Assessment of the Microbial Communities in Soil Contaminated with Petroleum Using Next-Generation Sequencing Tools
Abstract
1. Introduction
2. Materials and Methods
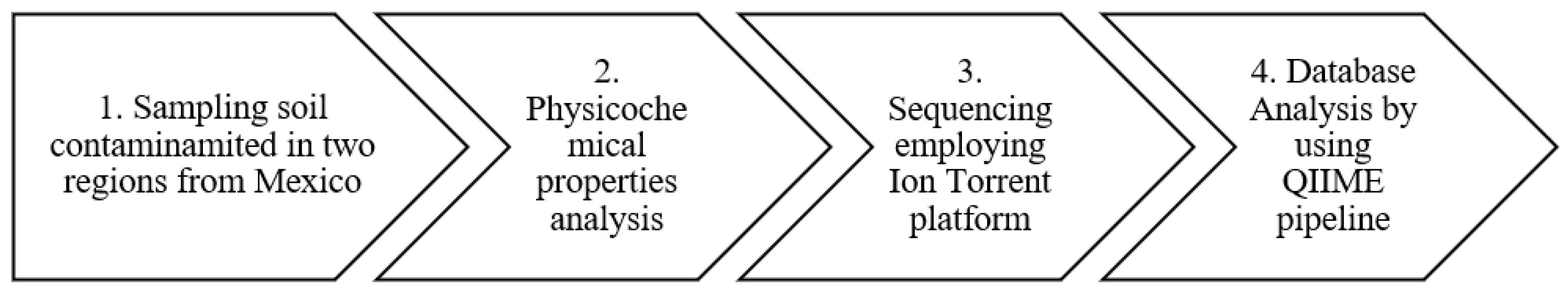
2.1. Research Design
- Step 1.
- The sampling collection was from soil contaminated in two regions of Mexico, Burgos and Tabasco.
- Step 2.
- The physicochemical analysis determined the principal properties of soil.
- Step 3.
- Sequencing was carried out using the ion Torrent Platform and subsequently trimming and cleaning the sequencing with low quality.
- Step 4.
- Data analysis was carried out with the QIMME software version 1.9, a next-genration platform. Alpha diversity, OTUS and beta diversity were computed. Finally, a heatmap was generated using R software version 3.3.3 and SRA sequences were uploaded at NCBI.
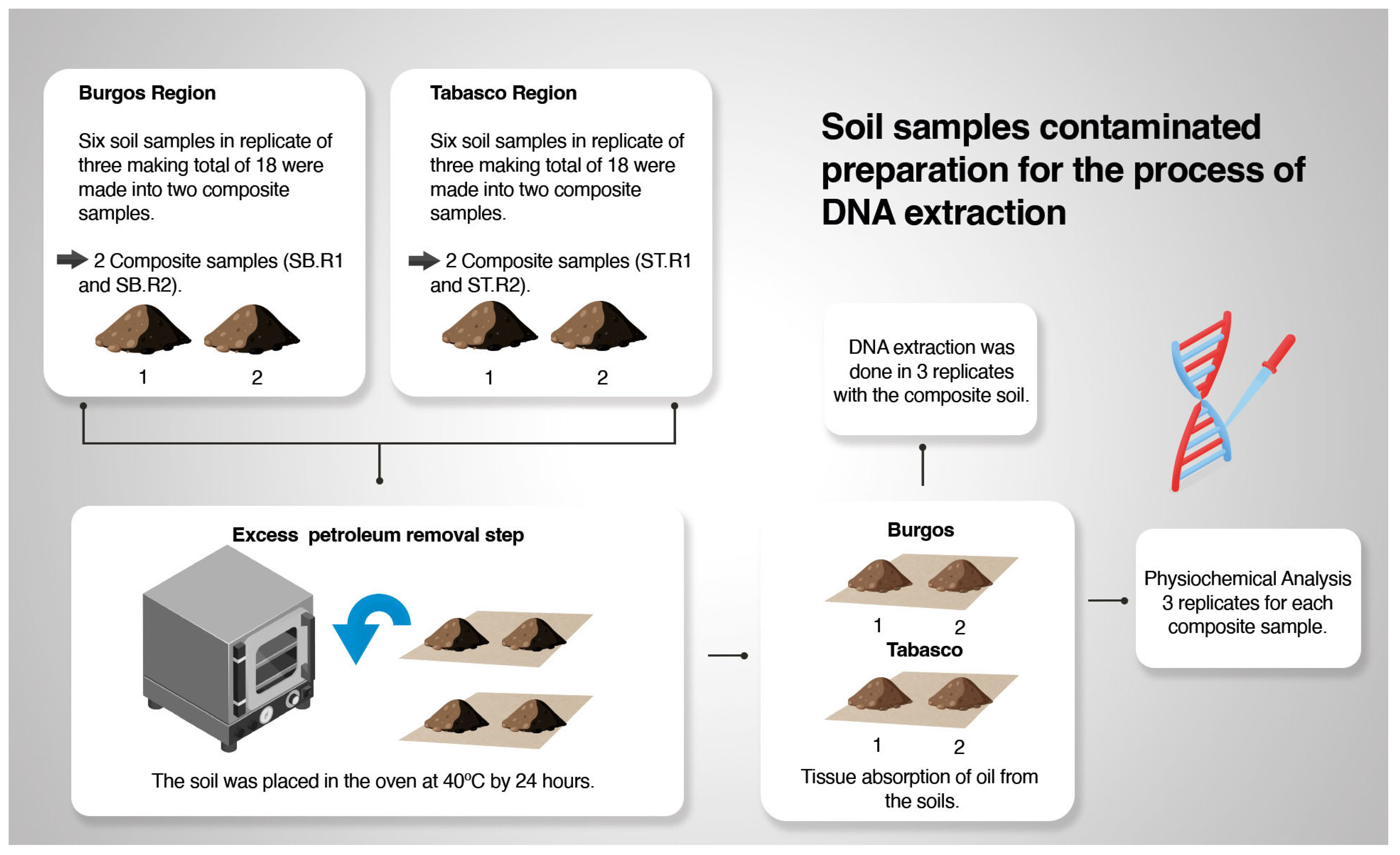
2.2. Sampling Site
2.3. DNA Extraction from Contaminated Soil Samples and Construction of the V2–V3-16S rDNA Libraries
2.4. Microbial Community Structure Analysis
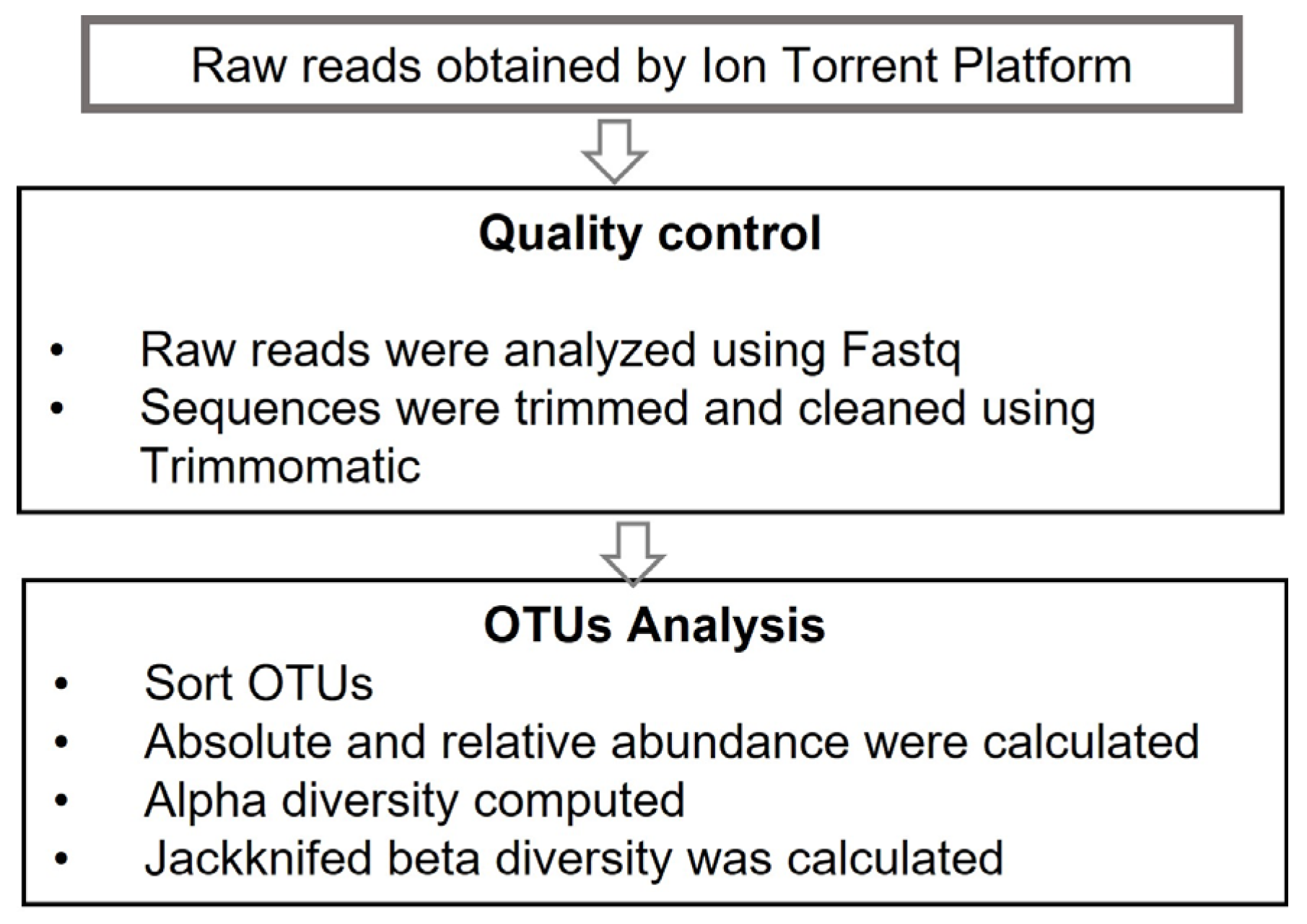
2.5. Diversity Computation and Bioinformatic Analysis
3. Results
3.1. Physicochemical Properties
3.2. Microbial Community Structure Analysis
3.2.1. Sequencing Analysis
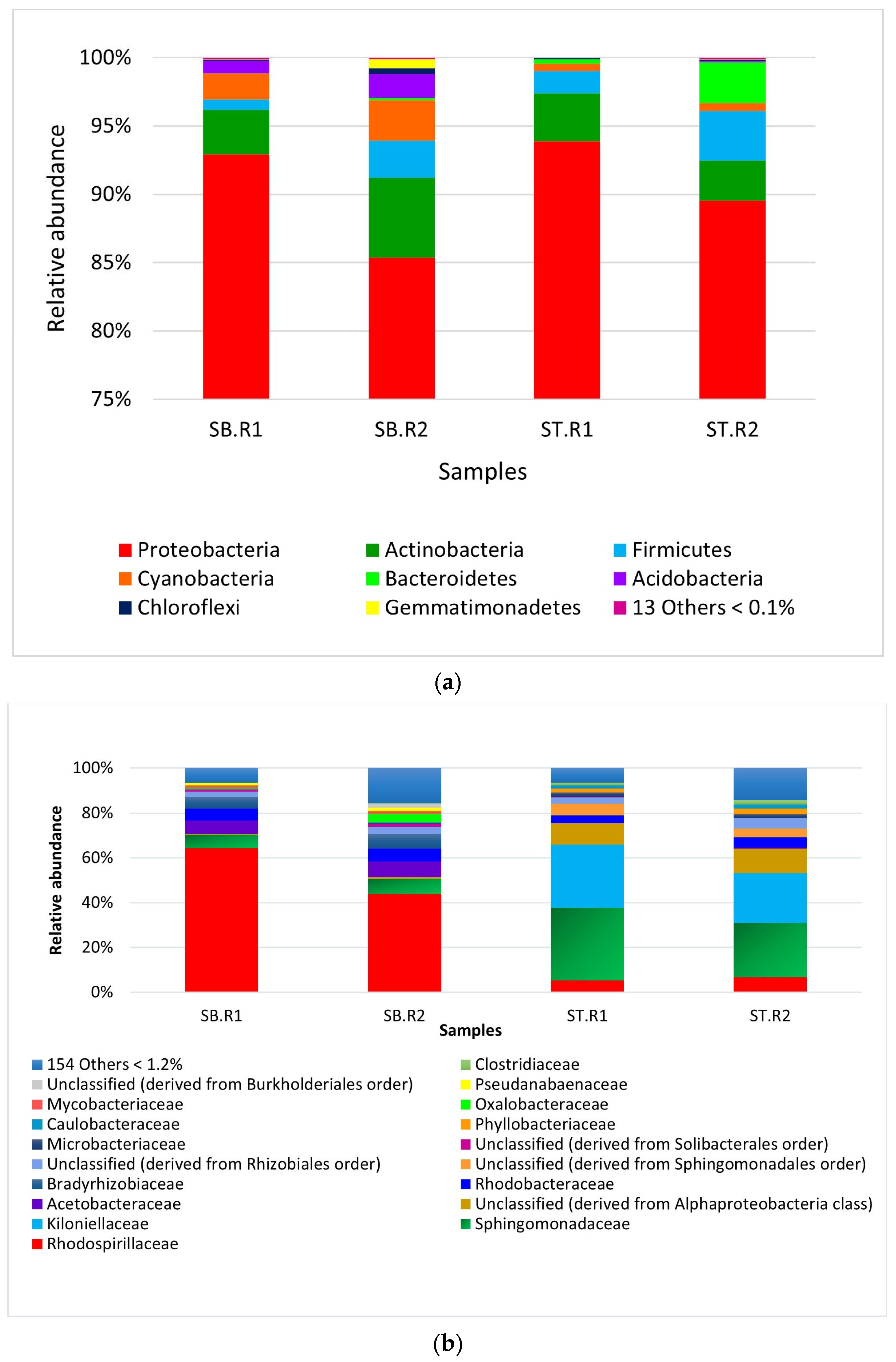
3.2.2. Relative Abundance
3.2.3. Alpha Diversity Analysis
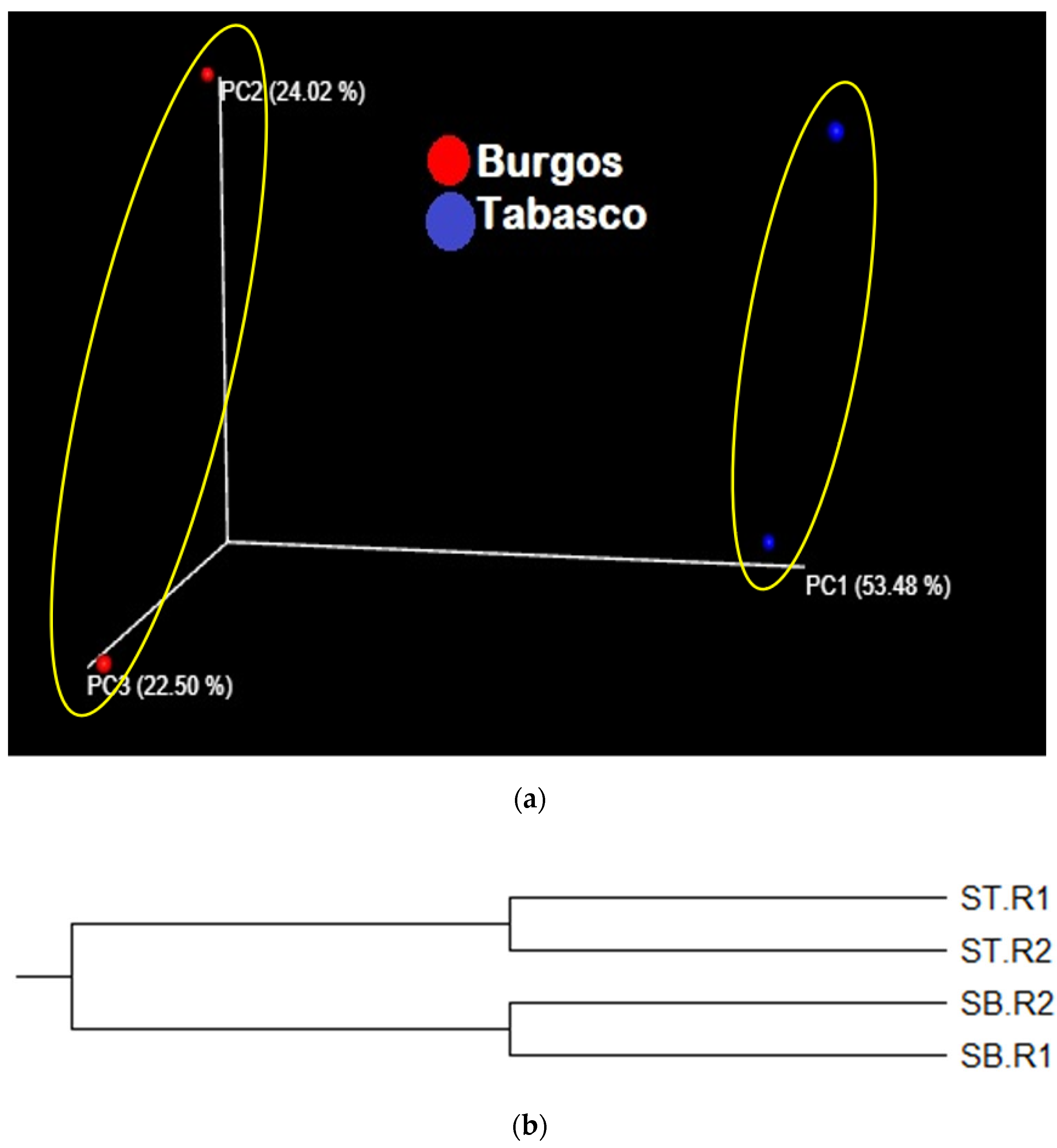
3.2.4. Beta Diversity Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoang, S.A.; Sarkar, B.; Seshadri, B.; Lamb, D.; Wijesekara, H.; Vithanage, M.; Liyanage, C.; Kolivabandara, P.A.; Rinklebe, J.; Lam, S.S.; et al. Mitigation of petroleum-hydrocarbon-contaminated hazardous soils using organic amendments: A review. J. Hazard. Mater. 2021, 416, 125702. [Google Scholar] [CrossRef] [PubMed]
- Khoshkholgh Sima, N.A.; Ebadi, A.; Reiahisamani, N.; Rasekh, B. Bio-based remediation of petroleum-contaminated saline soils: Challenges, the current state-of-the-art and future prospects. J. Environ. Manag. 2019, 250, 109476. [Google Scholar] [CrossRef] [PubMed]
- Thapa, B.; KC, A.K.; Ghimire, A. A Review on Bioremediation of Petroleum Hydrocarbon Contaminants in Soil. Kathmandu Univ. J. Sci. Eng. Technol. 2012, 8, 164–170. [Google Scholar] [CrossRef]
- Adedeji, J.A.; Tetteh, E.K.; Opoku Amankwa, M.; Asante-Sackey, D.; Ofori-Frimpong, S.; Armah, E.K.; Rathilal, S.; Mohammadi, A.H.; Chetty, M. Microbial Bioremediation and Biodegradation of Petroleum Products—A Mini Review. Appl. Sci. 2022, 12, 12212. [Google Scholar] [CrossRef]
- Song, Y.; Li, R.; Chen, G.; Yan, B.; Zhong, L.; Wang, Y.; Li, Y.; Li, J.; Zhang, Y. Bibliometric analysis of current status on bioremediation of petroleum contaminated soils during 2000–2019. Int. J. Environ. Res. Public Health. 2021, 18, 8859. [Google Scholar] [CrossRef]
- Teng, T.; Liang, J.; Wu, Z. Identification of pyrene degraders via DNA-SIP in oilfield soil during natural attenuation, bioaugmentation and biostimulation. Sci. Total Environ. 2021, 800, 149485. [Google Scholar] [CrossRef]
