Effect of H2O2 Treatment on Mechanical and Mechanochemical Properties of Fused Silica
Abstract
:1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Modified Adsorbed Water on Various FS Surfaces
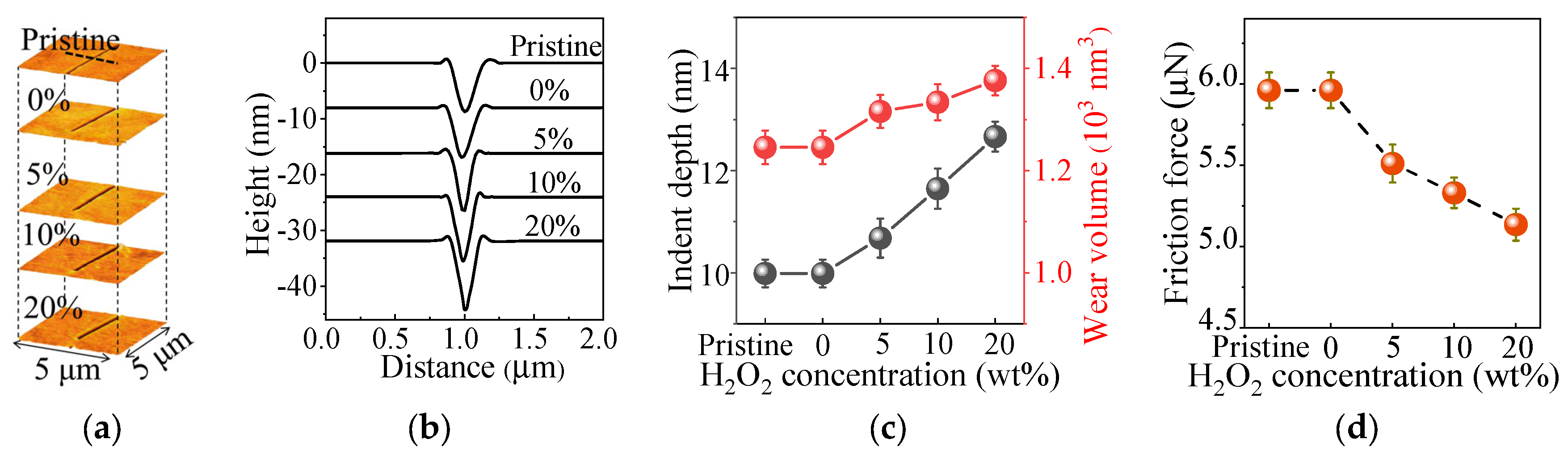
3.2. Suppressed Mechanical Properties of FS Surfaces
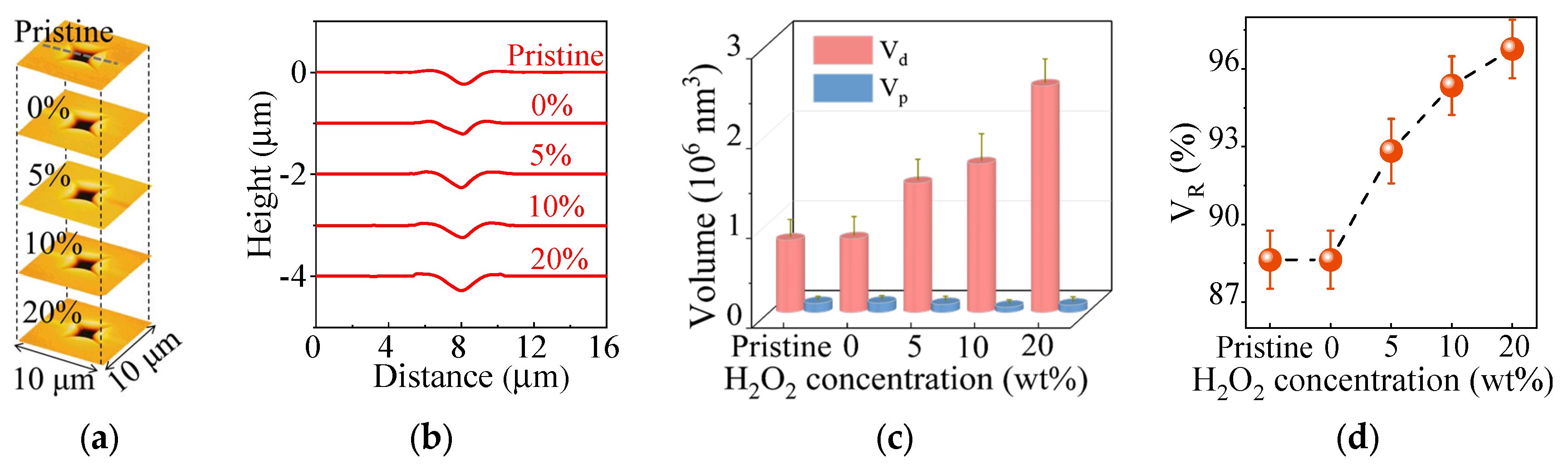
3.3. Enhanced Subsurface Deformation of FS upon Indentation and Nanowear
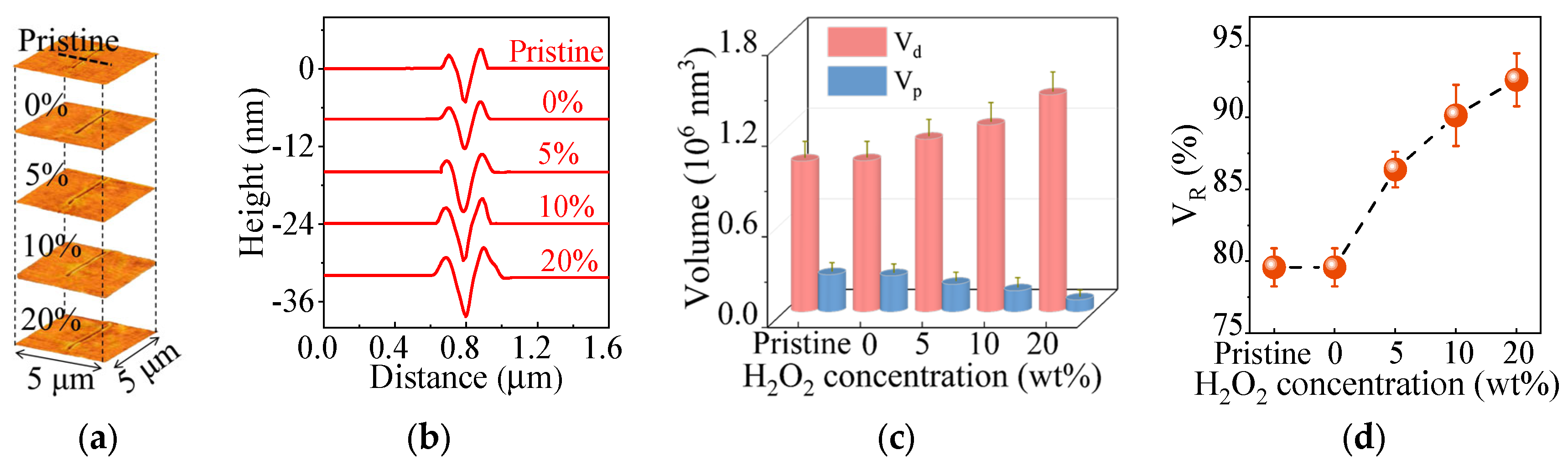
3.4. Suppressed Topography of Various FS Surfaces
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Moses, E.I. Ignition on the National Ignition Facility: A path towards inertial fusion energy. Nucl. Fusion 2009, 49, 104022. [Google Scholar] [CrossRef] [Green Version]
- Gibson, U.J.; Wei, L.; Ballato, J. Semiconductor core fibres: Materials science in a bottle. Nat. Commun. 2021, 12, 3990. [Google Scholar] [CrossRef] [PubMed]
- Kotz, F.; Arnold, K.; Bauer, W.; Schild, D.; Keller, N.; Sachsenheimer, K.; Nargang, T.M.; Richter, C.; Helmer, D.; Rapp, B.E. Three-dimensional printing of transparent fused silica glass. Nature 2017, 544, 337–339. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yan, H.; Yang, K.; Yao, C.; Wang, Z.; Zou, X.; Yan, C.; Yuan, X.; Ju, X.; Yang, L. Surface molecular structure defects and laser-induced damage threshold of fused silica during a manufacturing process. Sci. Rep. 2017, 7, 17870. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Hou, C.; Tian, C.; Guo, J.; Xiang, X.; Jiang, X.; Wang, H.; Liao, W.; Yuan, X.; Jiang, X.; et al. Layer by layer exposure of subsurface defects and laser-induced damage mechanism of fused silica. Appl. Surf. Sci. 2020, 508, 145186. [Google Scholar] [CrossRef]
- Quenneville, J.; Taylor, R.S.; Van Duin, A.C.T. Reactive molecular dynamics studies of DMMP adsorption and reactivity on amorphous silica surfaces. J. Phys. Chem. C 2010, 114, 18894–18902. [Google Scholar] [CrossRef]
