Abstract
The beneficial nutrients and biologically active ingredients extracted from plants have received great attention in the prevention and treatment of several diseases, including hypercholesterolemic, cancer, diabetes, cardiovascular disorders, hypoglycemic, hypolipidemic, edema, joint pain, weight control, eye vision problems, neuroprotective effects, and asthma. Highly active ingredients predominantly exist in fruit and cladodes, known as phytochemicals (rich contents of minerals, betalains, carbohydrates, vitamins, antioxidants, polyphenols, and taurine), which are renowned for their beneficial properties in relation to human health. Polyphenols are widely present in plants and have demonstrated pharmacological ability through their antimicrobial, anti-inflammatory, anti-bacterial, and antioxidant capacity, and the multi-role act of Opuntia ficus indica makes it suitable for current and future usage in cosmetics for moisturizing, skin improvement, and wound care, as healthful food for essential amino acids, as macro and micro elements for body growth, in building materials as an eco-friendly and sustainable material, as a bio-composite, and as an insulator. However, a more comprehensive understanding and extensive research on the diverse array of phytochemical properties of cactus pear are needed. This review therefore aims to gather and discuss the existing literature on the chemical composition and potential applications of cactus pear extracts, as well as highlight promising directions for future research on this valuable plant.
1. Introduction
The natural extracts obtained from seeds and plants that are present in natural surroundings, finding the novelty of advantages linked to their consumption, supported, promoted, and organized studies in the field of natural extraction and its uses. Therefore, extracts from natural plants and their components from family units of Opuntia have been explored [1]. Opuntia ficus indica (OFI) is a major source of fruit and is found in semiarid and arid regions of several countries in the world, including Italy, Spain, Mexico, South Africa, the Middle East, Australia, and North, Central, and South America. For industrial purposes, it is also cultivated on a suitable surface [2]. Cactus fruit produced by OFI is called nopal fruit or cactus figs. It has an oval shape, and the length is up to 3 cm, generally (Figure 1).

Figure 1.
Representation of (A) fruit, (B) flower, and (C) OFI plant.
Opuntia is a xerophyte plant (that means it is able to survive in conditions poor in liquid water) that contains 200 to 300 species. The three most important types are produced in Italy and Spain. In South African kinds, cladodes with a variety of shapes exist, while in Chile, a popular variety of fruit is green in color, even when it is ripe [3,4]. The color shades of OFI fruits are generally red, orange, magenta, and lime green. The difference in color is due to the inequality in the constituent pigments present in it [5]. The plant is able to survive in harsh environments due to its succulent leaves, which play an important role in thermal regulation, drought resistance, and water storage ability. The plant is appealing as food because of its productivity in changing into dry matter; therefore, it gives edible energy. OFI species succulent pads act as a source of mineral water in dry areas for livestock across the globe and provide essential feed supply to fodder [6]. OFI crops are important food resources in forest-adjacent areas, especially during drought periods, supporting both humans and local mammals. These crops are highly valuable due to their abundance of antioxidants like ascorbic acid and flavonoids, which offer significant health benefits [7]. Due to being fleshy and sweet, OFI is a source of refreshment, jams, and juices. The fermented juice of OFI is used to produce C2H5OH [8], and many types of alcoholic compounds are also obtained from the Opuntia plant [9]. Moreover, in the last decade, OFI derivatives have been investigated for application in packaging materials. Indeed, the incorporation of nano-emulsified bioactive compounds into gelatine films improves mechanical properties such as Young’s modulus, tensile strength, and percentage elongation [10]. Usually, the cactus plant is considered a source of fruits and vegetables, a polymer for cosmetic and medicinal purposes, erosion control, and the bioremediation of wastewater, and as a coagulant, natural color, biofuel, and building material. However, its uses are often restricted to countries of origin [11]. The common names used for Opuntia ficus indica (L.) Miller are nopal, spinless cacti, cactus-pear, tuna cactus, Indian Figure, and prickly pear [12,13]. OFI crops are a major food resource during drought periods in areas near forests, where humans and local mammals rely on them for sustenance. Its consumption as food makes it a valuable source of pure antioxidants, such as ascorbic acid and flavonoids.
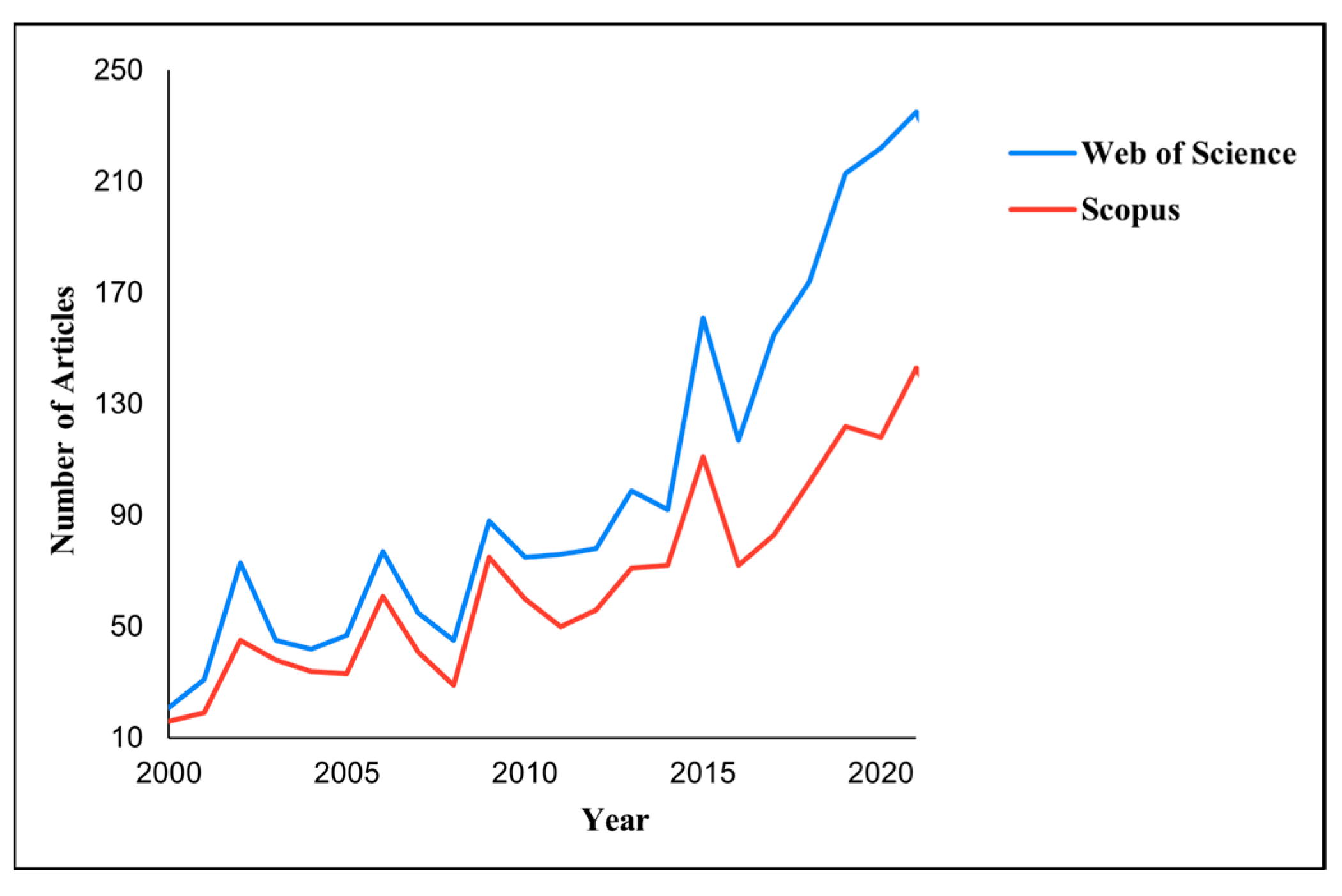
The keywords Opuntia ficus indica were applied to analyze literature in two familiar databases, which have shown the rising number of scientific articles on this subject in the most recent decade, as reported in Figure 2. The trend in the number of scientific articles related to OFI highlights the large and increasing interest in this topic.

Figure 2.
Articles published on Opuntia ficus indica vs. years in renowned technical databases.
This review covers the developments in the area of the chemical and pharmacological properties of relevant compounds separated from the anti-aerial components (cladodes, fruits, and flowers) of OFI. Furthermore, the main current and future uses in human foods, medicinal applications, disease prevention and rehabilitation, cosmetics, the bioremediation of wastewaters, building materials, and clean-energy production will be highlighted.
2. Nutritional and Phytochemicals
Though morphological standards do not match, in the literature, the term cactus leaves is commonly used for flattened stem segments of the OFI plant (see Figure 1). Stems are generally made of the colorless medullar parenchyma, with the pale green khloros-phyllon enclosing the photosynthetic efficient agent. The latter is enclosed together with the spines (altered leaves) and the trichomes or numerous cellular hairs, both producing the named areole, which is a typical fellows feature in forming the Cactaceae unit family. Opuntioideae is a subfamily depicted with harsh, short thorns and spines, named glochids. The areole is the flower source [4]. Glochids are made of purely 100% crystal-clear cellulose [14]. Generally, spines are composed of 96.00% polysaccharides, which are further divided: 49.70% cellulose and 50.30% arabinan. The rest are fats, ash-powder, and crude-waxes, and the residual is lignin. The length of cellulose μ-fibril is 0.04 mm, and the diameter is 6–10 μm; it is loosely imbedded and exists parallelly in an arabinan matrix. The latter is found in the solid gel, which is partly strongly woven with cellulose [15]. This polymer was a 50:50 combination of natural arabinan cellulose composite and swollen gel, without the natural components of hemicelluloses [16]. The spine’s length is 3.0 cm, and it represents 8.40% of the overall cladode mass. They have automated safety functions for the phytophage, light replication, stem shielding, and, therefore, decreasing water dropping [4].
The composition of cladodes depends on multiple factors, such as edaphic factors (physical and chemical characteristics of soil, including pH, salinity, and structure) in the farming area, the weather conditions during the growth season, and the availability of nutrient levels during growth [17,18,19]. The different varieties and breeds have different nutritional contents and vary across different regions. There is no universally accepted standard for determining their specific nutritive values. The composition of de-barded cladodes has been studied by Mohamed E. Malainine [20]. The sample of 100.00 g of dehydrated matter has 19.60 g of ash, 3.60 g of lignin, 7.20 g of lipids and waxes, 21.60 g of cellulose, and 48.00 g of the additional polysaccharides, although unrefined proteins were not evaluated. Researchers [17,20,21,22] stated that 100.00 g dry cladodes include 64.00–71.00 g of carbohydrates, 19.00–23.50 g of ash, 18.00 g of fibers, 1.00–4.00 g of lipids, and 4.00–10.00 g of proteins (complex molecules), including 1.00–2.00 g of digestive proteins. The quantity of active compounds in 100.00 g of fresh cladode is 3.00–7.00 g of carbohydrates, 1.0–2.00 g of minerals, 0.20 g of lipids, 0.50–1.00 g of proteins, and 1.00 g of fibrous substances [17,23]. Fresh cladodes showed greater protein and H2O contents. Remarkably, the 112.00 kg/ha of phosphate supplementation (e.g., Diammonium Phosphate (DAP), Monoammonium Phosphate (MAP), and NPKs) enhanced the minimal cladodes phosphate content, which plays a key role in the development and metabolism of the plant [24]. The fibrous structure of a plant during growth is formed in the cortex and decayed in the core. Overall, fibers and proteins decrease with time [17,24,25]. The large fiber contents and calcium are valuable. Moreover, cladodes are determined to be more beneficial than lettuce due to their natural composition pattern [23,24]. Furthermore, the 88.00–95.00% water content makes cladodes a low-calorie diet, with 27 kcal/100 g [26]. The corresponding compounds given in the literature are summarized in Table 1.

