Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties
Abstract
1. Introduction
2. Materials and Methods
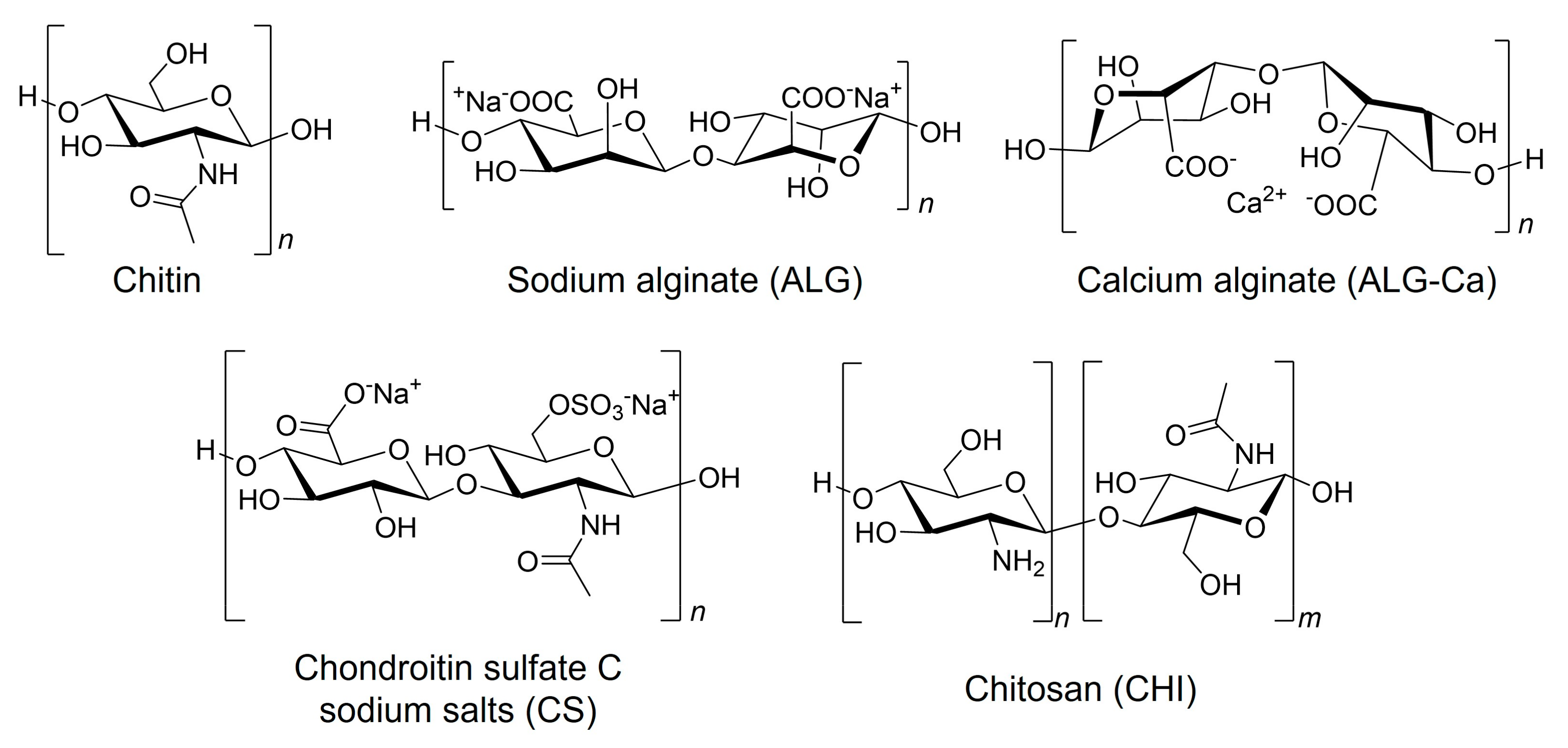
2.1. Materials
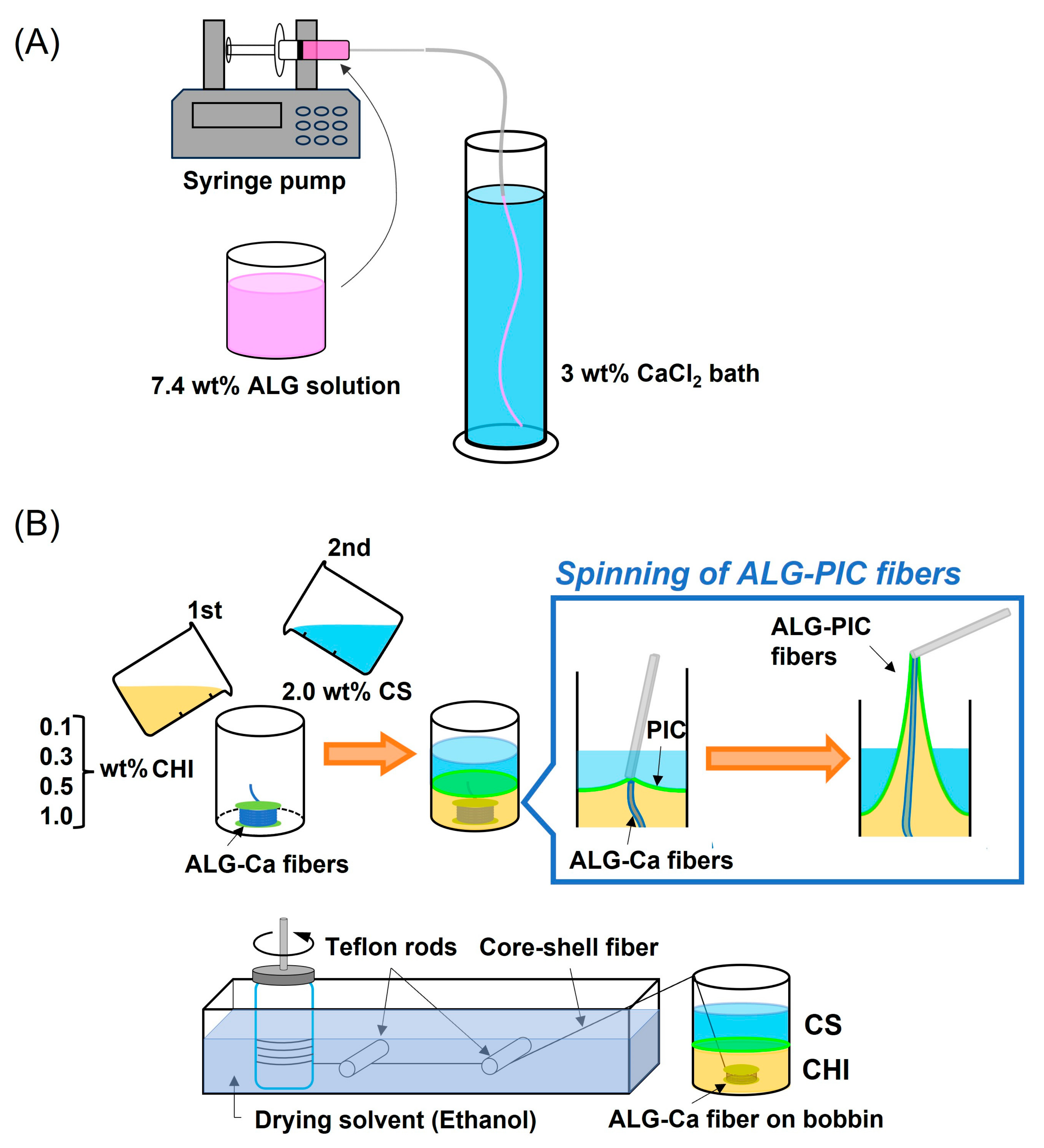
2.2. Preparation of ALG-Ca Fibers
2.3. Preparation of ALG-PIC Fibers
2.4. Preparation of FITC-Conjugated BSA (FITC-BSA)
2.5. Fabrication of FITC-BSA-Loaded ALG-Ca Fibers and FITC-BSA-Loaded ALG-PIC Fibers
2.6. Characterization
2.7. Release Experiments
3. Results
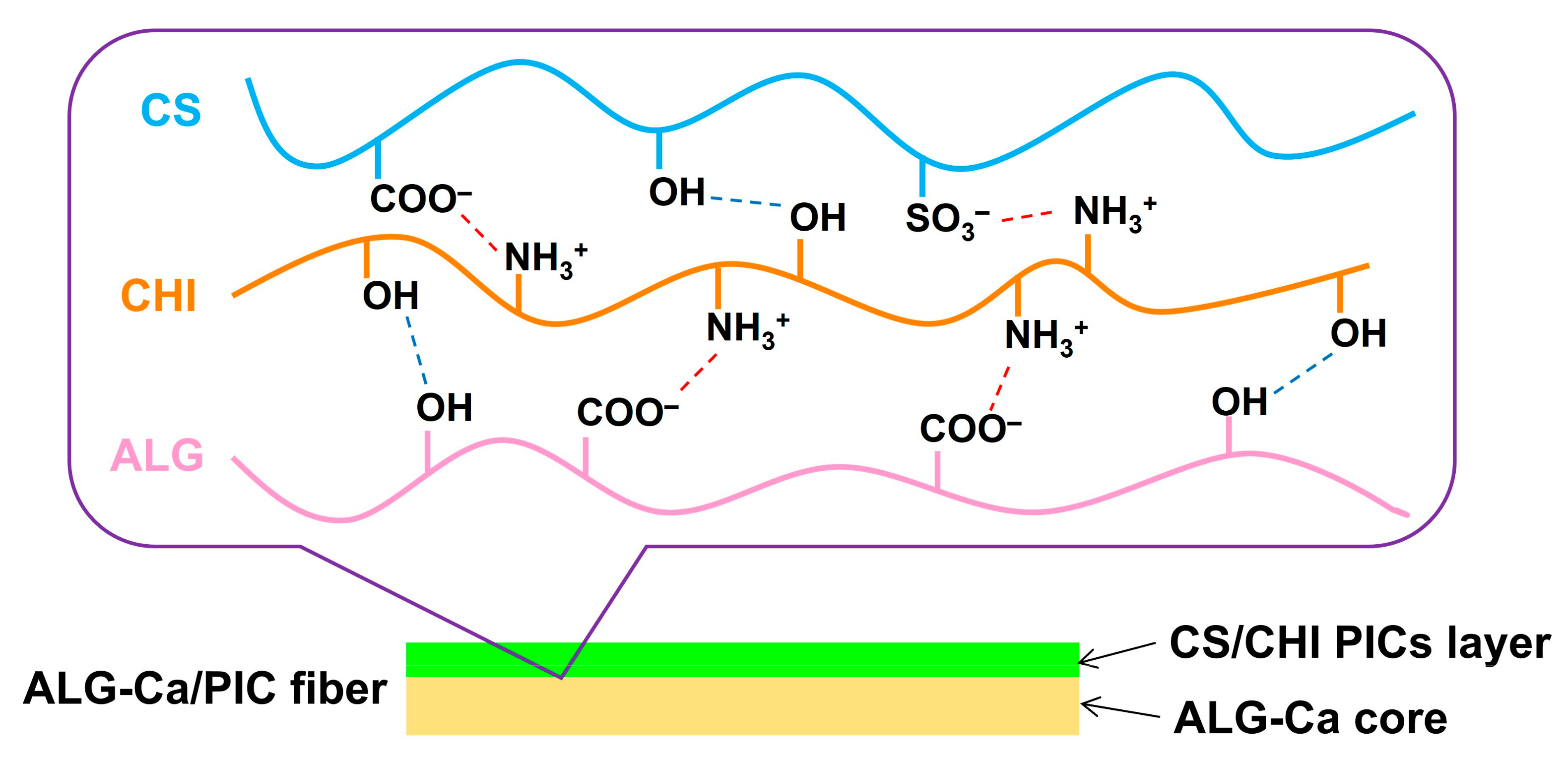
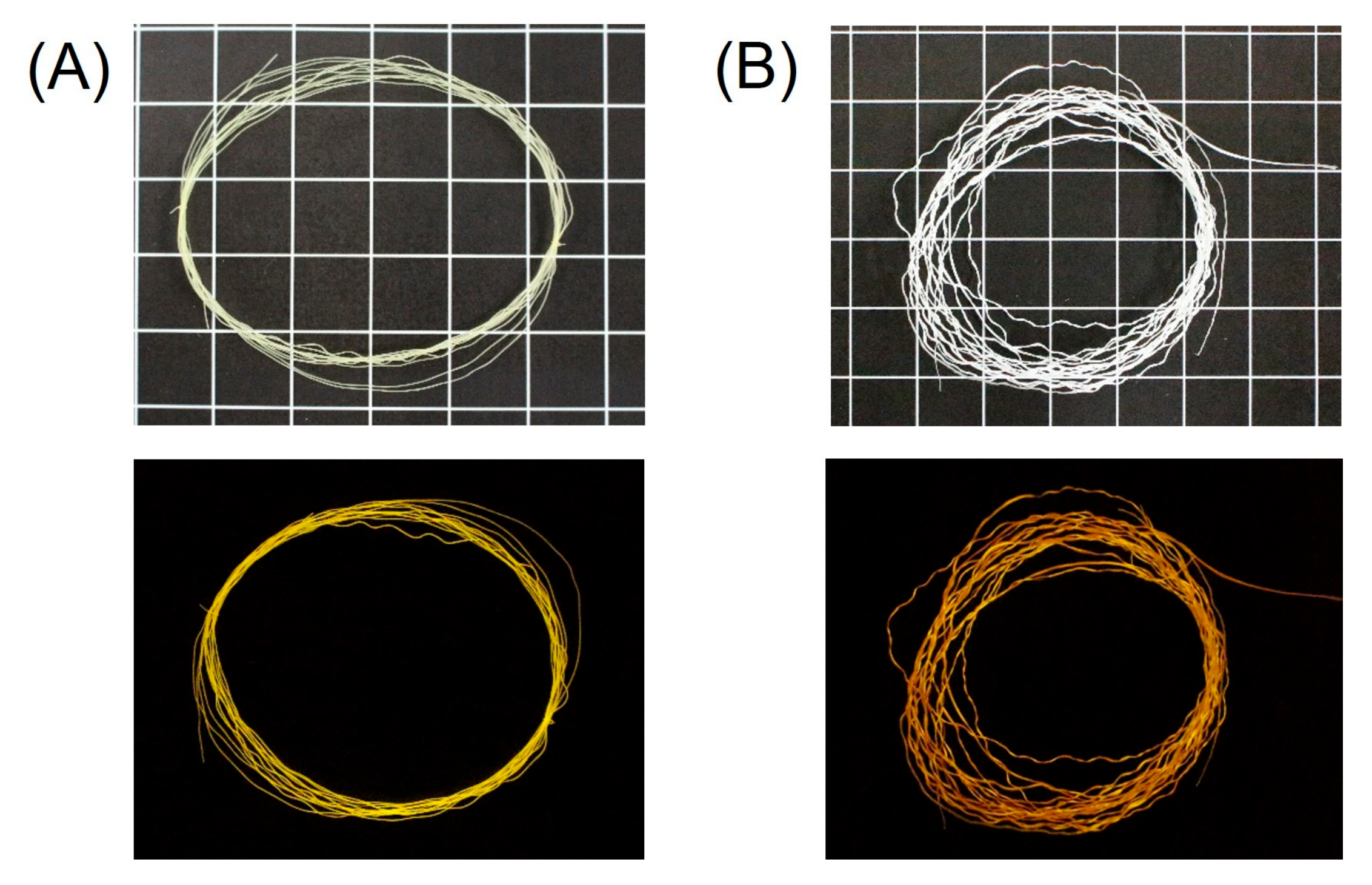
3.1. Fabrication of ALG-PIC Fibers by Wet Spinning
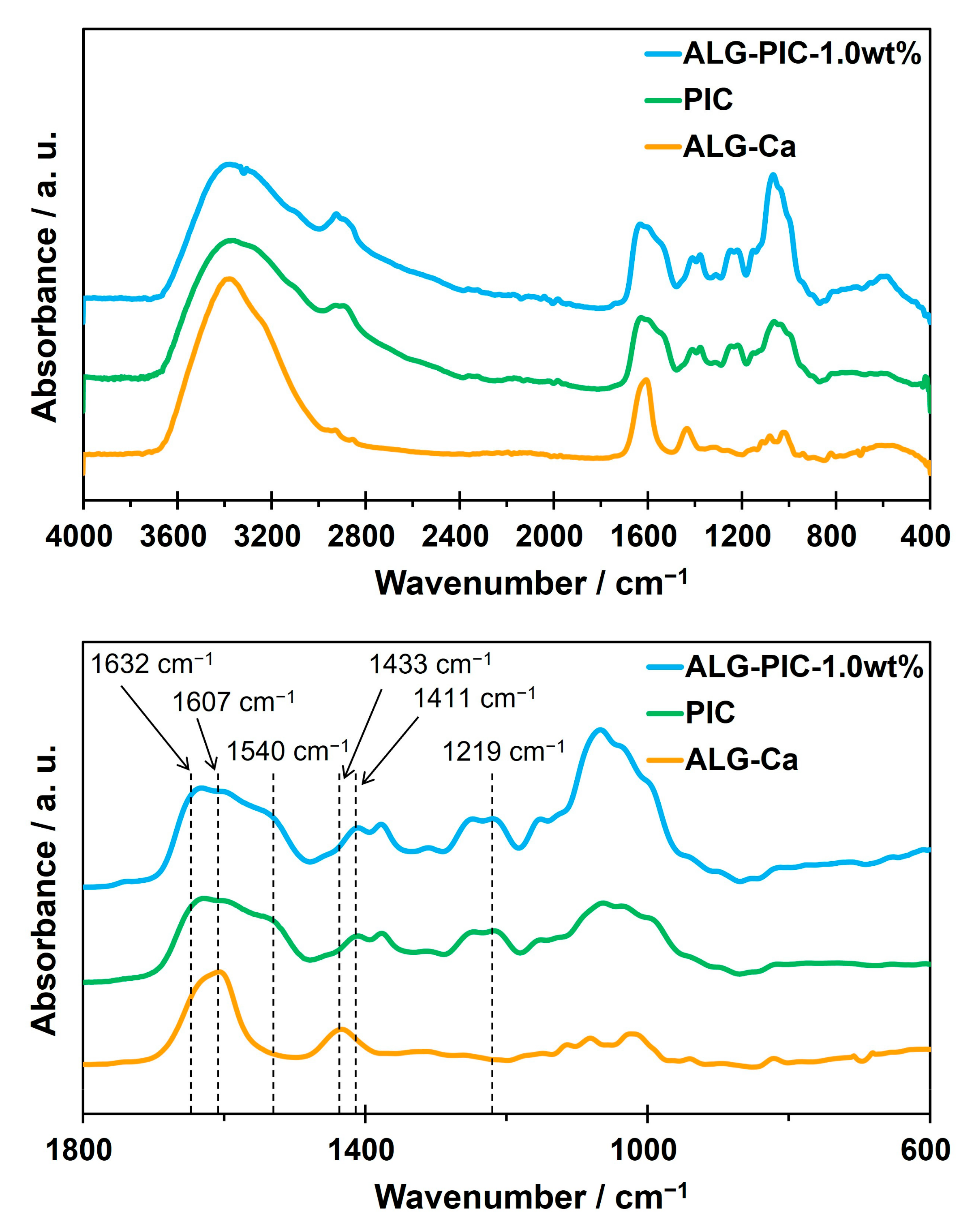
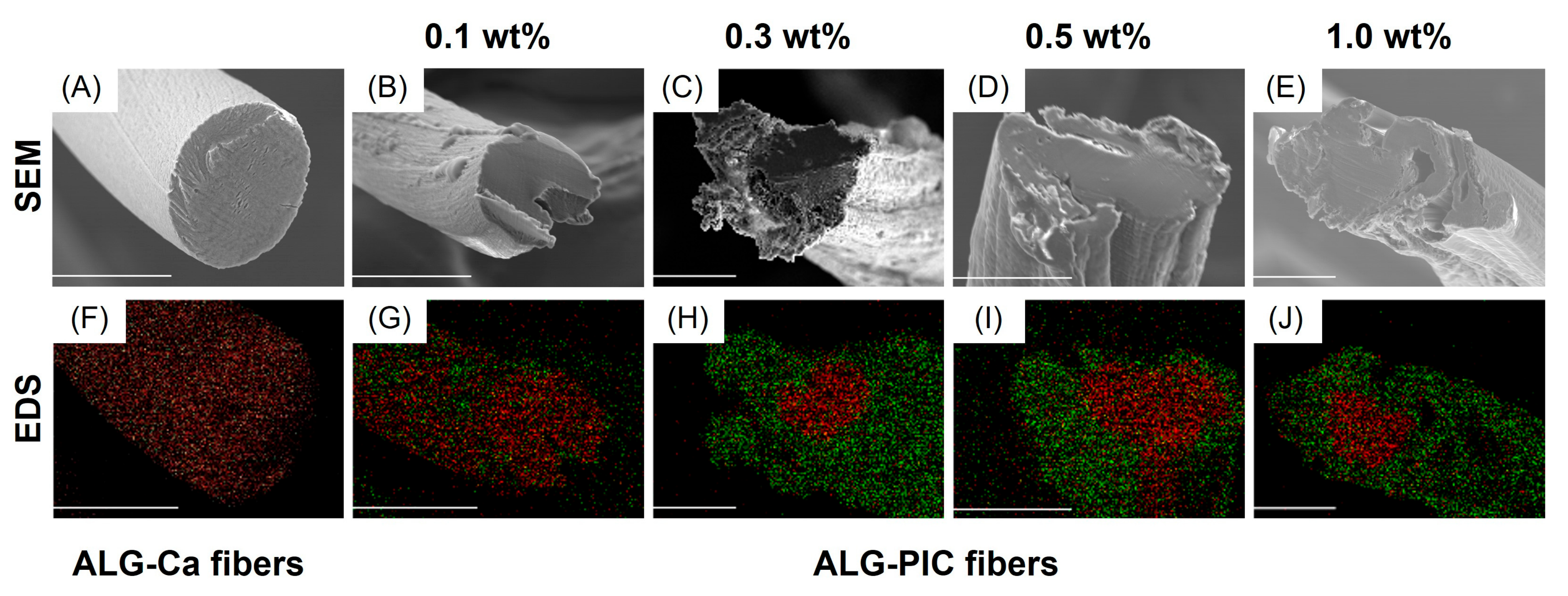
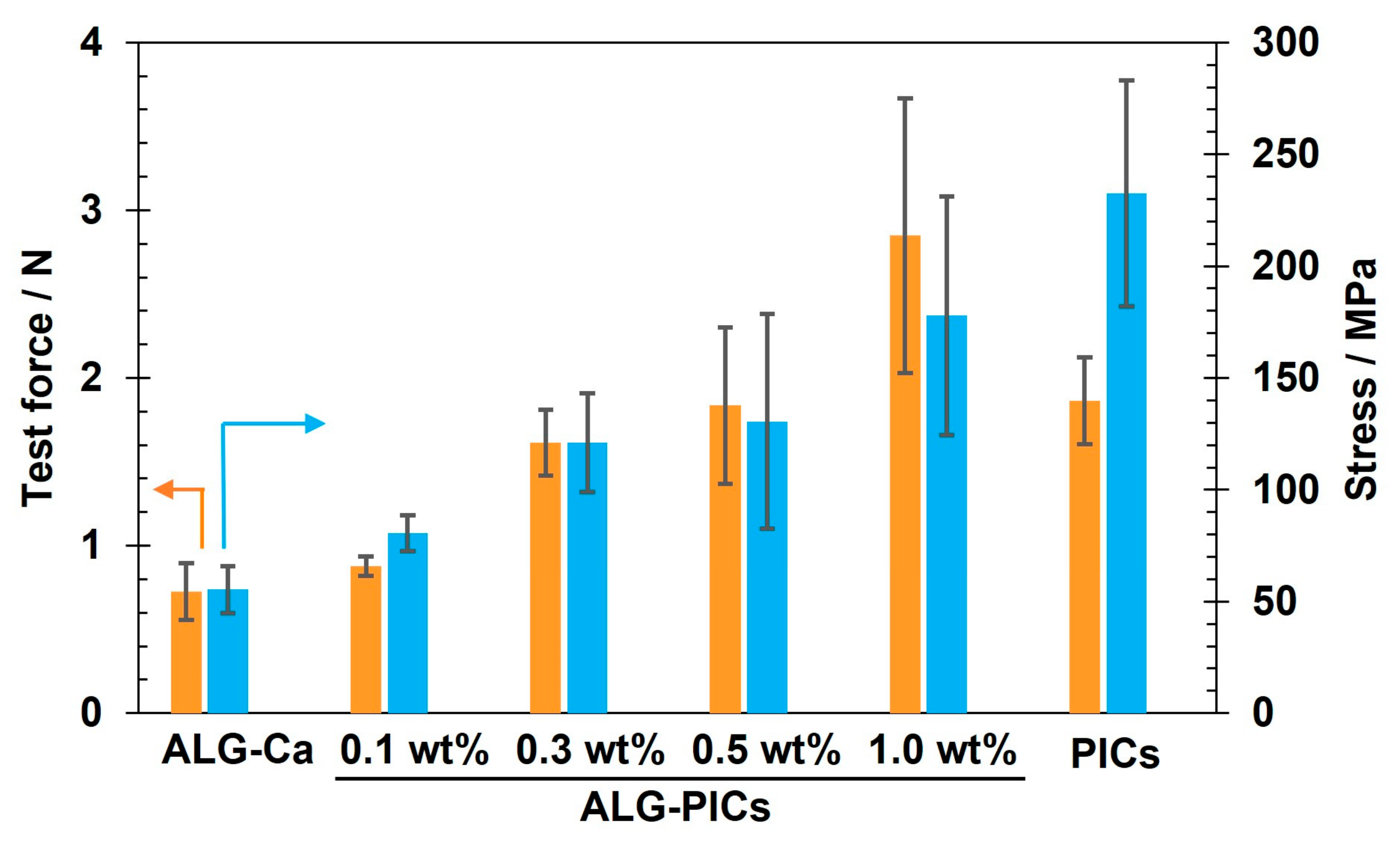
3.2. Characterization and Evaluation of ALG-PIC Fibers
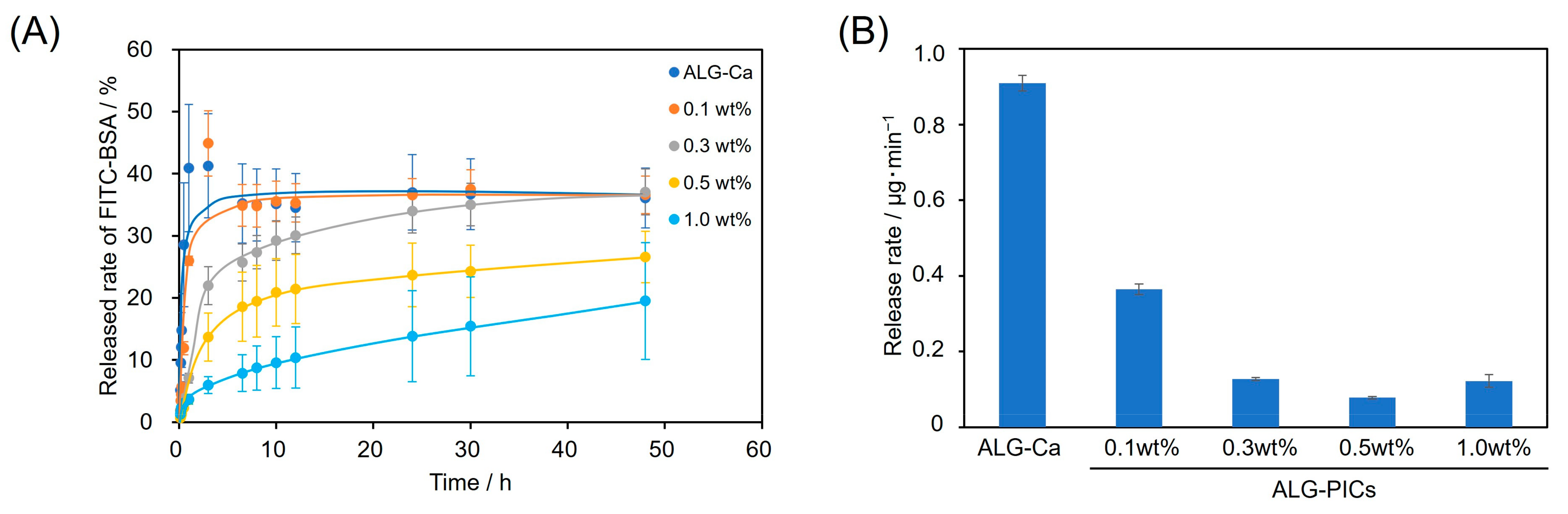
3.3. Utilization of ALG-PIC Fibers as a DDS Carrier
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Silva, A.C.Q.; Silvestre, A.J.D.; Vilela, C.; Freire, C.S.R. Natural Polymers-Based Materials: A Contribution to a Greener Future. Molecules 2022, 27, 94. [Google Scholar] [CrossRef] [PubMed]
- Müller, K.; Zollfrank, C.; Schmid, M. Natural Polymers from Biomass Resources as Feedstocks for Thermoplastic Materials. Macromol. Mater. Eng. 2019, 304, 1800760. [Google Scholar] [CrossRef]
- Liu, Z.; Jiao, Y.; Wang, Y.; Zhou, C.; Zhang, Z. Polysaccharides-based nanoparticles as drug delivery systems. Adv. Drug Deliv. Rev. 2008, 60, 1650–1662. [Google Scholar] [CrossRef] [PubMed]
- García-González, C.A.; Alnaief, M.; Smirnova, I. Polysaccharide-based aerogels—Promising biodegradable carriers for drug delivery systems. Carbohydr. Polym. 2011, 86, 1425–1438. [Google Scholar] [CrossRef]