- Mound, B.A.; Pharr, G.M. Nanoindentation of fused quartz at loads near the cracking threshold. Exp. Mech. 2019, 59, 369–380. [Google Scholar] [CrossRef]
- Hirao, K.; Tomozawa, M. Microhardness of SiO2 glass in various environments. J. Am. Ceram. Soc. 1987, 70, 497–502. [Google Scholar] [CrossRef]
- He, H.; Qian, L.; Pantano, C.G.; Kim, S.H. Mechanochemical wear of soda lime silica glass in humid environments. J. Am. Ceram. Soc. 2014, 97, 2061–2068. [Google Scholar] [CrossRef]
- Yu, J.; Qian, L.; Yu, B.; Zhou, Z. Effect of surface hydrophilicity on the nanofretting behavior of Si(100) in atmosphere and vacuum. J. Appl. Phys. 2010, 108, 034314. [Google Scholar] [CrossRef]
- Wang, X.; Kim, S.H.; Chen, C.; Chen, L.; He, H.; Qian, L. Humidity dependence of tribochemical wear of monocrystalline silicon. ACS Appl. Mater. Int. 2015, 7, 14785–14792. [Google Scholar] [CrossRef]
- Chen, C.; Xiao, C.; Wang, X.; Zhang, P.; Chen, L.; Qi, Y.; Qian, L. Role of water in the tribochemical removal of bare silicon. Appl. Surf. Sci. 2016, 390, 696–702. [Google Scholar] [CrossRef]
- Wang, M.; Duan, F.; Mu, X. Effect of surface silanol groups on friction and wear between amorphous silica surfaces. Langmuir 2019, 35, 5463–5470. [Google Scholar] [CrossRef]
- Bakos, T.; Rashkeev, S.N.; Pantelides, S.T. Reactions and diffusion of water and oxygen molecules in amorphous SiO2. Phys. Rev. Lett. 2002, 88, 55508. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Huang, J.; Yuan, S.; Chen, C.; Jin, Z.; Kang, R.; Guo, D. Effect of surface hydroxylation on ultra-precision machining of quartz glass. Appl. Surf. Sci. 2020, 501, 144170. [Google Scholar] [CrossRef]
- Barnette, A.L.; Bradley, L.C.; Veres, B.D.; Schreiner, E.P.; Park, Y.B.; Park, J.; Park, S.; Kim, S.H. Selective detection of crystalline cellulose in plant cell walls with Sum-Frequency-Generation (SFG) vibration spectroscopy. Biomacromolecules 2011, 12, 2434–2439. [Google Scholar] [CrossRef]
