Particles in Amino Acid-Based Catanionic Surfactant Systems. Langmuir 2006, 22, 5588–5596. [Google Scholar] [CrossRef]
- Feitosa, E. Spontaneous vesicles of sodium dihexadecylphosphate in HEPES buffer. J. Colloid Interface Sci. 2008, 320, 608–610. [Google Scholar] [CrossRef]
- González-Pérez, A.; Schmutz, M.; Waton, G.; Romero, M.J.; Krafft, M.P. Isolated Fluid Polyhedral Vesicles. J. Am. Chem. Soc. 2007, 129, 756–757. [Google Scholar] [CrossRef]
- Zhang, J.; Xu, G.; Song, A.; Wang, L.; Lin, M.; Dong, Z.; Yang, Z. Faceted fatty acid vesicles formed from single-tailed perfluorinated surfactants. Soft Matter 2015, 11, 7143–7150. [Google Scholar] [CrossRef]
- Noguchi, H. Polyhedral vesicles: A Brownian dynamics simulation. Phys. Rev. E 2003, 67, 041901. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Kawasak, H.; Fuse, M.; Umakoshi, H.; Kuboi, R. Membrane fusion mediated by phospholipase C under endo-somal pH conditions. Coll. Surf. B 2013, 103, 75–83. [Google Scholar] [CrossRef]
- Tribet, C.; Vial, F. Flexible macromolecules attached to lipid bilayers: Impact on fluidity, curvature, permeability and stability of the membranes. Soft Matter 2007, 4, 68–81. [Google Scholar] [CrossRef] [PubMed]
- Castangia, I.; Manca, M.L.; Caddeo, C.; Maxia, A.; Murgia, S.; Pons, R.; Demurtas, D.; Pando, D.; Falconieri, D.; Peris, J.E.; et al. Faceted phospholipid vesicles tailored for the delivery of Santolina insularis essential oil to the skin. Colloids Surf. B Biointerfaces 2015, 132, 185–193. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Ou-Yang, Z.-C.; Hao, J.; Lin, H.; Jiang, L.; Liu, Z.; Gao, X. The mechanism for the transition from vesicles to punctured lamellae and faceted vesicles in cationic and anionic fluorinated surfactant mixture. Colloids Surf. A Physicochem. Eng. Asp. 2016, 500, 40–44. [Google Scholar] [CrossRef]
- Nanikashvili, P.M.; Butenko, A.V.; Deutsch, M.; Lee, D.; Sloutskin, E. Salt-induced stability and modified interfacial energetics in self-faceting emulsion droplets. J. Colloid Interface Sci. 2022, 621, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Berton, C.; Genot, C.; Guibert, D.; Ropers, M.-H. Effect of lateral heterogeneity in mixed surfactant-stabilized interfaces on the oxidation of unsaturated lipids in oil-in-water emulsions. J. Colloid Interface Sci. 2012, 377, 244–250. [Google Scholar] [CrossRef]
- Hayashi, K.; Iwai, H.; Shimanouchi, T.; Umakoshi, H.; Iwasaki, T.; Kato, A.; Nakamura, H. Formation of lens-like vesicles induced via microphase separations on a sorbitan monoester membrane with different headgroups. Colloids Surf. B Biointerfaces 2015, 135, 235–242. [Google Scholar] [CrossRef]
- Chen, Y.; Qiao, F.; Fan, Y.; Han, Y.; Wang, Y. Interactions of Phospholipid Vesicles with Cationic and Anionic Oligomeric Surfactants. J. Phys. Chem. B 2017, 121, 7122–7132. [Google Scholar] [CrossRef]
- Kunstsche, J.; Horst, J.; Bunjes, H. Cryogenic transimission electron microscopy (cryo-TEM) for studying the morphology of colloidal drug delivery systems. Int. J. Pharm. 2011, 417, 120–137. [Google Scholar] [CrossRef]
- Lasic, D.D. Mechanisms of Liposome Formation. J. Liposome Res. 1995, 5, 431–441. [Google Scholar] [CrossRef]
- Zook, J.M.; Vreeland, W.N. Effects of temperature, acyl chain length, and flow-rate ratio on liposome formation and size in a microfluidic hydrodynamic focusing device. Soft Matter 2010, 6, 1352–1360. [Google Scholar] [CrossRef]