- Prasher, P.; Sharma, M.; Mehta, M.; Satija, S.; Aljabali, A.A.; Tambuwala, M.M.; Anand, K.; Sharma, N.; Dureja, H.; Jha, N.K.; et al. Current-status and applications of polysaccharides in drug delivery systems. Colloids Interface Sci. Commun. 2021, 42, 100418. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, H.; Wang, X.; Yu, B.; Cong, H.; Shen, Y. Polysaccharide-based nanocarriers for efficient transvascular drug delivery. J. Control. Release 2023, 354, 167–187. [Google Scholar] [CrossRef]
- Zhu, T.; Mao, J.; Cheng, Y.; Liu, H.; Lv, L.; Ge, M.; Li, S.; Huang, J.; Chen, Z.; Li, H.; et al. Recent Progress of Polysaccharide-Based Hydrogel Interfaces for Wound Healing and Tissue Engineering. Adv. Mater. Interfaces 2019, 6, 1900761. [Google Scholar] [CrossRef]
- Yang, J.; Wang, S. Polysaccharide-Based Multifunctional Hydrogel Bio-Adhesives for Wound Healing: A Review. Gels 2023, 9, 138. [Google Scholar] [CrossRef]
- Croisier, F.; Atanasova, G.; Poumay, Y.; Jérôme, C. Polysaccharide-Coated PCL Nanofi bers for Wound Dressing Applications. Adv. Healthc. Mater. 2014, 3, 2032–2039. [Google Scholar] [CrossRef]
- Miraftaba, M.; Qiao, Q.; Kennedy, J.F.; Anand, S.C.; Groocock, M.R. Fibres for wound dressings based on mixed carbohydrate polymer fibres. Carbohydr. Polym. 2003, 86, 225–231. [Google Scholar] [CrossRef]
- Ju, S.; Zhang, F.; Duan, J.; Jiang, J. Characterization of bacterial cellulose composite films incorporated with bulk chitosan and chitosan nanoparticles: A comparative study. Carbohydr. Polym. 2020, 237, 116167. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, M.; Vahidi, O.; Moghbeli, M.R.; Ahmadi, S.; Asadnia, M.; Akhavan, O.; Seidi, F.; Rabiee, M.; Saeb, M.R.; Webster, T.J.; et al. Customizing nano-chitosan for sustainable drug delivery. J. Control. Release 2022, 350, 175–192. [Google Scholar] [CrossRef]
