1. Introduction
Renewable energy sources are of increased interest due to the high global demand for energy, unstable prices for fossil fuels, and significant climate change [
1,
2]. The consumption of fossil fuels has dramatically changed the balance of the carbon cycle and exacerbated pollution. The availability of sustainable and carbon-neutral biofuels will largely solve the identified problems. One of the promising renewable energy sources is biomass, which can be used to produce biofuels and valuable chemicals [
3]. Compared to coal or fuel oil, biomass-to-energy technologies are more environmentally friendly in terms of emissions of harmful substances (e.g., soot, nitrogen oxides, sulfur, and carbon). In terms of availability, cost, and specifics of reproduction, biomass can be considered a renewable raw material [
4]. Agricultural waste such as straw, sawdust, coffee, and rice husks are biomass varieties of fairly high calorific value. More than 3 billion tons of such waste can be produced annually in the world [
5].
In thermochemical or biochemical processing, biomass can be converted directly into heat and electricity, or into co-products such as biochar, bio-oil, and biogas [
6,
7]. These products can be used as fuel or for the synthesis of new materials. For example, pyrolysis gas can be burned or converted to liquid fuel via Fischer–Tropsch synthesis [
8].
Pyrolysis is a fairly simple and proven technology with a wide range of applications. In traditional pyrolysis, the biomass particle absorbs heat through the surface through conduction, convection, and radiation. During microwave pyrolysis, energy is transferred directly to the core of the particle, forming a temperature gradient in the volume of the particle without physical contact between the raw material and the heat source. Such a heating mechanism releases volatile substances that diffuse from the core to the outer surface, from the high-temperature region to the low-temperature region, significantly affecting the characteristics of the raw material conversion products [
9]. Microwave pyrolysis is a promising approach in terms of increasing the yield and quality of the final liquid biofuel. Bio-oil contains many valuable compounds and can be used not only as a liquid fuel, but also as a resource for the extraction of chemicals [
10]. Microwave pyrolysis is of increasing interest due to its low thermal inertia, uniform and volumetric heating, and high conversion rate, which together can increase productivity and energy efficiency [
11]. The choice of the operating mode of microwave reactors is an important task both in the design of equipment and directly in the operation of a process plant.
Microwave heating does not require the sample to come into contact with any surface to transfer energy. When a sample is irradiated with microwaves, heating is realized due to the rotation of the dipole and the ionic conductivity of polar molecules and/or ions of the sample [
12]. The sample is heated in volume due to the direct and uniform penetration of microwaves into the deep layers, thereby forming a temperature gradient. In the case of pyrolysis based on contact heat transfer, it is difficult to achieve the same high heating rates [
13]. Uneven heating during traditional pyrolysis can lead to overheating of the sample surface and overcooling of the active zone [
14]. However, microwave pyrolysis does not have much flexibility in the choice of feedstock and is only suitable for polar materials and substances.
The yield of pyrolysis products significantly depends on the physicochemical properties of the biomass and the process parameters [
15]. The pyrolysis temperature is one of the main factors influencing the properties of the final products and their yield. Dominguez et al. [
13] studied the characteristics of coffee husk pyrolysis under two heating modes. An increase in temperature increased the yield of biogas and reduced its calorific value. The effect of temperature on biogas yield was more significant with microwave heating than with conventional pyrolysis. At the same time, the biochar yield decreased with increasing temperature, especially during microwave heating since the decomposition of coffee husks into volatile substances was promoted by high temperature and energy supply, which slowed down the carbonization process [
16]. With microwave heating, the liquid yield first increased and then decreased, and with electrical heating, it continuously decreased with increasing temperature.
