Abstract
Background: Diabetes mellitus (DM) has become a disease prevalent worldwide. Honey, which comprises predominantly bioactive constituents, has anti-inflammatory, antioxidant, and immunomodulating properties. Aim: Recent developments and benefits of natural products in treating various diseases have caught the attention of researchers. This study aims to investigate the antidiabetic effect of bee honey extract on induced diabetic Swiss mice. Materials and Methods: Fifty Swiss male mice were randomly assigned to five groups of 10 mice each. Group I served as the negative control; in group II, the mice received 2 mg/kg/b.wt of honey extract only; and groups III, IV, and V received cyclosporine (CsA) (20 mg/kg/day, s.c.) daily for 10 days prior to receiving streptozotocin (STZ) inoculated at multiple low doses (MLDSTZ) (30 mg/kg/day, i.p.) for five consecutive days. Group IV was administered with insulin initiated at a dose of 0.5 U/kg/b.wt as a standard treatment (positive control). Group V was administered 2 mg/kg/b.wt of honey extract, while group III received no treatment. Results: The results showed a significant hypoglycemic effect, increased body weight, increased liver glycogen levels, and the amelioration of antioxidant activities in groups IV and V compared with the diabetic group III. Moreover, serum matrix metalloproteinase (MMP-9) concentrations were significantly reduced in the mice treated with the insulin and honey extract in groups IV and V and the tissue inhibitor metalloproteinase-1 (TIMP-1) levels were significantly higher than the serum levels in group III. Furthermore, the histopathological examination of groups IV and V revealed regenerative changes with the restoration of normal islet cell architecture, as compared to the diabetic mice in group III. Compared to group I, group II showed no changes and exhibited non-significant data. Conclusion: Honey extract plays an effective role in improving all biomarkers in treated group V. Furthermore, MMP-9 and TIMP-1 are considered prognostic markers in the progression, severity, diagnosis, and treatment of type 1 DM. This may play an important role for the treatment of individuals in the future.
1. Introduction
The prevalence of diabetes mellitus (DM), which is currently regarded as a public health problem and may soon represent a global threat, has steadily increased. By 2030, more than 500 million individuals are expected to have DM [1]. Most of this diabetic population will be diagnosed in developing countries [2].
DM frequently results in cardiovascular complications, the greatest cause of death worldwide, as well as diabetic nephropathy and diabetic retinopathy [3]. These paradigms include the genetic risk variants and behavioral and environmental factors contributing to DM. However, DM can be controlled with a good diet, exercise, and medication, although lifelong maintenance is required [4]. Despite the availability of numerous antidiabetic medications, DM remains a major factor in both morbidity and mortality worldwide [5]. There is treatment available, but there is no known cure for DM. Some diabetic patients have turned to complementary and alternative therapies, such as acupuncture, chiropractic care, natural products, and herbal remedies [6].
Additionally, individuals with diabetes are more likely to experience recurrent coronary episodes, die from myocardial infarction, and develop heart insufficiency. This poor prognosis could be brought on by the abnormal production and activity of metalloproteinases (MMPs) [7]. Furthermore, it is unclear how diabetic angiopathy is related to MMPs and their inhibitors (specifically, tissue inhibitor matrix metalloproteinases (TIMP)).
Hyperglycemia may increase MMP activity and production in major arteries, either directly or indirectly (for example, through oxidative stress or advanced glycation products) [8]. Due to the increased glycation-related activity of TIMP-2, the primary MMP-2 inhibitor, diabetic nephropathy may be brought on by MMPs’ decreased proteolytic activity [9]. The matrix of atherosclerotic plaques may deteriorate because of an imbalance between MMPs and TIMPs, thereby raising the danger of plaque rupture. MMPs may also play a role in the developing acute thrombotic artery blockages and subsequent cardiovascular events, because they increase blood coagulability [10]. Moreover, it is unclear how MMPs contribute to the emergence of problems in DM. Evidence suggests that the venous monocytes of DM patients produce MMPs in higher quantities, but there is no evidence of enhanced MMP activity in the diabetic vasculature or skin fibroblasts [11].
Research on the health advantages of herbs and natural products, such as honey, in treating DM has received renewed attention. Honey is a natural product made by bees. It is one of the last unprocessed natural dietary ingredients [12]. Geographical origin, botanical sources of nectar, ambient and meteorological circumstances, and processing methods all impact honey composition [13]. Honey can be divided into monofloral and multifloral varieties [14], depending on whether it has a dominant pollen grain from a single source. According to several studies, honey reduces oxidative damage and hyperglycemia caused by DM [15].
In addition, honey has been shown to lower hepatic transaminases and raise high-density lipoprotein (HDL) [16]. There are at least 181 ingredients in honey, primarily monosaccharides and oligosaccharides [17]. Moreover, ascorbic acid, nitric oxide (NO) metabolites, carotenoid-derived chemicals, phenolic compounds, flavonoids, organic acids, aromatic compounds, trace elements, vitamins, amino acids, proteins, and additional bioactive components have also been discovered in honey [12]. A tryptophan metabolite with neuroactive properties, kynurenic acid, has been found in some types of honey, which may be responsible for the substance’s antinociceptive and antibacterial characteristics [18].
Honey has also been found to include several enzymes, including glucose oxidase, diastase, invertase, phosphatase, catalase, and peroxidase [19]. Honey has been used in folk medicine since 2100–2000 BC [20]. In the past, most perceived health advantages of honey were based on generalizations or simple observations without scientific support [21]. However, the health benefits of raw, natural honey in treating various disorders have been the subject of more research in recent years. Due to this interest, numerous medical benefits of honey have been discovered.
This study aims to investigate the antidiabetic effect of bee honey extract on type 1 diabetes induced in Swiss mice, as well as the roles of matrix metalloproteinase-9 and TIMP-1 levels as prognostic indicators. The study focused on antioxidants activities and their role in reducing glucose levels. In addition, histopathological changes in pancreatic islet cells were observed, and their significance was confirmed in relation to type 1 diabetes.
2. Materials and Methods
2.1. Honey Extract
The raw honey extract was prepared using the modified method of Mohapatra et al. [22]. In brief, methanol, ethanol, and ethyl acetate were used to extract the active components of honey based on polarity. Raw honey (10 g), sourced from a local market, was placed in a test tube, and 25 mL of methanol was added at room temperature (25 °C). The solution was then thoroughly mixed using a vortex before being centrifuged for 10 min at 3000 rpm. Filtration was performed to remove the supernatant from the test tube and transfer it to a stoppered test tube. The resultant supernatant was dissolved in 10 mL of dimethyl sulfoxide and thoroughly mixed by vortexing before being gently evaporated to dryness with a stream of nitrogen. Ethyl acetate and ethanol were treated in the same way. The extract was dried in a hot air oven at 40–45 °C until a solid or semi-solid mass was obtained, and it was then kept in a refrigerator at 8 °C in an airtight container. During the experiment, the suspension was prepared in saline each day for 24 days. Preliminary assessment of extraction methods indicated that the ethyl acetate is the best solvent for extraction of bioactive molecules from the honey. Thus, we used ethyl acetate extract for the subsequent intraperitoneal treatment. A dose of 2 mg/kg/b.wt of bee honey extract was chosen based on the time-and dose-dependent curve.
