The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée
Abstract
1. Introduction
2. Materials and Methods
2.1. Material
2.2. Preparation of Solutions and Film
2.3. Analytical Methods
2.3.1. Determination of Flow Curves and Gelation Temperature of Tested Composite Protein and Polysaccharide Film-Forming Solutions
2.3.2. Study of the Structure of Films
2.4. Computational and Statistical Methods
2.4.1. Calculation Methods
2.4.2. Statistical Methods
3. Results and Discussion
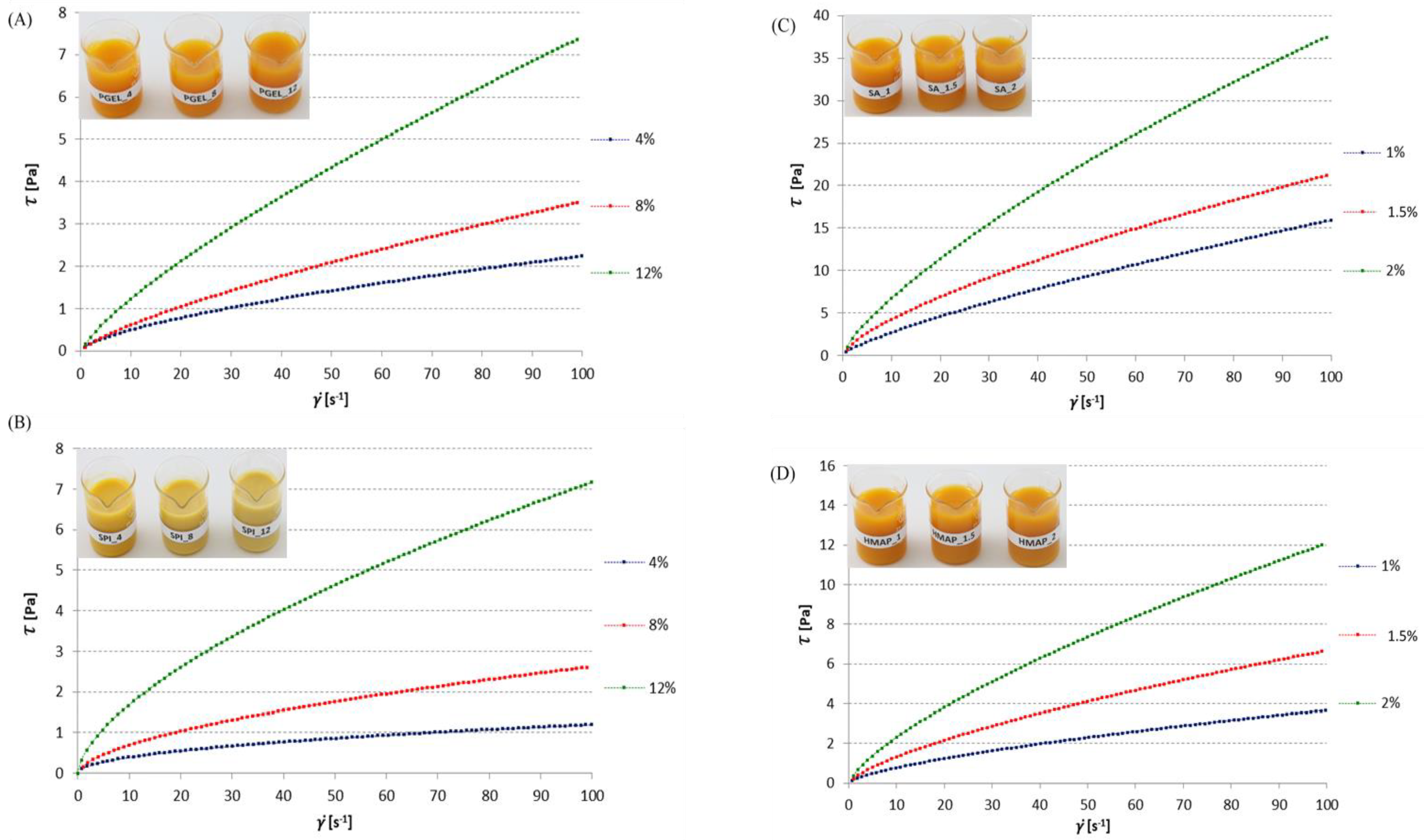
3.1. Flow Curves of Tested Film-Forming Solutions
3.1.1. Gelatine Solutions with Pumpkin Purée
3.1.2. Soy Protein Isolate Solutions with Pumpkin Purée
3.1.3. Sodium Alginate Solutions with Pumpkin Purée
3.1.4. High-Methylated Apple Pectin Solutions with Pumpkin Purée
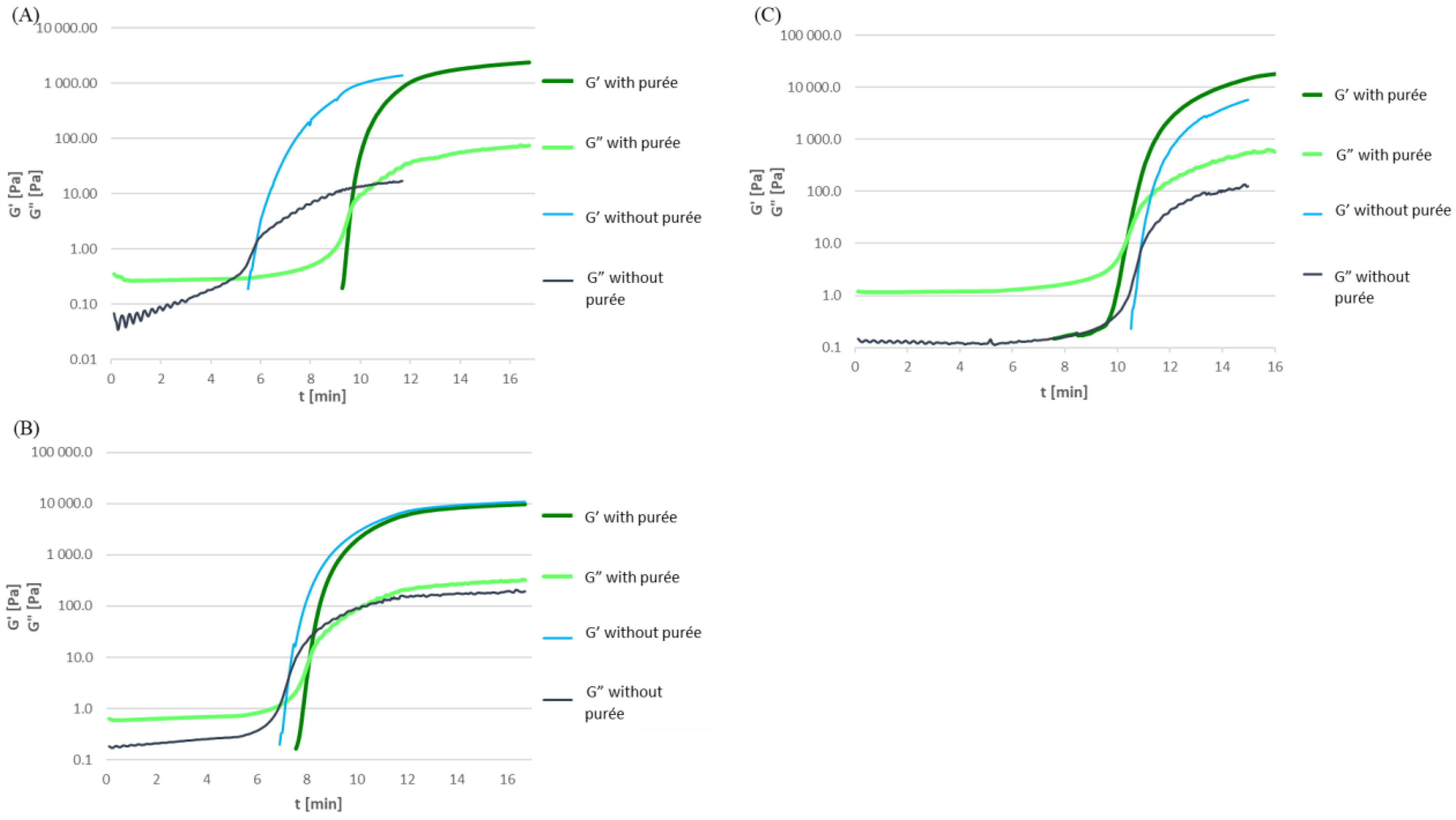
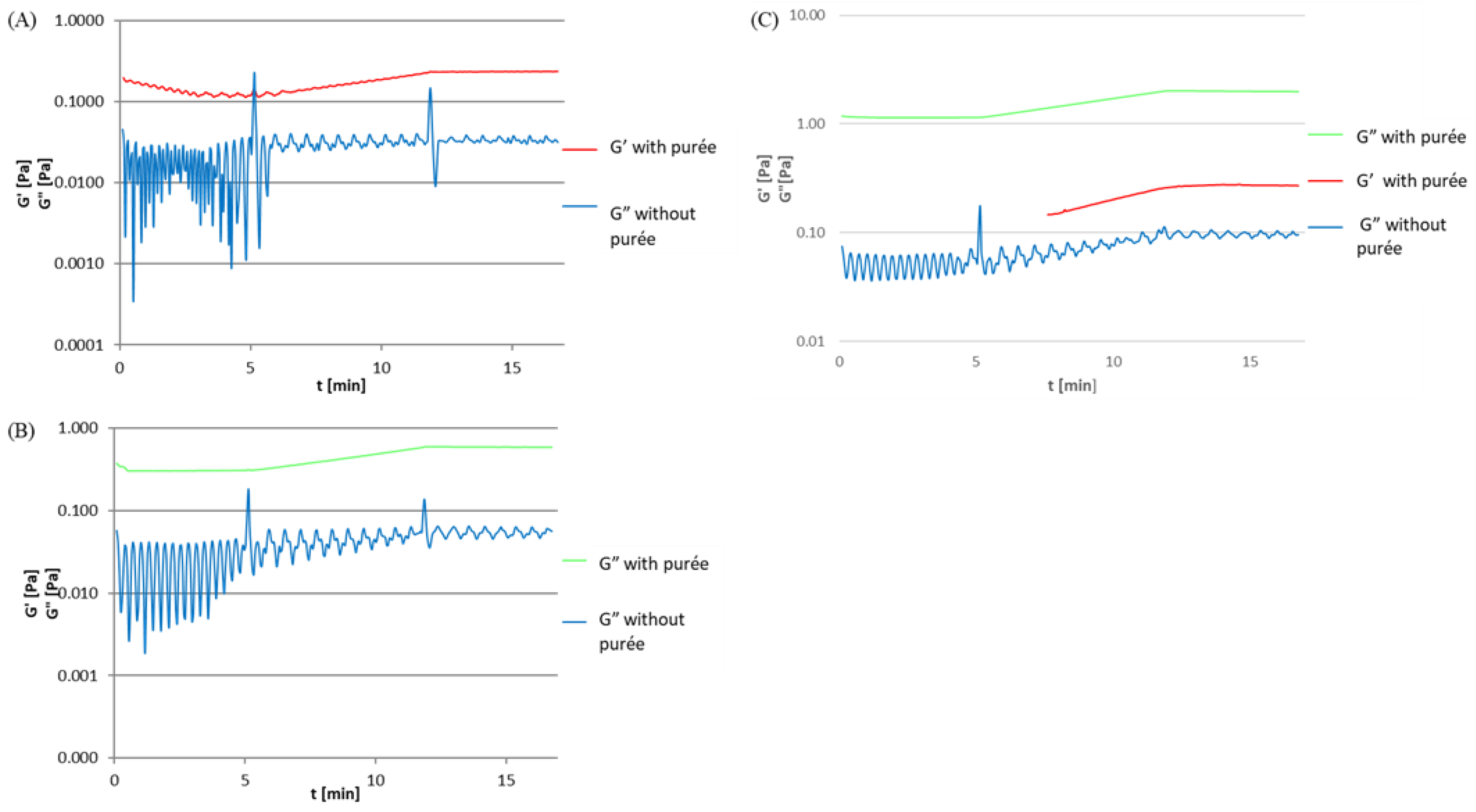
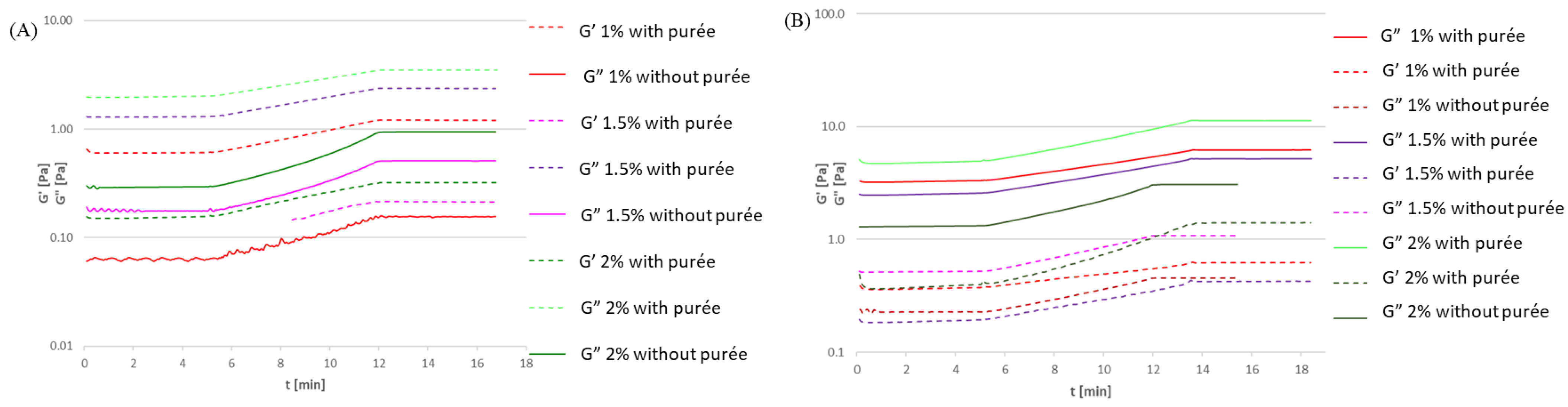
3.2. Effect of Purée Addition on Gelation Temperature
3.2.1. Gelatine Solutions with Pumpkin Purée