During microwave pyrolysis, the irradiation power significantly affects the yield of products. The higher the power, the higher the heating rate of the raw material. In [
17], waste tires were pyrolyzed in a microwave reactor at various powers, and the reaction temperature in the experiment was set to 500 °C with a retention time of 20 min. The high microwave power allowed the raw material surface temperature to reach about 500 °C in a short time, resulting in a longer reaction time than at lower microwave power. The biogas yield increased with increasing microwave power. The yield of bio-oil also increased and reached a maximum at 560 W; it was about 46 wt% and then decreased. Moreover, biochar yield tended to decrease with increasing microwave power. In [
18], rice straw, rice husks, corn straw, sugar cane cake, sugar cane peel, coffee ground waste, and bamboo leaves were used for microwave pyrolysis. Before pyrolysis, the components were air-dried, milled, and sieved. The moisture content of the biomass was 5–10 wt%. Microwave pyrolysis was carried out in a single-mode microwave oven at a frequency of 2.45 GHz. The biomass was added to a quartz crucible and then placed inside a quartz tube located opposite the magnetron. After purging with an inert gas, the power source was turned on at the specified microwave power level for 30 min. Then, the power was turned off. For all agricultural residues, the maximum temperature increased with increasing microwave power. The maximum temperatures (320, 420, and 530 °C) were reached at certain microwave powers (300, 400 and 500 W), regardless of which feedstock was pyrolyzed. During microwave pyrolysis of typical agricultural waste with a power of 300 W, approximately half of all pyrolysis products were in liquid form, while 26–31 wt% and 18–26 wt% were solid and non-condensable gas, respectively. With an increase in the microwave power from 300 W to 500 W, the gas yield increased by 12–15 wt%, but the yield of both solid and liquid decreased by 7–9 and 3–8 wt%, respectively [
18]. Menéndez et al. [
18] found that a higher level of microwave power can contribute to the formation of non-condensable gas, which is due to the self-gasification of the char [
19]. Zabaniotou et al. [
20] studied the effect of microwave power radiation on the characteristics of the pyrolysis of waste tires. The setup consisted of a microwave oven with a maximum output power of 900 W, a quartz reactor, and a product collection unit. The pyrolysis time was 30 min. As the power increased, the yield of solid products decreased from 48.85% at 9 W/g to 43% at 15 W/g.
With a further increase in specific power to 24 W/g, the solids yield continued to decrease. Final temperatures were 415 °C, 498 °C and 574 °C at 9 W/g, 15 W/g and 24 W/g, respectively. Liquid yield increased from 37% at 9 W/g to 45% at 15 W/g. The increase in liquid yield indicated that the temperature increased at higher power densities, resulting in the formation of more oily substances. With a further increase in specific power, the liquid yield decreased since some of the oil molecules continued to decompose into lighter gaseous molecules through secondary reactions [
20,
21]. Pyrolysis gases typically contain quite a lot of H
2, CH
4, and C
2H
4, as well as certain hydrocarbons such as C
2H
6, and CO, CO
2, and a trace amount of H
2S. In general, it can be assumed that microwave heating promotes deep transformation of macromolecules, so that a high percentage of light molecular gases, such as CH
4 and H
2, is formed.
This study is motivated by the need to find relationships between the power of microwave irradiation of raw materials and the quantity and quality of the products obtained. The scientific novelty lies in a comprehensive approach to studying the effect of irradiation power on woody biomass during microwave pyrolysis. For the first time, using multiple-criteria decision analysis, an assessment of a set of indicators was carried out, considering the energy consumption for pyrolysis.
2. Materials, Experimental Setup, and Procedures
Pine sawdust was used as raw material. For the experiments, sawdust of one tree species (Pínus sibírica) was used. Sawdust came from one of the local woodworking enterprises. The woody biomass was clean (no oil, paint, glue, or other contaminants).
Unlike conventional thermal pyrolysis, microwave pyrolysis is based on the principle of interaction of electromagnetic waves with matter. Therefore, this type of conversion does not require external drying of the fuel. Microwave pyrolysis provides fairly fast volumetric heating, when, in fact, there is a conversion of electrical energy into thermal energy. The efficiency of microwave pyrolysis depends on the dielectric properties of the raw material. Water is a special substance characterized by an extremely high permittivity. Therefore, in microwave pyrolysis, for efficient energy conversion, it is expedient to use moistened raw materials in order to improve the absorption of microwaves.