- Secretaría de Energía (SENER). Sistema de Información Energética. Dirección General de Planeación e Información Energéticas.Producción de Petróleo Crudo Por Región y En Activos Integrales. Available online: https://sie.energia.gob.mx/ (accessed on 1 March 2023).
- Lopez, E.S.; Elufisan, T.O.; Bustos, P.; Charles, C.P.M.; Mendoza-Herrera, A.; Guo, X. Complete Genome Report of a Hydrocarbon-Degrading Sphingobium yanoikuyae S72. Appl. Sci. 2022, 12, 6201. [Google Scholar] [CrossRef]
- Chicca, I.; Becarelli, S.; Di Gregorio, S. Microbial Involvement in the Bioremediation of Total Petroleum Hydrocarbon Polluted Soils: Challenges and Perspectives. Environments 2022, 9, 52. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.; Zhao, N.; Guo, J.; Xu, W.; Ma, M.; Li, X. Remediation of Crude Oil-Polluted Soil by the Bacterial Rhizosphere Community of Suaeda Salsa Revealed by 16S rRNA Genes. Int. J. Environ. Res. Public Health 2020, 17, 1471. [Google Scholar] [CrossRef] [PubMed]
- Ambaye, T.G.; Chebbi, A.; Formicola, F.; Prasad, S.; Gomez, F.H.; Franzetti, A.; Vaccari, M. Remediation of soil polluted with petroleum hydrocarbons and its reuse for agriculture: Recent progress, challenges, and perspectives. Chemosphere 2022, 293, 133572. [Google Scholar] [CrossRef]
- Zhuang, X.; Wang, Y.; Wang, H.; Dong, Y.; Li, X.; Wang, S.; Fan, H.; Wu, S. Comparison of the efficiency and microbial mechanisms of chemical- and bio-surfactants in remediation of petroleum hydrocarbon. Environ. Pollut. 2022, 314, 120198. [Google Scholar] [CrossRef]
- Chunyan, X.; Qaria, M.A.; Qi, X.; Daochen, Z. The role of microorganisms in petroleum degradation: Current development and prospects. Sci Total Environ. 2023, 865, 161112. [Google Scholar] [CrossRef] [PubMed]
- Ojuederie, O.B.; Babalola, O.O. Microbial and plant-assisted bioremediation of heavy metal polluted environments: A review. Int. J. Environ. Res. Public Health 2017, 14, 1504. [Google Scholar] [CrossRef] [PubMed]
- Eze, M.O. Metagenome analysis of a hydrocarbon-degrading bacterial consortium reveals the specific roles of btex biodegraders. Genes 2021, 12, 98. [Google Scholar] [CrossRef]
- Wang, S.-Y.; Kuo, Y.-C.; Hong, A.; Chang, Y.-M.; Kao, C.-M. Bioremediation of diesel and lubricant oil-contaminated soils using enhanced landfarming system. Chemosphere 2016, 164, 558–567. [Google Scholar] [CrossRef] [PubMed]
- Santisi, S.; Zoccali, M.; Catania, V.; Quatrini, P.; Mondello, L.; Genovese, M.; Cappello, S. Biodegradation Potential of Oil-degrading Bacteria Related to the Genus Thalassospira Isolated from Polluted Coastal Area in Mediterranean Sea. Soil Sediment Contam. Int. J. 2022, 31, 3. [Google Scholar] [CrossRef]
- Elufisan, T.O.; Lozano, L.; Bustos, P.; Rodríguez-Luna, I.C.; Sánchez-Varela, A.; Oyedara, O.O.; Villalobos-López, M.Á.; Guo, X. Complete Genome Sequence of Stenotrophomonas maltophilia Strain SVIA2, Isolated from Crude Oil-Contaminated Soil in Tabasco, Mexico. Microbiol. Resour. Announc. 2019, 8, e00529-19. [Google Scholar] [CrossRef] [PubMed]