2.2. Chemicals
Sigma Chemicals Co., Ltd. (St. Louis, MO, USA) provided cyclosporine A (CsA)—Sandimmune®—injection and streptozotocin (STZ). Bioassay Technology Laboratory (Harborne, UK), Insulin (glargine; Lantus, Sanofi-Aventis, Bridgewater, NJ, USA) and Life TechnologiesTM (BioSource, Nivelles, Belgium) provided the MMP-9 and TIMP-1 assay kits. Sigma Co., Ltd. supplied all the other chemicals used in this study.
2.3. Animals
Fifty adult male Swiss albino mice weighing (21–26 g) were obtained from King Abdulaziz University’s animal house in Jeddah, Saudi Arabia. The mice were housed in a pathogen-free environment at the Faculty of Medicine, Al-Baha University, Saudi Arabia, with a temperature of 22 ± 2 °C and a 12/12-h light/dark cycle. They had unlimited access to food and water. The mice were kept in the laboratory for seven days prior to the experiment to acclimate them and rule out any illness. All the procedures involving animals and their care adhered to institutional guidelines while complying with the national and international legal guidelines governing the care and use of laboratory animals. Approval for the research study was obtained from Al-Baha University (Ethical approval No. (REC/PEA/BU-FM/2023/22. Pr. No. 5/1437).
2.4. Induction of Autoimmune Type 1 DM
Cold 0.01 M citrate buffer, pH 4.5, freshly prepared for use, was used to dissolve the STZ. CsA (20 mg/kg/day, s.c.) was administered to mice in groups III, IV, and V daily for 10 consecutive days [23]. This was followed by intraperitoneal multiple low doses of streptozotocin (MLDSTZ) (30 mg/kg/day) for five consecutive days. Blood samples were taken via tail bleeding in heparinized tubes twice a week. Random glucose concentrations in the plasma samples were identified using a one-touch ultra-2 glucometer (LifeScan Europe, 6300, Zug, Switzerland) and suitable blood glucose strips. When their random blood glucose levels (BGL) exceeded 200 mg/dL in two successive readings, the mice were considered diabetic [24].
2.5. Study Design
Seven days after the last dose of MLDSTZ administration, mice with fasting BGLs of more than 200 mg/dL were selected as diabetic groups III, IV, and V. Ten normal mice were assigned as negative control group (group I), and group II was treated with 2 mg/kg/b.wt of honey extract only for twelve intraperitoneal injections every other day. The diabetic mice were divided into three equal groups of 10 mice each: the untreated diabetic group (group III) and the diabetic mice (group IV) treated with insulin as a standard treatment (positive control) for twelve injections every other day at a dose of 0.5 U/kg/b.wt. The dosage of insulin administration (range, 0.2 to 1 U/kg/b.wt.) was increased or decreased in response to a combination of measured glucose and body weight. The diabetic mice (group V) were treated with 2 mg/kg/b.wt of honey extract for twelve intraperitoneal injections every other day. The mice were anesthetized with ether at the end of the treatment period, and blood was collected via cardiac puncture. Samples were delivered in plastic tubes and allowed to clot. Subsequently, the samples were centrifuged at 3000 rpm for 5 min to obtain serum, which was then used to calculate glucose levels and other parameters. Each mouse’s pancreas was removed and cut in half longitudinally. One half was thoroughly dried with filter paper before being homogenized in PBS to yield a 10% homogenate. Supernatants were extracted to determine the levels of superoxide dismutase (SOD), reduced glutathione (GSH), and catalase (CAT) [25]. The remaining half of each excised pancreas was fixed in paraffin and set in 10% paraformaldehyde. The sections were stained with hematoxylin and eosin. The morphological changes were evaluated under a microscope (Nikon’s Eclipse 80i, made in Japan), and video cameras were used to record the images (DS-Fi1 digital microscope camera, Nikon, Japan). Each mouse’s liver was removed for the measurement of glycogen content.
2.6. Determination of Glucose Levels
To identify the diabetic mice, blood samples were taken from the tail tip veins seven days after the last dosage of STZ. At the end of the trial, blood samples were taken using a heart puncher by using a colorimetric technique [26].
2.7. Determination of Glycogen Content in Liver Tissue
Using the Glycogen Assay Kit (ab65620), the glycogen content in the livers of groups I, II, III, IV, and V was determined. In the assay, glucoamylase hydrolyzed glycogen to produce glucose, which was then specifically oxidized to produce a product that reacted with the OxiRedTM probe to produce coloration (570 nm) [27].
2.8. Determination of SOD, GSH, and Catalase Activity in Pancreatic Homogenate
The pancreases were finely sliced and homogenized in 10% (w/v) phosphate buffered saline at pH 7.8. The homogenates were centrifuged at 3000 rpm at 4 °C for 20 min using a high-speed cooling centrifuge. The clear supernatant was used to assay the levels of antioxidant activity, including SOD, reduced GSH, and catalase using a spectrophotometer (Philip Harris). SOD activity was measured using Nishikimi et al.’s method [28]. Reduced GSH levels were determined using Prins and Loose’s method [29]. CAT was measured by spectrophotometric assay using the BioVision Catalase Assay Kit. In the assay, catalase first reacts with H₂O₂ to produce water and oxygen. The unconverted H₂O₂ reacts with the OxiRed™ probe to produce a product, which was measured at 570 nm (colorimetric method) [30].
2.9. Determination of the Serum Levels of Matrix Metalloproteinase (MMP-9) and Tissue Inhibitors of Metalloproteinase 1 (TIMP1)
We used a competitive enzyme-linked immunosorbent assay (ELISA) for the quantitative determination of MMP-9 and TIMP-1, which measured the natural and recombinant forms of cytokines (Cytoimmune Sciences Inc., Rockville, MD, USA). For each sample, 100 μL of serum was added to the designated wells. This assay employed a quantitative sandwich [31].
Statistical Analysis
The data were expressed as the mean ± standard error of the mean (SEM). The statistical analysis was conducted using one-way ANOVA (analysis of variance) followed by a post-hoc Tukey’s test for multiple comparisons. Values were considered significant at p < 0.05. SPSS software, version 23, was used.
3. Results
3.1. Effect of Honey Extract on Body Weight
Seven days after the final STZ injection, after day 24, a significant reduction in body weight was evident in the diabetic group III (17.6 ± 0.42 gm) compared to the control group I (26.4 ± 0.55 gm). However, the treated diabetic group IV with insulin exhibit regained body weight significantly (23.4 ± 0.57). Moreover, the treated group V with honey extract utilizes significant regain of body weight (22.8 ± 0.48 gm) compared to the diabetic group III (Table 1). No significant changes were observed in group II, which received honey extract only, compared with group I.

Table 1.
Effect of honey extract 2 mg/kg/b.wt on mice body weight.