- Bazmandeh, A.Z.; Mirzaei, E.; Fadaie, M.; Shirian, S.; Ghasemi, Y. Dual spinneret electrospun nanofibrous/gel structure of chitosan-gelatin/chitosan-hyaluronic acid as a wound dressing: In-vitro and in-vivo studies. Int. J. Biol. Macromol. 2020, 162, 359–373. [Google Scholar] [CrossRef] [PubMed]
- Mazaheri, M.; Akhavan, O.; Simchi, A. Flexible bactericidal graphene oxide–chitosan layers for stem cell proliferation. Appl. Surf. Sci. 2014, 301, 456–462. [Google Scholar] [CrossRef]
- Yousefiasl, S.; Manoochehri, H.; Makvandi, P.; Afshar, S.; Salahinejad, E.; Khosraviyan, P.; Saidijam, M.; Asl, S.S.; Sharif, E. Chitosan/alginate bionanocomposites adorned with mesoporous silica nanoparticles for bone tissue engineering. J. Nanostruct. Chem. 2023, 13, 389–403. [Google Scholar] [CrossRef]
- Rahighi, R.; Panahi, M.; Akhavan, O.; Mansoorianfar, M. Pressure-engineered electrophoretic deposition for gentamicin loading within osteoblast-specific cellulose nanofiber scaffolds. Mater. Chem. Phys. 2021, 272, 125018. [Google Scholar] [CrossRef]
- Alvarez-Lorenzo, C.; Blanco-Fernandez, B.; Puga, A.M.; Concheiro, A. Crosslinked ionic polysaccharides for stimuli-sensitive drug delivery. Adv. Drug Deliv. Rev. 2013, 65, 1148–1171. [Google Scholar] [CrossRef]
- Dumitriu, S. Chornet, Inclusion and release of proteins from polysaccharide-based polyion complexes. Adv. Drug Deliv. Rev. 1998, 31, 223–246. [Google Scholar] [CrossRef]
- Sun, X.; Liu, C.; Omer, A.M.; Yang, L.; Ouyang, X. Dual-layered pH-sensitive alginate/chitosan/kappa-carrageenan microbeads for colon-targeted release of 5-fluorouracil. Macromolecules 2019, 132, 487–494. [Google Scholar] [CrossRef]