- Cabral, L.; Giovanella, P.; Pellizzer, E.P.; Teramoto, E.H.; Kiang, C.H.; Sette, L.D. Microbial communities in petroleum-contaminated sites: Structure and metabolisms. Chemosphere 2022, 286, 131752. [Google Scholar] [CrossRef]
- Nzila, A.; Musa, M.M. Current status of and future perspectives in bacterial degradation of benzo[a]pyrene. Int. J. Environ. Res. Public Health 2020, 18, 262. [Google Scholar] [CrossRef]
- Elufisan, T.O.; Rodríguez-Luna, I.C.; Oyedara, O.O.; Sánchez-Varela, A.; Hernández-Mendoza, A.; Dantán Gonzalez, E.; Paz-González, A.D.; Muhammad, K.; Rivera, G.; Villalobos-Lopez, M.A.; et al. The Polycyclic Aromatic Hydrocarbon (PAH) degradation activities and genome analysis of a novel strain Stenotrophomonas sp. Pemsol isolated from Mexico. PeerJ 2020, 8, e8102. [Google Scholar] [CrossRef]
- Das, N.; Chandran, P. Microbial Degradation of Petroleum Hydrocarbon Contaminants: An Overview. Biotechnol. Res. Int. 2011, 2011, 941810. [Google Scholar] [CrossRef] [PubMed]
- Sui, X.; Wang, X.; Li, Y.; Ji, H. Remediation of Petroleum-Contaminated Soils with Microbial and Microbial Combined Methods: Advances, Mechanisms, and Challenges. Sustainability 2021, 13, 9267. [Google Scholar] [CrossRef]
- Fernandes, G.M.; Martins, D.A.; dos Santos, R.P.; de Santiago, I.S.; Nascimento, L.S.; Oliveira, A.H.B.; Yamamoto, F.Y.; Cavalcante, R.M. Levels, source appointment, and ecological risk of petroleum hydrocarbons in tropical coastal ecosystems (northeast Brazil): Baseline for future monitoring programmes of an oil spill area. Environ. Pollut. 2022, 296, 118709. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Liang, K.; Huang, G.; Wang, X.; Lin, M.; Chen, Y.; Zhou, Z. Soil Bacterial Community Shifts Are Driven by Soil Nutrient Availability along a Teak Plantation Chronosequence in Tropical Forests in China. Biology 2021, 10, 1329. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, S.; Guo, P.; Guo, S. Characteristics of organic carbon metabolism and bioremediation of petroleum-contaminated soil by a mesophilic aerobic biopile system. Chemosphere 2021, 264, 128521. [Google Scholar] [CrossRef]
- Marshall, T.; Paschos, A.; Marangoni, A.G.; Yang, F.; Pensini, E. Injectable cationic traps and sticky bacterial emulsifiers: A safe alliance during diesel bioremediation. Colloids Surf. A Physicochem. Eng. Asp. 2021, 613, 126051. [Google Scholar] [CrossRef]
- Zheng, J.; Feng, J.Q.; Zhou, L.; Mbadinga, S.M.; Gu, J.D.; Mu, B.Z. Characterization of bacterial composition and diversity in a long-term petroleum contaminated soil and isolation of high-efficiency alkane-degrading strains using an improved medium. World J. Microbiol. Biotechnol. 2018, 34, 34. [Google Scholar] [CrossRef]
- Lüneberg, K.; Schneider, D.; Siebe, C.; Daniel, R. Drylands soil bacterial community is affected by land use change and different irrigation practices in the Mezquital Valley, Mexico. Sci. Rep. 2018, 8, 1413. [Google Scholar] [CrossRef]