3.2. Effects of Honey Extract on Blood Glucose Level (BGLs (mg/dL) and Liver Glycogen (mg/gm Tissue)
The results obtained in Table 2 demonstrated that the diabetic group III exhibited significantly higher BGLs. However, BGLs in the treated group IV and V were much lower (132 ± 6.45, 149 ± 4.63 mg/dL), respectively, than those in group III (233 ± 6.72 mg/dL). Furthermore, the liver glycogen content significantly dropped in the diabetic group III (4.6 ± 0.18 mg/gm tissue) compared to that of group I (7.13 ± 0.16 mg/gm tissue). Nevertheless, treatment with insulin and honey extract in group IV and V significantly restored declined glycogen content to (7.06 ± 0.29, 6.86 ± 0.13 mg/gm tissue), respectively, compared to diabetic group III and showed insignificant changes in comparison with groups I and II.

Table 2.
Effect of honey extract 2 mg/kg/b.wt on blood glucose level and liver glycogen.
3.3. Effects of Honey Extract on SOD (unit/min/mg Protein), Reduced GSH (µmol/gm Wet Tissue) and CAT (µmol/gm Wet Tissue) Activity of Pancreatic Tissue
The activity of SOD, GSH, and CAT in the diabetic group III was significantly reduced compared with the normal group I. Treatment with insulin and honey extract group IV and V revealed a significant amelioration of SOD, GSH and CAT activity compared with diabetic group III. This amelioration of GSH activity was high enough to achieve insignificant changes compared to that in group I, while CAT and SOD enzyme activity were still considerably lower (Table 3).

Table 3.
Effect of the honey extract 2 mg/kg/b.wt on antioxidant activity.
3.4. Effects of Honey Extract on Serum MMP-9 and TIMP-1
A significantly higher MMP-9 and lower TIMP-1 levels were found in the diabetic group III compared with those of group I, while the treated with insulin and honey extract group IV and V demonstrated a significant amelioration compared to the untreated group III (Table 4).

Table 4.
Effect of honey extract 2 mg/kg/b.wt on serum level of MMP-9 and TMP-1.
3.5. Histopathological Examination of the Pancreas
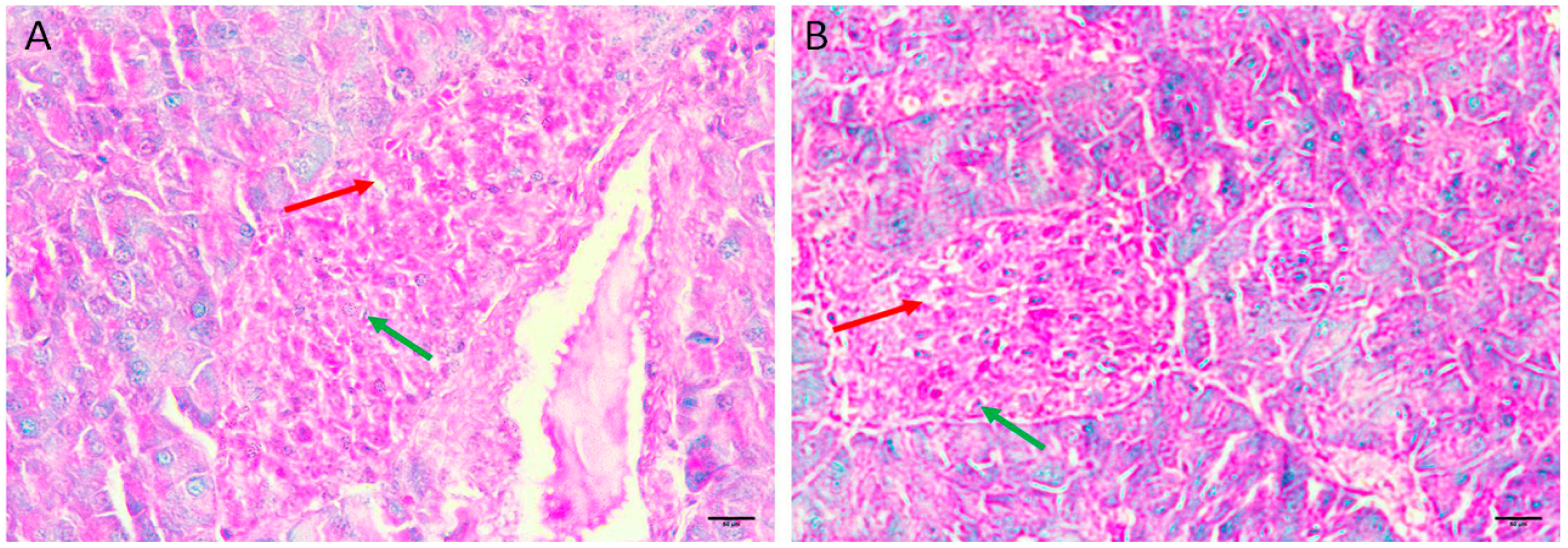
3.5.1. Normal Group I
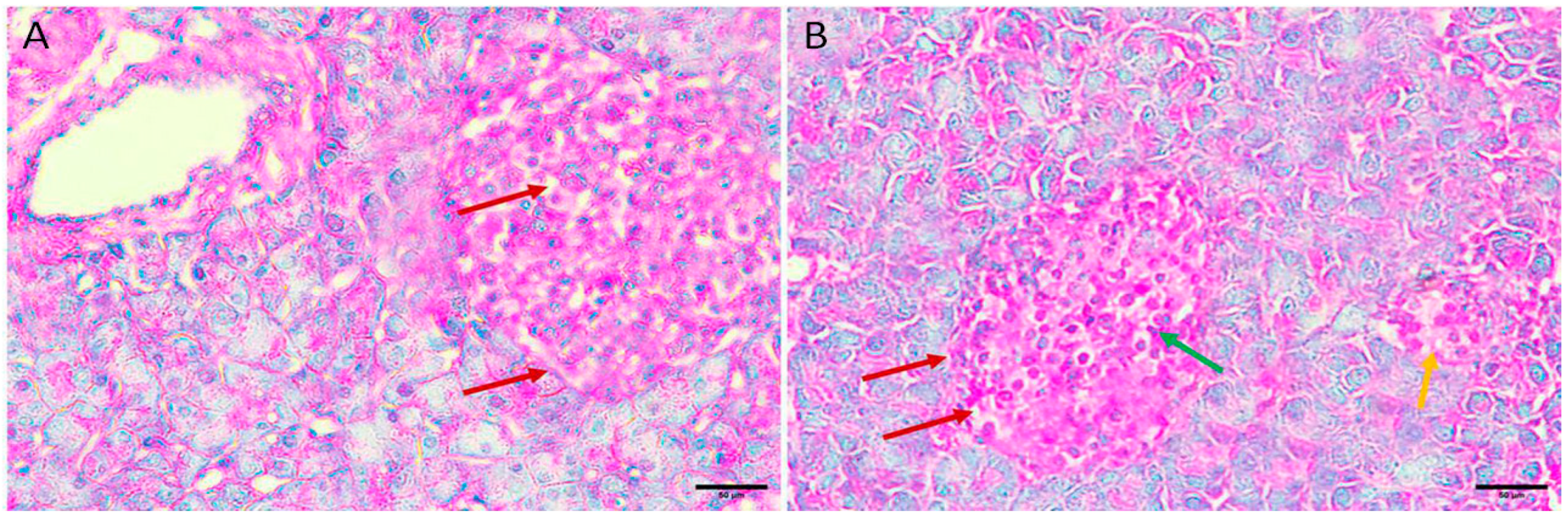
The normal histological structure of islet cells showed no pathological changes. The Langerhans islets were normal in size, distribution, and number and showed pale, rounded, and ovoid β-cells in the center (Figure 1A,B).