Table 1.
Opuntia cladodes chemical composition (g/100 g).
2.1. Nutritional Profile
2.1.1. Carbohydrates
The OFI contains several kinds of carbohydrates. One of the sugars found in OFI is a form of fructose named inulin, a soluble fiber that exists in many plant-based foods. Inulin works as a prebiotic, improves Ca absorption ability, has cholesterol-lowering effects and a low glycemic index, boosts heart health, decreases general calorie intake, and is effective for weight control. The important carbohydrates stated in OFI are galactose, galacturonic acid, and glucose. The high sugar contents observed are due to the presence of glucose (9.30 to 12.00 g/100 g), oxidized d-galactose (6.5 to 8.8 g/100 g), pectinose (1.5 to 2 g/100 g), and D-xylose (1.9 to 2.01 g/100 g), which were detected at lower levels, while rhamnose (0.35 to 0.79 g/100 g) was found in trace amounts. The monomeric sugar content analysis from the dry sample of cladode obtained from south Italy has the same report, confirming the large content of dextrose (15.3 g/100 g), hexuronic acid (9.6 g/100 g), and glucose lactique (3.3 g/100 g) [27]. These special properties make cactus pear sweet, a very good natural food, and an additive in different food materials.
2.1.2. Proteins
Amino acids present very important functions in human beings’ fitness and biology. They create proteins, which are essential for organ and tissue development, repair, and preservation. They are involved in metabolic activities (making energy, hormones, and neurotransmitters), immune functions, wound healing, muscle development and repair, adjusting appetite, feeling, and sleep [28,29,30]. The majority of amino acids observed in the cactus cladode are glutamine, and the rest are lysine, valine, leucine, arginine, isoleucine, and phenylalanine. In seed, glutamic acid is found as the major amino acid, varying in percentage from 15.73% to 20.27%, followed by arginine, (4.81% to 14.62%) [28,29]. By contrast, the main amino acids in cactus fruits of total amino acid contents are proline (46%) and taurine (15.78%), while ornithine is the only amino acid that is present in trace amounts in cladode, seed, and fruit. Therefore, pulp, fruit, and seeds can be counted as extremely great sources of amino acids [28,30,31,32] (see Table 2). Humans need to consume a balanced diet to obtain key amino acids, especially lysine, isoleucine, valine, and leucine, which are essential to the human body.

Table 2.
The compositions of the amino acids content in OFI fruit, cladodes, and seeds in (g/100 g).
2.1.3. Fats
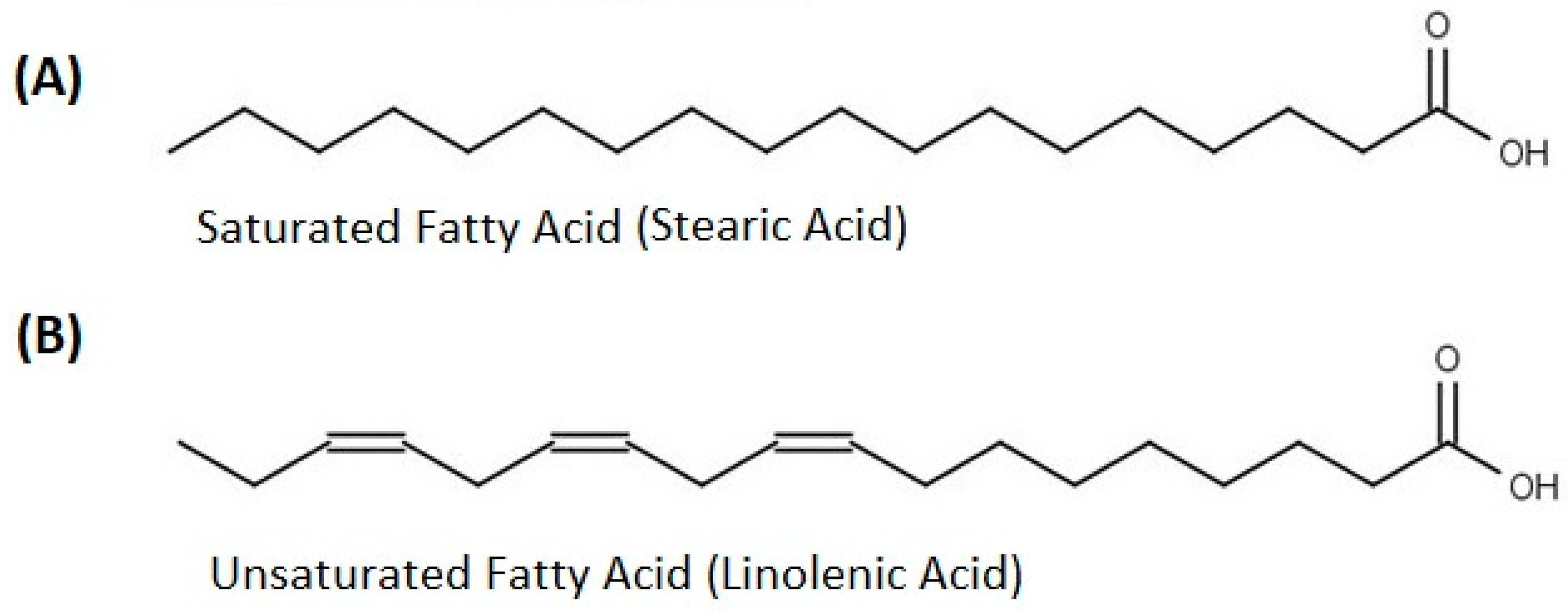
The fatty acids are essential for human beings’ health and are present in a lot of food stuff (plant-based and animal goods). Fatty acids help maintain good eye health, reduce age-related vision difficulties, lower joint pain, enhance joint flexibility, and are beneficial for brain function and growth. A general representation of fatty acids is reported in Figure 3.
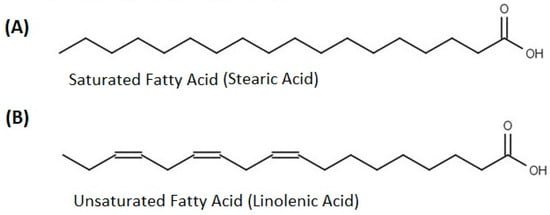
Figure 3.
General representation of (A) saturated and (B) un-saturated fatty acids.
Studies on the extracted lipids from the seed, pulp, and skin of OFI [33] reported that the maximum quantity of palmitic acid (C16:0) was found in cladode skin (20.76 g/100 g). A large quantity of oleic acid (C18:1) exists in pulp (23.26 g/100 g); the maximum amount of linoleic acid (C18:2) was found in pulp (48.86 g/100 g), while a quantity of 11.44 g/100 g of linolenic acid (C18:3) was found in skin (Table 3). Trace amounts of polyunsaturated fatty acids, eicosenoic acids, and eicosatetraenoic acids were observed in the skin, seed, and pulp, respectively. The unsaturated acids (Z)-octadec-9-enoic acid (C18:1) and cis, cis-9,12-Octadecadienoic acid (C18:2) represent 90% of all fatty acids calculated [31,33].

Table 3.
Fatty acid (g/100 g) distribution in the pulp, skin, and seed of the OFI.
2.1.4. Vitamins
Vitamins play a key role in human growth, immune system protection, and metabolic stability. The basic vitamins necessary for human health are Vitamin A (which stabilizes vision and promotes healthy skin), Vitamin C (which is an antioxidant and helps the immune system), Vitamin D (which enhances calcium absorption and bone strength), Vitamin E (which protects cells from destruction), and the B-complex vitamins, which maintain good nerve function and energy consumption. The OFI contains a significant quantity of vitamins (see Table 4). The concentration of vitamins in different portions of OFI varies and depends on the area of harvest [27]. The fruit, specifically its pulp, is enriched in ascorbic acid at levels up to 478.82 mg/100 g (Table 5); however, the skin from the fruits has a low concentration of σ-tocopherol (26 mg/100 g) [34,35]. A rich quantity of α-Tocopherol (1760 mg/100 g) is also extracted from the fruit’s skin (Table 5). The amount of ascorbic acid in cactus pear is 7 to 22 mg/100 g. Ascorbic acid, α-Tocopherol, and other tocopherols are present in all parts of the plant [35,36,37]. Folic acid, thiamine, pyridoxine, riboflavin, niacin, and lycopene are present in trace amounts only in the fruit pulp, while in cladodes, thiamine and niacin are present in trace amounts. [36]. Since vitamins are significant molecules for humans and adequate consumption is necessary for the correct implementation of many physiological activities, OFI represents a good source of them.

Table 4.
Vitamins (mg/100 g) present in different components of OFI.

Table 5.
Inorganic mineral composition of Opuntia peels, cladodes, fruits, and seeds (mg/100 g).
2.1.5. Inorganic Minerals
Inorganic minerals play an essential role in human physical health and physiological activities and keep many bodily activities within limits. The basic inorganic minerals and nutrients are needed on a small scale to sustain a good physical condition. These minerals are taken from numerous dietary nutritional sources. From the micro element, Zn is good for oxidative and antibacterial action. Co is for red blood cell formation, and Fe is for genetics, cytokine secretion, proteins, and pharmacological actions [38]. From the macro element, Mg is good for nerve regulation and muscles, Ca is for bone strength, and Na is for fluid balance. A significant number of inorganic minerals are present in OFI cladodes, seeds, and fruit. Table 5 shows the composition of the mineral content of OFI. Calcium and potassium are the main minerals, amounting, in the total ash content in dry matter, to 316.5 mg/100 g and 108.8 mg/100 g, respectively [39,40], while in seeds, potassium is 304.51 mg/100 g and calcium is 480.93 mg/100 g. Copper is the mineral with the lowest amount in total dry matter: 0.01 mg/100 g in cladodes, 0.21 mg/100 g in fruit, 9.47 mg/100 g in the peel, and 2.1 mg/100 g in seeds, respectively. Chromium and nickel are present in seeds only [41,42]. The considered standard values should be precise figures because the minerals and concentration differ with classes, cultivation spots, and the biological condition of the cladode tissue and seeds.