- Peng, M.; Zi, X.; Wang, Q. Bacterial community diversity of oil-contaminated soils assessed by high throughput sequencing of 16s rRNA genes. Int. J. Environ. Res. Public Health. 2015, 12, 12002–12015. [Google Scholar] [CrossRef]
- Wang, B.; Chu, C.; Wei, H.; Zhang, L.; Ahmad, Z.; Wu, S.; Xie, B. Ameliorative effects of silicon fertilizer on soil bacterial community and pakchoi (Brassica chinensis L.) grown on soil contaminated with multiple heavy metals. Environ. Pollut. 2020, 267, 115411. [Google Scholar] [CrossRef]
- Scagliola, M.; Valentinuzzi, F.; Mimmo, T.; Cesco, S.; Crecchio, C.; Pii, Y. Bioinoculants as Promising Complement of Chemical Fertilizers for a More Sustainable Agricultural Practice. Front. Sustain. Food Syst. 2021, 4, 622169. [Google Scholar] [CrossRef]
- Borowik, A.; Wyszkowska, J.; Kucharski, M.; Kucharski, J. Implications of soil pollution with diesel oil and BP petroleum with ACTIVE technology for soil health. Int. J. Environ. Res. Public Health 2020, 16, 2474. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Sanz, D.; Redondo-Nieto, M.; Guirado, M.; Pindado Jiménez, O.; Millán, R.; Martin, M.; Rivilla, R. Metagenomic insights into the bacterial functions of a diesel-degrading consortium for the rhizoremediation of diesel-polluted soil. Genes 2019, 10, 456. [Google Scholar] [CrossRef] [PubMed]
- Hoang, S.A.; Lamb, D.; Seshadri, B.; Sarkar, B.; Cheng, Y.; Wang, L.; Bolan, N.S. Petroleum hydrocarbon rhizoremediation and soil microbial activity improvement via cluster root formation by wild proteaceae plant species. Chemosphere 2021, 275, 130135. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.F.; Yang, Z.H.; Chen, Y.L.; Lo, K.H.; Kao, C.M. Remediation of weathered diesel-oil contaminated soils using biopile systems: An amendment selection and pilot-scale study. Sci. Total Environ. 2021, 786, 147395. [Google Scholar] [CrossRef]
- Hossain, F.; Akter, A.; Sohan, S.R.; Sultana, N.; Reza, A.; Hoque, F. Bioremediation potential of hydrocarbon degrading bacteria: Isolation, characterization, and assessment. Saudi J. Biol. Sci. 2022, 29, 211–216. [Google Scholar] [CrossRef]
- Wang, M.; Zhao, S.; Wang, L.; Chen, S.; Li, S.; Lei, X.; Sun, X.; Qin, L. Salt stress-induced changes in microbial community structures and metabolic processes result in increased soil cadmium availability. Sci. Total Environ. 2021, 782, 147125. [Google Scholar] [CrossRef]
- Shar, S.; Reith, F.; Ball, A.S.; Shahsavari, E. Long-term Impact of Gold and Platinum on Microbial Diversity in Australian Soils. Microb. Ecol. 2021, 81, 977–989. [Google Scholar] [CrossRef]
- Shahi, A.; Aydin, S.; Ince, B.; Ince, O. Reconstruction of bacterial community structure and variation for enhanced petroleum hydrocarbons degradation through biostimulation of oil contaminated soil. Chem. Eng. J. 2016, 306, 60–66. [Google Scholar] [CrossRef]
- Chopkova, V.; Petkova, M.; Shilev, S. Uncovering Bacterial Diversity during Mesophilic and Thermophilic Phases of Biowaste Composting through Next-Generation Sequencing. Appl. Sci. 2023, 13, 3111. [Google Scholar] [CrossRef]