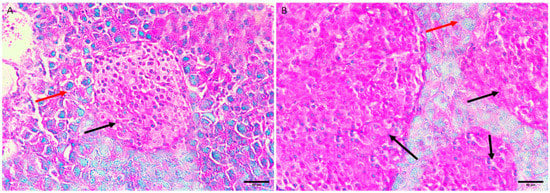
Figure 1.
Pancreas histopathology. (A,B) Normal negative control mouse pancreas showing normal sized, distribution, and number islets of Langerhans with pale, rounded, and ovoid β-cells in the center (black arrow), embedded in exocrine portion of pancreas (red arrow) (H&E, ×400).
3.5.2. Honey Extract Treated Group II
The histological structure of islet cells exhibits no pathological changes. The Langerhans islets were normal in size, distribution, and number and showed pale, rounded, and ovoid β-cells in the center (Figure 2A,B).
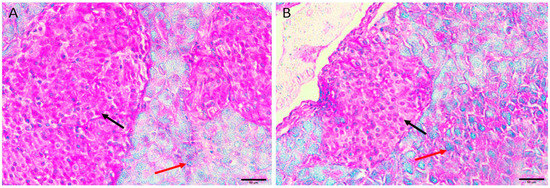
Figure 2.
Pancreas histopathology. (A,B) Normal mouse treated honey extract 2 mg/kg/b.wt showing normal sized, distribution, and number islets of Langerhans with pale, rounded, and ovoid β-cells in the center (black arrow), embedded in exocrine portion of pancreas (red arrow) (H&E, ×400).
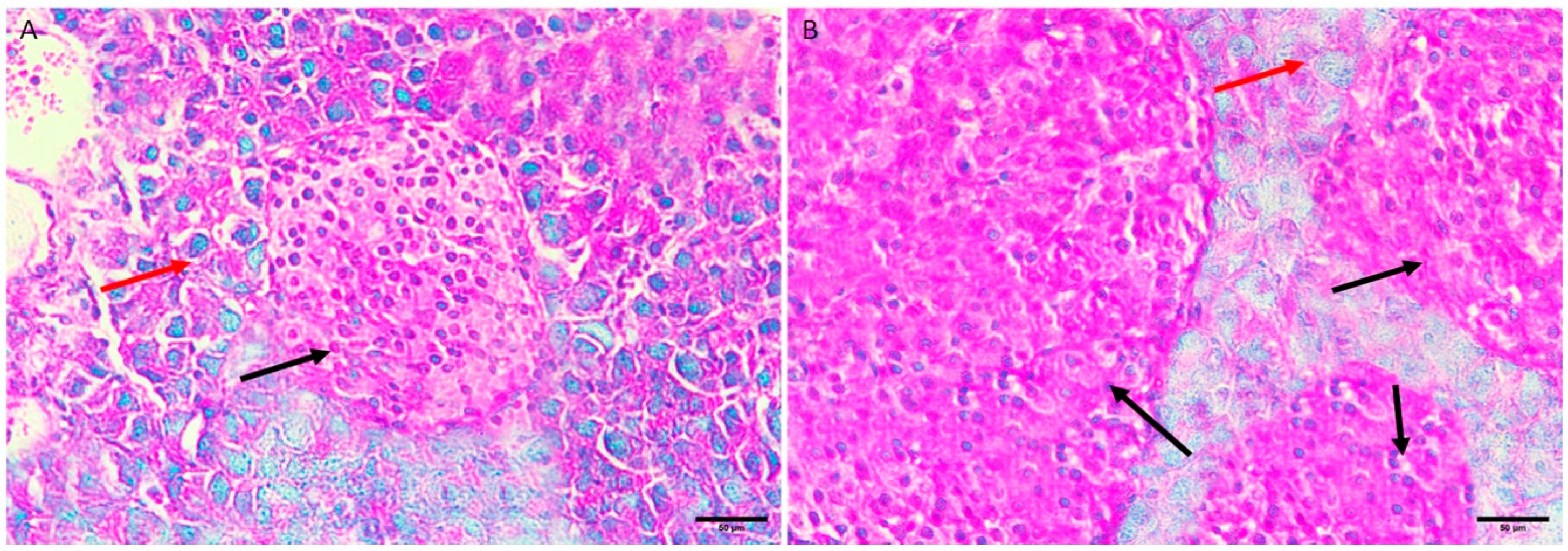
3.5.3. Diabetic Group III
Langerhans islets showed severe destruction, appearing as shrinkage and shape distortion, with marked edema. There was also degeneration and necrosis of component cells, where the nucleus appeared densely basophilic. A noticeable inflammatory infiltration, primarily made up of lymphocytes and plasma cells, was observed (Figure 3A,B).
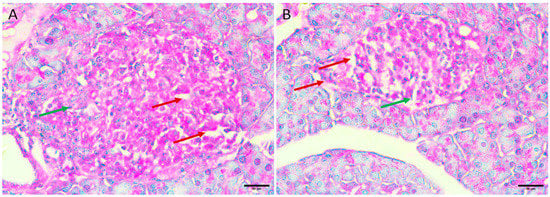
Figure 3.
Pancreas histopathology. (A,B) Diabetic mouse pancreas showing shrinkage and shape distortion of islets of Langerhans with marked oedema (dark red arrow) and degeneration and necrosis of components cells where its nucleus appeared densely basophilic (green arrow) (H&E, ×400).