3.2.2. Soy Protein Isolate Solutions with Pumpkin Purée
3.2.3. High-Methylated Apple Pectin Solutions with Pumpkin Purée
3.2.4. Sodium Alginate Solutions with Pumpkin Purée
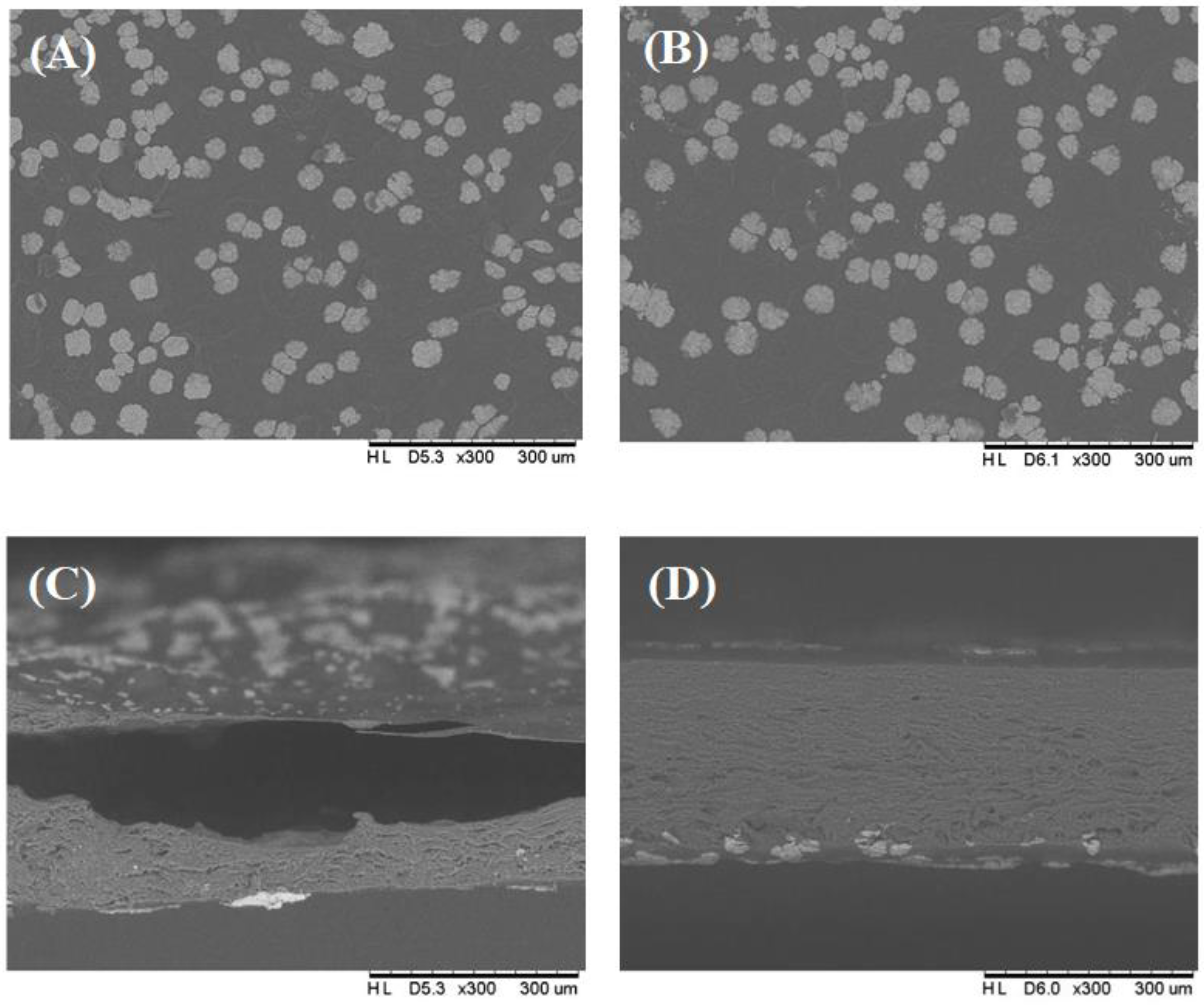
3.3. Effect of Degassing of Film-Forming Solutions on the Matrix and Surface of Edible Films
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Plazzotta, S.; Manzocco, L.; Nicoli, M.C. Fruit and Vegetable Waste Management and the Challenge of Fresh-Cut Salad. Trends Food Sci. Technol. 2017, 63, 51–59. [Google Scholar] [CrossRef]
- Laufenberg, G.; Kunz, B.; Nystroem, M. Transformation of Vegetable Waste into Value Added Products: (A) the Upgrading Concept; (B) Practical Implementations. Bioresour. Technol. 2003, 87, 167–198. [Google Scholar] [CrossRef] [PubMed]
- Directive 2008/98/EC Directive 2008/98/EC of the European Parliament and of the Council of 19 November 2008 on Waste and Repealing Certain Directives. Off. J. Eur. Union L 2008, 312, 22.