- Gao, L.; Huang, Y.; Liu, Y.; Mohamed, O.A.A.; Fan, X.; Wang, L.; Li, L.; Ma, J. Bacterial Community Structure and Potential Microbial Coexistence Mechanism Associated with Three Halophytes Adapting to the Extremely Hypersaline Environment. Microorganisms 2022, 10, 1124. [Google Scholar] [CrossRef]
- Kulik, K.; Lenart-boro, A.; Wyrzykowska, K. Impact of Antibiotic Pollution on the Bacterial Population within Surface Water with Special Focus on Mountain Rivers. Water 2023, 15, 975. [Google Scholar] [CrossRef]
- Gałązka, A.; Gawryjołek, K.; Grządziel, J.; Frac, M.; KsieZak, J. Microbial community diversity and the interaction of soil under maize growth in different cultivation techniques. Plant Soil Environ. 2017, 63, 264–270. [Google Scholar] [CrossRef]
- Vital, L.; Narvaez, J.A.; Cruz, M.A.; Ortiz, E.L.; Sanchez, E.; Mendoza, A. Unravelling the composition of soil belowground microbial community before sowing transgenic cotton. Plant Soil Environ. 2017, 63, 11. [Google Scholar] [CrossRef]
- Yu, G.; Fadrosh, D.; Goedert, J.J.; Ravel, J.; Goldstein, A.M. Nested PCR biases in interpreting microbial community structure in 16S rRNA gene sequence datasets. PLoS ONE 2015, 10, e0132253. [Google Scholar] [CrossRef]
- Grifoni, A.; Bazzicalupo, M.; Di Serio, C.; Fancelli, S.; Fani, R. Identification of Azospirillum strains by restriction fragment length polymorphism of the 16S rDNA and of the histidine operon. FEMS Microbiol. Lett. 1995, 127, 85–91. [Google Scholar] [CrossRef]
- Wuyts, J.; Van De Peer, Y.; Winkelmans, T.; De Wachter, R. The European database on small subunit ribosomal RNA. Nucleic Acids Res. 2002, 30, 183–185. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- DeSantis, T.Z.; Hugenholtz, P.; Larsen, N.; Rojas, M.; Brodie, E.L.; Keller, K.; Huber, T.; Dalevi, D.; Hu, P.; Andersen, G.L. Greengenes, a chimera-checked 16S rRNA gene database and workbench compatible with ARB. Appl. Environ. Microbiol. 2006, 72, 5069–5072. [Google Scholar] [CrossRef] [PubMed]
- Thompson, L.R.; Sanders, J.G.; McDonald, D.; Amir, A.; Ladau, J.; Locey, K.J.; Prill, R.J.; Tripathi, A.; Gibbons, S.M.; Ackermann, G. A communal catalogue reveals Earth’s multiscale microbial diversity. Nature 2017, 551, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Murugesan, S.; Ulloa-Martínez, M.; Martínez-Rojano, H.; Galván-Rodríguez, F.M.; Miranda-Brito, C.; Romano, M.C.; Piña-Escobedo, A.; Pizano-Zárate, M.L.; Hoyo-Vadillo, C.; García-Mena, J. Study of the diversity and short-chain fatty acids production by the bacterial community in overweight and obese Mexican children. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1337–1346. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Zhou, X.; Guo, D.; Zhao, J.H.; Yan, L.; Feng, G.Z.; Gao, Q.; Yu, H.; Zhao, L.P. Soil pH is the primary factor driving the distribution and function of microorganisms in farmland soils in northeastern China. Ann. Microbiol. 2019, 69, 1461–1473. [Google Scholar] [CrossRef]