2.2. Phytochemicals
2.2.1. Polyphenolic Compounds
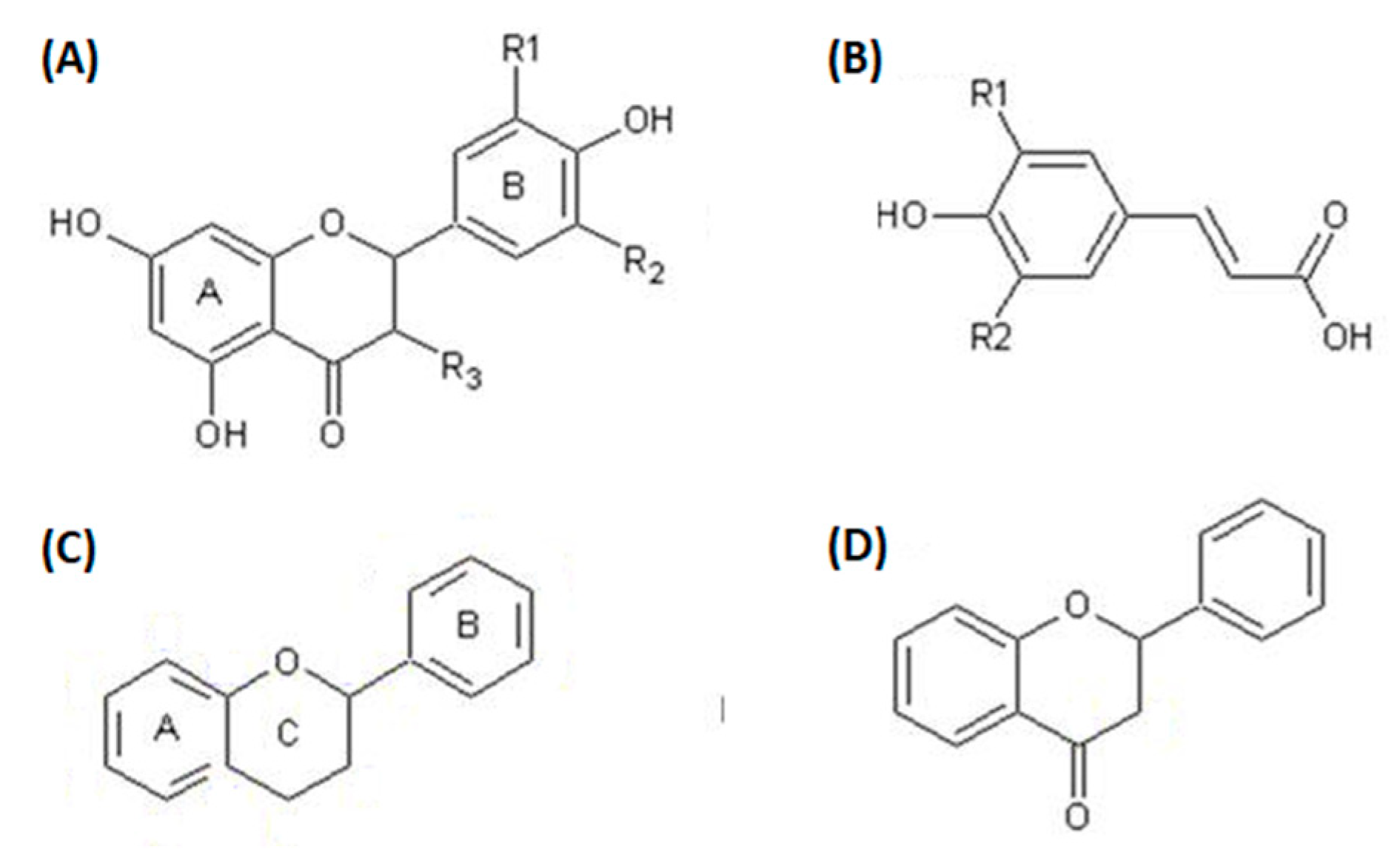
Polyphenolic compounds belong to a class of organic compounds commonly found in the plant kingdom. This class is divided into four major groups, which are stilbenes, flavonoids, lignans, and suberin-acids. These compounds control cell destruction initiated by oxidative stress and free radicals, enhance blood flow, minimize the threat of heart disease, decrease neurodegenerative ailments, and improve cognitive function. The common assembly of OFI polyphenols is represented in Figure 4.
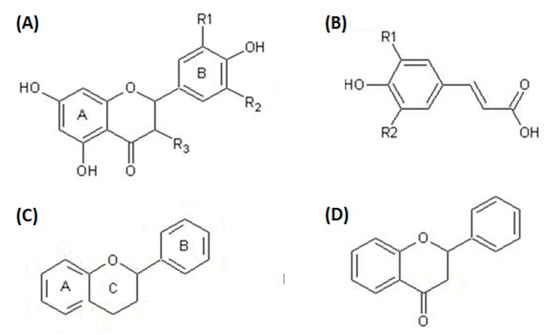
Figure 4.
General forms of OFI polyphenols: (A) general form of flavonols, (B) general form of hydroxycinnamates, (C) general form of flavonoids, (D) general form of flavones.
As the name suggests, polyphenolic compounds are characterized by the presence of various phenolic groups, which may be linked with low- or high-molecular-weight groups of chemicals to form their structures [44]. These compounds are by-products of plant metabolism [45]. The growing interest in polyphenolic compounds is due to their antioxidant ability [46] and health benefits [47]. Polyphonic compounds are present in all components of the cactus plant, such as a variety of polyphenolic acids and flavonoids (Table 6). The flower contains gallic acid as the main compound of dry matter (4900 mg/100 g), along with 6-isorhamnetin-3-O-robinobioside (C28H32O16) at a concentration of 4269.00 mg/100 g [48,49,50]. The remaining polyphenolic compounds have appeared on a small scale (Table 4). The pulp of OFI fruit is a rich source of phenolic content, with 218.80 mg/100 g present in the pulp [51]. Additionally, the isorhamnetin glycoside is present in a significant amount, 50.60 mg/100 g, in comparison to other flavonoids [52,53,54,55]. The fruit seeds are also high in polyphenolic compounds, including tannins and feruloyl derivatives [56] (Table 4). Notably, the fruit crust contains a remarkable (45.7 g/100 g) phenolic content; among the various phenolic compounds present, many have been found to have bioactive properties, particularly the derivatives of flavonoid quercetin and kaempferol (both are beneficial for heart diseases, Alzheimer diseases, anti-cancer effects, arthritis, and diabetes and improve memory function); the contents are 0.22 mg/100 g and 4.32 mg/100 g, respectively [7,53,57]. The highest significant source of flavonoids and polyphenolic compounds is the cactus flower. In particular, some types of cacti that have cladodes yield a diverse range of phenolic compounds. The Phaeacantha cactus contains a rich quantity of rare compounds of flavonoids such as narcissin (137.10 mg/0.1 kg) and nicotiflorin (146.50 mg/0.1 kg) (see Table 6). In addition, it contains a significant amount of isoquercetin (39.70 mg/100 g) and ferulic acid (34.8 mg/100 g) [55,58,59]. The variations in the polyphenolic contents of cacti can be explained by the nature of the soil, climate, cladode age, and environment. It is valuable to take a diet with wealthy polyphenolic compounds to obtain a full array of advantages.

Table 6.
Polyphenolic contents (mg/100 g) of the seed, skin, pulp, flower, and cladode of OFI.
2.2.2. Betalains
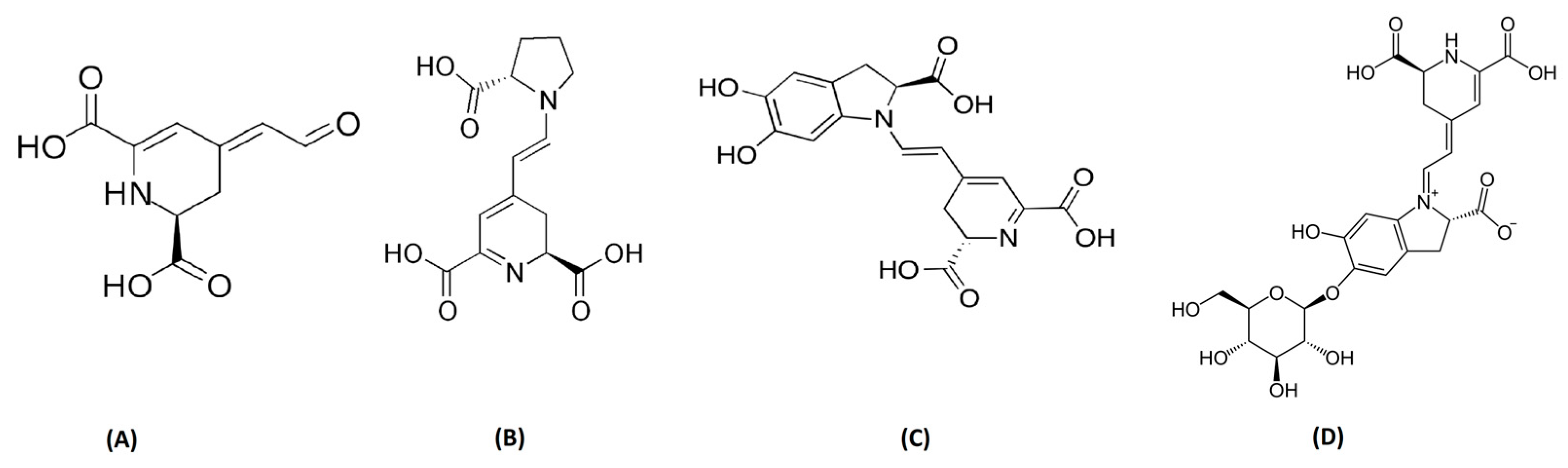
The presence of betalains is restricted to a limited number of plant species; beets, and cacti are the primary sources of this type of pigment. They are recognized for their cheerful and bright hues. Recent analyses have shown that OFI betalains have various interesting properties that are valuable for human health. Indeed, potentially, betalains are used as antimicrobials, they have cardiovascular benefits (they improve cholesterol levels and reduce blood pressure), they have anti-cancer benefits (especially against colon cancer cells), they act as antioxidants (they protect cells from damage), and they have been found to improve insulin sensitivity in the human body [63]. The core structure of betalain is made of nitrogen and betalamic acid, as reported in Figure 5. The derivates of amino acids and imino compounds react with betalamic acid to make betaxanthins and betacyanins pigments, which are yellow and violet, respectively. Amazingly, a large number of betalains are present in the skin and pulp of OFI. The color variation of the fruit depends on the concentration of betaxanthins, betacyanins, and their derivatives. Furthermore, indicaxanthin, betanin, betanidin, neobetanin, and isobetanin are also produced by OFI fruit pulp [64,65]. Betanin and indicaxanthin are also identified in the skin [66]. The presence of Vulgaxanthin IV, (S)-serine-betaxanthin, Vulgaxanthin II, Vulgaxanthin I, Miraxanthin II, Portulacaxanthin III, Portulacaxanthin I, muscaaurin, (S)-valine-betaxanthin’s, (S)-isoleucine and (S)-Phenylalaine betaxanthin, and gomphrenin I has also been reported [66,67,68,69].
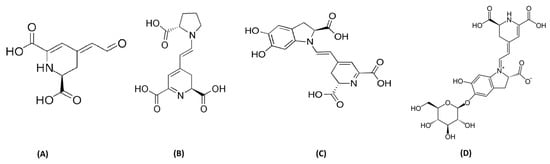
Figure 5.
General representation of (A) betalamic acid, (B) indicaxanthin, (C) betanidin, and (D) betanin.
At low concentrations, betacyanin-derived pigments including betanin, phyllocactin, and betanidin exhibited antioxidant activity, manifesting as yellow and red colors The presence of catechol groups in betanidin structures involved in free radical nitrogen scavenging activity has significant antioxidant capacity. The natural pigment betacyanins are useful for maintaining physiological ability under oxidative stress [70]. Additionally, indicaxanthin was presented as less active with respect to betanin in the free radical scavenging reactions [71].