- Nisha, S.; Howzen, A.; Campbell, A.; Spengler, S.; Liu, H.; Pantano, C.G.; Kim, S.H. Effects of tempering and heat strengthening on hardness, indentation fracture resistance, and wear of soda lime float glass. Int. J. Appl. Glass Sci. 2019, 10, 431–440. [Google Scholar] [CrossRef]
- Gibbs-Davis, J.M.; Hayes, P.L.; Scheidt, K.A.; Geiger, F.M. Anion chelation by amido acid functionalized fused quartz/water interfaces studied by nonlinear optics. J. Am. Chem. Soc. 2007, 129, 7175–7184. [Google Scholar] [CrossRef]
- Grundner, M.; Jacob, H. Investigations on hydrophilic and hydrophobic silicon (100) wafer surfaces by X-ray photoelectron and high-resolution electron-energy loss-spectroscopy. Appl. Phys. A-Mater. 1986, 39, 73–82. [Google Scholar] [CrossRef]
- Liu, W.; Yuan, S.; Guo, X. Atomic understanding of the densification removal mechanism during chemical mechanical polishing of fused glass. Appl. Surf. Sci. 2022, 591, 153166. [Google Scholar] [CrossRef]
- Xiao, X.; Qian, L. Investigation of humidity-dependent capillary force. Langmuir 2000, 16, 8153–8158. [Google Scholar] [CrossRef]
- Pasquarello, A.; Car, R. Identification of Raman defect lines as signatures of ring structures in vitreous silica. Phys. Rev. Lett. 1998, 80, 5145–5147. [Google Scholar] [CrossRef]
- Rouxel, T.; Ji, H.; Hammouda, T.; Moréac, A. Poisson’s ratio and the densification of glass under high pressure. Phys. Rev. Lett. 2008, 100, 225501. [Google Scholar] [CrossRef] [PubMed]
- Thomas, R. Determination of water contents of granite melt inclusions by confocal laser Raman microprobe spectroscopy. Am. Mineral. 2000, 85, 868–872. [Google Scholar] [CrossRef]
- Kuhar, N.; Sil, S.; Verma, T.; Umapathy, S. Challenges in application of Raman spectroscopy to biology and materials. RSC Adv. 2018, 8, 25888–25908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnette, A.L.; Asay, D.B.; Kim, S.H. Average molecular orientations in the adsorbed water layers on silicon oxide in ambient conditions. Phys. Chem. Chem. Phys. 2008, 10, 4981–4986. [Google Scholar] [CrossRef]