- Grasdalen, H.; Larsen, B.; Smidsrød, O. A p.m.r. study of the composition and sequence of uronate residues in alginates. Carbohydr. Res. 1979, 68, 23–31. [Google Scholar] [CrossRef]
- Hecht, H.; Srebnik, S. Structural Characterization of Sodium Alginate and Calcium Alginate. Biomacromolecules 2016, 17, 2160–2167. [Google Scholar] [CrossRef]
- Kumar, M.N.V.R. A review of chitin and chitosan applications. React. Funct. Polym. 2000, 46, 1–27. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Alimirzaei, F.; Vasheghani-Farahani, E.; Ghiaseddin, A.; Soleimani, M.; Pouri; Zeinab, N. pH-Sensitive Chitosan Hydrogel with Instant Gelation for Myocardial Regeneration. J. Tissue Sci. Eng. 2017, 8, 1000212. [Google Scholar]
- Denuziere, A.; Ferrier, D.; Domard, A. Chitosan-chondroitin sulfate and chitosan-hyaluronate polyelectrolyte complexes. Physico-chemical aspects. Carbohydr. Polym. 1996, 29, 317–323. [Google Scholar] [CrossRef]
- Hamman, J.H. Chitosan Based Polyelectrolyte Complexes as Potential Carrier Materials in Drug Delivery Systems. Mar. Drugs 2010, 8, 1305–1322. [Google Scholar] [CrossRef]
- Hashizume, M.; Kobayashi, H.; Ohashi, M. Preparation of Free-Standing Films of Natural Polysaccharides Using Hot Press Technique and Their Surface Functionalization with Biomimetic Apatite. Colloids Surf. B Biointerfaces 2011, 88, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Ohashi, M.; Kobayashi, H.; Tsuji, Y.; Iijima, K. Free-standing Polysaccharide Composite Films: Improved Preparation and Physical Properties. Colloids Surf. A Physicochem. Eng. Asp. 2015, 484, 18–24. [Google Scholar] [CrossRef]