2.2.3. Sterols
Sterols are a type of molecule present in plant-based foods, such as nuts and oils, and are also found in animals and dairy products. They are effective for human health, as they help to generate and stabilize hormones in the body, boost the body’s defense mechanisms, lower inflammation, and have anticancer abilities. From OFI fruits, seeds, and skin, the extracted sterol in prominent concentrations is β-sitosterol, which varies between 6.75 and 21.10 g/1000 g [72] (see Table 7). Further, a Campesterol quantity of 1.66–8.76 g/1000 g is claimed to be found in the skin, seed, and pulp. Additionally, glycine max (19.00 to 23.00 g/1000 g) and argan oil (4.00 g/1000 g) have similar Campesterol compositions in the seed, pulp, and skin. In addition, Δ7-Avenasterol, stigmasterol, and lanosterol exist on a small scale, while ergosterol exists in trace content in the peel. Sterols in flowers and cladodes are still unidentified. The schottenol and spina also exist in argan oil [73].

Table 7.
The sterols content (g/1000 g) of OFI consists of pulp, seed, peel, and skin.
3. Applications
Opuntia ficus indica is highly valued worldwide due to its wide range of applications. It is utilized as an effective tool in medicine, in cosmetic ingredients, in human nutrition as food, in livestock feed as forage, in wastewater treatment, in fuel production, and in sustainable and eco-friendly building materials. Its remarkable versatility and adaptability have made it invaluable in various industries and culture practices around the world.
3.1. Uses of Opunia in the Bioremediation of Wastewaters
Due to the increase in global population and pollution problems, providing safe drinking water is becoming an increasingly important issue. For this reason, many methods have been studied for removing contaminants from wastewater, including irradiation, biosorption, deionization, flotation, coagulation, microfiltration, membrane filtration, oxidation, ion exchange, ozonation, and electrochemical treatment. The elimination of organic and inorganic pollutants from wastewater by biosorption using OFI has advantages due to its low cost, its great ability for pollutant binding, its quick elimination of pollutants, and the easy accessibility of material [75]. The seeds of OFI and Moringa Oleifera were used as bio-coagulants in comparison to Alum, removing 100% water turbidity at pH 7.5. The OFI coagulation–flocculation active molecules, especially polysaccharides, work as inter particles binding in polluted H2O treatment [76]. Kinetic and thermodynamic studies and equilibrium models are utilized to determine the interfaces between pollutants and biosorbents. OFI pads, both in their raw and chemically and physically treated forms, are useful for removing chemical oxygen demand (COD) dyes, pesticides, turbidness, negative-ions, and metal species from wastewater [75,76,77]. Except for the skin, various parts of OFI show coagulation action with Moringa Oleifera, which is useful for the elimination of turbidity in synthetic clay solutions, as reported by Miller [77]. The turbidity is reduced by 92–99 percent with a natural coagulant. The presence of galacturonic acid and other active molecules in OFI makes it efficient for treating wastewater turbidity. Galactose, arabinose, and rhamnose combined with galacturonic acid account for fifty percent of all coagulation processes, indicating that other components of OFI also contribute to the coagulation process [74,77]. The biocoagulation–flocculation process was utilized to remove heavy metals from contaminated natural samples collected from the Mukuvisi River in Zimbabwe under standard conditions with high OFI powder activity. The optimal conditions for the process were 35 °C, 5 pH, and a 180 min contact time. At standard conditions, even in the presence of other ions, Pb(II) is easily eliminated with OFI powder [78].
3.2. Usage as Forage
The OFI is a highly drought-resistant species with a deep-rooted mechanism, well fitted in arid conditions. The OFI fruits and pads have high levels of protein, water, soluble sugar, and nutrients, making them ideal for use as forage crops. Cactus cladodes are used as fodder for goats, sheep, and cows in different regions of Africa, Asia, America, and Europe. They cover a significant quantity of recommended minerals, proteins, water, and nutrition for animals. Since its laxative effect is attributed to the high-level content of oxalic acid, a blend with straw is suggested. Moreover, the low tannin and phenolic contents of cactus stems aid in digestion, improve protein and fats, and enhance the meat production [79,80] and milk yield [81]. Cactus cladodes combined with sugarcane bagasse are utilized as a significant dairy supplement in semi-arid regions. This alternative to traditional lipid components enhances milk production, improves the milk fat content, and shifts the composition of milk fat towards a more favorable fatty acid profile, promoting a healthier outcome [82]. In this study, the dry matter digestibility is an important factor. Livestock are more likely to acquire the necessary nutrients for growth when there is an increase in the digestibility of dry matter as forage in drought [83].
3.3. Fuel Fabrication
As an alternate combustible material, the possible production of biomethane (biogas), electrical power energy, and heat energy from cactus pear using the Anaerobic Digestion method is possible. G.I.S (Geographic Information System) Dufour 2.0 software shows that 600,000.00 ha. ca. produced 612,115 × 103 m3 of biogas, resulting in the production of 342,784 × 103 m3 of bio CH4, 67,038,000 KWh of electric power energy, and 70,390,000 KWh of heat energy. Further, the obtained digestate can also be treated as a bio-fertilizer for natural and regular farming [84]. For green energy, a bioelectrochemical cell is frequently employed in extremely water-saturated environments. A novel plug-in integrated porcelain-based fuel cell was assembled, resulting in a typical energy density of 103.60 mW/m3 in a device applying O. albicarpa, with Opuntia (10.6 mW/m3) > Opuntia. robusta (7.5 mW/m3) > Opuntia. joconostle (0.46 mW/m3), accompanied by a resistance of 103 Ω. The 285.12 J electricity was attained in 4 weeks from O. albicarpa [85].
3.4. Pharamcological Ability
In recent years, the OFI has been considered an active pharmacological compound source. Findings have indicated that OFI contains betalains, which are molecules with great antioxidant abilities that protect from oxidative stress and lessen inflammation. OFI seeds contain glucuronoxylans, which act as biological hypoglycemic natural agents and have shown antidiabetic effects. The biological natural agents obtained from Opuntia through a natural and easy procedure also show incredible power against chronic diseases with no side-effects [86]. Generally, the mixture of OFI fruit and cladode extract is used to control the hypoglycemic impact in pre-diabetic overweight humans. The extract, when processed before the dextrose tolerance examination, reduces the blood glucose levels [87]. Several chemotherapeutic medicines have been derived from more than 3000 plants and artificial derivatives for cancer therapy, including carotenoids, terpenoids, and alkaloids as the most important components in cancer treatment. According to international reports, the fruit, stem, and cladodes of Opuntia species have a noteworthy approach for anticancer activity [88]. Giglio and co-workers stated in their work that the extract obtained from OFI decreases the atherogenicity, increases metabolic parameters, and decreases lipoproteins rarity in examinees with metabolic risk aspects [89].
3.4.1. Antioxident Capacity
The total polyphenolic compounds, obtained from the aqueous extract of dehydrated flowers and peels from OFI, have shown activity in controlling radical scavenging against hydroxyl anions and superoxide. When the antioxidant activity of dehydrated flower and peel extract was observed, the 2,2-diphenyl-1-picrylhydrazyl (DPPH) scavenging action was lower in the peel (5.17 g G.A.E. kg/D.W.) (Gallic acid equivalent per kg of dry weight) than in the flower (30.40 g G.A.E. kg/D.W.). These results showed that the antioxidant action of OFI peels was six times lower than that of the flower [90]. The antioxidant action of seed oil from OFI extraction was found in three different solvents, C6H14, C2H5OH, and C2H5-acetate, exhibiting noteworthy scavenging motion towards free radicals (DPPH). The oil extract in C2H5-acetate has a maximum antioxidant action of 274 (μmole TE/20 mg), followed by C2H5OH 247.00 (μmole TE/20 mg) and C6H14, which have the lowest values. The antioxidant action of oil is greatly influenced by the solvent for extraction [91]. In addition, several typical models showed that Betalain dyes have notable antioxidant action. The UV–Visible spectroscopy technique was used to analyze the antioxidant action of different samples simply and quickly by examining the change in the color of the DPPH radical scavenging activity assay from a dark purple hue to a bright yellow. The highest contents of polyphenolic compounds in the purple peel of Opuntia spp. exhibited the maximum scavenging activity as compared to yellow and others. Furthermore, the antioxidant activity increased when the number of polyphenolic compounds increased [92]. According to Oliveira [93], the use of Opuntia polyphenolic compounds causes a decrease in rat liver damage. Moreover, the oven drying peel is used in baking goods as an additive due to its antioxidant ability [94].
3.4.2. Anti-Inflammatory Capacity
OFI has a long history of traditional uses for many illnesses. In the last decade, its potential anti-inflammatory effects have been the focus of several works of research. Inflammation is a natural reaction of the body to infection or injury, but many diseases (such as cancer, diabetes, and heart problems) are developed due to chronic inflammation. Studies have indicated that OFI contains anti-inflammatory compounds. The pro-inflammatory cytokines, which play a significant role in the inflammatory reaction, are also produced in the OFI plant. The anti-inflammatory ability of Opuntia has been presented in a Moroccan study [95]. Anti-inflammatory activity has been also assessed in a recent experiment on chemical injury induced in Swiss rats. The seed oil used in this study was extracted from 20 g powder by the Soxhlet apparatus and concentrated in a rotary vacuum evaporator at 40–60 °C under low pressure. The 200 mg/kg dose was given daily for 14 days. This study’s results prove the seed oil OFI’s effectiveness as an anti-inflammatory agent, and this plant is also used in traditional medicines as an edematous agent [96]. Extracted seed oil from OFI and Punica granatum was used to experimentally evaluate the anti-inflammatory effect of carrageenan and the induced-trauma inflammatory in a female rat with an edema paw. The study shows that the seed oils of both plants had significant anti-inflammatory activity for the drugs used in the reference models and had no effect on the general behavior of the tested female animal [93,95]. The strongest anti-inflammatory and antioxidant activities are presented by OFI betalain-pure extract rather than betalains obtained from other sources by in vitro, cell-based, and cell-free assays. Both betalains and OFI betalain-pure extract reduced the release of oxygen species (R.O.S.) and important inflammatory markers (IL-6, IL-8, and NO), and they were more effective in reducing the intestinal inflammation than the reference drugs dexamethasone and trolox [97].
3.4.3. Antibacterial Effect
Antibacterial activity typically relies on factors such as the nature of the biological sample and the extraction method employed. Notably, oven-dried fruits exhibited superior activity compared to other samples [98]. In the extract analysis, the findings indicated that OFI seed oil isolated with C2H5OH and acidic methanol has valuable antibacterial activity in many bacterial strains and at dissimilar inhibition zone distances. It covers a broad range of antibacterial effects versus bacteria such as Klebsiella pneumonia (20.00 mm) (20.00–34.00 mm), MRSA (35.1 mm), and L. monocytogenes (24 mm) [99]. The biological activity of Opuntia dillenii seed oils and the OFI antibacterial activity were examined on 01 Gram +ve and 02 Gram −ve bacteria, respectively. The statistics of this analysis demonstrated that seed oil has no antibacterial action for Pseudomonas aeruginosa, while Staphylococcus aureus and Escherichia coli exhibited antibacterial properties [100]. The antimicrobial assays conducted in this study focused on specific bacterial strains, including Gram-negative bacteria such as Agrobacterium tumefaciens and Escherichia coli as well as Gram-positive bacteria like Micrococcus aureus, on 96-well microplates by the broth microdilution method for 24 h at 37 °C, inhibiting growth in minimum extract concentrations [101].