- Bradley, L.C.; Dilworth, Z.R.; Barnette, A.L.; Hsiao, E.; Barthel, A.J.; Pantano, C.G.; Kim, S.H. Hydronium ions in soda-lime silicate glass surfaces. J. Am. Ceram. Soc. 2013, 96, 458–463. [Google Scholar] [CrossRef]
- Ngo, D.; Liu, H.; Chen, Z.; Kaya, H.; Zimudzi, T.J.; Gin, S.; Mahadevan, T.; Du, J.; Kim, S.H. Hydrogen bonding interactions of H2O and SiOH on a boroaluminosilicate glass corroded in aqueous solution. NPJ Mater. Degrad. 2020, 4, 1. [Google Scholar] [CrossRef] [Green Version]
- Asanuma, H.; Noguchi, H.; Uosaki, K.; Yu, H.-Z. Water structure at superhydrophobic quartz/water interfaces: A vibrational sum frequency generation spectroscopy study. J. Phys. Chem. C 2009, 113, 21155–21161. [Google Scholar] [CrossRef] [Green Version]
- Wei, X.; Miranda, P.B.; Zhang, C.; Shen, Y.R. Sum-frequency spectroscopic studies of ice interfaces. Phys. Rev. B 2002, 66, 085401. [Google Scholar] [CrossRef] [Green Version]
- Voyiadjis, G.Z.; Yaghoobi, M. Review of nanoindentation size effect: Experiments and atomistic simulation. Crystals 2017, 7, 321. [Google Scholar] [CrossRef] [Green Version]
- Tao, Y.; Wang, X. Revealing the atomic-scale origin of simultaneously enhanced hardness and crack resistance in a single phase material. J. Appl. Phys. 2021, 129, 155107. [Google Scholar] [CrossRef]
- Sawamura, S.; Limbach, R.; Behrens, H.; Wondraczek, L. Lateral deformation and defect resistance of compacted silica glass: Quantification of the scratching hardness of brittle glasses. J. Non-Cryst. Solids 2018, 481, 503–511. [Google Scholar] [CrossRef]
- Michel, M.D.; Serbena, F.C.; Lepienski, C.M. Effect of temperature on hardness and indentation cracking of fused silica. J. Non-Cryst. Solids 2006, 352, 3550–3555. [Google Scholar] [CrossRef]
