Infrared Spectroscopy to Assess Manufacturing Procedures of Bone Artefacts from the Chalcolithic Settlement of Vila Nova de São Pedro (Portugal)
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
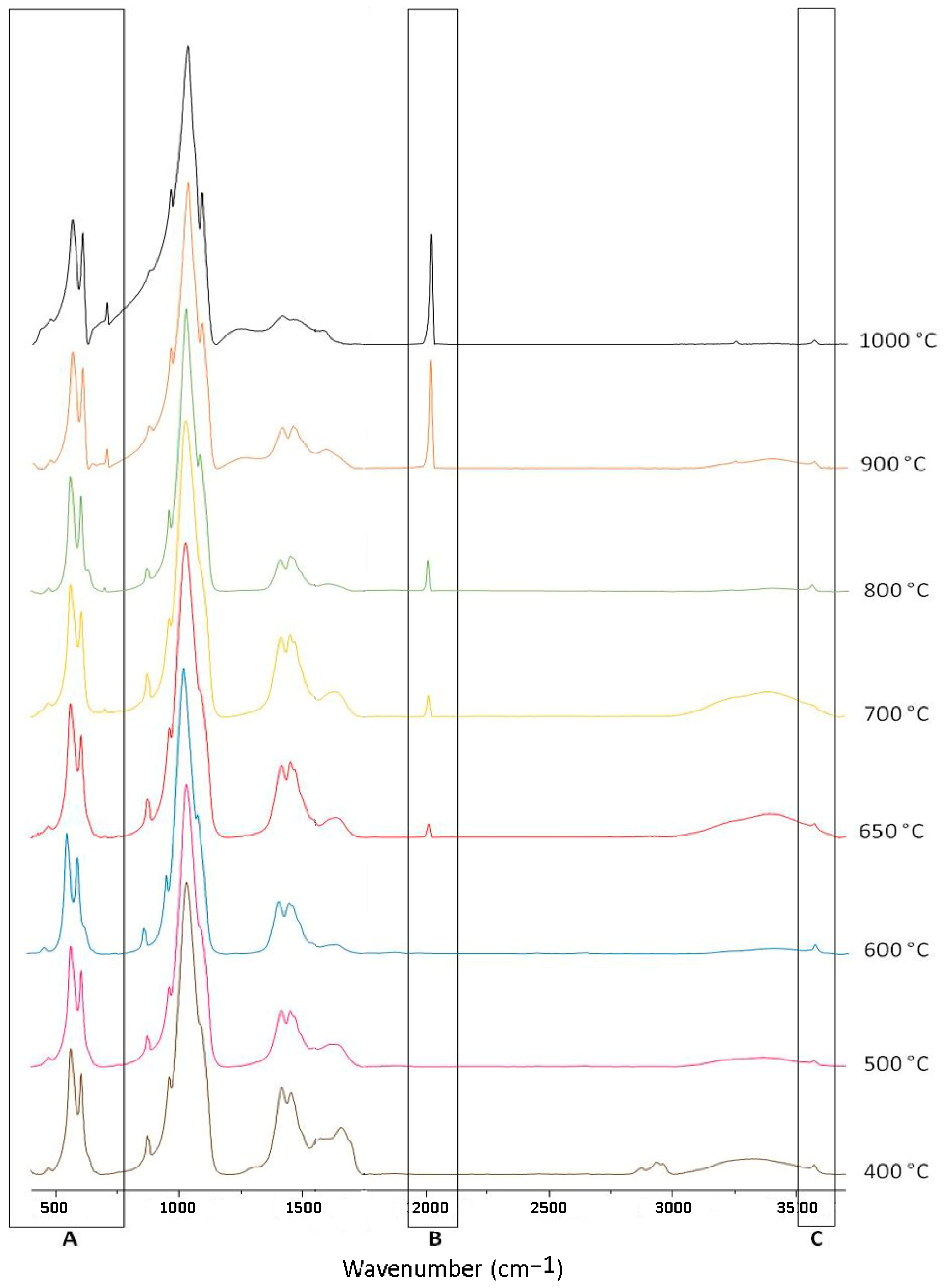
3.1. Experimental Analysis
- the absence of bands for the OH libration at ~630 cm−1 (Figure 3A), in clear contrast to the sharp peak usually observed in bone burnt aerobically at temperatures above 600 °C [21,25,34,35,38]. Possibly, this is explained by the OH libration being a lattice mode which is visible only when the system has other neighbouring OH groups (within a network). When considerable CN22− for OH substitutions take place, only the OH stretching (at ca. 3500 cm−1) is visible.