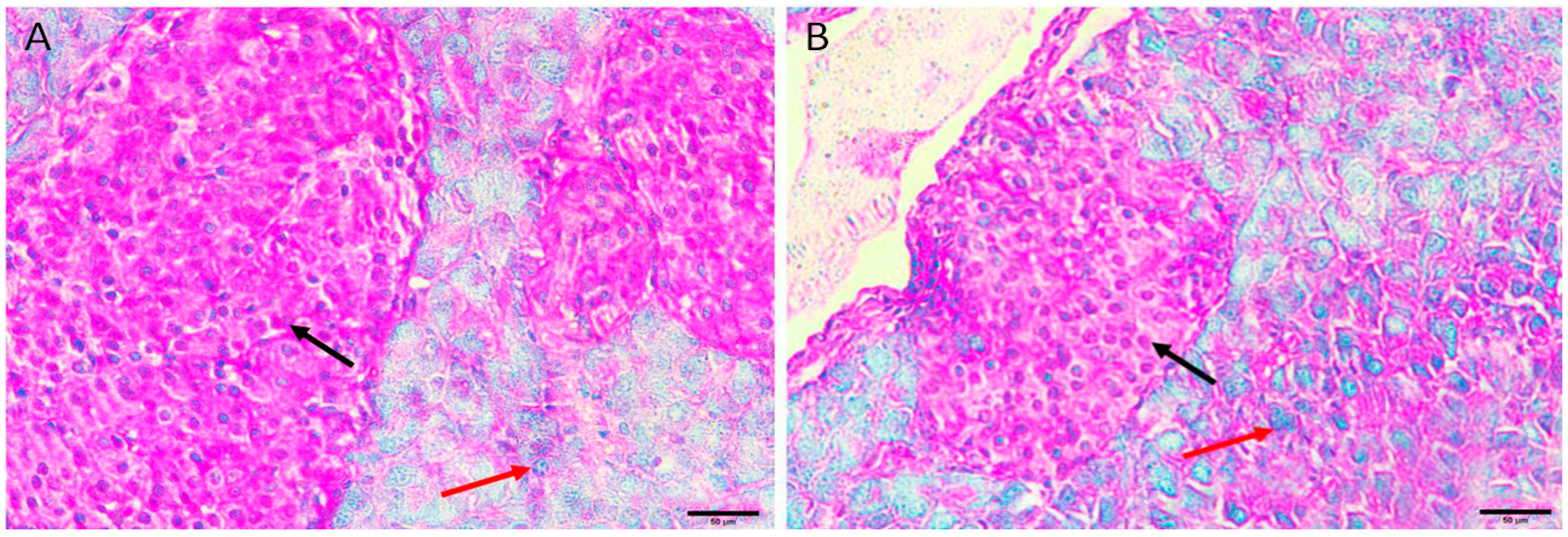
3.5.4. Diabetic Mice Treated with Insulin 0.5 U/kg/b.wt in Group IV
Diabetic mice treated with insulin, the pancreas showing restoration of the normal size and contour of islets of Langerhans with less oedema and less necrotic cells (Figure 4A,B).
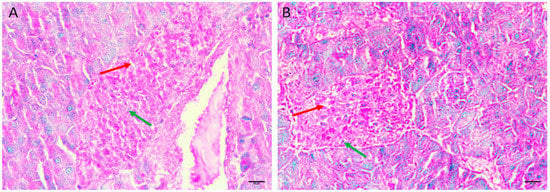
Figure 4.
Pancreas histopathology. (A,B) Diabetic mice treated with insulin, the pancreas showing restoration of the normal size and contour of islets of Langerhans with less oedema (dark red arrow) and less necrotic cells (green arrow). (H&E, ×400).
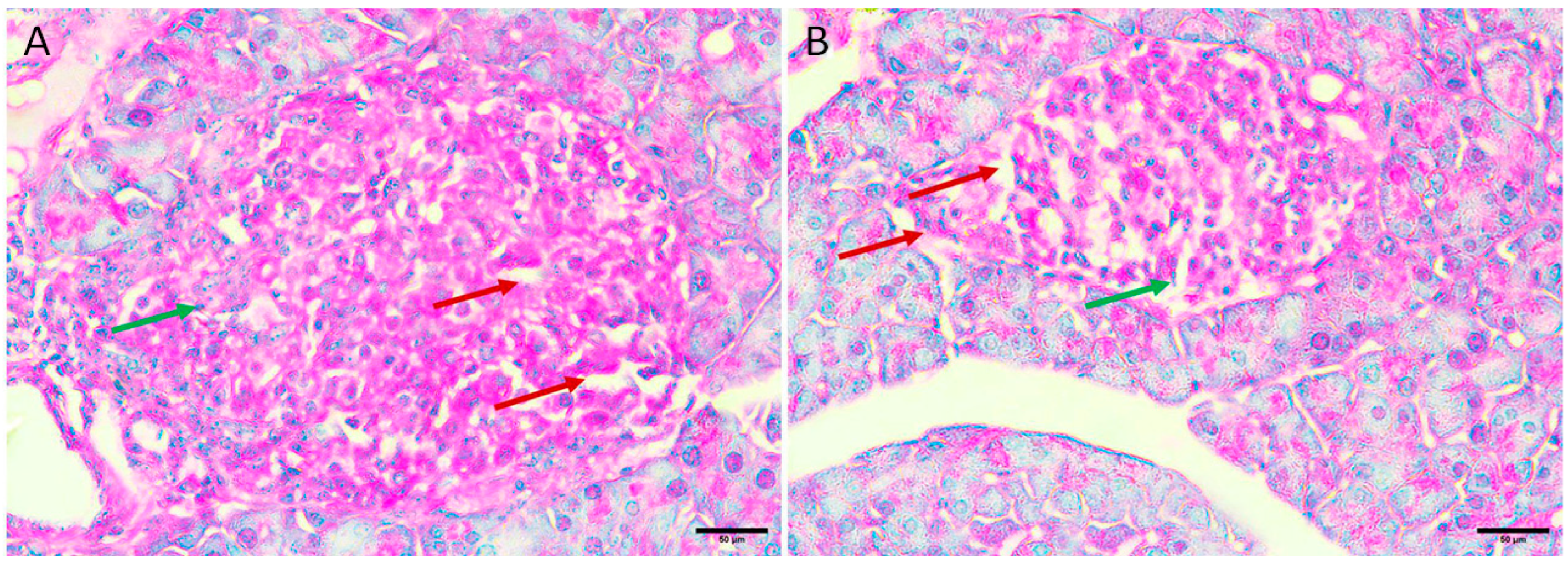
3.5.5. Diabetic Mice Treated with the Honey Extract 2 mg/kg/b.wt in Group V
Regenerative changes appeared as the normal size and contour of the Langerhans islets were restored, and edema was reduced. In addition, there were fewer necrotic cells and small regenerative islets (Figure 5A,B).
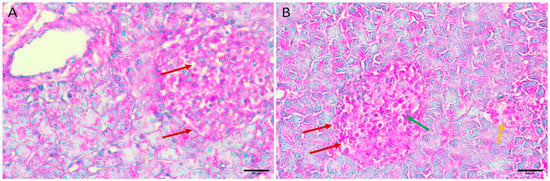
Figure 5.
Pancreas histopathology. (A,B) Diabetic mouse treated honey extract 2 mg/kg/b.wt pancreas showing restoration of the normal size and contour of islets of Langerhans with less oedema (dark red arrow), less necrotic cells (green arrow), and small regenerative islets (orange arrow) (H&E, ×400).
4. Discussion
DM is a condition characterized by insulin resistance or insufficiency, which causes an increase in BGLs. According to previous research, there have been 366 million verified cases of DM, and this number will surpass 500 million by 2030 [32]. Over the last decade, the occurrence of DM has significantly increased in developed countries. Moreover, this disease may spread and become a serious threat in the US, the Middle East, Africa, and Latin America in the future [33].
Honey comprises more than 200 ingredients, including fructose, glucose, and water. When honey was first utilized in folk medicine in ancient times, its medicinal advantages were entirely based on observation and lacked scientific support. The scientific community has only recently become interested in researching and describing the advantages of honey. These studies have mainly explained the medicinal benefits of honey, including its therapeutic effects on inflammation, diabetes, and oxidative stress. The contribution of the immune system to the development of type 1 diabetes mellitus (T1DM) is widely acknowledged by several theories.
