Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling
Abstract
1. Introduction
2. Materials and Methods
2.1. Experiment Design
2.2. Technical Material
- MP—Mineral plaster without biocide additives;
- MPGS—Mineral plaster with primer and silicone paint, without biocide additives;
- S—Silicone plaster without biocide additives;
- SGS—Silicone plaster with primer and silicone paint, without biocide additives.
2.3. Biological Material
- Stichococcus bacilliaris (CCAP, Culture Collection of Algae and Protozoa, Dunbeg, Scotland, UK);
- Nostoc commune (CCAP, Culture Collection of Algae and Protozoa, Dunbeg, Scotland, UK);
- Pseudochlorella signiensis (Environmental isolate);
- Coenochloris signiensis (Environmental isolate).
2.4. Experiment 1: Selection of Methods Used for Assessing Photoautotrophic Growth on Plaster Coatings
2.4.1. Inoculation Mixture
2.4.2. Inoculation and Incubation Procedure
2.4.3. Biofilm Cell Enumeration
2.4.4. Luminometric ATP Measurement
2.4.5. Chlorophyll a Determination
2.4.6. Visual Assessment
2.4.7. Spectrophotometric Color Change Evaluation
2.4.8. Correlation Analysis
2.5. Experiment 2: Assessment of the Inoculation and Incubation Conditions of Samples Tested for Resistance against Photoautotrophic Growth
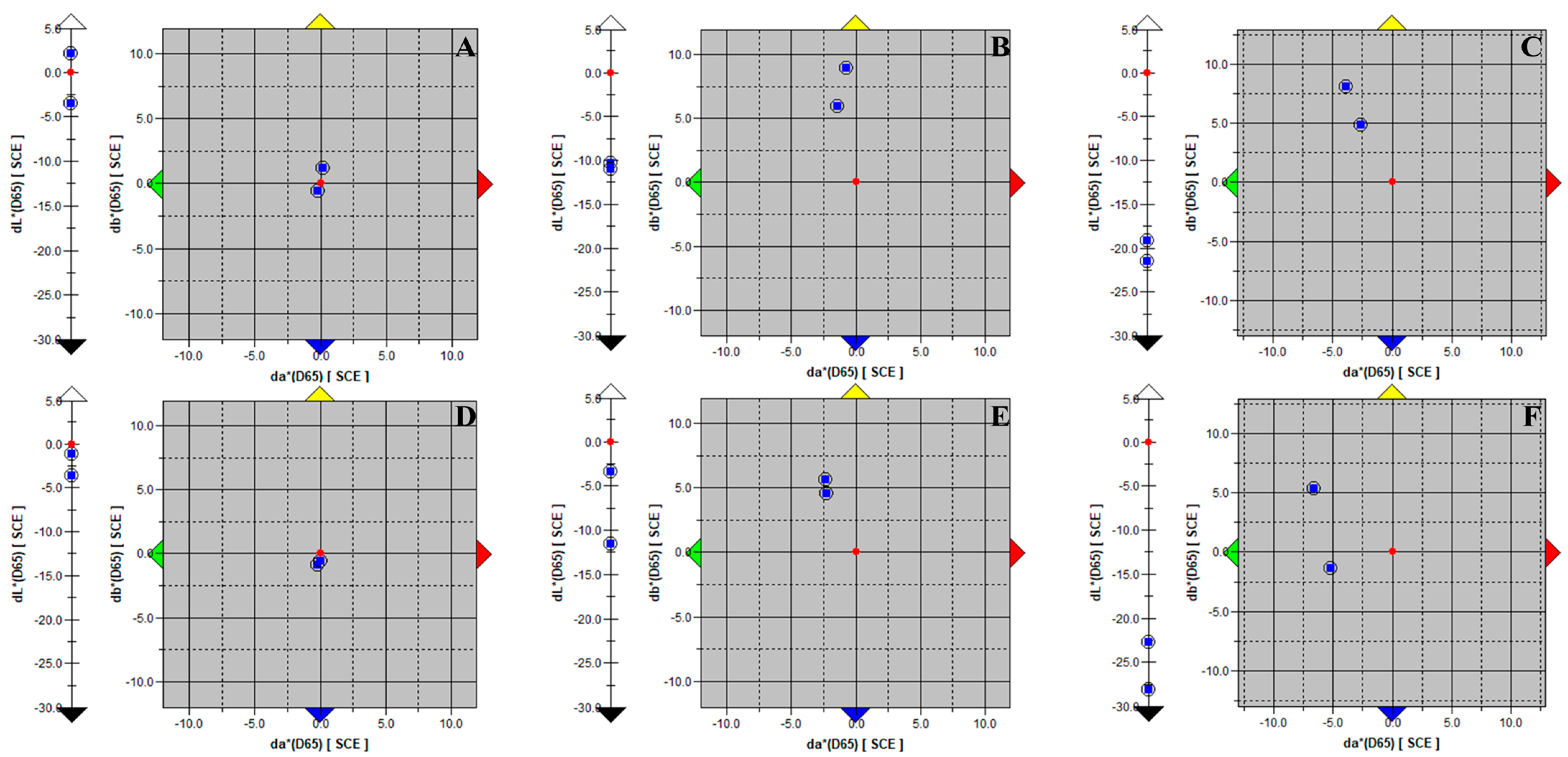
3. Results
3.1. Experiment 1: Selection of Methods Used for Assessing Photoautotrophic Growth on Plaster Coatings
3.2. Experiment 2: Assessment of the Inoculation and Incubation Conditions of Samples Tested for Resistance against Photoautotrophic Growth
4. Discussion
4.1. Selection of Methods Used for Assessing Photoautotrophic Growth on Plaster
4.2. Assessment of the Inoculation and Incubation Conditions of Samples Tested for Resistance against Photoautotrophic Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hueck, H.J. The biodeterioration of materials as a part of hylobiology. Mater. Org. 1965, 1, 5–34. [Google Scholar]
- Allsopp, D.; Seal, K.J.; Gaylarde, C.C. Introduction to Biodeterioration, 2nd ed.; Cambridge University Press: Cambridge, UK, 2004; ISBN 9780521821353. [Google Scholar]
- Nowicka-Krawczyk, P.; Komar, M.; Gutarowska, B. Towards understanding the link between the deterioration of building materials and the nature of aerophytic green algae. Sci. Total Environ. 2022, 802, 149856. [Google Scholar] [CrossRef] [PubMed]
- Häubner, N.; Schumann, R.; Karsten, U. Aeroterrestrial microalgae growing in biofilms on facades—Response to temperature and water stress. Microb. Ecol. 2006, 51, 285–293. [Google Scholar] [CrossRef]
