Influence of Al Doping on the Physical Properties of CuO Thin Films
Abstract
:1. Introduction
2. Materials and Methods
2.1. Thin Film Deposition
2.2. Structural, Morphological, Optical and Electrical Analysis
3. Results and Discussion
3.1. Structural Analysis
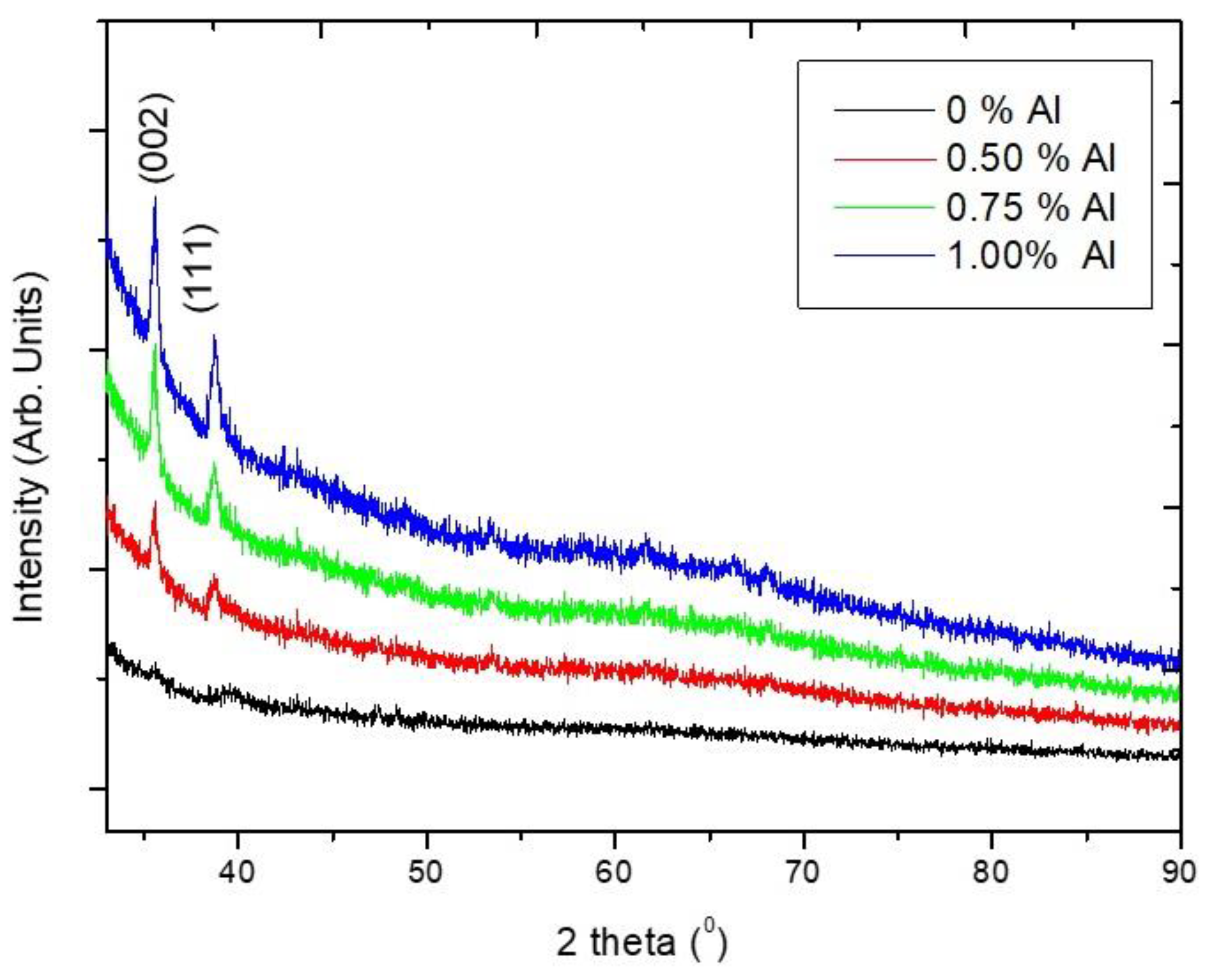
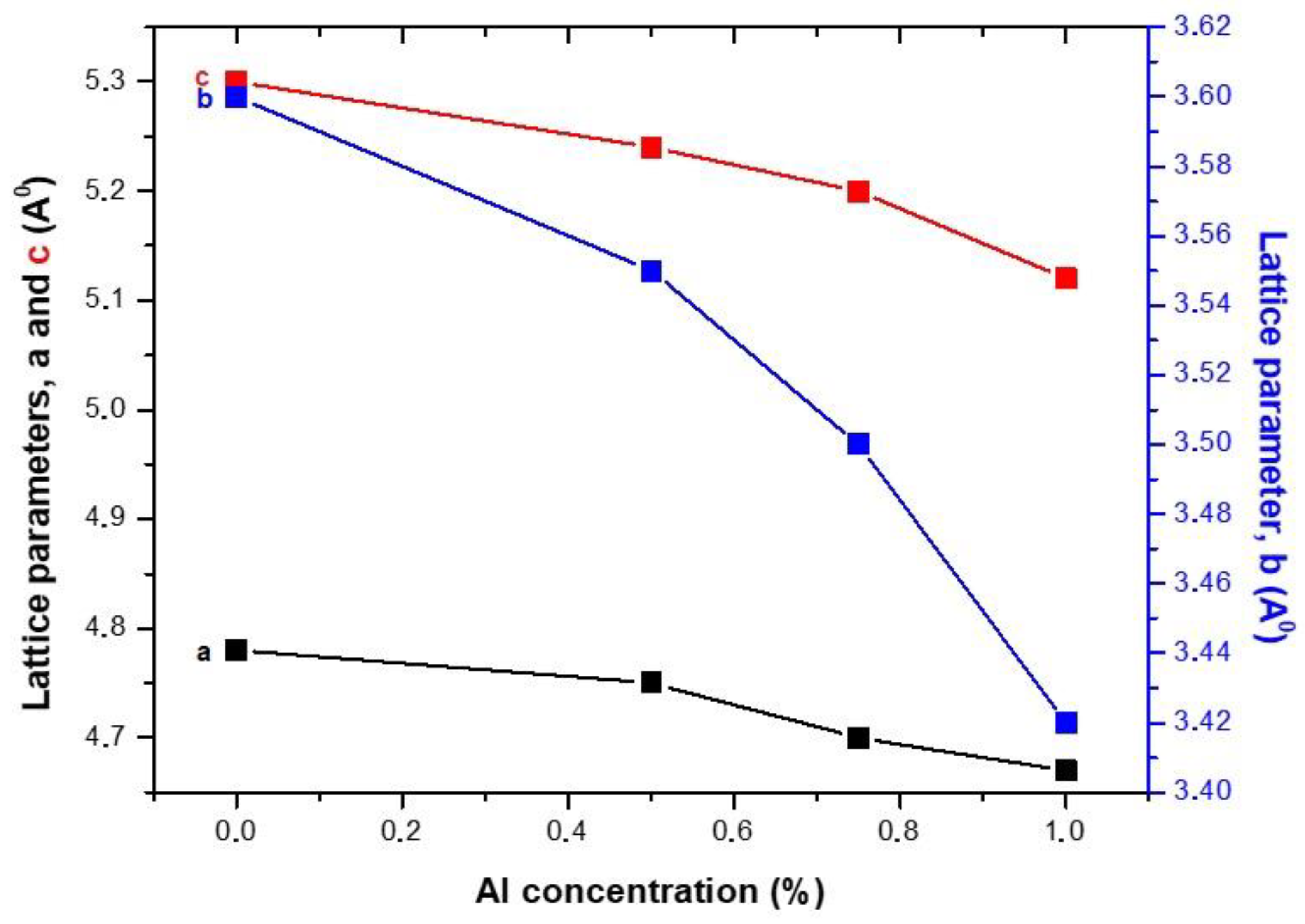
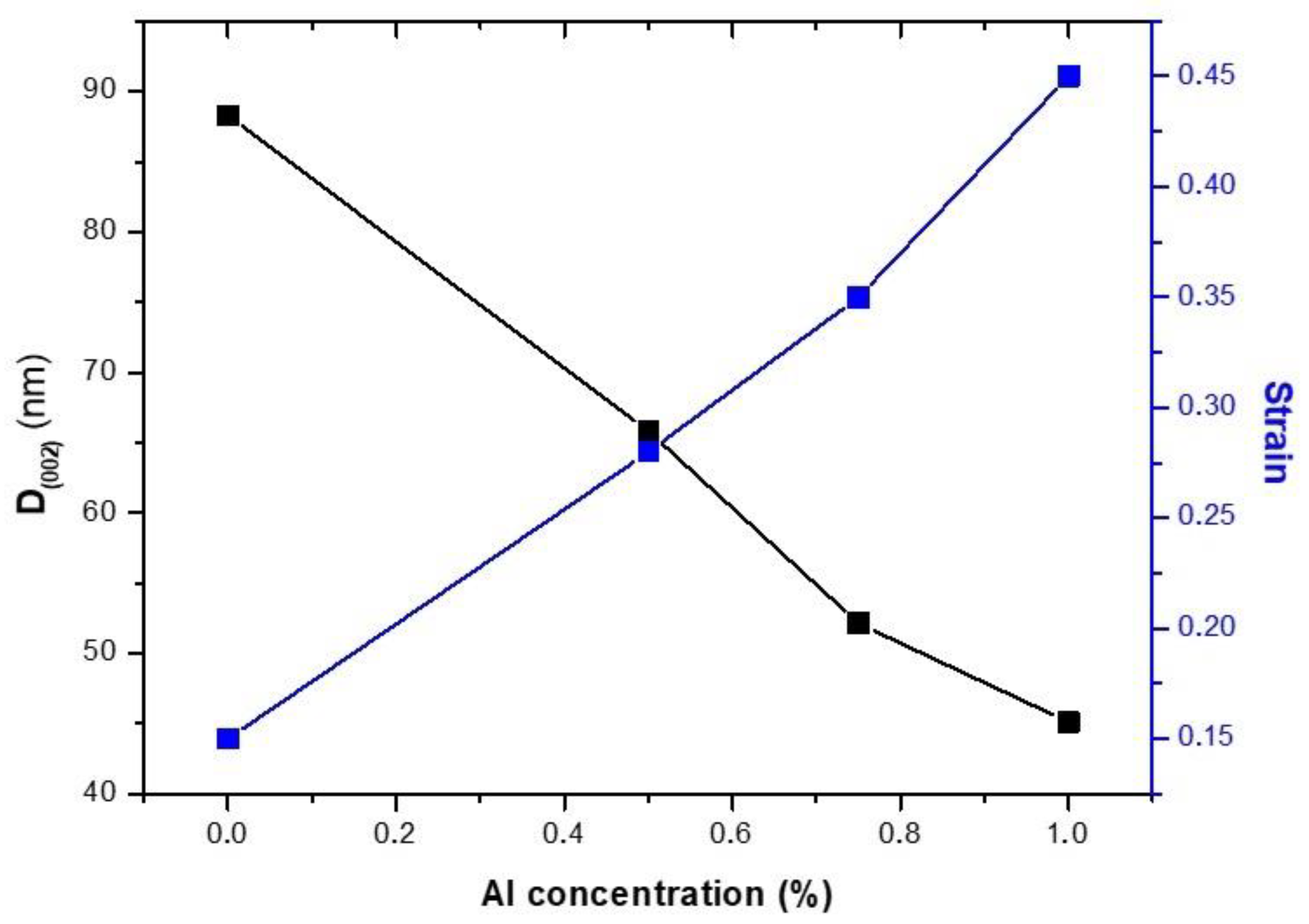
3.1.1. X-ray Diffraction
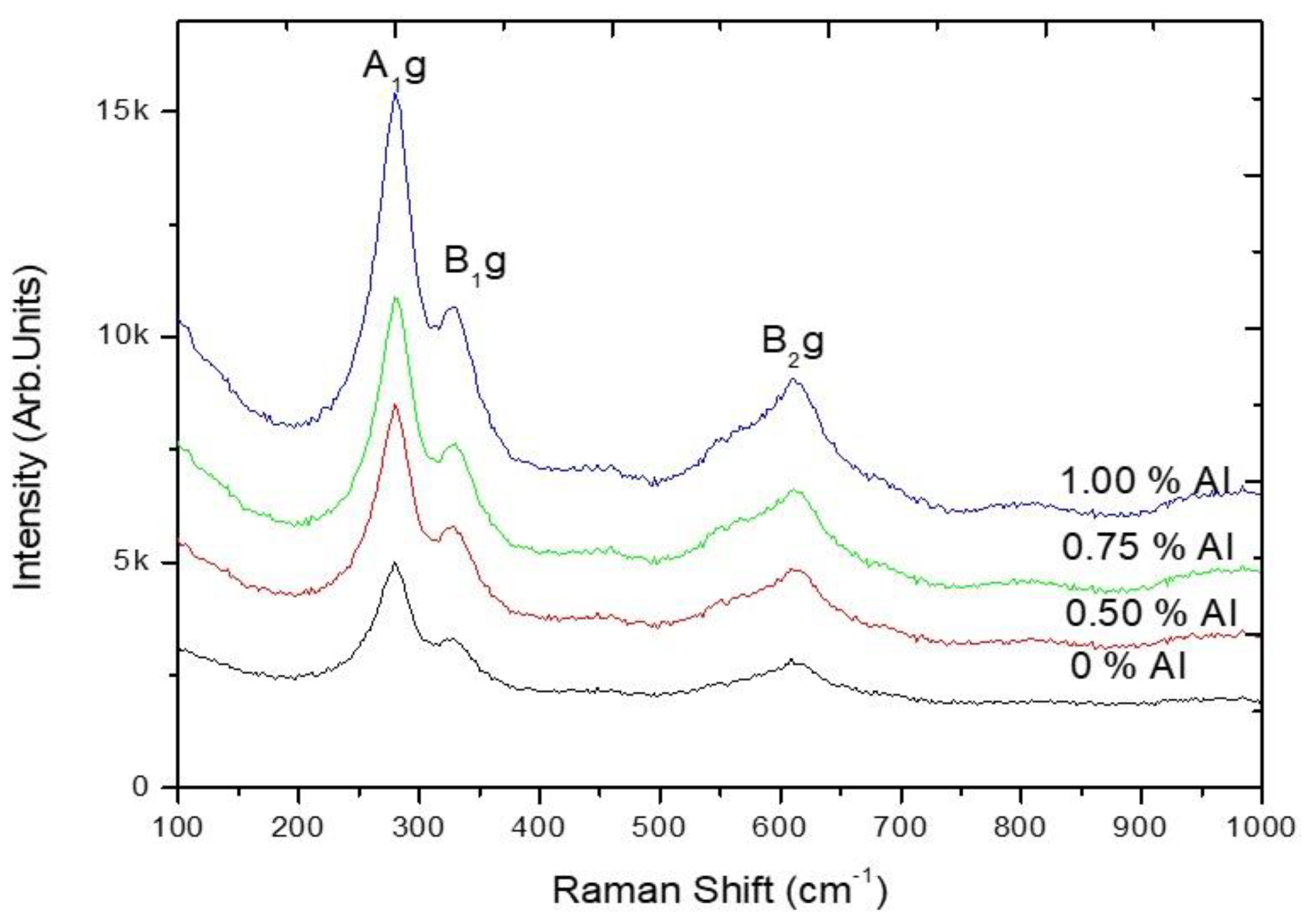
3.1.2. Raman Spectroscopy
3.2. Electrical Properties
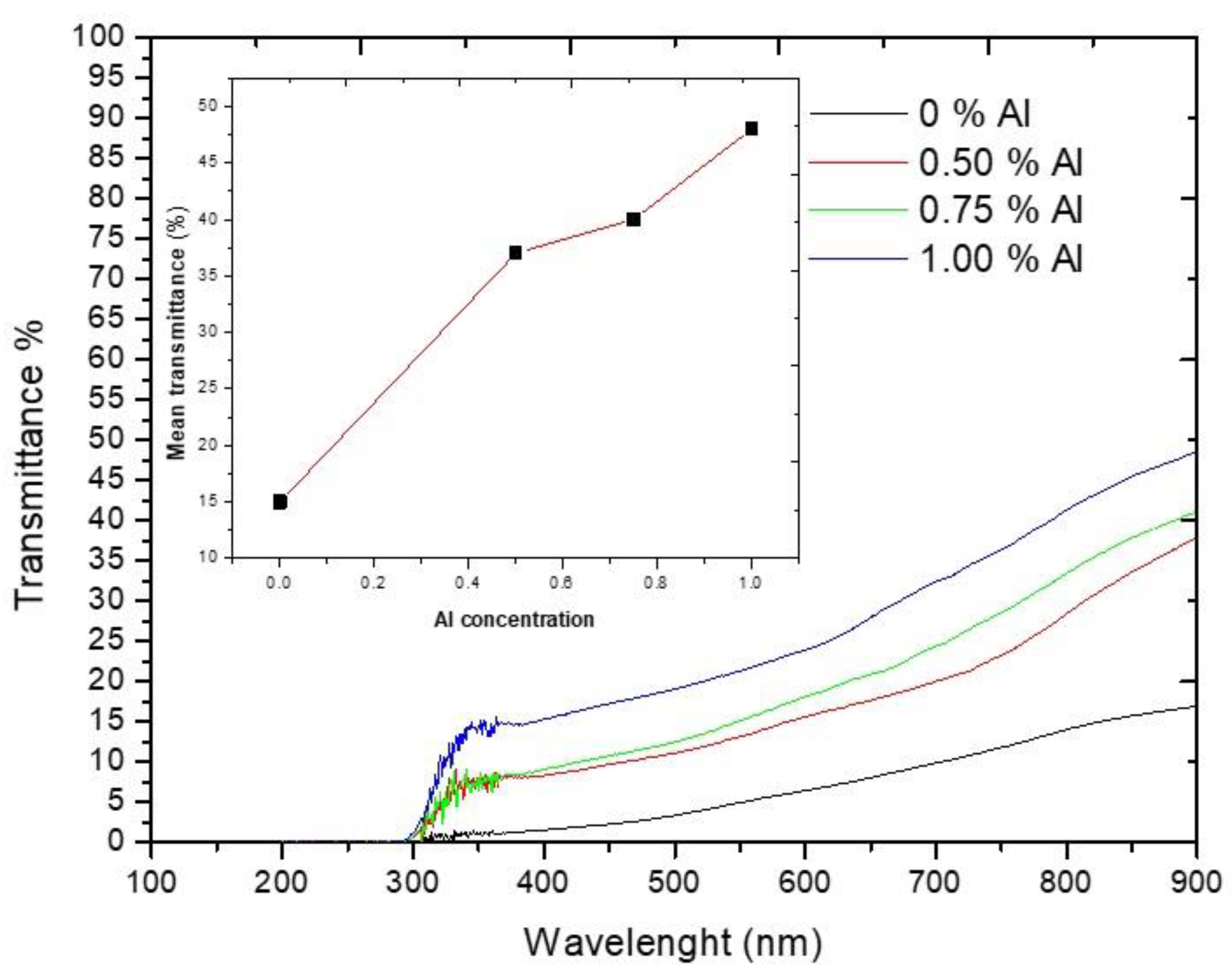
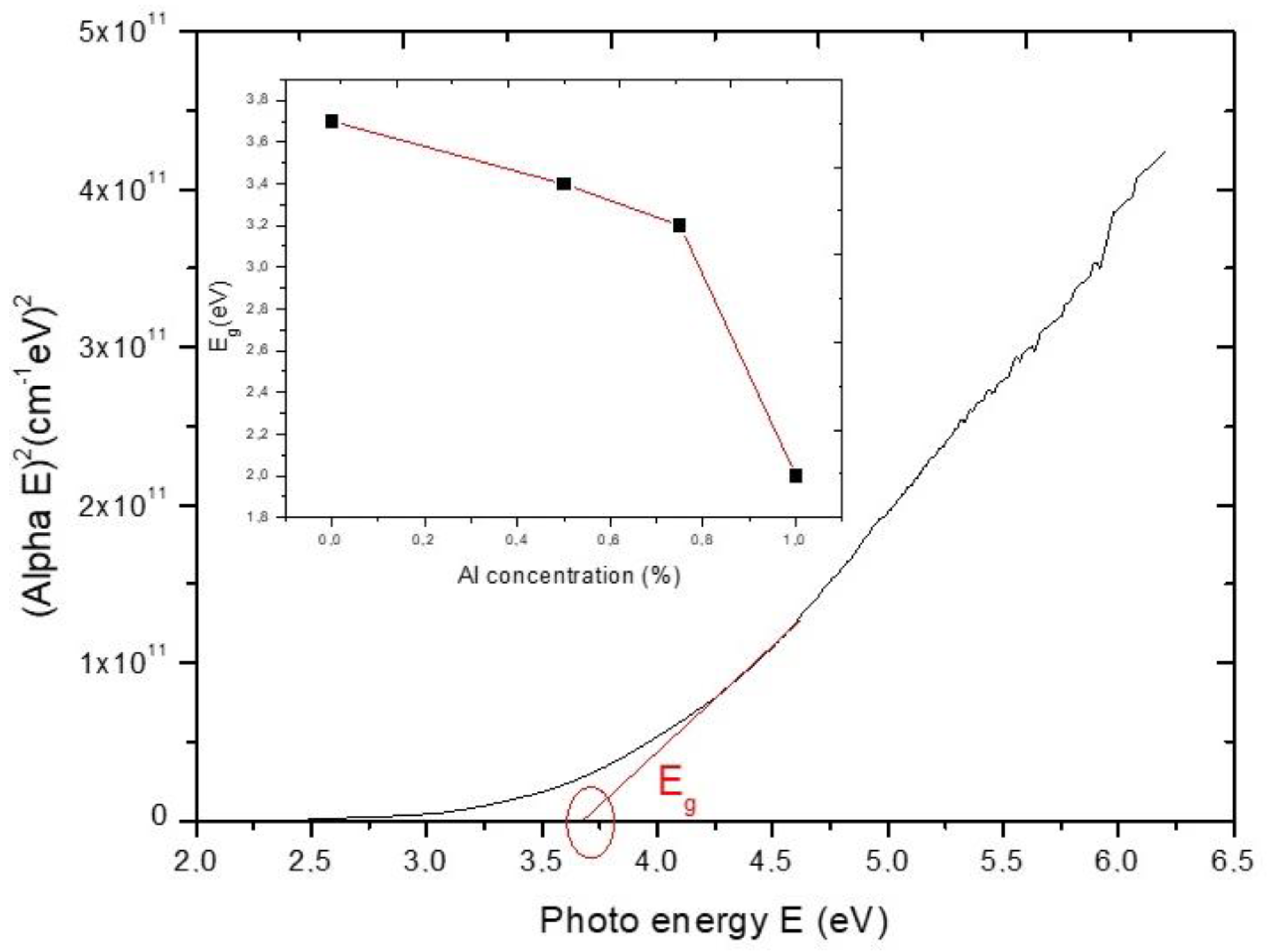
3.3. Optical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balamurugan, B. Optical and structural properties of nanocrystalline copper oxide thin films prepared by activated reactive evaporation. Thin Solid Film. 2001, 396, 90–96. [Google Scholar] [CrossRef]
- Lange, R. Photoelements and Their Applications; Reinhold Publishing Co.: New York, NY, USA, 1939. [Google Scholar]
- Seung-Deok, S.; Yun-Ho, J.; Seung-Hun, L.; Hyun-Woo, S.; Dong Wan, K. Low-temperature synthesis of CuO-interlaced nanodiscs for lithium ion battery electrodes. Nano Scale Res. Lett. 2011, 6, 2. [Google Scholar]