In the current study, a model of type 1 autoimmune diabetes with an immune-mediated component was induced by MLDSTZ after CsA administration. This was supported by biochemical testing and histopathological analysis of pancreatic tissues from diabetic mice in group III, which revealed moderate insulitis. The tested mice were healthy and free of any underlying immunological disorders that could have impeded the experimental process.
To investigate the effects and mechanism of honey extract, we conducted an in vivo experiment with the induction of diabetes in mice. Our findings indicated that the honey extract intraperitoneally injected at 2 mg/kg/b.wt, day after day, was beneficial for the mice. After the treatment period, the diabetic group V treated with honey extract exhibited significant body weight increases compared to the diabetic group III (Table 1), indicating a noticeable weight gain induced by the honey extract. Furthermore, the honey extract demonstrated antidiabetic activity, as evidenced by a significant reduction in BGL in group V 149 ± 4.63 compared to that in the diabetic group III (Table 2). These findings are consistent with those of Meo et al., who conducted several studies on honey and DM in both animals and humans, and reported that honey ameliorates BGL in people with diabetes [34].
Additionally, according to Reddy et al. honey’s efficacy is based on its nature and composition. Honey was found to improve BGL and body weight, whether taken alone or in conjunction with dietary supplements [35]. Moreover, consistent with the review by Bobiş et al. the findings point to honey as a potential medication that will help regulate blood glucose levels and prevent other associated disorders [16]. Moreover, Syarifuddin et al. demonstrated that different varieties of honey mitigate BGLs during pregnancy [36]. The diabetes model applied in this study had many benefits. First, the lesion of pancreatic islets in the model resembled those found in clinically diagnosed type 1 DM [37,38].
The results showed that the CsA/MLDSTZ co-treatment constituted an immune-mediated type 1 autoimmune DM. Hepatic glycogen levels, which serve as the biological indicator of insulin action, support this conclusion [39]. This was accomplished with BGL decreases in group V, which was treated with honey extract, compared to the significant increases in untreated group III. The results also revealed a significant reduction in hepatic glycogen levels (4.6 ± 0.18) in the diabetes-induced mice in group III (Table 1). The administration of honey extract appears to have the potential to improve insulin levels or sensitivity, as evidenced by the improvement in glycogen content to 6.86 ± 0.13 in the treated group V, as shown in Table 2. Cellular damage may result from oxidant stress, a crucial element in the pathogenesis of diabetes [40]. The body creates more free radicals when hyperglycemic, which might lead to an increase in oxidative stress and an increase in the likelihood of vascular problems [41].
The current study examined the effects of honey extract in mice with T1DM more closely by assessing the antioxidant activity. In our experiment, the administration of 2 mg/kg/b.wt honey extract to group V significantly increased the SOD, GSH, and CAT activities in the T1DM mice after 24 days, which were significantly decreased in the diabetic group III (Table 3). The amelioration of the activities of those enzymes can efficiently reduce oxidative stress, indicating that the utilization of honey extract enhanced the power of scavenging ROS. In addition, honey extract has significant antioxidant properties and is high in polyphenols, which are thought to be one of the potential mechanisms for protecting enzyme activity. However, in the current study, the diabetes-induced mice in group V treated with honey extract expressed higher SOD, GSH, and CAT activities. Moreover, other studies have reported similar results [42,43].
The findings of the present study showed significantly increased serum MMP-9 levels in mice with diabetes compared to those in normal mice in group I, mainly due to the triggering of more ROS production by hyperglycemia, which then activates the pathway that increases the expression of MMP-9. Although research has shown that hyperglycemia can affect many intracellular signaling processes, whether these changes are related to vascular disease is still unknown. In T1DM animal models, serum MMP-9 activity was assessed. Therefore, plasma MMP-9 levels could serve as a reasonable indicator of the intensity and stability of diabetic plaques, considering the growing evidence for their involvement in the development of atherogenesis. This is in agreement with Yildirim et al. who discovered that diabetes patients’ plasma had much higher levels of MMP-9 than in the normal group [44].
In the present study, the administration of 2 mg/kg/b.wt honey extract to group V significantly reduced serum MMP-9 activity, either directly or through the improvement of BGLs or antioxidant enzyme activities. Cardiovascular diseases are the primary cause of mortality globally; thus, mechanism may significantly reduce the frequently occurring cardiovascular complications associated with diabetes.
TIMPs regulate MMPS activation and have a stronger affinity for MMP-9 than any other MMP [45]. TIMP-1 level estimation is a marker for identifying the aberrant regulation of tissue remodeling (TIMP-1a) [46]. In our study, T1DM, either directly or indirectly through oxidation or inflammatory changes, significantly decreased serum TIMP-1 levels. This finding is supported by Kowluru, who found that the gene expression of TIMP-1 in the retina was reduced by about 35% compared with the results of control rats [47]. However, Maxwell et al. noted that plasma TIMP-1 concentrations were noticeably increased in people with diabetics compared to controls. Treatment with honey extract helped slow down this reduction, although it was still noticeably lower than in normal mice [48].
In the current study, pancreas histopathology in group I and treated honey extract only in group II revealed normal-sized, distributed, and numbered islets of Langerhans with pale, rounded, and ovoid β-cells in the center (Figure 1A,B and Figure 2A,B). In contrast, the diabetic mice in group III became more condensed, mainly in the perivascular area, showing shrinkage and shape distortion of islets of Langerhans with marked edema and degeneration and necrosis of components cells where its nucleus appeared densely basophilic (Figure 3A,B). Group IV diabetic mice treated with insulin at a dose of 0.5 U/kg/b.wt as a standard treatment (positive control) showing restoration of the normal size and contour of islets of Langerhans with less oedema and necrotic cells (Figure 4A,B). The histopathological outcomes confirmed the efficacy of the honey extract in treating T1DM group V, the changes appeared in the form of the renovation of normal islet cell construction, an increase in the number of reformative cells, a slight disappearance of glycogenic vacuolization, and the minimization of edematous stroma (Figure 5A,B). In addition, the utilization of honey extract in group V led to decreased cellular infiltration compared to group III. Honey extract treatment showed marked regenerative changes in the form of the complete restoration of the normal architecture of islet cells and their numbers along the cut sections. Only a few lymphocytic infiltrates were observed in the perivascular area. These findings support those of El-Kordy and Alshahrani, who demonstrated, through histological analysis, that genistein greatly reduced cell loss and enhanced glucose and insulin levels in diabetic mice [49].
5. Conclusions
Considering the results of this study, we conclude that honey extract is a potential antidiabetic agent. The administration of honey improved type 1 DM features by downregulating proinflammatory MMP-9 levels in association with the elevation of TIMP-1 levels. Moreover, the administration of honey improved the antioxidant activities of pancreatic tissue, attenuated BGLs, and completely restored islet cells. Therefore, treatment with honey has the potential to resolve, assist, and ameliorate T1DM severity. Future experimental studies, clinical trials, and innovative research activities are recommended to further determine the specific clinical effects of honey on the treatment of T1DM.