3.5. Role of Opuntia Regarding Bodyweight and Bone Health
Alloxan, a diabetogenic compound, is commonly tested for the study of body weight loss. Through tests conducted on a sample of laboratory mice, it has been demonstrated that when alloxan is used in combination with OFI seed oil, more encouraging results are achieved as compared to those achieved with alloxan alone [102]. Seed oil at a concentration of 0.025% per kilogram in a high-fat diet for four consecutive weeks resulted in a significant weight gain compared to a basic diet [103]. Moreover, the hepatoprotective effects of the seed oil Opuntia dilleniid (SOD) on CCl4-provoked injury in rat livers have been studied. The rat was treated with SOD 2 mL/kg regularly for two weeks. The weight gain, plasmatic glucose level, and liver injury decreased significantly. The SOD has a protective effect against medicated-CCl4 injury [104]. In male rats at the growing stage, it was found that the final growth stage of OFI cladodes played a role in bone development. Additionally, during the initial and final periods of cladode maturity, soluble fibers demonstrated good bone development properties, including the Ca content, micro-architecture, and fracture resistance, as evaluated in a study where rats were given insoluble cladode fibers [102].
3.6. Cosmetic Applications
As already mentioned above, OFI has a rich variety of useful compounds, including antioxidants, essential fatty acids, and polyphenols. It provides help in skin improvement, moisturizing, and wound care. The OFI (1%) extract in the oil-to-water base nano-emulsion can increase the water content for five hours in the corneum stratum, demonstrating significant improvement over the vehicle formulation. The excellent cleansing and moistening ability of the current formulation, due to the presence of carbohydrates in OFI, has stability and a soothing effect with potential in cosmetics [105]. High-power microwave treatment was applied to obtain O. humifusa extract (MA-OHE) with good viscosity, a high antioxidant capacity, and reduced consequences of particulate matter. So, MA-OHE is a prospective component in cosmetics for stopping/avoiding diseases [106].
3.7. Application of Opuntia in Building Materials
The application of OFI in construction materials has gained significant attention because of its ability to be a sustainable and eco-friendly alternative. OFI is easily cultivated in many areas, making it an instantly available resource for construction material applications. Findings have shown that Opuntia-dried pads, spines, and fibrous materials are used in roofs, furniture, natural adhesives in traditional construction, joining adobe bricks, making household objects, and in many other building raw materials, including wall panels, bio-composites, and insulations. The cladodes and stems are utilized to make insulating material with a high thermal ability and fire resistance. Further, plant fibers and bio-degradable materials are combined to make bio-composites with good mechanical properties. The admixture of resin with bio-silica grains (0.5% Vol.) was poured on a fixed, coated wax rubber mold; then, a layer of short OFI fiber (30% by volume) was carefully added to obtain composite material at 25 °C for 24 h and, subsequently, at 120 °C for 48 h. The results showed an enhancement in toughness strength, improved wear resistance, high tensile strength, and a significant increase in energy capacity storage (4.34 GPa and 34,371 life counts fatigue with a 0.71 loss factor) [107]. In another work, the OFI fiber of cladode (10% wt.) treated with alkali was applied in a (HDPE) high-density polyethylene matrix and demonstrated a rise in rigidity and the modulus of elasticity, while the addition of the maleic anhydride grafted 3, block C8H8–(C2H4–C4H8)–C8H8 copolymer also enhanced the plasticity, ductility, and heat properties. The green composite OFI/HDPE is applied as an alternative material in construction products [108]. For mortar preparation, OFI mucilage (1:1 cladode/H2O ratio), Mexican standard cement (1:3 ratio with silica sand), and liquid (H2O + mucilage) (650 mL) are used with different concentrations of the total volume of OFI mucilage (1.5 to 95%). The results show that OFI mucilage decreases mortar (cement-based) porosity, enhances durability, and increases compressive strength and electrical resistivity. Additionally, it improves mechanical strength and increases its lifespan [109,110]. The OFI mucilage, cooked OFI mucilage and exudate OFI mucilage (4%, 8%, 15%, 30% w/m concentration), and OFI dehydrated powder (1%, 2%, 4% cement by sand mass replacement) are used to calculate the durability of concrete for 30, 90, 180, and 400 days. The OFI dehydrated powder improved chloride transport and decreased the RCP (rapid chloride permeability) index by 10%. The exudate OFI mucilage improved the 30% RCP index and 20% durability index, while for mixture controlling, cooked OFI mucilage showed excellent results. OFI derivatives work like biopolymers (clogging sponges) in the matrix pores of cement, stopping the transport of H2O and chloride in concrete [111]. The cactus extract solution contains polysaccharides with a gluey character which were used in mortar to improve the sustainability, water absorption resistance, and plasticity and significantly reduce water absorption in the concrete. The cactus 100% solution was shown to be more effect in mortar and concrete as compared to the cactus 50% solution. The study demonstrates that natural biopolymers sugarcane bagasse [112], straw, wood, stalks with lime, bamboo, and animal dung [113]—were utilized in construction materials as reinforcing agents to enhance the strength and durability of structures like adobe buildings, mud bricks, and wall plasters in ancient times [114].
In Table 8, we have compiled a comprehensive summary of the diverse applications of various parts of Opuntia ficus indica, including cladodes, fruits, flowers, seeds, skins, and mucilage.

Table 8.
Opuntia ficus indica applications.
4. Conclusions
In conclusion, the accumulated evidence substantiated that the chemical composition of nopal cactus contains abundant sets of macro and micro molecules and a rich chemistry of bioactive compounds—particularly, polyunsaturated-fatty acids, polyose, phytosterols, vitamins, tocopherols, and polyphenolic compounds. Cladode and fruit peel have more minerals and bio-active species compared to seed, which are useful for human beings’ health and as medication for numerous diseases (cancer, diabetes, skin, and cardiovascular diseases). In fact, fruit peels worked as organic dyestuffs, organic antioxidants, therapeutic agents, and additives. In agriculture, they worked as forage, an important crop in harsh environmental regions. The food industry took OFI as a beneficial ingredient in a functional diet. Its health-promoting abilities and great nutritional contents make it a favorable ingredient for use in food development products. Still, more analysis and study are needed to understand the plant chemistry and the role of environmental conditions regarding the chemical composition in OFI. The construction industry took OFI as a sustainable and eco-friendly bio-source polymer for making building raw materials such as roofs, adobe bricks, wall panels, insulation, and natural adhesives in traditional and modern construction to enhance the thermal and mechanical properties. Moreover, it is still required to conduct a deeper examination of the diverse series of bioactive compounds obtained from cactus pear plants and their beneficial usages in multiple innovative industries and efficient functional foods.
Author Contributions
Conceptualization, R.S., M.C., G.P. and L.P.; methodology, R.S., M.C., G.P. and L.P; validation, L.P. and G.P.; formal analysis, R.S. and M.C.; investigation, R.S. and L.P.; data curation, R.S.; writing—original draft preparation, R.S.; writing—review and editing, R.S., M.C., G.P. and L.P.; visualization, R.S., M.C., G.P. and L.P.; supervision, G.P. and L.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors gratefully acknowledge the Università degli Studi di Cagliari. R.S. performed his activity in the framework of the International Ph.D. in Innovation Sciences and Technologies at the University of Cagliari, Italy.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osorio-Esquivel, O.; Ortiz-Moreno, A.; Garduño-Siciliano, L.; Álvarez, V.B.; Hernández-Navarro, M.D. Antihyperlipidemic Effect of Methanolic Extract from Opuntia Joconostle Seeds in Mice Fed a Hypercholesterolemic Diet. Plant Foods Hum. Nutr. 2012, 67, 365–370. [Google Scholar] [CrossRef] [PubMed]
- Mounir, B.; Younes, E.G.; Asmaa, M.; Abdeljalil, Z.; Abdellah, A. Physico-Chemical Changes in Cladodes of Opuntia ficus-indica as a Function of the Growth Stage and Harvesting Areas. J. Plant Physiol. 2020, 251, 153196. [Google Scholar] [CrossRef] [PubMed]
- Moßhammer, M.R.; Stintzing, F.C.; Carle, R. Cactus Pear Fruits (Opuntia spp.): A Review of Processing Technologies and Current Uses. J. Prof. Assoc. Cactus Dev. 2006, 8, 1–25. [Google Scholar]
- Anderson, E.F. The Cactus Family. In The Cactus Family; Timber Press: Portland, OR, USA, 2001; pp. 15–72. [Google Scholar]