- Demirbas, A. Waste Management, Waste Resource Facilities and Waste Conversion Processes. Energy Convers. Manag. 2011, 52, 1280–1287. [Google Scholar] [CrossRef]
- Schneider, F.; Kearney, A.T. The Shadow Economy in Europe, 2013; Johannes Kepler Universitat: Linz, Austria, 2013. [Google Scholar]
- Piccinno, F.; Hischier, R.; Seeger, S.; Som, C. Life Cycle Assessment of a New Technology to Extract, Functionalize and Orient Cellulose Nanofibers from Food Waste. ACS Sustain. Chem. Eng. 2015, 3, 1047–1055. [Google Scholar] [CrossRef]
- Han, S.-K.; Shin, H.-S. Performance of an Innovative Two-Stage Process Converting Food Waste to Hydrogen and Methane. J. Air Waste Manag. Assoc. 2004, 54, 242–249. [Google Scholar] [CrossRef]
- Roy, S.; Priyadarshi, R.; Łopusiewicz, Ł.; Biswas, D.; Chandel, V.; Rhim, J.-W. Recent Progress in Pectin Extraction, Characterization, and Pectin-Based Films for Active Food Packaging Applications: A Review. Int. J. Biol. Macromol. 2023, 239, 124248. [Google Scholar] [CrossRef]
- McHugh, T.H.; Senesi, E. Apple Wraps: A Novel Method to Improve the Quality and Extend the Shelf Life of Fresh-Cut Apples. J. Food Sci. 2000, 65, 480–485. [Google Scholar] [CrossRef]
- McHugh, T.H.; Huxoll, C.C.; Krochta, J.M. Permeability Properties of Fruit Puree Edible Films. J. Food Sci. 1996, 61, 88–91. [Google Scholar] [CrossRef]
- McHugh, T.H.; de Bord, M.D.; Olsen, C.W. Fruit and Vegetable Films and Uses Thereof. Patent Number 8,048,466, 2011. Available online: https://patents.google.com/patent/US8715763B2/en (accessed on 17 May 2023).
- Otoni, C.G.; de Moura, M.R.; Aouada, F.A.; Camilloto, G.P.; Cruz, R.S.; Lorevice, M.V.; Soares, N.d.F.F.; Mattoso, L.H.C. Antimicrobial and Physical-Mechanical Properties of Pectin/Papaya Puree/Cinnamaldehyde Nanoemulsion Edible Composite Films. Food Hydrocoll. 2014, 41, 188–194. [Google Scholar] [CrossRef]
- Galus, S.; Kadzińska, J. Food Applications of Emulsion-Based Edible Films and Coatings. Trends Food Sci. Technol. 2015, 45, 273–283. [Google Scholar] [CrossRef]
- Kadzińska, J.; Janowicz, M.; Kalisz, S.; Bryś, J.; Lenart, A. An Overview of Fruit and Vegetable Edible Packaging Materials. Packag. Technol. Sci. 2019, 32, 483–495. [Google Scholar] [CrossRef]
- Otoni, C.G.; Avena-Bustillos, R.J.; Azeredo, H.M.C.; Lorevice, M.V.; Moura, M.R.; Mattoso, L.H.C.; McHugh, T.H. Recent Advances on Edible Films Based on Fruits and Vegetables-A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 1151–1169. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sun, X.; Liu, H.; Li, M.; Ma, Z. Barrier and Mechanical Properties of Carrot Puree Films. Food Bioprod. Process. 2011, 89, 149–156. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Teng, A.; Liu, A. Mechanical Reinforcement of Gelatin Hydrogel with Nanofiber Cellulose as a Function of Percolation Concentration. Int. J. Biol. Macromol. 2017, 103, 226–233. [Google Scholar] [CrossRef]
- Ninčević Grassino, A.; Rimac Brnčić, S.; Badanjak Sabolović, M.; Šic Žlabur, J.; Marović, R.; Brnčić, M. Carotenoid Content and Profiles of Pumpkin Products and By-Products. Molecules 2023, 28, 858. [Google Scholar] [CrossRef]
- Lalnunthari, C.; Devi, L.M.; Badwaik, L.S. Extraction of Protein and Pectin from Pumpkin Industry By-Products and Their Utilization for Developing Edible Film. J. Food Sci. Technol. 2020, 57, 1807–1816. [Google Scholar] [CrossRef]