Author Contributions
Methodology, A.H.A., I.M.S., S.S., A.H.K. and M.F.E.-R.; Investigation, S.S. and A.H.K.; Resources, A.H.A.; Data curation, I.M.S. and M.F.E.-R.; Writing—original draft, M.F.E.-R. and I.M.S.; Writing—review & editing, A.H.A. and I.M.S.; Visualization, I.M.S. All authors have read and agreed to the published version of the manuscript.
Funding
This study was sponsored by Deanship of Scientific Research in Al Baha University, Kingdom of Saudi Arabia, for their financial and logistical support and for providing necessary guidance concerning project implementation. Project No: 5/1437.
Institutional Review Board Statement
This study complied with the Scientific Research and Ethics committee Guidelines. Ethical approval committee of faculty of Medicine, Al-Baha University, approval number (REC/PEA/BU-FM/2023/22).
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, El-Refaei, M.F. The data are not publicly available for the time being and will be available in demand.
Acknowledgments
Authors would like to thank Ihab S. Atta. Faculty of Medicine, Al-Azhar University for his assistance in the histopathological part of this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rowley, W.R.; Bezold, C.; Arikan, Y.; Byrne, E.; Krohe, S. Diabetes 2030: Insights from Yesterday, Today, and Future Trends. Popul. Health Manag. 2017, 20, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Fan, W. Epidemiology in diabetes mellitus and cardiovascular disease. Cardiovasc. Endocrinol. 2017, 6, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Asif, M. The prevention and control the type-2 diabetes by changing lifestyle and dietary pattern. J. Educ. Health Promot. 2014, 3, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, J.; Zhang, B.; Li, X.; Li, Y. Diabetes Mellitus and Cause-Specific Mortality: A Population-Based Study Diabetes. Metab. J. 2019, 43, 319–341. [Google Scholar] [CrossRef]
- Zargar, A.H.; Wani, A.I.; Masoodi, S.R.; Laway, B.A.; Bashir, M.I. Mortality in diabetes mellitus—Data from a developing region of the world. Diabetes Res. Clin. Pract. 1999, 43, 67–74. [Google Scholar] [CrossRef]
- Pandey, A.; Tripathi, P.; Pandey, R.; Srivatava, R.; Goswami, S. Alternative therapies useful in the management of diabetes: A systematic review. J. Pharm. Bioallied Sci. 2011, 3, 504–512. [Google Scholar]
- Li, T.; Li, X.; Feng, Y.; Dong, G.; Wang, Y.; Yang, J. The Role of Matrix Metalloproteinase-9 in Atherosclerotic Plaque Instability. Mediat. Inflamm. 2020, 2020, 3872367. [Google Scholar] [CrossRef]
- Uemura, S.; Matsushita, H.; Li, W.; Glassford, A.J.; Asagami, T.; Lee, K.H.; Harrison, D.G.; Tsao, P.S. Diabetes Mellitus Enhances Vascular Matrix Metalloproteinase Activity: Role of Oxidative Stress. Circ. Res. 2001, 88, 1291–1298. [Google Scholar] [CrossRef]
- Cabral-Pacheco, G.A.; Garza-Veloz, I. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Papazafiropoulou, A.K.; Tentolouris, N. Matrix metalloproteinases and cardiovascular diseases. Hippokratia 2009, 13, 76–82. [Google Scholar]
- Ayuk, S.M.; Abrahamse, H.; Houreld, N.N. The Role of Matrix Metalloproteinases in Diabetic Wound Healing in relation to Photobiomodulation. J. Diabetes Res. 2016, 2016, 2897656. [Google Scholar] [CrossRef] [PubMed]
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and Health: A Review of Recent Clinical Research. Pharmacogn. Res. 2017, 9, 121–127. [Google Scholar]
- Owen, R.E. Geographical, Entomological and Botanical Origins of Honey. In Honey—Composition and Properties; Intech: London, UK, 2022; p. 106414. [Google Scholar]
- Mureșan, C.I.; Cipcigan, M.C.; Suharoschi, R.; Erler, S.; Mărgăoan, R. Honey botanical origin and honey-specific protein pattern: Characterization of some European honeys. LWT 2022, 154, 112883. [Google Scholar] [CrossRef]
- Omotayo, E.O.; Gurtu, S.; Sulaiman, S.A.; Wahab, M.S.; Sirajudeen, K.N.S.; Salleh, S. Hypoglycemic and Antioxidant Effects of Honey Supplementation in Streptozotocin-induced Diabetic Rats. Int. J. Vitam. Nutr. Res. 2013, 80, 1. [Google Scholar] [CrossRef] [PubMed]
- Bobiş, O.; Dezmirean, D.S.; Moise, A.R. Honey and Diabetes: The Importance of Natural Simple Sugars in Diet for Preventing and Treating Different Type of Diabetes. Oxid. Med. Cell. Longev. 2018, 2018, 4757893. [Google Scholar] [CrossRef]
- Erejuwa, O.O.; Sulaiman, S.A.; Wahab, M.S. Honey—A Novel Antidiabetic Agent. Int. J. Biol. Sci. 2012, 8, 913–934. [Google Scholar] [CrossRef]
- Turski, M.P.; Turska-Kozłowska, M.; Zgrajka, W.; Turski, W.A. Presence of kynurenic acid in food and honeybee products Amino Acids. Amino Acids 2008, 36, 75–80. [Google Scholar] [CrossRef]
- Sahin, H.; Kolayli, S.; Beykaya, M. Investigation of Variations of Invertase and Glucose Oxidase Degrees against Heating and Timing Options in Raw Honeys. J. Chem. 2020, 2020, 5398062. [Google Scholar] [CrossRef]
- Tashkand, H. Honey in wound healing: An updated review. Open Life Sci. 2021, 16, 1091–1100. [Google Scholar] [CrossRef]
- Azman, K.F.; Zakaria, R. Honey as an antioxidant therapy to reduce cognitive ageing. Iran. J. Basic Med. Sci. 2019, 22, 1368–1377. [Google Scholar]
- Mohapatra, D.P.; Thakur, V.; Brar, S.K. Antibacterial Efficacy of Raw and Processed Honey. Biotechnol. Res. Int. 2011, 2011, 917505. [Google Scholar] [CrossRef]
- Helmy, M.H.; Helmy, M.M.; El-Mas, M.M. Enhanced lipoxygenase/LTD4 signaling accounts for the exaggerated hypertensive and nephrotoxic effects of cyclosporine plus indomethacin in rats. Biomed. Pharmacother. 2018, 102, 309–316. [Google Scholar] [CrossRef]
- Mathews, C.E.; Xue, S.; Posgai, A.; Lightfoot, Y.L.; Li, X.; Lin, A.; Wasserfall, C.; Haller, M.J.; Schatz, D.; Atkinson, M.A. Acute Versus Progressive Onset of Diabetes in NOD Mice: Potential Implications for Therapeutic Interventions in Type 1. Diabetes 2015, 64, 3885–3890. [Google Scholar] [CrossRef]