Water provides rapid heating of the raw material layer due to the very high dielectric constant. Upon reaching the boiling point, water turns into water vapor. The permittivity of water vapor is many times lower than that of water: at a temperature of 373 K in the state of saturation, water vapor has a permittivity of 1.006, and water has a permittivity of about 55. However, water molecules not only receive energy from an external source, but also transfer molecules of the pyrolyzed raw material throughout its volume. Thus, more efficient heat transfer is achieved in the first conversion stage. Shepherd et al. [
22] showed that a liquid can be effectively used to “pump” energy into a material during microwave pyrolysis. But, of course, the role of water can be ambiguous at subsequent stages, when water vapor can participate in the reactions. During pyrolysis, moisture in the biomass contributes to the formation of a vapor–air mixture in the reaction zone. A high steam concentration promotes pyrolysis towards the generation of more H
2 and CO
2 [
23]: CO + H
2O → CO
2 + H
2. In [
2], a more humid fraction gave a higher quality gas product in terms of the yield of carbon monoxide and hydrogen. However, many studies on steam gasification (for example, [
24,
25]) indicate that an excess amount of water vapor can worsen the reactions, lower the temperature, and, as a result, worsen the composition of the synthesis gas.
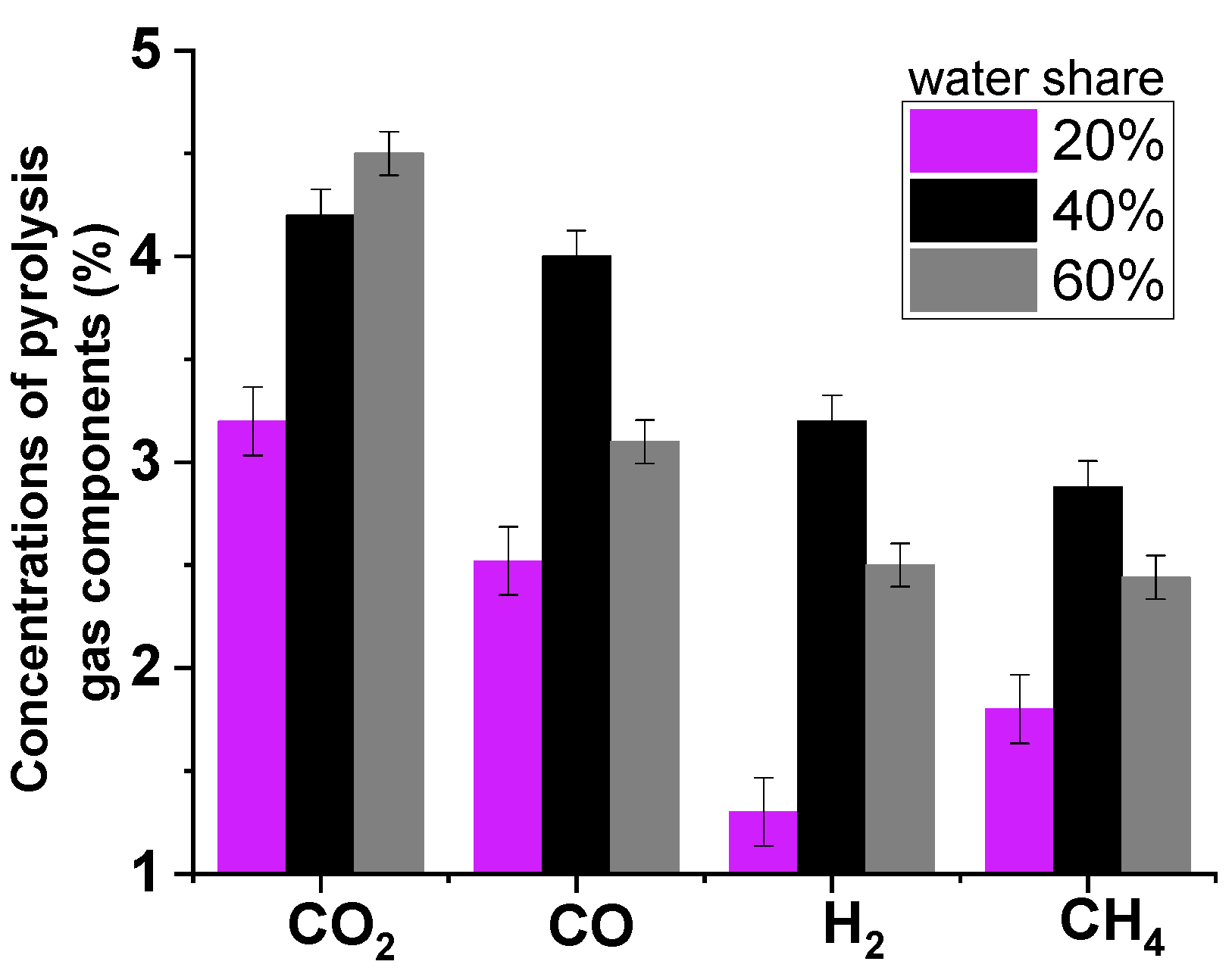
In the present study, the wood raw material was mixed with water to provide microwave volumetric heating. The composition 60% sawdust–40% water was prepared. Pine sawdust absorbs and retains water very well. Therefore, in the mixture used, the proportion of water was large enough (40 wt%) to ensure uniform saturation of sawdust with water throughout the volume. In addition, experiments with a gas analyzer were performed to assess the influence