- Jiang, Y.; Decker, S.; Mohs, C.; Klabunde, K.J. Catalytic solid state reactions on the surface of nanoscale metal oxide particles. J. Catal. 1998, 180, 24. [Google Scholar] [CrossRef]
- Chowdhuri, A.; Gupta, V.; Sreenivas, K.; Kumar, R.; Mozumdar, S.; Patanjali, P.K. Response speed of SnO2-based H2S gas sensors with CuO nanoparticles. Appl. Phys. Lett. 2004, 84, 1180–1182. [Google Scholar] [CrossRef]
- Hoa, N.D.; An, S.Y.; Dung, N.D.; Quy, N.V.; Kim, D. Synthesis of p-type Semiconducting Cupric Oxide Thin Films and Their Application to hydrogen detection. Sens. Actuators B Chem. 2010, 146, 239–244. [Google Scholar] [CrossRef]
- Kari, E.R.; Brown, K.S. Electrochemical synthesis and characterization of transparent nanocrystalline Cu2O films and their conversion to CuO films. Chem. Commun. 2006, 31, 3311–3313. [Google Scholar]
- Maruyama, T. Copper oxide thin films prepared from copper dipivaloylmethanate and oxygen by chemical vapor deposition. Jpn. J. Appl. Phys. 2011, 37, 4099–4102. [Google Scholar] [CrossRef]
- Oral, A.Y.; Mensur, E.; Aslan, M.H.; Basaran, E. The preparation of copper (II) oxide thin films and the study of their microstructures and optical properties. Mater. Chem. Phys. 2004, 83, 140. [Google Scholar] [CrossRef]