- Tomozawa, M.; Kim, D.-L.; Agarwal, A.; Davis, K.M. Water diffusion and surface structural relaxation of silica glasses. J. Non-Cryst. Solids 2001, 288, 73–80. [Google Scholar] [CrossRef]
- Wang, Y.; He, H.; Yu, J.; Zhang, Y.; Hu, H. Effect of absorbed water on the adhesion, friction, and wear of phosphate laser glass at nanoscale. J. Am. Ceram. Soc. 2017, 100, 5075–5085. [Google Scholar] [CrossRef]
- He, H.; Hahn, S.H.; Yu, J.; Qian, L.; Kim, S.H. Factors governing wear of soda lime silicate glass: Insights from comparison between nano- and macro-scale wear. Tribol. Int. 2022, 171, 107566. [Google Scholar] [CrossRef]
- He, H.; Kim, S.H.; Qian, L. Effects of contact pressure, counter-surface and humidity on wear of soda-lime-silica glass at nanoscale. Tribol. Int. 2016, 94, 675–681. [Google Scholar] [CrossRef] [Green Version]
- Yu, J.; Kim, S.H.; Yu, B.; Qian, L.; Zhou, Z. Role of tribochemistry in nanowear of single-crystalline silicon. ACS Appl. Mater. Int. 2012, 4, 1585–1593. [Google Scholar] [CrossRef]
- Yoshida, S.; Sanglebœuf, J.C.; Rouxel, T. Quantitative evaluation of indentation-induced densification in glass. J. Mater. Res. 2005, 20, 3404–3412. [Google Scholar] [CrossRef]
- Januchta, K.; Smedskjaer, M.M. Indentation deformation in oxide glasses: Quantification, structural changes, and relation to cracking. J. Non-Cryst. Solids X 2019, 1, 100007. [Google Scholar] [CrossRef]
- He, H.; Hahn, S.H.; Yu, J.; Qiao, Q.; van Duin, A.C.T.; Kim, S.H. Friction-induced subsurface densification of glass at contact stress far below indentation damage threshold. Acta Mater. 2020, 189, 166–173. [Google Scholar] [CrossRef]
- He, H.; Chen, Z.; Lin, Y.; Hahn, S.H.; Yu, J.; van Duin, A.C.T.; Gokus, T.D.; Rotkin, S.V.; Kim, S.H. Subsurface structural change of silica upon nanoscale physical contact: Chemical plasticity beyond topographic elasticity. Acta Mater. 2021, 208, 116694. [Google Scholar] [CrossRef]