of the moisture factor on the composition of the conversion products (the procedure is described below). Experiments were carried out with sawdust with a water content of 20%, 40%, and 60%. The results showed that the best composition of the pyrolysis gas was obtained when the mixture “60% sawdust, 40% water” was used (
Figure 1). For these reasons, this ratio was used for further experiments.
A typical industrial microwave installation consists of three main components: a microwave generator (magnetron), guiding systems (waveguide), and a resonator. In industry, microwave generators with operating frequencies of 433, 915, and 2375 MHz are most often used [
26]. Household microwave devices use an operating frequency of 2375 MHz, which corresponds to an electromagnetic wavelength of 12.2 cm. Below is a brief overview of the reactors used in microwave pyrolysis research. In [
27], microwave pyrolysis of corn cobs was carried out using a microwave installation, consisting of a modified household microwave oven, a condenser tube, and a quartz flask. The microwave power was 450 and 600 W. In [
28], an experimental microwave system Makewave was used for biomass conversion. The output power of the microwaves was about 600 W. The setup consisted of a gas cylinder for purging with argon, a gas meter, a preheating chamber, a microwave oven, a control unit, a gas analyzer, a cooling system, and a gas collection system. In [
29], household microwave ovens with a power of 1000 W and 800 W at a frequency of 2.4 GHz were used for the experimental production of pyrolysis gas from palm date seeds. The installation included a thermocouple, a gas analyzer, a gas cylinder, and a water filter.
Figure 2 shows the diagram of the experimental setup used in the present study. A microwave reactor (Menumaster Commercial, model DEC21E2) and a gas analyzer (Test-1, Boner-VT) were the base of the experimental rig.