- Benjamin, J.H.; Nikolai, K.; Ganhua, L.; Khan, L.I.; Junhong, C.; Xin, Z. Transport analyte detection and optoelectronic response of p-type CuO nanowires. J. Phys. Chem. C 2010, 114, 2440–2447. [Google Scholar]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208. [Google Scholar] [CrossRef]
- Chen, A.; Long, H.; Yang, G.; Lu, P. Controlled growth and characteristics of single-phase Cu2O and CuO films by pulsed laser deposition. Vacuum 2009, 83, 927. [Google Scholar] [CrossRef]
- Chen, F.; Zhang, J.; Wang, G.; Zhang, B.; Miao, X.; Fan, J. CuO nanowires synthesized by thermal oxidation route. J. Alloys Compd. 2008, 454, 268. [Google Scholar] [CrossRef]
- Boudjema, B.; Daira, R.; Kabir, A.; Djebien, R. Physico-Chemical Properties of CuO Thin Films Deposited by Spray Pyrolysis. Mater. Sci. Forum 2017, 895, 33–36. [Google Scholar] [CrossRef]
- Daira, R.; Kabir, A.; Boudjema, B.; Sedreati, C. Structural and optical transmittance analysis of CuO thin film deposited by the spray pyrolysis method. Solid State Sci. 2020, 104, 106254. [Google Scholar] [CrossRef]
- Tan, T.Y. Handbook of Solid State Diffusion; Elsevier Inc.: Amsterdam, The Netherlands, 2017; Volume 1, ISBN 978-0-12-804287-8. [Google Scholar]