- Iijima, K.; Tsuji, Y.; Kuriki, I.; Kakimoto, A.; Nikaido, Y.; Ninomiya, R.; Iyoda, T.; Fukai, F.; Hashizume, M. Control of Cell Adhesion and Proliferation Utilizing Polysaccharide Composite Film Scaffolds. Colloids Surf. B Biointerfaces 2017, 160, 228–237. [Google Scholar] [CrossRef] [PubMed]
- Hashizume, M.; Murata, Y.; Iijima, K.; Shibata, T. Drug Loading and Release Behaviors of Freestanding Polysaccharide Composite Films. Polym. J. 2016, 48, 545–550. [Google Scholar] [CrossRef]
- Iijima, K.; Kimura, T.; Sato, R.; Takahashi, T.; Hashizume, M. Kinetic Analysis of Molecular Permeabilities of Free-standing Polysaccharide Composite Films. Macromol. Chem. Phys. 2017, 218, 1600391. [Google Scholar] [CrossRef]
- Sagawa, T.; Sakakibara, M.; Iijima, K.; Yataka, Y.; Hashizume, M. Preparation and physical properties of free-standing films made of polyion complexes of carboxymethylated hyaluronic acid and chitosan. Polymer 2022, 253, 125033. [Google Scholar] [CrossRef]
- Sagawa, T.; Nikaido, Y.; Iijima, K.; Sakaguchi, M.; Yataka, Y.; Hashizume, M. Preparation of Mechanically Anisotropic Polysaccharide Composite Films Using Roll-Press Techniques. ACS Omega 2023, 8, 5607–5616. [Google Scholar] [CrossRef] [PubMed]
- Decher, G. Fuzzy Nanoassemblies: Toward Layered Polymeric Multicomposites. Science 1997, 277, 1232–1237. [Google Scholar] [CrossRef]
- Decher, G.; Schlenoff, J.B. (Eds.) Multilayer Thin Films, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2012. [Google Scholar]
- Moreira, J.; Vale, A.C.; Pires, R.A.; Botelho, G.; Reis, R.L.; Alves, N.M. Spin-Coated Polysaccharide-Based Multilayered Freestanding Films with Adhesive and Bioactive Moieties. Molecules 2020, 25, 840. [Google Scholar] [CrossRef]
- Tamura, H.; Tsuruta, Y.; Itoyama, K.; Worakitkanchanakul, W.; Rujiravanit, R.; Tokura, S. Preparation of chitosan filament applying new coagulation system. Carbohydr. Polym. 2004, 56, 205–211. [Google Scholar] [CrossRef]