To place a raw material in the reactor, we used a ceramic crucible (made of red clay). This material is heat-resistant, microwave-neutral, cheap, and readily available. Ceramic crucibles can be used for the pyrolysis of many organic materials. The power (
P) of the magnetron was regulated in the range from 400 to 2200 W. The reactor was hermetically sealed with a door into which a gas sampling hose was built. To determine the composition of the pyrolysis gas, a gas analyzer with the following set of elements was used: a modular probe, a condensate collector, a sample filtration system, a computing unit, a flow meter, a pump, and electrochemical sensors (O
2, CO, SO
2, NO, NO
2, H
2S, HCl). The gas analyzer also had optical sensors for CO
2 and CH
4 and a polarographic sensor for H
2. The characteristics of the measuring channels are presented in
Table 1. The gas analyzer was connected to a computer via the RS232 interface. The auxiliary software displayed real-time measurements and also exported the resulting data to standard text and graphic formats.
The experimental procedure included the following steps:
Preparation of biomass. Dry sawdust was crushed using a high-speed rotary mill and then sieved. The average dispersion of woody particles was 2000 μm. Water was then added to the biomass to obtain a “60% sawdust–40% water” mixture. The mass of the pyrolyzed sample was 30 g in each experiment.
The fuel sample was evenly distributed in a ceramic heat-resistant crucible, which was then tightly closed with a ground-in ceramic lid to prevent loss of pyrolysis gas. The crucible was placed in the reactor. The door was hermetically sealed.
Magnetron and gas analyzer turned on. The gas mixture passed through a condensate collector, where moisture was deposited. The gas mixture purified from moisture was supplied to the sensors.
Microwave irradiation was carried out until the concentration of pyrolysis gas components decreased at powers of 840 W, 1760 W, and 2200 W. After the maximum concentrations were reached, the magnetron was turned off. Further, the release of pyrolysis gas continued without energy supply from an external source.
After the experiment, the solid pyrolysis product was weighed. Before starting the next experiment, the reactor and all gas channels were purged with compressed air. In one experimental set, 3–5 experiments were performed under identical conditions.
Gross errors were excluded and experimental results were averaged. Calculation of average concentrations was performed using the trapezoidal method. The details of the method are given in [
30]. The basis of the method was that the area under the concentration trend was approximated by rectangular trapezoids with a step of 1 s. Next, the area of each trapezoid was determined. Then, the ratio of the sum of the areas of all trapezoids to the time of emission of the gas component was calculated. After averaging the results, confidence intervals (error bars) were determined.
To date, approaches to pyrolysis in microwave reactors can vary significantly in different studies. Previously, it was found (for example, in [
27,
31,
32]) that the time of exposure to microwaves significantly affects the yield of pyrolysis products. Depending on the objectives of the research and the characteristics of the equipment, the time of exposure to microwaves can be a variable factor. In the present study, the magnetron worked for a limited time in the experiments. Since the experiment was carried out on a laboratory scale with a limited mass of raw materials, this was expedient. The main criterion was the reduction in CO
2 concentration. Verification tests showed that the time of exposure to microwaves is sufficient for the material to self-heat and further release of the pyrolysis gas components occurs due to the thermal energy accumulated in the layer. In addition, the factor of the magnetron operation time was further considered when calculating the pyrolysis efficiency index, when the consumed energy was one of the main influencing factors (
Section 3).
Figure 3 shows an example of the CH
4 emission trend during microwave pyrolysis. The increase in concentration began at the start of the experiment. The time of the CH
4 release was about 1863 s. The integral area under the curve was 4278.4 s·%. Therefore, the average value of the methane concentration in this experiment was 2.3%.
3. Results and Discussion
Due to their abundance and availability, woody biomass is promising for the production of carbon-neutral energy. Sawdust or wood shavings are a cheap lignocellulosic compound. Sawdust as industrial and agricultural waste finds many applications in vast areas [
33]. Sawdust or wood waste mainly consists of cellulose (45–50%), lignin (23–30%), hemicellulose (20–30%), and other substances (acids, soluble sugar, resins, wax, and oil) [
34,
35]. Sawdust has good mechanical stability, high porosity and water-holding capacity, and sufficiently high carbon content and calorific value [
36]. The main properties of the sawdust used are given in
Table 2.