- Arifin, N.; Izyan, S.N.; Huda-Faujan, N. Physical Properties and Consumer Acceptability of Basic Muffin Made from Pumpkin Puree as Butter Replacer. Food Res. 2019, 3, 840–845. [Google Scholar] [CrossRef]
- Dhiman, A.K.; Sharma, K.; Attri, S. Functional Constituents and Processing of Pumpkin: A Review. J. Food Sci. Technol. 2009, 45, 411–417. [Google Scholar]
- Kaur, S.; Panghal, A.; Garg, M.K.; Mann, S.; Khatkar, S.K.; Sharma, P.; Chhikara, N. Functional and Nutraceutical Properties of Pumpkin—A Review. Nutr. Food Sci. 2019, 50, 384–401. [Google Scholar] [CrossRef]
- Provesi, J.G.; Dias, C.O.; Amante, E.R. Changes in Carotenoids during Processing and Storage of Pumpkin Puree. Food Chem. 2011, 128, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C.; Miranda, K.W.E.; Ribeiro, H.L.; Rosa, M.F.; Nascimento, D.M. Nanoreinforced Alginate–Acerola Puree Coatings on Acerola Fruits. J. Food Eng. 2012, 113, 505–510. [Google Scholar] [CrossRef][Green Version]
- Sharma, M.; Kristo, E.; Corredig, M.; Duizer, L. Effect of Hydrocolloid Type on Texture of Pureed Carrots: Rheological and Sensory Measures. Food Hydrocoll. 2017, 63, 478–487. [Google Scholar] [CrossRef]
- Wang, H.; Wan, L.; Chen, D.; Guo, X.; Liu, F.; Pan, S. Unexpected Gelation Behavior of Citrus Pectin Induced by Monovalent Cations under Alkaline Conditions. Carbohydr. Polym. 2019, 212, 51–58. [Google Scholar] [CrossRef] [PubMed]
- Janowicz, M.; Sitkiewicz, I.; Ciurzyńska, A.; Galus, S. Rheological Properties of Film-Forming Dispersions of Selected Biopolymers Used for Packaging Films or Food Coating. Coatings 2022, 12, 1704. [Google Scholar] [CrossRef]
- Sitkiewicz, I.; Pałacha, Z. Rheological Properties of Fruit Fillings to Yoghourts. Technol. Prog. Food Process. 2010, 2, 37–41. [Google Scholar]
- Martinez, M.J.; Pizones Ruiz-Henestrosa, V.M.; Carrera Sánchez, C.; Rodríguez Patino, J.M.; Pilosof, A.M.R. Foaming and Surface Properties of Casein Glycomacropeptide–Gelatin Mixtures as Affected by Their Interactions in the Aqueous Phase. Food Hydrocoll. 2013, 33, 48–57. [Google Scholar] [CrossRef]
- Echeverría, I.; Eisenberg, P.; Mauri, A.N. Nanocomposites Films Based on Soy Proteins and Montmorillonite Processed by Casting. J. Memb. Sci. 2014, 449, 15–26. [Google Scholar] [CrossRef]
- Xiao, Q.; Tong, Q.; Lim, L.T. Pullulan-Sodium Alginate Based Edible Films: Rheological Properties of Film Forming Solutions. Carbohydr. Polym. 2012, 87, 1689–1695. [Google Scholar] [CrossRef]
- Falguera, V.; Mengual, A.; Vicente, M.; Ibarz, A. Effect of Calcium Pidolate on the Rheological Characteristics of Jams and Gelatins. Food Res. Int. 2010, 43, 882–885. [Google Scholar] [CrossRef]
- Cai, L.; Feng, J.; Regenstein, J.; Lv, Y.; Li, J. Confectionery Gels: Effects of Low Calorie Sweeteners on the Rheological Properties and Microstructure of Fish Gelatin. Food Hydrocoll. 2017, 67, 157–165. [Google Scholar] [CrossRef]
- Pang, Z.; Deeth, H.; Sopade, P.; Sharma, R.; Bansal, N. Rheology, Texture and Microstructure of Gelatin Gels with and without Milk Proteins. Food Hydrocoll. 2014, 35, 484–493. [Google Scholar] [CrossRef]
- Tiziani, S.; Vodovotz, Y. Rheological Effects of Soy Protein Addition to Tomato Juice. Food Hydrocoll. 2005, 19, 45–52. [Google Scholar] [CrossRef]