- Leng, J.; Wang, Z.; Wang, J.; Wu, H.H.; Yan, G.; Li, X.; Guo, H.; Liu, Y.; Zhang, Q.; Guo, Z. Advances in nanostructures fabricated via spray pyrolysis and their applications in energy storage and conversion. Chem. Soc. Rev. 2019, 48, 3015–3072. [Google Scholar] [CrossRef]
- Falcony, C.; Aguilar-Frutis, M.A.; García-Hipólito, M. Spray Pyrolysis Technique; High-K Dielectric Films and Luminescent Materials: A Review. Micromachines 2018, 9, 414. [Google Scholar] [CrossRef] [Green Version]
- Moumen, A.; Hartiti, B.; Thevenin, P.; Siadat, M. Synthesis and characterization of CuO thin films grown by chemical spray pyrolysis. Opt. Quant. Electron. 2017, 49, 70. [Google Scholar] [CrossRef]
- Aroussi, S.; Dahamni, M.A.; Ghamnia, M.; Tonneau, D.; Fauquet, C. Characterization of Some Physical and Photocatalytic Properties of CuO Nanofilms Synthesized by a Gentle Chemical Technique. Condens. Matter 2022, 7, 37. [Google Scholar] [CrossRef]
- Tripathi, A.; Dixit, T.; Agrawal, J.; Singh, V. Bandgap engineering in CuO nanostructures: Dual-band, broadband, and UV-C photodetectors. Appl. Phys. Lett. 2020, 116, 111102. [Google Scholar] [CrossRef]
- Baturay, S.; Tombak, A.; Batibay, D.; Ocak, Y.S. n-Type conductivity of CuO thin films by metal doping. Appl. Surf. Sci. 2019, 477, 91. [Google Scholar] [CrossRef]