- Perrin, N.; Mohammadkhani, G.; Homayouni, M.F.; Delattre, C.; Zamani, A. Biocompatible fibers from fungal and shrimp chitosans for suture application. Curr. Opin. Biotechnol. 2022, 4, 530–536. [Google Scholar] [CrossRef]
- Onoe, H.; Okitsu, T.; Itou, A.; Kato-Negishi, M.; Gojo, R.; Kiriya, D.; Sato, K.; Miura, S.; Iwanaga, S.; Kuribayashi-Shigetomi, K.; et al. Metre-long cell-laden microfibres exhibit tissue morphologies and functions. Nat. Matter. 2013, 12, 584–590. [Google Scholar] [CrossRef]
- Zhang, H.; Cheng, J.; Ao, Q. Preparation of Alginate-Based Biomaterials and Their Applications in Biomedicine. Mar. Drugs 2021, 19, 264. [Google Scholar] [CrossRef]
- Desai, K.; Kit, K.; Li, J.; Zivanovic, S. Morphological and Surface Properties of Electrospun Chitosan Nanofibers. Biomacromolecules 2008, 9, 1000–1006. [Google Scholar] [CrossRef]
- Okada, T.; Nobunaga, Y.; Konishi, T.; Yoshioka, T.; Hayakawa, S.; Lopes, M.A.; Miyazaki, T. Preparation of chitosan-hydroxyapatite composite mono-fiber using coagulation method and their mechanical properties. Carbohydr. Polym. 2017, 175, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Hachisu, M.; Ohkawa, K.; Yamamoto, H. Preparation of Silk-Like Fibers Designed by Self-Assembled Ionic Polypeptides. Macromol. Biosci. 2003, 3, 92–99. [Google Scholar] [CrossRef]
- Yamamoto, H.; Senoo, Y. Polyion complex fiber and capsule formed by selfassembly of chitosan and gellan at solution interfaces. Macromol. Chem. Phys. 2000, 201, 84–92. [Google Scholar] [CrossRef]
- Chen, W.-B.; Wang, L.-F.; Chen, J.-S.; Fan, S.-Y. Characterization of polyelectrolyte complexes between chondroitin sulfate and chitosan in the solid state. J. Biomed. Mater. Res. A 2005, 75, 128–137. [Google Scholar] [CrossRef]