- Miller, A.Z.; Sanmartín, P.; Pereira-Pardo, L.; Dionísio, A.; Saiz-Jimenez, C.; Macedo, M.F.; Prieto, B. Bioreceptivity of building stones: A review. Sci. Total Environ. 2012, 426, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sanmartín, P.; Miller, A.Z.; Prieto, B.; Viles, H.A. Revisiting and reanalysing the concept of bioreceptivity 25 years on. Sci. Total Environ. 2021, 770, 145314. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, E.; Vázquez-Nion, D.; Prieto, B. Laboratory development of subaerial biofilms commonly found on buildings. A methodological review. Build. Environ. 2022, 223, 109451. [Google Scholar] [CrossRef]
- Manso, S.; De Muynck, W.; Segura, I.; Aguado, A.; Steppe, K.; Boon, N.; De Belie, N. Bioreceptivity evaluation of cementitious materials designed to stimulate biological growth. Sci. Total Environ. 2014, 481, 232–241. [Google Scholar] [CrossRef]
- Sanmartín, P.; Grove, R.; Carballeira, R.; Viles, H. Impact of colour on the bioreceptivity of granite to the green alga Apatococcus lobatus: Laboratory and field testing. Sci. Total Environ. 2020, 745, 141179. [Google Scholar] [CrossRef]
- Gambino, M.; Sanmartín, P.; Longoni, M.; Villa, F.; Mitchell, R.; Cappitelli, F. Surface colour: An overlooked aspect in the study of cyanobacterial biofilm formation. Sci. Total Environ. 2019, 659, 342–353. [Google Scholar] [CrossRef]
- Stanaszek-Tomal, E. The problem of biological destruction of façades of insulated buildings—Causes and effects. IOP Conf. Ser. Mater. Sci. Eng. 2017, 245, 32012. [Google Scholar] [CrossRef]
- Nugari, M.P.; Pietrini, A.M.; Caneva, G.; Imperi, F.; Visca, P. Biodeterioration of mural paintings in a rocky habitat: The Crypt of the Original Sin (Matera, Italy). Int. Biodeter. Biodegr. 2009, 63, 705–711. [Google Scholar] [CrossRef]
- Moreau, C.; Vergès-Belmin, V.; Leroux, L.; Orial, G.; Fronteau, G.; Barbin, V. Water-repellent and biocide treatments: Assessment of the potential combinations. J. Cult. Herit. 2008, 9, 394–400. [Google Scholar] [CrossRef]
- Munafò, P.; Goffredo, G.B.; Quagliarini, E. TiO2-based nanocoatings for preserving architectural stone surfaces: An overview. Constr. Build. Mater. 2015, 84, 201–218. [Google Scholar] [CrossRef]
- Carrillo-González, R.; Martínez-Gómez, M.A.; González-Chávez, M.D.C.A.; Mendoza Hernández, J.C. Inhibition of microorganisms involved in deterioration of an archaeological site by silver nanoparticles produced by a green synthesis method. Sci. Total Environ. 2016, 565, 872–881. [Google Scholar] [CrossRef] [PubMed]