Table 3 shows the maximum concentrations of the gas mixture components recorded in the experimental sets. It was found that the peak concentrations of the pyrolysis gas increased with increasing magnetron power. The maximum values were recorded at a microwave power of 2200 W, and the minimum peaks were observed at 840 W. With an increase in microwave power from 840 W to 2200 W, the maximum yield of CO increased 4 times; H
2, 8 times; CH
4, 3 times; and CO
2, 2 times.
It is clearly seen from the quantitative data that gaseous products with a higher calorific value can be obtained at higher microwave power values. It can be assumed that increasing the power of microwave heating contributes to a deep transformation of wood macromolecules so that a higher percentage of light gases such as CH
4 and H
2 is formed. An increase in the power of microwaves provokes a more intense increase in temperature in the layer. Accordingly, the peak concentrations of gases increase. This is in good agreement with the data of earlier experimental studies [
16,
17,
18,
19,
20,
21]. Pan et al. [
37] used kinetic models to calculate the yield of gas, tar, and char during secondary reactions of the components with an increase in temperature and heating rate during pyrolysis. Upon reaching a heating rate of 10 °C/min, the gas output increased and remained almost constant at temperatures above 700 °C. Resin yield increased below 350 °C. Then, the yield of pyrolysis gas decreased. Tar decomposition, which may be one of the secondary charring reactions, occurred at a temperature of about 400 °C. During pyrolysis, heat and mass transfer is intensified, and the initial biomass undergoes various primary and secondary conversion reactions. Primary reactions include the decomposition of cellulose, hemicellulose, and lignin present in the biomass, leading to the formation of primary and intermediate products. Intermediate products are involved in secondary cracking. Primary reactions include dehydration and charring, while secondary reactions include decomposition and volatilization of intermediates.
The general trend was an increase in the average yield of pyrolysis gas components with an increase in the power of microwave irradiation (
Figure 4a). However, an increase in power affected the yield of individual gases non-linearly and on a different scale. In particular, experiments have shown that the average yield of CO and CO
2 remained practically unchanged when the microwave power was increased from 840 to 1760 W. However, with a further increase in power to 2200 W, there was a significant increase in the average yield of CO and CO
2 (2.5 and 1.4 times, respectively). The formation of methane and hydrogen (according to the average yield) showed a higher sensitivity to an increase in the microwave power. An increase in power by 2.6 times contributed to an increase in the average yield of CH
4 by 5 times and H
2 by 3.8 times. A significant difference between the average yields of pyrolysis gas is explained by the different intensities of wood decomposition at different microwave exposure powers and, as a result, different reaction times (
Figure 4b). At 2200 W, the process time was about 30 min, while at 840 W, the release of pyrolysis gases was completed in 50 min.
To compare the results of microwave and conventional pyrolysis of biomass, [
38] was considered. In [
38], the traditional pyrolysis of sawdust was performed in a laboratory reactor. The average yield of conversion gas products was as follows: CO
2—14%; CO—10%; H
2—3.2%; CH
4—less than 0.4%. These values are comparable to those obtained for microwave pyrolysis of sawdust. Microwave pyrolysis provided a significantly lower release of carbon dioxide (an undesirable conversion product); however, the average yield of CO and H
2 was lower than that in conventional pyrolysis (1.2 and 4 times, respectively). Also, during conventional pyrolysis, the average methane yield was significantly lower than that during microwave conversion at 2200 W. However, the differences in the experimental conditions should be considered. In particular, in [
38], the heating of the raw material was gradual, from 25 °C to 500 °C for about 50 min, while microwave pyrolysis required about 600 s of operation of the magnetron at a power of 2200 W.