- Huang, H.; Trentle, M.; Liu, Z.; Xiang, K.; Higgins, W.; Wang, Y.; Xue, B.; Yang, S. Polymer Complex Fiber: Property, Functionality, and Applications. ACS Appl. Mater. Interfaces 2023, 15, 7639–7662. [Google Scholar] [CrossRef] [PubMed]
- Iijima, K.; Yuyama, K.; Asaine, K.; Irie, K.; Hashizume, M. Preparation of Chondroitin Sulfate/Chitosan Composite Fibers by Spinning from Aqueous Solution Interfaces. Kobunshi Ronbunshu 2014, 71, 11–16. [Google Scholar] [CrossRef]
- Iijima, K.; Hashizume, M. Application of Polysaccharides as Structural Materials. Trends Glycosci. Glycotechnol. 2015, 27, 67–79. [Google Scholar] [CrossRef]
- Iijima, K.; Ohyama, S.; Yuyama, K.; Shono, A.; Hashizume, M. Selective fabrication of hollow and solid polysaccharide composite fibers using a microfluidic device by controlling polyion complex formation. Polym. J. 2018, 50, 1187–1198. [Google Scholar] [CrossRef]
- Iijima, K.; Ichikawa, S.; Ishikawa, S.; Matsukuma, D.; Yataka, Y.; Otsuka, H.; Hashizume, M. Preparation of Cell-Paved and -Incorporated Polysaccharide Hollow Fibers Using a Microfluidic Device. ACS Biomater. Sci. Eng. 2019, 5, 5688–5697. [Google Scholar] [CrossRef]
- Hermanson, G.T. Bioconjugate Techniques, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Brzezińska, M.; Szparaga, G. The Effect of Sodium Alginate Concentration on the Rheological Parameters of Spinning Solutions. Autex Res. J. 2015, 15, 123–126. [Google Scholar] [CrossRef]