- Tobaldi, D.M.; Tucci, A.; Camera-Roda, G.; Baldi, G.; Esposito, L. Photocatalytic activity for exposed building materials. J. Eur. Ceram. Soc. 2008, 28, 2645–2652. [Google Scholar] [CrossRef]
- Bondioli, F.; Taurino, R.; Ferrari, A.M. Functionalization of ceramic tile surface by sol–gel technique. J. Colloid Interface Sci. 2009, 334, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Folli, A.; Pade, C.; Hansen, T.B.; De Marco, T.; Macphee, D.E. TiO2 photocatalysis in cementitious systems: Insights into self-cleaning and depollution chemistry. Cem. Concr. Res. 2012, 42, 539–548. [Google Scholar] [CrossRef]
- Dybowska-Józefiak, M.; Wesołowska, M. Internal abiotic components that influence the development of biocorrosion on ETICS plasters. Materials 2022, 15, 127. [Google Scholar] [CrossRef]
- PN-EN 15458; Paints and Varnishes—Laboratory Method for Testing the Efficacy of Film Preservatives in a Coating against Algae. European Standards: Brussels, Belgium, 2014.
- Wiejak, A. Ocena skuteczności działania środków ochrony powłok elewacyjnych przed grzybami pleśniowymi i glonami. Pr. Inst. Tech. Bud. 2011, 40, 15–25. [Google Scholar]
- Miller, A.Z.; Rogerio-Candelera, M.A.; Laiz, L.; Wierzchos, J.; Ascaso, C.; Sequeira Braga, M.A.; Hernández-Mariné, M.; Maurício, A.; Dionísio, A.; Macedo, M.F.; et al. Laboratory-induced endolithic growth in calcarenites: Biodeteriorating potential assessment. Microb. Ecol. 2010, 60, 55–68. [Google Scholar] [CrossRef]
- Prieto, B.; Vázquez-Nion, D.; Silva, B.; Sanmartín, P. Shaping colour changes in a biofilm-forming cyanobacterium by modifying the culture conditions. Algal Res. 2018, 33, 173–181. [Google Scholar] [CrossRef]
- Ramil, A.; Vázquez-Nion, D.; Pozo-Antonio, J.S.; Sanmartín, P.; Prieto, B. Using hyperspectral imaging to quantify phototrophic biofilms on granite. J. Environ. Inform. 2018, 35, 34–44. [Google Scholar] [CrossRef]
- European Organization for Technical Assessment (EOTA). External Thermal Insulation Composite Systems (ETICS) with Renderings; EAD (European Assessment Document) 040083-00-0404; EOTA: Brussels, Belgium, 2020. [Google Scholar]
- Andersen, R. Algal Culturing Techniques; Elsevier: Amsterdam, The Netherlands, 2005. [Google Scholar]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Dudek, M.; Gutarowska, B. Biodeterioration potential of algae on building materials—Model study. Int. Biodeter. Biodegr. 2023, 180, 105593. [Google Scholar] [CrossRef]
- PN-EN 15457; Paints and Varnishes—Laboratory Method for Testing the Efficacy of Film Preservatives in a Coating against Fungi. European Standards: Brussels, Belgium, 2014.