- Mashhad-toroghi, A.H.; Shahtahmasebia, N.; Azhira, E.; Madahia, P.; Mashreghi, M. Deposition of Copper Oxide Nanostructured Thin films and Study of the Influence of Fe3+ Doped on the Structural, Optical and Antibacterial Properties of CuO Thin Films. In Proceedings of the 4th International Conference on Nanostructures ICNS4, Kish Island, Iran, 12–14 March 2012; pp. 852–854. [Google Scholar]
- Volanti, D.P.; Keyson, D.; Cavalcante, L.S.; Simões, A.Z.; Joya, M.R.; Longo, E.; Varela, J.A.; Pizani, P.S.; Souza, A.G. Synthesis and characterization of CuO flower-nanostructure processing by a domestic hydrothermal microwave. J. Alloys Compd. 2008, 459, 537–542. [Google Scholar] [CrossRef]
- Yu, T.; Sow, C.H.; Gantimahapatruni, A.; Cheong, F.C.; Zhu, Y.; Chin, K.C.; Xu, X.; Lim, C.T.; Shen, Z.; Thong, J.T.L.; et al. Patterning and fusion of CuO nanorods with a focused laser beam. Nanotechnology 2005, 16, 1238. [Google Scholar] [CrossRef]
- Dennis, B. Elements of X-ray Diffraction, 3rd ed.; Prentice-Hall International: Upper Saddle River, NJ, USA, 2000. [Google Scholar]