- Plazinski, W. Conformational properties of acidic oligo- and disaccharides and their ability to bind calcium: A molecular modeling study. Carbohydr. Res. 2012, 357, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ho, E.; Deng, Y.; Akbar, D.; Da, K.; Létourneau, M.; Morshead, C.M.; Chatenet, D.; Shoichet, M.S. Tunable Surface Charge Enables the Electrostatic Adsorption-Controlled Release of Neuroprotective Peptides from a Hydrogel−Nanoparticle Drug Delivery System. ACS. Appl. Mater. Interfaces 2023, 15, 91–105. [Google Scholar] [CrossRef]
- Khosravikia, M.; Rahbar-Kelishami, A. A simulation study of an applied approach to enhance drug recovery through electromembrane extraction. J. Mol. Liq. 2022, 3585, 119210. [Google Scholar] [CrossRef]
- Sagawa, T.; Oishi, M.; Yataka, Y.; Sato, R.; Iijima, K.; Hashizume, M. Control of the molecular permeability of polysaccharide composite films utilizing a molecular imprinting approach. Polym. J. 2022, 54, 571–579. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sagawa, T.; Morizumi, H.; Iijima, K.; Yataka, Y.; Hashizume, M. Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties. Appl. Sci. 2023, 13, 8053. https://doi.org/10.3390/app13148053
Sagawa T, Morizumi H, Iijima K, Yataka Y, Hashizume M. Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties. Applied Sciences. 2023; 13(14):8053. https://doi.org/10.3390/app13148053
Chicago/Turabian StyleSagawa, Takuya, Hiroki Morizumi, Kazutoshi Iijima, Yusuke Yataka, and Mineo Hashizume. 2023. "Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties" Applied Sciences 13, no. 14: 8053. https://doi.org/10.3390/app13148053
APA StyleSagawa, T., Morizumi, H., Iijima, K., Yataka, Y., & Hashizume, M. (2023). Fabrication of Polysaccharide-Based Coaxial Fibers Using Wet Spinning Processes and Their Protein Loading Properties. Applied Sciences, 13(14), 8053. https://doi.org/10.3390/app13148053