- Evans, J.D. Straightforward Statistics for the Behavioral Sciences; Thomson Brooks/Cole Publishing Co.: Belmont, CA, USA, 1996; ISBN 0-534-23100-4. [Google Scholar]
- Negi, A.; Sarethy, I.P. Microbial biodeterioration of cultural heritage: Events, colonization, and analyses. Microb. Ecol. 2019, 78, 1014–1029. [Google Scholar] [CrossRef]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Konca, P.; Gutarowska, B. Metabolomic analysis of photosynthetic biofilms on building façades in temperate climate zones. Int. Biodeter. Biodegr. 2022, 169, 105374. [Google Scholar] [CrossRef]
- Rindi, F. Terrestrial green algae: Systematics, biogeography and expected responses to climate change. In Climate Change, Ecology and Systematics; Cambridge University Press: Cambridge, UK, 2011; pp. 201–227. ISBN 9780511974540. [Google Scholar]
- Zarrinmehr, M.J.; Farhadian, O.; Heyrati, F.P.; Keramat, J.; Koutra, E.; Kornaros, M.; Daneshvar, E. Effect of nitrogen concentration on the growth rate and biochemical composition of the microalga, Isochrysis galbana. Egypt. J. Aquat. Res. 2020, 46, 153–158. [Google Scholar] [CrossRef]
- Choudhury, N.K.; Behera, R.K. Photoinhibition of photosynthesis: Role of carotenoids in photoprotection of chloroplast constituents. Photosynthetica 2001, 39, 481–488. [Google Scholar] [CrossRef]

| Time (d) | MP | MPGS | S | SGS | |
|---|---|---|---|---|---|
| Cell density (cfu/cm2) | 0 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 14 | 3.33 × 107 ± 9.43 × 106 | 3.67 × 107 ± 4.71 × 106 | 2.00 × 107 ± 9.43 × 106 | 2.67 × 107 ± 9.43 × 106 | |
| 28 | 6.33 × 107 ± 4.71 × 106 | 4.67 × 107 ± 1.89 × 107 | 4.00 × 107 ± 9.43 × 106 | 4.67 × 107 ± 9.43 × 106 | |
| Chl-a (mg/cm2) | 0 | 1.00 × 10−2 ± 1.00 × 10−2 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 | 0.00 × 100 ± 0.00 × 100 |
| 14 | 4.00 × 10−2 ± 0.00 × 100 | 2.80 × 10−1 ± 8.00 × 10−2 | 0.00 × 100 ± 0.00 × 100 | 4.00 × 10−2 ± 0.00 × 10−1 | |
| 28 | 4.40 × 10−1 ± 4.00 × 10−2 | 3.30 × 10−1 ± 3.00 × 10−2 | 2.10 × 10−1 ± 2.00 × 10−2 | 5.00 × 10−2 ± 1.00 × 10−2 | |
| ATP (RLU) | 0 | 1.41 × 101 ± 4.22 × 100 | 1.56 × 101 ± 2.53 × 100 | 1.47 × 101 ± 1.28 × 100 | 1.19 × 101 ± 5.24 × 100 |
| 14 | 1.09 × 101 ± 7.42 × 101 | 8.79 × 102 ± 4.60 × 102 | 7.15 × 101 ± 4.23 × 101 | 9.33 × 101 ± 4.00 × 101 | |
| 28 | 2.58 × 102 ± 9.02 × 101 | 2.82 × 102 ± 4.91 × 101 | 7.67 × 101 ± 5.76 × 101 | 1.65 × 102 ± 1.23 × 102 | |
| ΔE (-) | 0 | 2.93 × 100 ± 1.03 × 100 | 2.51 × 100 ± 1.67 × 100 | 1.61 × 100 ± 1.91 × 10−1 | 2.84 × 100 ± 1.50 × 100 |
| 14 | 1.32 × 101 ± 7.50 × 10−1 | 9.60 × 100 ± 4.87 × 100 | 5.97 × 100 ± 1.46 × 100 | 1.02 × 101 ± 1.41 × 100 | |
| 28 | 2.16 × 101 ± 2.33 × 100 | 2.65 × 101 ± 3.08 × 100 | 1.21 × 101 ± 3.96 × 100 | 1.20 × 101 ± 2.93 × 100 |