- Khodair, T.Z.; Shallal, N. Effect of Aluminum Doping on Structural Properties of CuO Thin Films Prepared by Chemical Spray Pyrolysis (CSP) Technique. Diyala J. Pure Sci. 2017, 13, v198–v208. [Google Scholar] [CrossRef]
- Pankove, J.I. Optical Processing in Semiconductors; Dover: New York, NY, USA, 1971. [Google Scholar]
- Moumen, A.; Hartiti, B.; Comini, E.; El Khalidi, Z.; Arachchige, H.M.M.; Fadili, S.; Thevenin, P. Preparation and characterization of nanostructured CuO thin films using spray pyrolysis technique. Superlattice Microst. 2019, 127, 2–10. [Google Scholar] [CrossRef]
- Ravichandran, A.T.; Dhanabalan, K.; Valanarasu, S.; Vasuhi, A.; Kathalingam, A.J. Role of immersion time on the properties of SILAR deposited CuO thin films. Mater. Sci. Mater. Electron. 2015, 26, 921. [Google Scholar] [CrossRef]
- Jundale, D.M.; Joshi, P.B.; Sen, S.; Patil, V.B. Nanocrystalline CuO thin films: Synthesis, microstructural and optoelectronic properties. J. Mater. Sci. Mater. Electron. 2012, 23, 1492. [Google Scholar] [CrossRef]
- Daira, R.; Boudjema, B. Application of laser technique for the calculating the speckle size and object incidence angle. J. Intense Pulsed Lasers Appl. Adv. Phys. 2015, 5, 1–4. [Google Scholar]