- Almilaibary, A.; Abdallah, E.A.A.; El-Refaei, M.F. Fagonia indica attenuates chromium-induced nephrotoxicity via antioxidant and anti-inflammatory activities in mice. Heliyon 2022, 27, e10373. [Google Scholar] [CrossRef]
- Singleton, J.R.; Smith, A.G.; Bromberg, M.B. Increased prevalence of impaired glucose tolerance in patients with painful sensory neuropathy. Diabetes Care 2001, 24, 1448–1453. [Google Scholar] [CrossRef]
- Xirouchaki, C.E.; Mangiafico, S.P.; Bate, K.; Ruan, Z.; Huang, A.M.; Tedjosiswoyo, B.W.; Lamont, B.; Pong, W.; Favaloro, J.; Blair, A.R.; et al. Impaired glucose metabolism and exercise capacity with muscle-specific glycogen synthase 1 (gys1) deletion in adult mice. Mol. Metab. 2016, 5, 221–232. [Google Scholar] [CrossRef] [PubMed]
- Nishikimi, M.; Rao, N.A.; Yagi, K. The occurrence of superoxide anion in the reaction ofreduced phenazinemethosulfate and molecular oxygen. Biochembiophys. Res. Commun. 1972, 46, 849–854. [Google Scholar] [CrossRef] [PubMed]
- Prins, G.; Loose, J. Glutathione. In Biochemical Methods in Red Cell Genetics; Yunis, J.J., Ed.; Academic Press: New York, NY, USA, 1969; pp. 126–129. [Google Scholar]
- Walton, P.A.; Pizzitelli, M. Effects of peroxisomal catalase inhibition on mitochondrial function. Front. Physiol. 2012, 3, 108. [Google Scholar] [CrossRef] [PubMed]
- El-Refaei, M.F.; El-Naa, M.M. Inhibitory effect of caffeic acid phenethyl ester on mice bearing tumor involving angiostatic and apoptotic activities. Chemico-Biol. Interact. 2010, 186, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Alhomayani, F.K.H.; Alotibi, Y.Z.M.; NasserAlharbi, A.A.; Alsuwat, H.A.M.; Altowairqi, M.H.A.; Alotaibi, H.A.A. Knowledge and attitude toward diabetes mellitus complications in Saudi Arabia; a systematic review. Int. J. Med. Dev. Ctries. 2020, 4, 498–503. [Google Scholar] [CrossRef]
- Azar, W.S.; Njeim, R.; Fares, A.H.; Azar, N.S.; Azar, S.T.; El-Sayed; Eid, A.A. COVID-19 and diabetes mellitus: How one pandemic worsens the other. Rev. Endocr. Metab. Disord. 2020, 21, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Meo, S.A.; Al-Asiri, S.A.; Mahesar, A.; Ansari, M.J. Honey and diabetes mellitus: Obstacles and challenges—Road to be repaired. Saudi J. Biol. Sci. 2017, 24, 1030–1033. [Google Scholar] [CrossRef]
- Reddy, S.H.; Alhabsi, F.S.; Aldohli, H.M.; Almusallami, S.T.; Al Sharji, W.H. A comparative study on the role of Omani honey with various food supplements on diabetes and wound healing. J. King Saud Univ. Sci. 2020, 32, 2122–2128. [Google Scholar]
- Syarifuddin, S.; Hadju, H.; Inriasari, R. Effect of Honey Variation on Blood Glucose Level in Pregnant Wistar Rats (Rattus norvegicus). Maced. J. Med. Sci. 2020, 25, 98–103. [Google Scholar] [CrossRef]
- Somoza, N.; Vargas, F.; Roura-Mir, C.; Vives-Pi, M.; Fernández-Figueras, M.T.; Ariza, A.; Gomis, R.; Bragado, R.; Marti, M.; Jaraquemada, D. Pancreas in recent onset insulin-dependent diabetes mellitus. J. Immunol. 1994, 15, 1360–1377. [Google Scholar] [CrossRef]
- Serreze, D.V.; Gaskins, H.R.; Leiter, E.H. Defects in the differ- entiation and function of antigen presenting cells in NOD/Lt mice. J. Immunol. 1993, 150, 2534–2543. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, W.; Zhao, H.; Guo, S.; Wang, Q.; Cheng, N.; Bai, N. Protective Mechanism of Fagopyrum esculentum Moench. Bee Pollen EtOH Extract Against Type II Diabetes in a High-Fat Diet/Streptozocin-Induced C57BL/6J Mice. Front. Nutr. 2022, 9, 925351. [Google Scholar] [CrossRef] [PubMed]
- Papachristoforou, E.; Lambadiari, V.; Maratou, E.; Makrilakis, K. Association of Glycemic Indices (Hyperglycemia, Glucose Variability, and Hypoglycemia) with Oxidative Stress and Diabetic Complications. J. Diabetes Res. 2020, 2020, 7489795. [Google Scholar] [CrossRef] [PubMed]
- Rumpagaporn, P.; Reuhs, B.L.; Kaur, A.; Patterson, J.A.; Keshavarzian, A.; Hamaker, B.R. Structural features of soluble cereal arabinoxylan fibers associated with a slow rate of in vitro fermentation by human fecal microbiota. Carbohydr. Polym. 2015, 130, 191–197. [Google Scholar] [CrossRef]
- Galal, R.M.; Zaki, H.F.; Seif El-Nasr, M.M.; Agha, A.M. Potential protective effect of honey against paracetamol-induced hepatotoxicity. Arch. Iran. Med. 2012, 15, 674–680. [Google Scholar]
- Kaya, E.; Yılmaz, S.; Ceribasi, S. Protective role of propolis on low and high dose furan-induced hepatotoxicity and oxidative stress in rats. J. Vet. Res. 2019, 63, 423–431. [Google Scholar] [CrossRef]
- Yildirim, N.; Sahin, A.; Erol, N.; Kara, S.; Uslu, S.; Topbas, S. The relationship between plasma MMP-9 and TIMP-2 levels and intraocular pressure elevation in diabetic patients after intravitreal triamcinolone injection. J. Glaucoma 2008, 17, 253–256. [Google Scholar] [CrossRef] [PubMed]
- Brew, K.; Dinakarpandian, D.; Nagase, H. Tissue inhibitors of metalloproteinases: Evolution, structure and function. Biochim. Biophys. Acta 2000, 1477, 267–283. [Google Scholar] [CrossRef]
- Papadopoulou-Marketou, N.; Whiss, P.A.; Eriksson, A.C.; Hyllienmark, L.; Papassotiriou, I.; Wahlberg, J. Plasma levels of tissue inhibitor of metalloproteinase-1 in patients with type 1 diabetes mellitus associate with early diabetic neuropathy and nephropathy. Diab. Vasc. Dis. Res. 2021, 18, 14791641211002470. [Google Scholar] [CrossRef]
- Kowluru, R.A. Role of matrix metalloproteinase-9 in the development of diabetic retinopathy and its regulation by H-Ras. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4320–4326. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, P.R.; Timms, P.M.; Chandran, S.; Gordon, D. Peripheral blood level alterations of TIMP-1, MMP-2 and MMP-9 in patients with Type 1 diabetes. Diabet. Med. 2001, 18, 777–780. [Google Scholar] [CrossRef] [PubMed]
- EL-Kordy, E.; Alshahrani, A.M. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. J. Microsc. Ultrastruct. 2015, 3, 108–119. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).