| Cell Density | Chl-a | ΔE | ATP | ||||
|---|---|---|---|---|---|---|---|
| MP | Cell density | 1.000 | 0.790 | 0.999 | 0.975 | 0.00 | |
| Chl-a | 0.790 | 1.000 | 0.770 | 0.902 | 0.20 | ||
| ΔE | 0.999 | 0.770 | 1.000 | 0.967 | 0.40 | ||
| ATP | 0.975 | 0.902 | 0.967 | 1.000 | 0.60 | ||
| MPGS | Cell density | 1.000 | 0.997 | 0.727 | 0.342 | 0.80 | |
| Chl-a | 0.997 | 1.000 | 0.674 | 0.995 | 1.00 | ||
| ΔE | 0.727 | 0.674 | 1.000 | 0.006 | |||
| ATP | 0.342 | 0.995 | 0.006 | 1.000 | |||
| S | Cell density | 1.000 | 0.750 | 0.660 | 0.812 | ||
| Chl-a | 0.750 | 1.000 | 0.660 | 0.318 | |||
| ΔE | 0.660 | 0.830 | 1.000 | 0.729 | |||
| ATP | 0.812 | 0.318 | 0.729 | 1.000 | |||
| SSS | Cell density | 1.000 | 0.746 | 0.933 | 0.998 | ||
| Chl-a | 0.746 | 1.000 | 0.997 | 0.939 | |||
| ΔE | 0.933 | 0.997 | 1.000 | 0.909 | |||
| ATP | 0.998 | 0.939 | 0.909 | 1.000 | |||
| Substrate Type | 0 Days | 14 Days | 28 Days | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MP | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 4 | 4 |
| MPGS | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 4 | 4 |
| S | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 4 | 4 |
| SGS | 1 | 1 | 1 | 3 | 3 | 3 | 4 | 4 | 4 |
| M1 | M2 | ||||
|---|---|---|---|---|---|
| Colonized Area | ΔE | Colonized Area | ΔE | ||
| MP | 1 | 85–90% | 15.46 ± 4.36 | 15–20% | 8.55 ± 3.73 |
| 2 | 85–90% | 10–15% | |||
| 3 | 80–85% | 80–85% | |||
| 4 | 90–95% | 5–10% | |||
| MPGS | 1 | 70–75% | 8.87 ± 4.37 | 0–1% | 3.35 ± 2.85 |
| 2 | 65–70% | 1–5% | |||
| 3 | 30–35% | 10–15% | |||
| 4 | 55–60% | 5–10% | |||
| S | 1 | 30–35% | 5.36 ± 1.60 | 5–10% | 3.50 ± 1.05 |
| 2 | 20–25% | 1–5% | |||
| 3 | 35–40% | 1–5% | |||
| 4 | 25–30% | 5–10% | |||
| SGS | 1 | 15–20% | 6.81 ± 1.47 | 1–5% | 3.84 ± 1.32 |
| 2 | 5–10% | 0–1% | |||
| 3 | 5–10% | 1–5% | |||
| 4 | 10–15% | 5–10% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Komar, M.; Szulc, J.; Kata, I.; Szafran, K.; Gutarowska, B. Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling. Appl. Sci. 2023, 13, 8009. https://doi.org/10.3390/app13148009
Komar M, Szulc J, Kata I, Szafran K, Gutarowska B. Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling. Applied Sciences. 2023; 13(14):8009. https://doi.org/10.3390/app13148009
Chicago/Turabian StyleKomar, Michał, Justyna Szulc, Iwona Kata, Krzysztof Szafran, and Beata Gutarowska. 2023. "Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling" Applied Sciences 13, no. 14: 8009. https://doi.org/10.3390/app13148009
APA StyleKomar, M., Szulc, J., Kata, I., Szafran, K., & Gutarowska, B. (2023). Development of a Method for Assessing the Resistance of Building Coatings to Phoatoautotrophic Biofouling. Applied Sciences, 13(14), 8009. https://doi.org/10.3390/app13148009