- Wang, X.; He, Z.; Zeng, M.; Qin, F.; Adhikari, B.; Chen, J. Effects of the Size and Content of Protein Aggregates on the Rheological and Structural Properties of Soy Protein Isolate Emulsion Gels Induced by CaSO4. Food Chem. 2017, 221, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Muhoza, B.; Xia, S.; Zhang, X. Gelatin and High Methyl Pectin Coacervates Crosslinked with Tannic Acid: The Characterization, Rheological Properties, and Application for Peppermint Oil Microencapsulation. Food Hydrocoll. 2019, 97, 105174. [Google Scholar] [CrossRef]
- Ström, A.; Schuster, E.; Goh, S.M. Rheological Characterization of Acid Pectin Samples in the Absence and Presence of Monovalent Ions. Carbohydr. Polym. 2014, 113, 336–343. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a Rheology Modifier: Origin, Structure, Commercial Production and Rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Axelos, M.A.V.; Thibault, J.F. The Chemistry of Low-Methoxyl Pectin Gelation. Chem. Technol. Pectin. 1991, 6, 108–109. [Google Scholar]
- Taylor, S. The Chemistry and Technology of Pectin; Walter, R.H., Ed.; Academic Press: Cambridge, MA, USA, 2012. [Google Scholar]
- Loh, X.J. Polymers for Personal Care Products and Cosmetics; Loh, X.J., Ed.; The Royal Society of Chemistry: London, UK, 2016. [Google Scholar]
- Feng, Y.; Kopplin, G.; Sato, K.; Draget, K.I.; Vårum, K.M. Alginate Gels with a Combination of Calcium and Chitosan Oligomer Mixtures as Crosslinkers. Carbohydr. Polym. 2017, 156, 490–497. [Google Scholar] [CrossRef]

| Type of Hydrocolloid | Concentration of Hydrocolloid [%] | Glycerol [%] | Concentration of Pumpkin Purée [%] | Initial Temperature [°C] | Final Temperature [°C] |
|---|---|---|---|---|---|
| Gelatine PGEL | 4 * | 50 | 40 | 30 | 10 |
| 8 | 50 | 40 | 30 | 10 | |
| 12 | 50 | 40 | 40 | 10 | |
| Soy protein isolate SPI | 4 | 50 | 40 | 20 | 0 |
| 8 | 50 | 40 | 20 | 0 | |
| 12 | 50 | 40 | 20 | 0 | |
| Sodium alginate SA | 1 | 50 | 40 | 20 | 0 |
| 1.5 | 50 | 40 | 20 | 0 | |
| 2 | 50 | 40 | 20 | 0 | |
| High-methylated apple pectin HMAP | 1 | 50 | 40 | 25 | 0 |
| 1.5 | 50 | 40 | 25 | 0 | |
| 2 | 50 | 40 | 25 | 0 |
| Gelatin (PGEL) | |||||
|---|---|---|---|---|---|
| Concentration [%] | k | n | R2 | Apparent viscosity [Pa·s] | |
| 4 | 0.1101 | 0.6544 | 1 | 0.0285 ± 0.0000 | |
| 8 | 0.1098 | 0.7524 | 0.999 | 0.0417 ± 0.0000 | |
| 12 | 0.2096 | 0.7733 | 0.999 | 0.0860 ± 0.0001 | |
| Statistical analysis of the effect of pumpkin puree concentration and addition on the apparent viscosity of gelatine solutions | |||||
| SS | Degrees of freedom | MS | F | p-value | |
| The addition of pumpkin puree | 0.009547 | 1 | 0.009547 | 35305.94 | 0.000 |
| Solution concentration | 0.003437 | 2 | 0.001719 | 6354.92 | 0.000 |
| The addition of pumpkin puree concentration | 0.002092 | 2 | 0.001046 | 3868.56 | 0.000 |
| Soy protein isolate (SPI) | |||||
| Concentration [%] | k | n | R2 | Apparent viscosity [Pa·s] | |
| 4 | 0.1310 | 0.4816 | 0.999 | 0.0170 ± 0.0004 | |
| 8 | 0.1858 | 0.5737 | 0.999 | 0.0350 ± 0.0003 | |
| 12 | 0.3941 | 0.6301 | 1 | 0.0927 ± 0.0004 | |
| Statistical analysis of the effect of pumpkin puree concentration and addition on the apparent viscosity of soy protein isolate solutions | |||||
| SS | Degrees of freedom | MS | F | p-value | |
| The addition of pumpkin puree | 0.009073 | 1 | 0.009073 | 137739.3 | 0.000 |
| Solution concentration | 0.005185 | 2 | 0.002592 | 39352.9 | 0.000 |
| The addition of pumpkin puree concentration | 0.004227 | 2 | 0.002113 | 32083.4 | 0.000 |
| Sodium alginate (SA) | |||||
| Concentration [%] | k | n | R2 | Apparent viscosity [Pa·s] | |
| 1 | 0.4555 | 0.7718 | 1 | 0.1866 ± 0.0007 | |