- Daira, R.; Boudjema, B.; Mordjaoui, M.; Mezéri, M. Electrical properties of metallophthalocyanine thin films. J. Optoelectron. Adv. Mater. Rapid Commun. 2011, 5, 167–171. [Google Scholar]
- Konar, S.; Kalita, H.; Puvvada, N.; Tantubay, S.; Mahto, M.K.; Biswas, S.; Pathak, A. Shape-dependent catalytic activity of CuO nanostructures. J. Catal. 2016, 336, 11. [Google Scholar] [CrossRef]
- Dubal, D.P.; Gund, G.S.; Lokhande, C.D.; Holze, R. CuO cauliflowers for supercapacitor application: Novel potentiodynamic deposition. Mater. Res. Bull. 2013, 48, 923–928. [Google Scholar] [CrossRef]
- Li, J.; Su, Q.; Du, G. Facile synthesis of flowerlike CuO by double-hydroxides treatment and their electrochemical properties. Mater. Lett. 2012, 84, 97. [Google Scholar] [CrossRef]
- Patake, V.D.; Joshi, S.S.; Lokhande, C.D.; Joo, O.-S. Electrodeposited porous and amorphous copper oxide film for application in supercapacitor. Mater. Chem. Phys. 2009, 114, 6. [Google Scholar] [CrossRef]
- Wang, X.; Hu, C.; Liu, H.; Du, G.; He, X.; Xi, Y. Synthesis of CuO nanostructures and their application for nonenzymatic glucose sensing. Sens. Actuators B Chem. 2010, 144, 220. [Google Scholar] [CrossRef]