- Sanders, C.R.; Landis, G.C. Reconstitution of Membrane Proteins into Lipid-Rich Bilayered Mixed Micelles for NMR Studies. Biochemistry 1995, 34, 4030–4040. [Google Scholar] [CrossRef]
- Vold, R.R.; Prosser, R. Magnetically Oriented Phospholipid Bilayered Micelles for Structural Studies of Polypeptides. Does Ideal Bicelle Exist? J. Magn. Reson. Ser. B 1996, 113, 267–271. [Google Scholar] [CrossRef]
- Dugourc, E. Bicelles and nanodiscs for biophysical chemistry. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183478. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Umakoshi, H.; Kuboi, R. Kinetic Study on Giant Vesicle Formation with Electroformation Method. Langmuir 2009, 25, 4835–4840. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Umakoshi, H.; Kuboi, R. Growth behavior of giant vesicles using the electroformation method: Effect of proteins on swelling and deformation. J. Colloid Interface Sci. 2013, 394, 269–276. [Google Scholar] [CrossRef]
- Bowick, M.J.; Sknepnek, R. Pathways to faceting of vesicles. Soft Matter 2013, 9, 8088–8095. [Google Scholar] [CrossRef]
- Olvera de la Cruz, M. Mesoscale studies of ionic closed membranes with polyhedral geometries. APL Mater. 2016, 4, 061102. [Google Scholar] [CrossRef]
- Guttman, S.; Ocko, B.M.; Deutsch, M.; Sloutskin, E. From faceted vesicles to liquid icoshedra: Where topology and crystallography meet. Curr. Opin. Colloid Interface Sci. 2016, 22, 35–40. [Google Scholar] [CrossRef]
- Shimanouchi, T.; Ishii, H.; Yoshimoto, N.; Umakoshi, H.; Kuboi, R. Calcein permeation across phosphatidylcholine bilayer membrane: Effects of membrane fluidity, liposome size, and immobilization. Colloids Surf. B Biointerfaces 2009, 73, 156–160. [Google Scholar] [CrossRef]
- Fukuma, S.; Shimanouchi, T.; Hayashi, K.; Kimura, Y. Calcein Leakage Behavior from Vesicles Induced by Protein–Vesicle Interaction: A Study by Surface Pressure–Area Isotherms. Chem. Lett. 2017, 46, 1036–1039. [Google Scholar] [CrossRef]
- Maherani, B.; Arab-Tehrany, E.; Kheirolomoom, A.; Geny, D.; Linder, M. Calcein release behavior from liposomal bilayer; influ-ence of physicochemical/mechanical/structural properties of lipids. Biochimie 2013, 95, 2018–2033. [Google Scholar] [CrossRef] [PubMed]
- Xiang, T.-X.; Anderson, B.D. Influence of Chain Ordering on the Selectivity of Dipalmitoylphosphatidylcholine Bilayer Membranes for Permeant Size and Shape. Biophys. J. 1998, 75, 2658–2671. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shimanouchi, T.; Komori, Y.; Toramoto, K.; Hayashi, K.; Yasuhara, K.; Jung, H.-S.; Kimura, Y. Hydrothermal Preparation of Faceted Vesicles Made of Span 40 and Tween 40 and Their Characterization. Appl. Sci. 2023, 13, 6893. https://doi.org/10.3390/app13126893
Shimanouchi T, Komori Y, Toramoto K, Hayashi K, Yasuhara K, Jung H-S, Kimura Y. Hydrothermal Preparation of Faceted Vesicles Made of Span 40 and Tween 40 and Their Characterization. Applied Sciences. 2023; 13(12):6893. https://doi.org/10.3390/app13126893
Chicago/Turabian StyleShimanouchi, Toshinori, Yui Komori, Kazuki Toramoto, Keita Hayashi, Kazuma Yasuhara, Ho-Sup Jung, and Yukitaka Kimura. 2023. "Hydrothermal Preparation of Faceted Vesicles Made of Span 40 and Tween 40 and Their Characterization" Applied Sciences 13, no. 12: 6893. https://doi.org/10.3390/app13126893
APA StyleShimanouchi, T., Komori, Y., Toramoto, K., Hayashi, K., Yasuhara, K., Jung, H.-S., & Kimura, Y. (2023). Hydrothermal Preparation of Faceted Vesicles Made of Span 40 and Tween 40 and Their Characterization. Applied Sciences, 13(12), 6893. https://doi.org/10.3390/app13126893