| 1.5 | 0.8642 | 0.6951 | 1 | 0.2621 ± 0.0011 | |
| 2 | 1.258 | 0.7385 | 1 | 0.4522 ± 0.0014 | |
| Statistical analysis of the effect of pumpkin puree concentration and addition on the apparent viscosity of sodium alginate solutions | |||||
| SS | Degrees of freedom | MS | F | p-value | |
| The addition of pumpkin puree | 0.211910 | 1 | 0.211910 | 343554.4 | 0.000 |
| Solution concentration | 0.084327 | 1 | 0.084327 | 136713.6 | 0.000 |
| The addition of pumpkin puree concentration | 0.028787 | 1 | 0.028787 | 46670.4 | 0.000 |
| High-methylated apple pectin (HMAP) | |||||
| Concentration [%] | k | n | R2 | Apparent viscosity [Pa·s] | |
| 1 | 0.1644 | 0.6746 | 0.9991 | 0.0460 ± 0.0001 | |
| 1.5 | 0.2694 | 0.6960 | 0.9995 | 0.0820 ± 0.0011 | |
| 2 | 0.4526 | 0.7122 | 0.9999 | 0.1468 ± 0.0010 | |
| Statistical analysis of the effect of pumpkin puree concentration and addition on the apparent viscosity of high-methylated apple pectin solutions | |||||
| SS | Degrees of freedom | MS | F | p-value | |
| The addition of pumpkin puree | 0.024983 | 1 | 0.024983 | 99200.0 | 0.000 |
| Solution concentration | 0.007904 | 1 | 0.007904 | 31383.5 | 0.000 |
| The addition of pumpkin puree concentration | 0.007332 | 1 | 0.007332 | 29114.0 | 0.000 |
| Statistical analysis of the correlation between the concentration of the tested hydrocolloid solutions and the apparent viscosity of the tested solutions | |||||
| Gelatin (PGEL) | 0.9649 | ||||
| Soy protein isolate (SPI) | 0.9864 | ||||
| Sodium alginate (SA) | 0.9608 | ||||
| High-methylated apple pectin (HMAP) | 0.9487 | ||||
| Gelatine (PGEL) | |||||
|---|---|---|---|---|---|
| Concentration [%] | Gelation temperature of the solution without puree [°C] | Gelation temperature of the solution with the addition of puree [°C] | |||
| 4 | 19.74 ± 0.30 b * | 16.64 ± 0.02 a | |||
| 8 | 23.21 ± 0.31 d | 21.26 ± 0.09 c | |||
| 12 | 22.97 ± 0.25 d | 24.31 ± 0.21 e | |||
| Statistical analysis of the effect of pumpkin puree concentration and addition on the apparent viscosity of gelatine solutions | |||||
| SS | Degrees of freedom | MS | F | p-value | |
| The addition of pumpkin puree | 96.155 | 2 | 48.078 | 749.5 | 0.0·1020 |
| Solution concentration | 6.944 | 1 | 6.944 | 108.3 | 0.0·1020 |
| The addition of pumpkin puree concentration | 15.614 | 2 | 7.807 | 121.7 | 0.0·1020 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Janowicz, M.; Kadzińska, J.; Ciurzyńska, A.; Szulc, K.; Galus, S.; Karwacka, M.; Nowacka, M. The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée. Appl. Sci. 2023, 13, 6959. https://doi.org/10.3390/app13126959
Janowicz M, Kadzińska J, Ciurzyńska A, Szulc K, Galus S, Karwacka M, Nowacka M. The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée. Applied Sciences. 2023; 13(12):6959. https://doi.org/10.3390/app13126959
Chicago/Turabian StyleJanowicz, Monika, Justyna Kadzińska, Agnieszka Ciurzyńska, Karolina Szulc, Sabina Galus, Magdalena Karwacka, and Małgorzata Nowacka. 2023. "The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée" Applied Sciences 13, no. 12: 6959. https://doi.org/10.3390/app13126959
APA StyleJanowicz, M., Kadzińska, J., Ciurzyńska, A., Szulc, K., Galus, S., Karwacka, M., & Nowacka, M. (2023). The Structure-Forming Potential of Selected Polysaccharides and Protein Hydrocolloids in Shaping the Properties of Composite Films Using Pumpkin Purée. Applied Sciences, 13(12), 6959. https://doi.org/10.3390/app13126959