- Huerta, E.; Rodriguez-Olan, J.; Evia-Castillo, I.; Montejo-Meneses, E.; de la Cruz-Mondragon, M.; Garcia-Hernández, R. Earthworms and soil properties in Tabasco, Mexico. Eur. J. Soil Biol. 2007, 43, S190–S195. [Google Scholar] [CrossRef]
- Rivera Ortiz, P.; Rivera Lárraga, J.E.; Andrade Limas, E.D.C.; Heyer Rodríguez, L.; De La Garza Requena, F.R.; Castro Meza, B.I. Bioestimulación y biorremediación de recortes de perforación contaminados con hidrocarburos. Rev. Int. Contam. Ambien. 2017, 34, 249–262. [Google Scholar] [CrossRef]
- Lin, M.-S.; Huang, C.-Y.; Lin, Y.-C.; Lin, S.-L.; Hsiao, Y.-H.; Tu, P.-C.; Cheng, P.-C.; Cheng, S.-F. Green Remediation Technology for Total Petroleum Hydrocarbon-Contaminated Soil. Agronomy 2022, 12, 2759. [Google Scholar] [CrossRef]
- Wang, M.; Garrido-Sanz, D.; Sansegundo-Lobato, P.; Redondo-Nieto, M.; Conlon, R.; Martin, M.; Mali, R.; Liu, X.; Dowling, D.N.; Rivilla, R.; et al. Soil Microbiome Structure and Function in Ecopiles Used to Remediate Petroleum-Contaminated Soil. Front. Environ. Sci. 2021, 9, 39. [Google Scholar] [CrossRef]
- Bao, Y.J.; Xu, Z.; Li, Y.; Yao, Z.; Sun, J.; Song, H. High-throughput metagenomic analysis of petroleum-contaminated soil microbiome reveals the versatility in xenobiotic aromatics metabolism. J. Environ. Sci. 2017, 56, 25–35. [Google Scholar] [CrossRef]
- Kim, J.-W.; Hong, Y.-K.; Kim, H.-S.; Oh, E.-J.; Park, Y.-H.; Kim, S.-C. Metagenomic analysis for evaluating change in bacterial diversity in tph-contaminated soil after soil remediation. Toxics 2021, 9, 319. [Google Scholar] [CrossRef]
- Kumar, V.; AlMomin, S.; Al-Aqeel, H.; Al-Salamen, F.; Nair, S.; Shajan, A. Metagenomic analysis of rhizosphere microflora of oil-contaminated soil planted with barley and alfalfa. PLoS ONE 2018, 13, e0202127. [Google Scholar] [CrossRef]
- Melekhina, E.N.; Belykh, E.S.; Markarova, M.Y.; Taskaeva, A.A.; Rasova, E.E.; Baturina, O.A.; Kabilov, M.R.; Velegzhaninov, I.O. Soil microbiota and microarthropod communities in oil contaminated sites in the European Subarctic. Sci. Rep. 2021, 11, 19620. [Google Scholar] [CrossRef] [PubMed]
- Bonomo, M.G.; Calabrone, L.; Scrano, L.; Bufo, S.A.; Di Tomaso, K.; Buongarzone, E.; Salzano, G. Metagenomic monitoring of soil bacterial community after the construction of a crude oil flowline. Environ. Monit. Assess. 2022, 194, 48. [Google Scholar] [CrossRef] [PubMed]
- Galazka, A.; Grzadziel, J.; Galazka, R.; Ukalska-Jaruga, A.; Strzelecka, J.; Smreczak, B. Genetic and functional diversity of bacterial microbiome in soils with long term impacts of petroleum hydrocarbons. Front. Microbiol. 2018, 9, 1923. [Google Scholar] [CrossRef]
- Steenhoudt, O.; Vanderleyden, J. Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: Genetic, biochemical and ecological aspects. FEMS Microbiol. Rev. 2000, 24, 487–506. [Google Scholar] [CrossRef] [PubMed]