According to the experimental data, approximation formulas (Equations (1)–(5)) were determined for calculating the yield of individual components of the pyrolysis gas from woody biomass:
The approximation formulas were obtained using OriginPro software. Curve fitting tools made it possible to calculate an equation that describes the experimental points according to the least squares rule (to minimize the total deviation of the curve from the points). It is important to note that the same experimental data can be described by different equations. Therefore, when analyzing the data, we were also guided by the fact that the equation should be fairly simple (not of a high order). This is important for further application of formulas, convenience of working with them, and reduction in computational costs. All approximation formulas given in the work describe specific experimental data obtained in experiments. The area of their proven applicability is the power variation range of 840–2200 W. With a certain degree of assumptions, the equations can be used to predict the characteristics of microwave pyrolysis over a wider range and to model processes. However, this requires experimental verification.
Figure 4b shows the characteristic times of the process under study at different microwave powers. The minimum total time of pyrolysis was recorded at 2200 W (1890 ± 40 s). The pyrolysis process was the longest (3948 ± 55 s) when using the minimum power of microwave processing of biomass. The increase in power contributed to a decrease in the duration of the inert period, i.e., the time when pyrolysis gas is not yet released. Increasing the microwave power from 840 W to 2200 W reduced the delay time of pyrolysis gas yield by about 20 times.
According to
Figure 4b, approximate formulas (Equations (6)–(8)) for the characteristic times of microwave pyrolysis of woody biomass are determined:
Figure 5 illustrates typical time trends in the concentrations of gaseous products from the pyrolysis of sawdust. The curves have some fluctuations as well as several extremes. The non-monotonicity of gas release is explained by the fact that the temperature in the layer increases during microwave pyrolysis. Therefore, the released gases enter into secondary reactions with the pyrolysis liquid and the resulting biochar at higher temperatures. Experiments have shown that microwave power is an important factor influencing the dynamics of pyrolysis gas formation. For example, at low microwave power, methane evolution was characterized by a single-peak mode, in which the maximum possible concentrations are released for a rather short time relative to the total pyrolysis time. For other components, a similar trend was observed—with an increase in power, both the maximum yield of combustible gases increased, and the conditional “density” of this yield increased, i.e., a large proportion of the total pyrolysis time included the useful time for the release of gases.
Figure 6 shows that with increasing microwave power, the relative yield of solid and liquid products decreased. The largest masses of solid and liquid products (6.4 ± 0.2 g and 5.5 ± 0.2 g, respectively) were recorded at 840 W, and the smallest (1.2 ± 0.1 g and 2.0 ± 0.1 g) were recorded at a power of 2200 W. The decrease in liquid yield is due to secondary cracking reactions at a high energy level. The yield of biochar decreased with increasing power since the decomposition of biomass into volatile substances was promoted by high temperature and energy supply, which inhibited the carbonization process. The decrease in the mass of solid and liquid residues with increasing power is due to more intense degassing of hemicellulose and lignin during wood pyrolysis. With intense exposure to microwaves and the corresponding self-heating of the biomass layer, side-chain cleavage reactions of hemicellulose and lignin occur, such as deacetylation and demethoxylation [
39]. With an increase in temperature, the processes of depolymerization of hemicellulose and lignin are intensified [
39], which leads to a decrease in the mass of solid and liquid residues and an increase in gas formation. According to the results obtained, an increase in the power of microwaves contributed to a decrease in the mass of the solid residue by 5.3 times. The mass of the liquid product decreased by 2.7 times. Based on experimental data, approximation formulas were obtained to predict the percentage yield of solid and liquid products obtained during microwave pyrolysis (relative to the initial dry weight of raw materials):
The integral efficiency of wood pyrolysis at various powers of the microwave reactor can be estimated through a set of indicators. Multiple-criteria decision analysis [
40] has been developed for such problems. In particular, the weighted sum method [
41] can be used. This method is based on comparing individual values with the best possible value and then summing up the individual components that characterize the process or project. According to the weighted sum method, the efficiency indicator can be calculated using the following formula:
where
is the normalized characteristic,
wi is the weight coefficient, and
n is the number of characteristics. The normalization of the values of each parameter (characteristic) is performed relative to the best-known value.