- Gu, F.; Qiao, Q.; Yu, J.; He, H. Effects of mechanical interactions on friction-induced subsurface densification of borosilicate glass. J. Non-Cryst. Solids 2021, 572, 121088. [Google Scholar] [CrossRef]
- Ilie, F.; Ipate, G. A modelling study of the correlation between the layer obtained by selective transfer and the dislocations movement at the friction surfaces limit. Metals 2022, 12, 180. [Google Scholar] [CrossRef]
- Wan, Z.; Wang, W.; Feng, J.; Dong, L.; Yang, S.; Jiang, Z. Effect of scratch direction on densification and crack initiation of optical glass BK7. Ceram. Int. 2020, 46, 16754–16762. [Google Scholar] [CrossRef]
- Kazembeyki, M.; Yang, K.; Mauro, J.C.; Smedskjaer, M.M.; Bauchy, M.; Hoover, C.G. Decoupling of indentation modulus and hardness in silicate glasses: Evidence of a shear-to densification-dominated transition. J. Non-Cryst. Solids 2021, 553, 120518. [Google Scholar] [CrossRef]
- Sun, L.; Zhang, Y.; Zhou, X.; Duan, M.; Ye, X.; Li, W.; Li, Y.; Yang, L. Towards investigating surface quality of single-crystal silicon optics polished with different processes. Coatings 2022, 12, 158. [Google Scholar] [CrossRef]
- Sun, L.; Shao, T.; Zhou, X.; Li, W.; Li, F.; Ye, X.; Huang, J.; Chen, S.; Li, B.; Yang, L.; et al. Understanding the effect of HF-based wet shallow etching on optical performance of reactive-ion-etched fused silica optics. RSC Adv. 2021, 11, 29323–29332. [Google Scholar] [CrossRef]
- Shao, T.; Shi, Z.; Sun, L.; Ye, X.; Huang, J.; Li, B.; Yang, L.; Zheng, W. Role of each step in the combined treatment of reactive ion etching and dynamic chemical etching for improving the laser-induced damage resistance of fused silica. Opt. Express 2021, 29, 12365. [Google Scholar] [CrossRef]
- Suratwala, T.; Steele, W.; Wong, L.; Feit, M.D.; Miller, P.E.; Dylla-Spears, R.; Shen, N.; Desjardin, R. Chemistry and formation of the beilby layer during polishing of fused silica glass. J. Am. Ceram. Soc. 2015, 98, 2395–2402. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y.; Yuan, Z.; Wang, J.; Xu, Q.; Yang, W. Improving UV laser damage threshold of fused silica optics by wet chemical etching technique. Proc. SPIE 2015, 9532, 953221. [Google Scholar] [CrossRef]