- Arfan, M.; Siddiqui, D.N.; Shahid, T.; Iqbal, Z.; Majeed, Y.; Akram, I.; Bagheri, R.; Song, Z.; Zeb, A. Tailoring of nanostructures: Al doped CuO synthesized by composite-hydroxide-mediated approach. Results Phys. 2019, 13, 102187. [Google Scholar] [CrossRef]
- Al-Kattan, A.; Grojo, D.; Drouet, C.; Mouskeftaras, A.; Delaporte, P.; Casanova, A.; Robin, J.D.; Magdinier, F.; Alloncle, P.; Constantinescu, C.; et al. Short-Pulse Lasers: A Versatile Tool in Creating Novel Nano-/Micro-Structures and Compositional Analysis for Healthcare and Wellbeing Challenges. Nanomaterials 2021, 11, 712. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Daira, R.; Boudjema, B.; Bououdina, M.; Aida, M.S.; Constantinescu, C.-D. Influence of Al Doping on the Physical Properties of CuO Thin Films. Appl. Sci. 2023, 13, 8193. https://doi.org/10.3390/app13148193
Daira R, Boudjema B, Bououdina M, Aida MS, Constantinescu C-D. Influence of Al Doping on the Physical Properties of CuO Thin Films. Applied Sciences. 2023; 13(14):8193. https://doi.org/10.3390/app13148193
Chicago/Turabian StyleDaira, Radouane, Bouzid Boudjema, Mohamed Bououdina, Mohamed Salah Aida, and Catalin-Daniel Constantinescu. 2023. "Influence of Al Doping on the Physical Properties of CuO Thin Films" Applied Sciences 13, no. 14: 8193. https://doi.org/10.3390/app13148193