- da Silva, M.L.B.; Mezzari, M.P.; Ibelli, A.M.G.; Gregory, K.B. Sulfide removal from livestock biogas by Azospirillum-like anaerobic phototrophic bacteria consortium. Int. Biodeterior. Biodegrad. 2014, 86, 248–251. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Mendoza-Herrera, A.; Bocanegra-García, V.; Rivera, G. Azospirillum spp. from Plant Growth-Promoting Bacteria to Their Use in Bioremediation. Microorganisms 2022, 10, 1057. [Google Scholar] [CrossRef] [PubMed]
- Zokaei, F.H.; Gharavi, S.; Asgarani, E.; Zarrabi, M.; Soudi, M. A comparative taxonomic profile of microbial polyethylene and hydrocarbon-degrading communities in diverse environments. Iran. J. Biotechnol. 2021, 19, 2. [Google Scholar] [CrossRef]
- Stepanova, A.Y.; Gladkov, E.A.; Osipova, E.S.; Gladkova, O.V.; Tereshonok, D.V. Bioremediation of Soil from Petroleum Contamination. Processes 2022, 10, 1224. [Google Scholar] [CrossRef]
- Liu, P.; Zhang, Y.; Tang, Q.; Shi, S. Bioremediation of metal-contaminated soils by microbially-induced carbonate precipitation and its effects on ecotoxicity and long-term stability. Biochem. Eng. J. 2021, 166, 107856. [Google Scholar] [CrossRef]
- Hentati, D.; Chebbi, A.; Mahmoudi, A.; Hadrich, F.; Cheffi, M.; Frikha, I.; Sayadi, S.; Chamkha, M. Biodegradation of hydrocarbons and biosurfactants production by a newly halotolerant Pseudomonas sp. strain isolated from contaminated seawater. Biochem. Eng. J. 2021, 166, 107861. [Google Scholar] [CrossRef]
- Cruz-Hernández, M.A.; Reyes-Peralta, J.; Mendoza-Herrera, A.; Rivera, G.; Bocanegra-García, V. Characterization of a Microbacterium sp. strain isolated from soils contaminated with hydrocarbons in the burgos basin, Mexico. Rev. Int. Contam. Ambient 2021, 37, 227–235. [Google Scholar] [CrossRef]


| Primer | Ion Torrent Linker Primer | Golay Barcode | Spacer | Linker-Primer Forward | Sample ID | Description |
|---|---|---|---|---|---|---|
| V2–V3_344F_BC19 | 5′-CCATCTCATCCCTGCGTGTCTCCGAC TCAG-3′ | TTAGTCGGAC | GAT | ACGGRAGGCAGCAG | SB.R1 | Burgos |
| V2–V3_344F_BC8 | 5′-CCATCTCATCCCTGCGTGTCTCCGAC TCAG-3′ | TTCCGATAAC | GAT | ACGGRAGGCAGCAG | SB.R2 | Burgos |
| V2–V3_344F_BC20 | 5′-CCATCTCATCCCTGCGTGTCTCCGAC TCAG-3′ | CAGATCCATC | GAT | ACGGRAGGCAGCAG | ST.R1 | Tabasco |
| V2–V3_344F_BC9 | 5′-CCATCTCATCCCTGCGTGTCTCCGAC TCAG-3′ | TGAGCGGAAC | GAT | ACGGRAGGCAGCAG | ST.R2 | Tabasco |
| V2–V3_E534R_trP1 | 3′-CCTCTCTATGGGCAGTCGGTGAT-5′ | NOT APPLICABLE | ATTACCGCGGCTGCTGGC | |||
| Property | Tabasco Soil | Burgos Soil | p-Value |
|---|---|---|---|
| Sand (%) | 60.2 ± 0.68 | 58.5 ± 0.68 | 0.03759 |
| Clay (%) | 15.44 ± 0.23 | 11.44 ± 0.25 | 0.000034 |
| Silt (%) | 22 ± 0.16 | 20 ± 0.21 | 0.000195 |
| pH | 5.66 ± 0.12 | 7.96 ± 0.12 | 0.00002 |
| Sample | Description | Number of Sequences | Quality Control (Length Mean from 150 to 200 bp) |
|---|---|---|---|
| SB.R1 | Burgos | 21,630 | 13,881 |
| SB.R2 | Burgos | 47,084 | 39,370 |
| ST.R1 | Tabasco | 7503 | 5576 |
| ST.R2 | Tabasco | 103,671 | 80,586 |
| Total | 179,888 | 139,413 |
| Taxon | Burgos | Tabasco | ||
|---|---|---|---|---|
| SB.R1 (%) | SB.R2 (%) | ST.R1 (%) | ST.R2 (%) | |
| Phyla | ||||
| Proteobacteria | 93 | 85.3 | 93.9 | 89.5 |