- Ye, H.; Li, Y.; Yuan, Z.; Wang, J.; Yang, W.; Xu, Q. Laser induced damage characteristics of fused silica optics treated by wet chemical processes. Appl. Surf. Sci. 2015, 357, 498–505. [Google Scholar] [CrossRef]
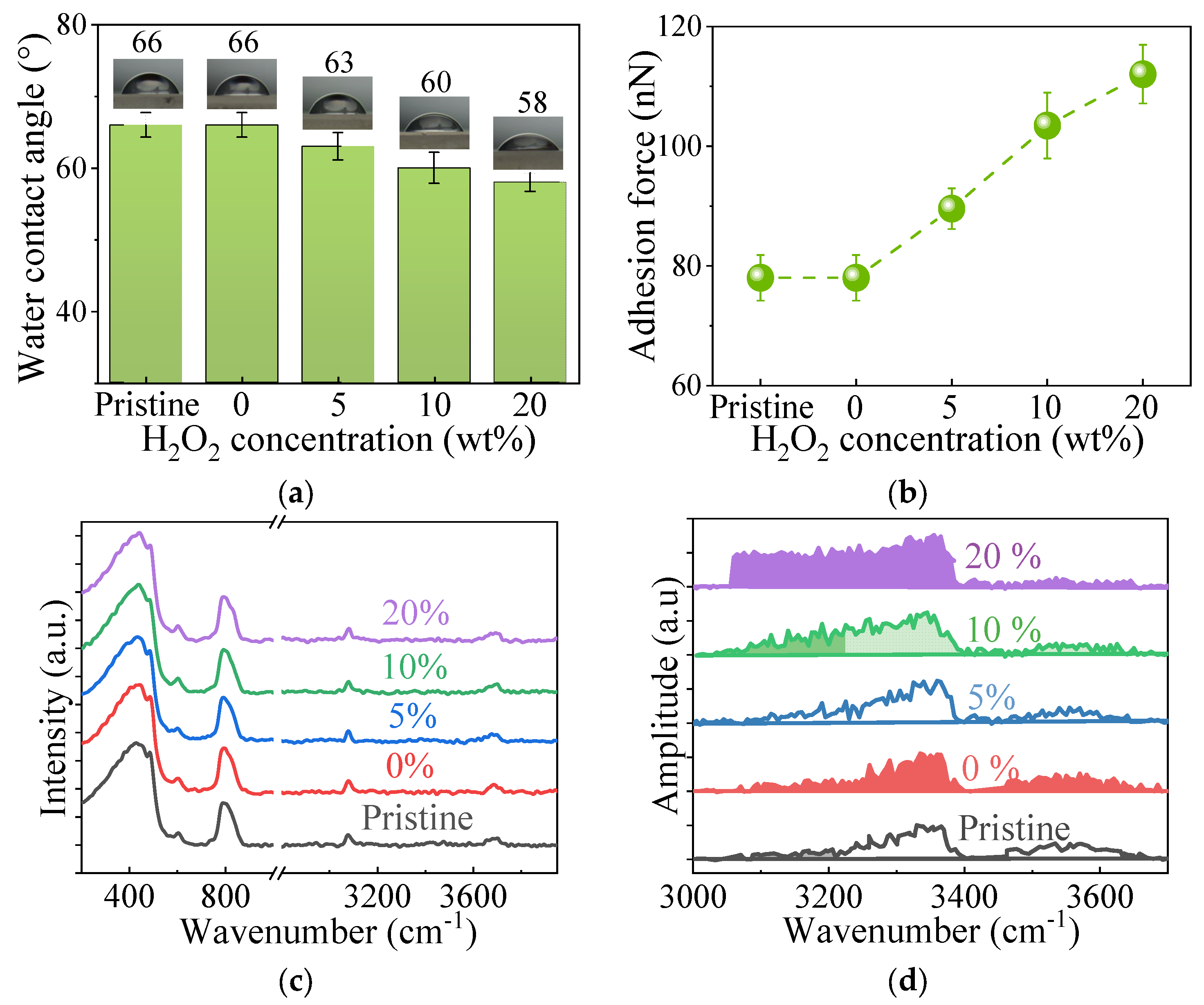



| Sample | Pristine | 0% | 5% | 10% | 20% |
|---|---|---|---|---|---|
| Water contact angle (°) | 66 | 66 | 63 | 60 | 58 |
| Thickness of water (nm) | 0.42 | 0.42 | 0.46 | 0.50 | 0.52 |
| Fc (nN) | 59.09 | 59.09 | 70.89 | 82.31 | 89.70 |
| Fvdw (nN) | 13.03 | 13.03 | 11.79 | 10.84 | 10.33 |
| Calculated Fa (nN) | 72.11 | 72.11 | 82.68 | 93.16 | 100.03 |
| Measured Fa (nN) | 78.01 | 78.01 | 89.56 | 103.45 | 112.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Yin, L.; He, H.; Ma, Y.; Zheng, Q.; Sun, L.; Wang, F.; Yu, J.; Cai, Y. Effect of H2O2 Treatment on Mechanical and Mechanochemical Properties of Fused Silica. Appl. Sci. 2023, 13, 7636. https://doi.org/10.3390/app13137636
Liu X, Yin L, He H, Ma Y, Zheng Q, Sun L, Wang F, Yu J, Cai Y. Effect of H2O2 Treatment on Mechanical and Mechanochemical Properties of Fused Silica. Applied Sciences. 2023; 13(13):7636. https://doi.org/10.3390/app13137636
Chicago/Turabian StyleLiu, Xinqi, Lingyu Yin, Hongtu He, Youze Ma, Qiuju Zheng, Laixi Sun, Fang Wang, Jiaxin Yu, and Yong Cai. 2023. "Effect of H2O2 Treatment on Mechanical and Mechanochemical Properties of Fused Silica" Applied Sciences 13, no. 13: 7636. https://doi.org/10.3390/app13137636
APA StyleLiu, X., Yin, L., He, H., Ma, Y., Zheng, Q., Sun, L., Wang, F., Yu, J., & Cai, Y. (2023). Effect of H2O2 Treatment on Mechanical and Mechanochemical Properties of Fused Silica. Applied Sciences, 13(13), 7636. https://doi.org/10.3390/app13137636