- Felker, P.; Stintzing, F.C.; Müssig, E.; Leitenberger, M.; Carle, R.; Vogt, T.; Bunch, R. Colour Inheritance in Cactus Pear (Opuntia ficus-indica) Fruits. Ann. Appl. Biol. 2008, 152, 307–318. [Google Scholar] [CrossRef]
- Gebremariam, T.; Melaku, S.; Yami, A. Effect of Different Levels of Cactus (Opuntia ficus-indica) Inclusion on Feed Intake, Digestibility and Body Weight Gain in Tef (Eragrostis Tef) Straw-Based Feeding of Sheep. Anim. Feed. Sci. Technol. 2006, 131, 43–52. [Google Scholar] [CrossRef]
- Kuti, J.O. Antioxidant Compounds from Four Opuntia Cactus Pear Fruit Varieties. Food Chem. 2004, 85, 527–533. [Google Scholar] [CrossRef]
- Turker, N.; Coşkuner, Y.; Ekiz, H.I.; Aksay, S.; Karababa, E. The Effects of Fermentation on the Thermostability of the Yellow-Orange Pigments Extracted from Cactus Pear (Opuntia ficus-indica). Eur. Food Res. Technol. 2001, 212, 213–216. [Google Scholar] [CrossRef]
- Chiteva, R.; Wairagu, N. Chemical and Nutritional Content of Opuntia ficus-indica (L.). Afr. J. Biotechnol. 2016, 12, 3309–3312. [Google Scholar]
- Espino-Manzano, S.O.; León-López, A.; Aguirre-Álvarez, G.; González-Lemus, U.; Prince, L.; Germán Campos-Montiel, R. Molecules Application of Nanoemulsions (W/O) of Extract of Opuntia oligacantha C.F. Först and Orange Oil in Gelatine Films. Molecules 2020, 25, 3487. [Google Scholar] [CrossRef]
- Vigueras, A.L.; Portillo, L. Uses of Opuntia Species and the Potential Impact of Cactoblastis Cactorum (Lepidoptera: Pyralidae) in Mexico. Fla. Entomol. 2001, 84, 493–498. [Google Scholar] [CrossRef]
- Bakar, B.; Çakmak, M.; Ibrahim, M.S.; Özer, D.; Saydam, S.; Karatas, F. Investigation of Amounts of Vitamins, Lycopene, and Elements in the Fruits of Opuntia ficus-indica Subjected to Different Pretreatments. Biol. Trace Elem. Res. 2020, 198, 315–323. [Google Scholar] [CrossRef] [PubMed]
- Lim, T.K. Opuntia ficus-indica. Edible Med. Non-Med. Plants 2012, 1, 660–682. [Google Scholar] [CrossRef]
- Pritchard, H.N.; Hall, J.A. The Chemical Composition of Glochids from Opuntia. Can. J. Bot. 2011, 54, 173–176. [Google Scholar] [CrossRef]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Mahrouz, M.; Vuong, R.; Vignon, M.R. Structure and Morphology of Cladodes and Spines of Opuntia ficus-indica. Cellulose Extraction and Characterisation. Carbohydr. Polym. 2003, 51, 77–83. [Google Scholar] [CrossRef]
- Vignon, M.R.; Heux, L.; Malainine, M.E.; Mahrouz, M. Arabinan–Cellulose Composite in Opuntia ficus-indica Prickly Pear Spines. Carbohydr. Res. 2004, 339, 123–131. [Google Scholar] [CrossRef]
- Rodriguez-Felix, A.; Cantwell, M. Developmental Changes in Composition and Quality of Prickly Pear Cactus Cladodes (Nopalitos). Plant Foods Hum. Nutr. 1988, 38, 83–93. [Google Scholar] [CrossRef]
- Retamal, N.; Durán, J.M.; Fernández, J. Seasonal Variations of Chemical Composition in Prickly Pear (Opuntia ficus-indica (L.) Miller). J. Sci. Food Agric. 1987, 38, 303–311. [Google Scholar] [CrossRef]
- Batista, A.M.; Mustafa, A.F.; McAllister, T.; Wang, Y.; Soita, H.; McKinnon, J.J. Effects of Variety on Chemical Composition, in Situ Nutrient Disappearance and in Vitro Gas Production of Spineless Cacti. J. Sci. Food Agric. 2003, 83, 440–445. [Google Scholar] [CrossRef]
- Malainine, M.E.; Dufresne, A.; Dupeyre, D.; Vignon, M.R.; Mahrouz, M. First Evidence for the Presence of Weddellite Crystallites in Opuntia ficus indica Parenchyma. Z. Für Nat. C 2003, 58, 812–816. [Google Scholar] [CrossRef]
- Mohamed-Yasseen, Y.; Barringer, S.A.; Splittstoesser, W.E. A Note on the Uses of Opuntia spp. in Central/North America. J. Arid. Env. 1996, 32, 347–353. [Google Scholar] [CrossRef]
- Mizrahi, Y.; Nerd, A. Climbing and Columnar Cacti: New Arid Land Fruit Crops. In Perspectives on New Crops and New Uses; Janick, J., Ed.; ASHS Press: Alexanddria, VA, USA, 1999; pp. 358–366. [Google Scholar]
- Retamal, N.; Durán, J.M.; Fernández, J. Ethanol Production by Fermentation of Fruits and Cladodes of Prickly Pear Cactus [Opuntia ficus-indica (L.) Miller]. J. Sci. Food Agric. 1987, 40, 213–218. [Google Scholar] [CrossRef]
- Nerd, A.; Afflalo, E.; Mizrahi, Y. Introduction of Cacti as Vegetable Crops for Israel. In Combating Desertification with Plants; Springer: Berlin/Heidelberg, Germany, 2001; pp. 249–255. [Google Scholar] [CrossRef]
- Sutton, B.G.; Ting, I.P.; Sutton, R. Carbohydrate Metabolism of Cactus in a Desert Environment. Plant Physiol. 1981, 68, 784–787. [Google Scholar] [CrossRef] [PubMed]
- Amador, B.M.; Diéguez, E.T.; Garibay, A.N.; García, M.A. El Nopal: Cultivo Forrajero Sostenible Para El Noroeste de México; Baja, L.P., Ed.; Centro de Investigaciones Biológicas del Noroeste: California Sur, Mexico, 2002; ISBN 9701865820. [Google Scholar]
- di Bella, G.; Vecchio, G.L.; Albergamo, A.; Nava, V.; Bartolomeo, G.; Macrì, A.; Bacchetta, L.; lo Turco, V.; Potortì, A.G. Chemical Characterization of Sicilian Dried Nopal [Opuntia ficus-indica (L.) Mill.]. J. Food Compos. Anal. 2022, 106, 104307. [Google Scholar] [CrossRef]
- Nassar, A.G. Chemical Composition and Functional Properties of Prickly Pear (Opuntia ficus indica) Seeds Flour and Protein Concentrate. World J. Dairy Food Sci. 2008, 3, 11–16. [Google Scholar]
- Uchoa, A.F.; Souza, P.A.S.; Zarate, R.M.L.; Gomes-Filho, E.; Campos, F.A.P. Seed Reserve Protein from O. Ficus-Indica Brazilian. J. Med. Biol. Res. 1998, 31, 757–761. [Google Scholar] [CrossRef] [PubMed]
- Zito, P.; Sajeva, M.; Bruno, M.; Rosselli, S.; Maggio, A.; Senatore, F. Essential Oils Composition of Two Sicilian Cultivars of Opuntia ficus-indica (L.) Mill. (Cactaceae) Fruits (Prickly Pear). Nat. Prod Res. 2013, 27, 1305–1314. [Google Scholar] [CrossRef]
- Sawaya, W.N.; Khalil, J.K.; Al-Mohammad, M.M. Nutritive Value of Prickly Pear Seeds, Opuntia ficus-indica. Qual. Plant. Plant Foods Hum. Nutr. 1983, 33, 91–97. [Google Scholar] [CrossRef]
- Feugang, J.M.; Konarski, P.; Zou, D.; Stintzing, F.C.; Zou, C. Nutritional and Medicinal Use of Cactus Pear (Opuntia Spp.) Cladodes and Fruits. Front. Biosci. 2006, 11, 2574–2589. [Google Scholar] [CrossRef]
- Albergamo, A.; Potortí, A.G.; Di Bella, G.; Ben Amor, N.; Vecchio, G.L.; Nava, V.; Rando, R.; Ben Mansour, H.; Turco, V.L. Chemical Characterization of Different Products from the Tunisian Opuntia ficus-indica (L.) Mill. Foods 2022, 11, 155. [Google Scholar] [CrossRef]
- Piga, A. Cactus Pear: A Fruit of Nutraceutical and Functional Importance | Journal of the Professional Association for Cactus Development. J. Prof. Assoc. Cactus Dev. 2004, 6, 9–22. [Google Scholar]
- Tesoriere, L.; Allegra, M.; Butera, D.; Gentile, C.; Livrea, M.A. Kinetics of the Lipoperoxyl Radical-Scavenging Activity of Indicaxanthin in Solution and Unilamellar Liposomes. Free. Radic. Res. 2007, 41, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Çakmak, M.; Bakar, B.; Özer, D.; Geckil, H.; Karatas, F.; Saydam, S. Investigation of some biochemical parameters of wild and cultured Myrtus communis L. fruits subjected to different conservation methods. Food Meas. 2021, 15, 983–993. [Google Scholar] [CrossRef]
- Khatabi, O.; Hanine, H.; Elothmani, D.; Hasib, A. Extraction and Determination of Polyphenols and Betalain Pigments in the Moroccan Prickly Pear Fruits (Opuntia ficus indica). Arab. J. Chem. 2016, 9, S278–S281. [Google Scholar] [CrossRef]
- El-Mostafa, K.; el Kharrassi, Y.; Badreddine, A.; Andreoletti, P.; Vamecq, J.; el Kebbaj, M.S.; Latruffe, N.; Lizard, G.; Nasser, B.; Cherkaoui-Malki, M. Nopal Cactus (Opuntia ficus-indica) as a Source of Bioactive Compounds for Nutrition, Health and Disease. Molecules 2014, 19, 14879–14901. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.R.; Akash, S.; Jony, M.H.; Nowrin, F.T.; Rahman, M.M.; Rauf, A.; Thiruvengadam, M. Exploring the Potential Function of Trace Elements in Human Health: A Therapeutic Perspective. Mol. Cell. Biochem. 2023, 13, 1–31. [Google Scholar] [CrossRef]
- Dahmardeh, M. The Effect of Polythene Colour Container and Three Spawn Rates on Production of Pleurotus Ostreatus Mushroom. Afr. J. Biotechnol. 2012, 11, 9373–9376. [Google Scholar] [CrossRef]
- Medina, E.M.D.; Rodríguez, E.M.R.; Romero, C.D. Chemical Characterization of Opuntia Dillenii and Opuntia ficus indica Fruits. Food Chem. 2007, 103, 38–45. [Google Scholar] [CrossRef]
- Ö-Zcan, M.M.; Al Juhaimi, F.Y. Nutritive Value and Chemical Composition of Prickly Pear Seeds (Opuntia ficus indica L.) Growing in Turkey. Int. J. Food Sci. Nutr. 2011, 62, 533–536. [Google Scholar] [CrossRef]
- Zou, D.; Brewer, M.; Garcia, F.; Feugang, J.M.; Wang, J.; Zang, R.; Liu, H.; Zou, C. Cactus pear: A natural product in cancer chemoprevention. Nutr. J. 2005, 4, 25. [Google Scholar] [CrossRef]
- Contreras-Padilla, M.; Pérez-Torrero, E.; Hernández-Urbiola, M.I.; Hernández-Quevedo, G.; del Real, A.; Rivera-Muñoz, E.M.; Rodríguez-García, M.E. Evaluation of Oxalates and Calcium in Nopal Pads (Opuntia ficus-indica Var. Redonda) at Different Maturity Stages. J. Food Compos. Anal. 2011, 1, 38–43. [Google Scholar] [CrossRef]
- Ayadi, M.A.; Abdelmaksoud, W.; Ennouri, M.; Attia, H. Cladodes from Opuntia ficus indica as a Source of Dietary Fiber: Effect on Dough Characteristics and Cake Making. Ind. Crops Prod. 2009, 30, 40–47. [Google Scholar] [CrossRef]