| Actinobacteria | 3.2 | 5.8 | 3.5 | 2.0 |
| Firmicutes | 0.8 | 2.7 | 1.6 | 3.6 |
| Cyanobacteria | 1.9 | 3.0 | 0.5 | 0.6 |
| Families | ||||
| Rhodospirillaceae | 64.2 | 43.8 | 5.3 | 6.6 |
| Sphingomonadaceae | 6.0 | 6.8 | 32.3 | 24.3 |
| Kiloniellaceae | 0.015 | 0.03 | 28.3 | 22.3 |
| Unclassified member of the Alphaproteobacteria | 0.5 | 0.7 | 9.6 | 11.0 |
| Families with abundances between 2 and 7% | ||||
| Acetobacteraceae | 5.9 | 7.0 | 0.1 | 0.4 |
| Rhodobacteraceae | 5.3 | 5.8 | 3.3 | 4.6 |
| Bradyrhizobiaceae | 5.2 | 6.5 | 0.1 | 0.04 |
| Unclassified derived from Sphingomonadales order | 0.04 | 0.1 | 5.2 | 3.9 |
| Unclassified derived from the order Rhizobiales | 2.5 | 3.1 | 2.8 | 4.7 |
| Microbacteriaceae | 0.1 | 0.2 | 0 | 1.6 |
| Phyllobacteriaceae | 0.1 | 0.1 | 1.8 | 2.6 |
| Oxalobacteraceae | 0.4 | 3.6 | 0 | 0.004 |
| Genera | ||||
| Skermanella | 35 | 22.8 | 0.3 | 0.5 |
| Azospirillum | 15.9 | 10.4 | 0.3 | 0.3 |
| Unclassified genus derived from Rhodospirillaceae | 10.8 | 9.2 | 3.9 | 4.6 |
| Thalassospira | 0.02 | 0.04 | 28.3 | 22.3 |
| Unclassified genus derived from Sphingomonadaceae family | 5.1 | 5.8 | 19.7 | 14.6 |
| Unclassified genus derived from Alphaproteobacteria | 0.5 | 0.7 | 9.6 | 11 |
| Overview | PERMANOVA Results |
|---|---|
| Method name | PERMANOVA |
| Test statistic name | Pseudo-F |
| Sample size | 4 |
| Number of groups | 2 |
| Test statistic | 9.306193 |
| p-value | 0.332 |
| Number of permutations | 999 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
García-García, R.; Bocanegra-García, V.; Vital-López, L.; García-Mena, J.; Zamora-Antuñano, M.A.; Cruz-Hernández, M.A.; Rodríguez-Reséndiz, J.; Mendoza-Herrera, A. Assessment of the Microbial Communities in Soil Contaminated with Petroleum Using Next-Generation Sequencing Tools. Appl. Sci. 2023, 13, 6922. https://doi.org/10.3390/app13126922
García-García R, Bocanegra-García V, Vital-López L, García-Mena J, Zamora-Antuñano MA, Cruz-Hernández MA, Rodríguez-Reséndiz J, Mendoza-Herrera A. Assessment of the Microbial Communities in Soil Contaminated with Petroleum Using Next-Generation Sequencing Tools. Applied Sciences. 2023; 13(12):6922. https://doi.org/10.3390/app13126922
Chicago/Turabian StyleGarcía-García, Raul, Virgilio Bocanegra-García, Lourdes Vital-López, Jaime García-Mena, Marco Antonio Zamora-Antuñano, María Antonia Cruz-Hernández, Juvenal Rodríguez-Reséndiz, and Alberto Mendoza-Herrera. 2023. "Assessment of the Microbial Communities in Soil Contaminated with Petroleum Using Next-Generation Sequencing Tools" Applied Sciences 13, no. 12: 6922. https://doi.org/10.3390/app13126922
APA StyleGarcía-García, R., Bocanegra-García, V., Vital-López, L., García-Mena, J., Zamora-Antuñano, M. A., Cruz-Hernández, M. A., Rodríguez-Reséndiz, J., & Mendoza-Herrera, A. (2023). Assessment of the Microbial Communities in Soil Contaminated with Petroleum Using Next-Generation Sequencing Tools. Applied Sciences, 13(12), 6922. https://doi.org/10.3390/app13126922