Table 4 shows the initial data for calculating the efficiency, as well as the criterion for normalizing the values included in the final performance indicator. The rationing logic is based on the fact that pyrolysis is more efficient when less energy is spent on the process and more biomass is converted into combustible gases. The calculations in this work are performed for three cases of priority distribution: (1) all characteristics have the same weight; (2) the main priority (
w = 0.5) is assigned to minimizing the energy consumed for pyrolysis, and the remaining coefficients are distributed evenly; (3) the main priority (
w = 0.5) is assigned to maximizing the yield of combustible gases (methane, hydrogen, and carbon monoxide), and the remaining coefficients are distributed evenly.
The results of the efficiency calculation are presented in
Figure 7. It is clearly seen that the efficiency is maximum at high microwave power. It is important that high performance was achieved regardless of the distribution of priority. So, for example, with a 50% dominance of the weight coefficient for minimizing energy costs for pyrolysis, the final efficiency indicator decreased by only 15% compared to the case where the weight coefficients were evenly distributed. That is, despite the fact that an increase in the reactor power entails an increase in the energy consumption for pyrolysis, these costs are integrally compensated by a more efficient physical and chemical conversion of the feedstock.
The overall efficiency of microwave pyrolysis was minimal at the lowest reactor power. At the same time, the efficiency increased as expected when the main priority was given to minimizing the energy consumed for pyrolysis. The efficiency indicator in this case increased by 50–60% in comparison with other priority options. In other cases (that is, at powers of 1760 W and 2200 W), varying the priority of certain parameters did not give such significant differences in the integral efficiency of microwave pyrolysis.
An increase in microwave power from 840 W to 2200 W contributed to an increase in the pyrolysis efficiency indicator by 1.3–2.2 times. In the case when the priority was given to maximizing the production of combustible gases, the increase in power showed the greatest impact on the integral biomass pyrolysis efficiency index.
The results of the study revealed the potential of microwave pyrolysis of woody biomass and made it possible to determine the extent of the effect of microwave exposure power on the composition and amount of conversion products. The increase in power, despite the associated costs, is very effective for cases where it is necessary to obtain a high yield of high-calorie pyrolysis gases. At subsequent stages of the technological process, the gas mixture can be usefully used, among other things, for conversion into pure liquid biofuels (in particular, when using the Fischer–Tropsch reactor).
4. Conclusions
With an increase in microwave power from 840 W to 2200 W, the generation of pyrolysis gas from sawdust was significantly intensified. In particular, the average yield of CO increased by 2.5 times, and the average yield of H2 and CH4 increased by 5 and 2.8 times, respectively. The yield of methane and hydrogen was the most sensitive to the variation in microwave power.
The power of microwave waves had a non-linear effect on the dynamics of pyrolysis gas formation. Increasing the microwave power from 840 W to 2200 W reduced the delay time of pyrolysis gas yield by about 20 times.
Intensive degassing of raw materials with increased microwave power contributes to the decarbonization of biomass. An increase in the power of microwaves (from 840 to 2200 W) contributed to a decrease in the mass of the solid residue and the liquid product by 5.3 and 2.7 times, respectively.
An increase in energy consumption for pyrolysis with an increase in microwave power reduced the integral efficiency of biomass conversion to a limited extent. The pyrolysis efficiency index calculated by the weighted sum method increased by a factor of 1.3–2.2 when the power was varied from 840 W to 2200 W.
The present study considers the effect of microwave exposure power on woody biomass conversion products. The results have applicability limitations since the power range of the magnetron was limited to 840–2200 W. For the subsequent development of the research, it is advisable to expand the range of influencing factors; in particular, it is advisable to experimentally determine the fuel heating rate in different zones of a layer, vary the residence time of the feedstock in the reactor, and quantitatively and qualitatively evaluate the effect of heat flows on biomass conversion. In addition, tests with other types of raw materials and adaptation of the experimental technique to a wider range of magnetron power to verify the obtained approximation formulas are the future development of this study.