- Ghazi, Z.; Ramdani, M.; Tahri, M.; Rmili, R.; Elmsellem, H.; Mahi, B.E.; Fauconnier, M.L. Chemical Composition and Antioxidant Activity of Seeds Oils and Fruit Juice of Opuntia ficus indica and Opuntia Dillenii from Morocco. J. Mater. Environ. Sci. 2015, 6, 2338–2345. [Google Scholar]
- Laughton, M.J.; Evans, P.J.; Moroney, M.A.; Hoult, J.R.S.; Halliwell, B. Inhibition of Mammalian 5-Lipoxygenase and Cyclo-Oxygenase by Flavonoids and Phenolic Dietary Additives. Biochem. Pharm. 1991, 42, 1673–1681. [Google Scholar] [CrossRef]
- Ammar, I.; Ennouri, M.; Khemakhem, B.; Yangui, T.; Attia, H. Variation in Chemical Composition and Biological Activities of Two Species of Opuntia Flowers at Four Stages of Flowering. Ind. Crops Prod. 2012, 37, 34–40. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Tanbouly, N.D.E.; Islam, W.T.; Sleem, A.A.; Senousy, A.S.E. Antiinflammatory Flavonoids from Opuntia Dillenii (Ker-Gawl) Haw. Flowers Growing in Egypt. Phytother. Res. 2005, 19, 807–809. [Google Scholar] [CrossRef] [PubMed]
- de Leo, M.; de Abreu, M.B.; Pawlowska, A.M.; Cioni, P.L.; Braca, A. Profiling the Chemical Content of Opuntia ficus-indica Flowers by HPLC–PDA-ESI-MS and GC/EIMS Analyses. Phytochem. Lett. 2010, 3, 48–52. [Google Scholar] [CrossRef]
- Fernández-López, J.A.; Almela, L.; Obón, J.M.; Castellar, R. Determination of Antioxidant Constituents in Cactus Pear Fruits. Plant Foods Hum. Nutr. 2010, 65, 253–259. [Google Scholar] [CrossRef]
- Tesoriere, L.; Fazzari, M.; Allegra, M.; Livrea, M.A. Biothiols, Taurine, and Lipid-Soluble Antioxidants in the Edible Pulp of Sicilian Cactus Pear (Opuntia ficus-indica) Fruits and Changes of Bioactive Juice Components upon Industrial Processing. J. Agric. Food Chem. 2005, 53, 7851–7855. [Google Scholar] [CrossRef]
- Moussa-Ayoub, T.E.; El-Samahy, S.K.; Kroh, L.W.; Rohn, S. Identification and Quantification of Flavonol Aglycons in Cactus Pear (Opuntia ficus indica) Fruit Using a Commercial Pectinase and Cellulase Preparation. Food Chem. 2011, 124, 1177–1184. [Google Scholar] [CrossRef]
- Salim, N.; Abdelwaheb, C.; Rabah, C.; Ahcene, B. Chemical Composition of Opuntia ficus-indica (L.) Fruit. Afr. J. Biotechnol. 2009, 8, 1623–1624. [Google Scholar]
- Bensadón, S.; Hervert-Hernández, D.; Sáyago-Ayerdi, S.G.; Goñi, I. By-Products of Opuntia ficus-indica as a Source of Antioxidant Dietary Fiber. Plant Foods Hum. Nutr. 2010, 65, 210–216. [Google Scholar] [CrossRef] [PubMed]
- Chougui, N.; Tamendjari, A.; Hamidj, W.; Hallal, S.; Barras, A.; Richard, T.; Larbat, R. Oil Composition and Characterisation of Phenolic Compounds of Opuntia ficus-indica Seeds. Food Chem. 2013, 139, 796–803. [Google Scholar] [CrossRef] [PubMed]
- Jorge, A.J.; de La Garza, T.H.; Alejandro, Z.C.; Ruth, B.C.; Noé, A.C. The Optimization of Phenolic Compounds Extraction from Cactus Pear (Opuntia ficus-indica) Skin in a Reflux System Using Response Surface Methodology. Asian Pac. J. Trop. Biomed. 2013, 3, 436. [Google Scholar] [CrossRef] [PubMed]
- Valente, L.M.M.; da Paixão, D.; do Nascimento, A.C.; dos Santos, P.F.P.; Scheinvar, L.A.; Moura, M.R.L.; Tinoco, L.W.; Gomes, L.N.F.; da Silva, J.F.M. Antiradical Activity, Nutritional Potential and Flavonoids of the Cladodes of Opuntia monacantha (Cactaceae). Food Chem 2010, 123, 1127–1131. [Google Scholar] [CrossRef]
- Guevara-Figueroa, T.; Jiménez-Islas, H.; Reyes-Escogido, M.L.; Mortensen, A.G.; Laursen, B.B.; Lin, L.W.; de León-Rodríguez, A.; Fomsgaard, I.S.; Barba de la Rosa, A.P. Proximate Composition, Phenolic Acids, and Flavonoids Characterization of Commercial and Wild Nopal (Opuntia spp.). J. Food Compos. Anal. 2010, 23, 525–532. [Google Scholar] [CrossRef]
- Ginestra, G.; Parker, M.L.; Bennett, R.N.; Robertson, J.; Mandalari, G.; Narbad, A.; lo Curto, R.B.; Bisignano, G.; Faulds, C.B.; Waldron, K.W. Anatomical, Chemical, and Biochemical Characterization of Cladodes from Prickly Pear [Opuntia ficus-indica (L.) Mill.]. J. Agric. Food Chem. 2009, 57, 10323–10330. [Google Scholar] [CrossRef]
- Clark, W.D.; Brown, G.K.; Mays, R.L. Flower Flavonoids of Opuntia Subgenus Cylindr Opuntia. Phytochemistry 1980, 19, 2042–2043. [Google Scholar] [CrossRef]
- Galati, E.M.; Mondello, M.R.; Giuffrida, D.; Dugo, G.; Miceli, N.; Pergolizzi, S.; Taviano, M.F. Chemical Characterization and Biological Effects of Sicilian Opuntia ficus indica (L.) Mill. Fruit Juice: Antioxidant and Antiulcerogenic Activity. J. Agric. Food Chem. 2003, 51, 4903–4908. [Google Scholar] [CrossRef]
- Calva-Estrada, S.J.; Jiménez-Fernández, M.; Lugo-Cervantes, E. Betalains and Their Applications in Food: The Current State of Processing, Stability and Future Opportunities in the Industry. Food Chem. Mol. Sci. 2022, 4, 100089. [Google Scholar] [CrossRef]
- Piattelli, M.; Minale, L. Pigments of Centrospermae—I.: Betacyanins from Phyllocactus Hybridus Hort. And Opuntia ficus-indica Mill. Phytochemistry 1964, 3, 307–311. [Google Scholar] [CrossRef]
- Minale, L.; Piattelli, M.; Nicolaus, R.A. Pigments of Centrospermae—IV: On the Biogenesis of Indicaxanthin and Betanin in Opuntia ficus-indica Mill. Phytochemistry 1965, 4, 593–597. [Google Scholar] [CrossRef]
- Yeddes, N.; Chérif, J.K.; Guyot, S.; Sotin, H.; Ayadi, M.T. Comparative Study of Antioxidant Power, Polyphenols, Flavonoids and Betacyanins of the Peel and Pulp of Three Tunisian Opuntia Forms. Antioxidants 2013, 2, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Strack, D.; Vogt, T.; Schliemann, W. Recent Advances in Betalain Research. Phytochemistry 2003, 62, 247–269. [Google Scholar] [CrossRef]
- Castellanos-Santiago, E.; Yahia, E.M. Identification and Quantification of Betalains from the Fruits of 10 Mexican Prickly Pear Cultivars by High-Performance Liquid Chromatography and Electrospray Ionization Mass Spectrometry. J. Agric. Food Chem. 2008, 56, 5758–5764. [Google Scholar] [CrossRef]
- Stintzing, F.C.; Schieber, A.; Carle, R.; Stintzing, F.C.; Schieber, A.; Carle, R. Phytochemical and Nutritional Significance of Cactus Pear. Eur. Food Res. Technol. 2001, 212, 396–407. [Google Scholar] [CrossRef]
- Taira, J.; Tsuchida, E.; Katoh, M.C.; Uehara, M.; Ogi, T. Antioxidant Capacity of Betacyanins as Radical Scavengers for Peroxyl Radical and Nitric Oxide. Food Chem. 2015, 166, 531–536. [Google Scholar] [CrossRef]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Purification and Antiradical Properties of the Structural Unit of Betalains. J. Nat. Prod 2012, 75, 1030–1036. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, M.F.; Mörsel, J.T. Recovered Lipids from Prickly Pear [Opuntia ficus-indica (L.) Mill] Peel: A Good Source of Polyunsaturated Fatty Acids, Natural Antioxidant Vitamins and Sterols. Food Chem. 2003, 83, 447–456. [Google Scholar] [CrossRef]
- Gharby, S.; Harhar, H.; Guillaume, D.; Haddad, A.; Matthäus, B.; Charrouf, Z. Oxidative Stability of Edible Argan Oil: A Two-Year Study. LWT-Food Sci. Technol. 2011, 44, 1–8. [Google Scholar] [CrossRef]
- Ramadan, M.F.; Mörsel, J.T. Oil Cactus Pear (Opuntia ficus-indica L.). Food Chem. 2003, 82, 339–345. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of Heavy Metal Ions from Wastewaters: A Review. J. Environ. Manag. 2011, 92, 407–418. [Google Scholar] [CrossRef] [PubMed]
- Hadadi, A.; Imessaoudene, A.; Bollinger, J.C.; Assadi, A.A.; Amrane, A.; Mouni, L. Comparison of Four Plant-Based Bio-Coagulants Performances against Alum and Ferric Chloride in the Turbidity Improvement of Bentonite Synthetic Water. Water 2022, 14, 3324. [Google Scholar] [CrossRef]
- Nharingo, T.; Moyo, M. Application of Opuntia ficus-indica in Bioremediation of Wastewaters. A Critical Review. J. Environ. Manag. 2016, 166, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Nharingo, T.; Zivurawa, M.T.; Guyo, U. Exploring the Use of Cactus Opuntia ficus indica in the Biocoagulation–Flocculation of Pb(II) Ions from Wastewaters. Int. J. Environ. Sci. Technol. 2015, 12, 3791–3802. [Google Scholar] [CrossRef]
- Kumar, S.; Palsaniya, D.R.; Kumar, T.K.; Misra, A.K.; Ahmad, S.; Rai, A.K.; Sarker, A.; Louhaichi, M.; Hassan, S.; Liguori, G.; et al. Survival, Morphological Variability, and Performance of Opuntia ficus-indica in a Semi-Arid Region of India. Arch. Agron. Soil Sci. 2022, 69, 708–725. [Google Scholar] [CrossRef]
- Licona Galeano, V.J.; Monteiro, C.C.F.; Carvalho, F.F.R.; Souza, A.F.; Souza, F.G.; Corrêa, A.M.N.; Vasconcelos, E.Q.L.; Mesquita, F.L.T.; Gama, M.A.S.; Ferreira, M.A. Productive Responses of Dairy Goats Fed on Diets Containing Elephant Grass (Pennisetum purpureum) Associated or Not with Cactus (Opuntia stricta) Cladodes, and Extra-Fat Whole Corn Germ as a Substitute for Corn. Small Rumin. Res. 2022, 207, 106609. [Google Scholar] [CrossRef]
- Sánchez, B.M.S.; Véras, A.S.C.; Freitas, E.V.; Farias, L.R.; Albuquerque, J.G.S.S.; Almeida, G.A.P.; Mora-Luna, R.E.; Monteiro, C.C.F.; Gama, M.A.S.; Ferreira, M.A.; et al. Partial Replacement of Sugarcane with Cactus (Opuntia stricta) Cladodes Improves Milk Yield and Composition in Holstein Dairy Cows. Anim. Prod Sci. 2022, 62, 691–699. [Google Scholar] [CrossRef]
- da Silva, C.S.; Gama, M.A.S.; Silva, E.A.M.; Ribeiro, E.F.; Souza, F.G.; Monteiro, C.C.F.; Mora-Luna, R.E.; Oliveira, J.C.V.; Santos, D.C.; Ferreira, M. de A. Nutritional Quality of Milk Fat from Cows Fed Full-Fat Corn Germ in Diets Containing Cactus Opuntia and Sugarcane Bagasse as Forage Sources. Animals 2023, 13, 568. [Google Scholar] [CrossRef]
- Samir, M.; Raul, B.; López, S. Potential of Opuntia ficus-indica Cladodes In M’sila (North ALGERIA) as Feed for Ruminants: Chemical Composition and in Vitro Assessment. Acta Agric. Scand. A Anim. Sci. 2023, 7, 1–7. [Google Scholar] [CrossRef]
- Comparetti, A.; Febo, P.; Greco, C.; Mammano, M.M.; Comparetti, A.; Mammano, M.M.; Orlando, S. Potential Production of Biogas from Prinkly Pear (Opuntia ficus-indica L.) in Sicilian Uncultivated Areas. Chem. Eng. Trans. 2017, 58, 559–564. [Google Scholar] [CrossRef]
- Apollon, W.; Kamaraj, S.K.; Silos-Espino, H.; Perales-Segovia, C.; Valera-Montero, L.L.; Maldonado-Ruelas, V.A.; Vázquez-Gutiérrez, M.A.; Ortiz-Medina, R.A.; Flores-Benítez, S.; Gómez-Leyva, J.F. Impact of Opuntia Species Plant Bio-Battery in a Semi-Arid Environment: Demonstration of Their Applications. Appl. Energy 2020, 279, 115788. [Google Scholar] [CrossRef]
- Dalila, M.; Soltane, R.; Chrouda, A.; Dhahri, A.; Pashameah, R.A.; Almulla, N.; Soltane, R.; Chrouda, A.; Dhahri, A.; Pashameah, R.A.; et al. Opuntia spp.: Chemistry, Bioactivity and Industrial Applications; Springer: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]
- Godard, M.P.; Ewing, B.A.; Pischel, I.; Ziegler, A.; Benedek, B.; Feistel, B. Acute Blood Glucose Lowering Effects and Long-Term Safety of OpunDia Supplementation in Pre-Diabetic Males and Females. J. Ethnopharmacol. 2010, 130, 631–634. [Google Scholar] [CrossRef] [PubMed]
- Abid, F.; Saleem, M.; Muller, C.D.; Asim, M.H.; Arshad, S.; Maqbool, T.; Hadi, F. Anti-Proliferative and Apoptosis-Inducing Activity of Acacia Modesta and Opuntia Monocantha Extracts on HeLa Cells. Asian Pac. J. Cancer Prev. 2020, 21, 3125. [Google Scholar] [CrossRef] [PubMed]
- Giglio, R.V.; Carruba, G.; Cicero, A.F.G.; Banach, M.; Patti, A.M.; Nikolic, D.; Cocciadiferro, L.; Zarcone, M.; Montalto, G.; Stoian, A.P.; et al. Pasta Supplemented with Opuntia ficus-indica Extract Improves Metabolic Parameters and Reduces Atherogenic Small Dense Low-Density Lipoproteins in Patients with Risk Factors for the Metabolic Syndrome: A Four-Week Intervention Study. Metabolites 2020, 10, 428. [Google Scholar] [CrossRef] [PubMed]
- Amrane-Abider, M.; Nerín, C.; Tamendjari, A.; Serralheiro, M.L.M. Phenolic Composition, Antioxidant and Antiacetylcholinesterase Activities of Opuntia ficus-indica Peel and Flower Teas after in Vitro Gastrointestinal Digestion. J. Sci. Food Agric. 2022, 102, 4401–4409. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Moreno, E.; Cariño-Cortés, R.; Cruz-Cansino, N.D.S.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Montañez-Izquierdo, V.Y.; Hernández-Herrero, M.M.; Filardo-Kerstupp, T. Antioxidant and Antimicrobial Properties of Cactus Pear (Opuntia) Seed Oils. J. Food Qual. 2017, 2017, 1–8. [Google Scholar] [CrossRef]
- Tsiailanis, A.D.; Chatzigiannis, C.M.; Papaemmanouil, C.D.; Chatziathanasiadou, M.V.; Chaloulos, P.; Riba, I.; Mullard, G.; Wiczkowski, W.; Koutinas, A.; Mandala, I.; et al. Exploration of Betalains and Determination of the Antioxidant and Cytotoxicity Profile of Orange and Purple Opuntia Spp. Cultivars in Greece. Plant Foods Hum. Nutr. 2022, 77, 198–205. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira e Silva, A.M.; Vidal-Novoa, A.; Batista-González, A.E.; Pinto, J.R.; Mancini, D.A.P.; Reina-Urquijo, W.; Mancini-Filho, J. In Vivo and in Vitro Antioxidant Activity and Hepatoprotective Properties of Polyphenols from Halimeda Opuntia (Linnaeus) Lamouroux. Commun. Free Radic. Res. 2013, 17, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Bouazizi, S.; Montevecchi, G.; Masino, F.; Antonelli, A.; Hamdi, M. Tunisian Opuntia ficus-indica fruit peels: Biochemical and microbiological characterization and possible applications. Ann. Univ. Dunarea Jos Galati Fascicle VI Food Technol. 2022, 46, 67–78. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, P.; You, S.; Zhao, D.; An, Q.; Wang, D.; Zhang, J.; Li, M.; Wang, C. Anti-Inflammatory Effects of Opuntia Milpa Alta Polysaccharides Fermented by Lactic Acid Bacteria in Human Keratinocyte HaCaT Cells. Chem. Biodivers. 2022, 19, e202100923. [Google Scholar] [CrossRef]
- el Hachimi, F.; Hajjaj, G.; Bendriss, A.; Cherrah, Y.; Alaoui, K. Anti-Inflammatory Activity of Seed Oils of Opuntia ficus-indica L. and Punica granatum L. from Morocco. World J. Pharm. Res. 2015, 4, 284–294. [Google Scholar]
- Smeriglio, A.; de Francesco, C.; Denaro, M.; Trombetta, D. Prickly Pear Betalain-Rich Extracts as New Promising Strategy for Intestinal Inflammation: Plant Complex vs. Main Isolated Bioactive Compounds. Front. Pharm. 2021, 12, 2067. [Google Scholar] [CrossRef]
- Giraldo-Silva, L.; Ferreira, B.; Rosa, E.; Dias, A.C.P. Opuntia ficus-indica Fruit: A Systematic Review of Its Phytochemicals and Pharmacological Activities. Plants 2023, 12, 543. [Google Scholar] [CrossRef] [PubMed]
- Aruwa, C.E.; Amoo, S.O.; Kudanga, T. Extractable and Macromolecular Antioxidants of Opuntia ficus-indica Cladodes: Phytochemical Profiling, Antioxidant and Antibacterial Activities. S. Afr. J. Bot. 2019, 125, 402–410. [Google Scholar] [CrossRef]
- Ortega-Ortega, M.D.L.A.; Cruz-Cansino, N.D.S.; Alanís-García, E.; Delgado-Olivares, L.; Ariza-Ortega, J.A.; Ramírez-Moreno, E.; Manríquez-Torres, J.D.J. Optimization of Ultrasound Extraction of Cactus Pear (Opuntia ficus indica) Seed Oil Based on Antioxidant Activity and Evaluation of Its Antimicrobial Activity. J. Food Qual. 2017, 72, 1–9. [Google Scholar] [CrossRef]
- Drira, M.; Ghanmi, S.; Zaidi, I.; Brini, F.; Miled, N.; Hanin, M. The Heat-Stable Protein Fraction from Opuntia ficus-indica Seeds Exhibits an Enzyme Protective Effect against Thermal Denaturation and an Antibacterial Activity. Biotechnol. Appl. Biochem. 2022, 70, 593–602. [Google Scholar] [CrossRef]
- Berraaouan, A.; Ziyyat, A.; Mekhfi, H.; Legssyer, A.; Sindic, M.; Aziz, M.; Bnouham, M. Evaluation of Antidiabetic Properties of Cactus Pear Seed Oil in Rats. Pharm. Biol. 2014, 52, 1286–1290. [Google Scholar] [CrossRef] [PubMed]
- Ennouri, M.; Fetoui, H.; Hammami, M.; Bourret, E.; Attia, H.; Zeghal, N. Effects of Diet Supplementation with Cactus Pear Seeds and Oil on Serum and Liver Lipid Parameters in Rats. Food Chem. 2007, 101, 248–253. [Google Scholar] [CrossRef]
- Bouhrim, M.; Ouassou, H.; Choukri, M.; Mekhfi, H.; Ziyyat, A.; Legssyer, A.; Aziz, M.; Bnouham, M. Hepatoprotective Effect of Opuntia Dillenii Seed Oil on CCl4 Induced Acute Liver Damage in Rat. Asian Pac. J. Trop. Biomed. 2018, 8, 260. [Google Scholar] [CrossRef]
- de Azevedo Ribeiro, R.C.; Barreto, S.M.A.G.; Ostrosky, E.A.; da Rocha-Filho, P.A.; Veríssimo, L.M.; Ferrari, M. Production and Characterization of Cosmetic Nanoemulsions Containing Opuntia ficus-indica (L.) Mill Extract as Moisturizing Agent. Molecules 2015, 20, 2492–2509. [Google Scholar] [CrossRef]
- Moon, J.Y.; Ngoc, L.T.N.; Chae, M.; van Tran, V.; Lee, Y.C. Effects of Microwave-Assisted Opuntia humifusa Extract in Inhibiting the Impacts of Particulate Matter on Human Keratinocyte Skin Cell. Antioxidants 2020, 9, 271. [Google Scholar] [CrossRef]
- Neopolean, P.; Karuppasamy, K. Characterization of Silane Treated Opuntia Short Fibre and Bagasse Biosilica Toughened Epoxy Resin Composite. Silicon 2022, 14, 9331–9340. [Google Scholar] [CrossRef]
- Ait Benhamou, A.; Boussetta, A.; Nadifiyine, M.; Moubarik, A. Effect of Alkali Treatment and Coupling Agent on Thermal and Mechanical Properties of Opuntia ficus-indica Cladodes Fibers Reinforced HDPE Composites. Polym. Bull. 2022, 79, 2089–2111. [Google Scholar] [CrossRef]
- Martínez-Molina, W.; Torres-Acosta, A.A.; Celis-Mendoza, C.E.; Alonso-Guzman, E. Physical Properties of Cement-Based Paste and Mortar with Dehydrated Cacti Additions. Int. J. Archit. Herit. 2015, 9, 443–452. [Google Scholar] [CrossRef]
- Martinez-Molina, W.; Torres-Acosta, A.A.; Martínez-Peña, G.E.I.; Guzmán, E.A.; Mendoza-Pérez, I.N. Cement-Based, Materials-Enhanced Durability from Opuntia ficus indica Mucilage Additions. ACI Mater. J. 2015, 112, 165–172. [Google Scholar] [CrossRef]
- Torres-Acosta, A.A.; Alejandra Díaz-Cruz, L. Concrete Durability Enhancement from Nopal (Opuntia ficus-indica) Additions. Constr. Build. Mater. 2020, 243, 118170. [Google Scholar] [CrossRef]
- Kusuma, H.S.; Permatasari, D.; Umar, W.K.; Sharma, S.K. Sugarcane Bagasse as an Environmentally Friendly Composite Material to Face the Sustainable Development Era. In Biomass Conversion and Biorefinery; Springer Science: Berlin/Heidelberg, Germany; Business Media Deutschland GmbH: Berlin, Germany, 2023; pp. 2190–6823. [Google Scholar] [CrossRef]
- Lu, J.; Yanan Jiang, S.; Chen, J.; Lee, C.-H.; Cai, Z.; Daniel Ruan, H. Fabrication of Superhydrophobic Soil Stabilizers Derived from Solid Wastes Applied for Road Construction: A Review. Transp. Geotech. 2023, 4, 100974. [Google Scholar] [CrossRef]
- Chandra, S.; Eklund, L.; Villarreal, R.R. Use of Cactus in Mortars and Concrete. Cem. Concr. Res. 1998, 28, 41–51. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).