- the OH stretching band at ~3572 cm−1 was clearly present in spectra from bones burnt at all investigated temperature thresholds (Figure 3C). The spectra reported by Reidsma et al. [20], showed no OH stretching under anaerobic conditions which is, contrastingly, a frequent observation in aerobically burnt bones (Figure 4). This suggests that some variability may be expected as well for the intensity of this feature or, that it instead reflects burns at shorter durations (60 min against the 120–211 mins durations used in the present study);
- progressively more intense bands from CN22− were present at ca. 700 cm−1 (N-C≡N bending, Figure 3A) and 2009 cm−1 (Figure 3B, C≡N stretching) in the spectra of bones burnt at temperatures above 650 °C. Cyanamide formation has been previously reported to be due to incomplete oxidation of organic matter [39], leading to the incorporation of CN22− ions into bone’s inorganic framework substituting for OH− and yielding cyanamidapatite (Ca10(PO4)6CN2) [19,20,21,22,23];
- additionally, between 600 and 700 °C, the formation of NH3/NH4+ was observed which resulted in broad signals at ca. 1700 and 3300 cm−1 due to NH deformation and stretching modes, respectively. As the temperature increased, more and more cyanamide was formed (from ammonia) and the characteristic NH signals gave way to those from cyanamide.
| Index | Bone Type | 400 °C | 500 °C | 550 °C | 600 °C | 650 °C | 700 °C | 750 °C | 800 °C | 900 °C | 1000 °C | [19-27 AP C16] | [19-26 AP C16] | [19-11 AP C16] | [19-23 AP C16] Lighter Region | [19-23 AP C16] Darker Region |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CI | An F | 3.85 | 3.87 | 3.53 | 3.66 | 3.53 | 3.51 | 3.42 | 3.87 | 4.81 | 4.30 | - | - | - | - | - |
| An T | 3.95 | 3.85 | - | - | - | 3.42 | - | - | 4.90 | 4.69 | ||||||
| Ae | 3.53 | 3.68 | - | 3.75 | - | 4.33 | - | 5.52 | 5.69 | 5.34 | - | - | - | - | - | |
| Ar | - | - | - | - | - | - | - | - | - | - | 5.07 | 4.58 | 3.72 | 3.20 | 3.56 | |
| BPI | An F | 0.86 | 0.58 | 0.73 | 0.64 | 0.71 | 0.77 | 0.85 | 0.43 | 0.42 | 0.26 | - | - | - | - | - |
| An T | 0.73 | 0.50 | - | - | - | 0.85 | - | - | 0.42 | 0.28 | ||||||
| Ae | 0.67 | 0.50 | - | 0.45 | - | 0.35 | - | 0.24 | 0.14 | 0.11 | - | - | - | - | - | |
| Ar | - | - | - | - | - | - | - | - | - | - | 0.12 | 0.26 | 0.51 | 1.59 | 0.88 | |
| C/C | An F | 0.95 | 0.99 | 0.98 | 0.99 | 1.04 | 1.03 | 1.04 | 1.22 | 1.03 | 0.88 | - | - | - | - | - |
| An T | 0.96 | 1.01 | - | - | - | 1.04 | - | - | 1.00 | 0.94 | ||||||
| Ae | 0.99 | 1.04 | - | 1.03 | - | 1.03 | - | 1.26 | 1.27 | 1.27 | - | - | - | - | - | |
| Ar | - | - | - | - | - | - | - | - | - | - | 0.91 | 0.98 | 0.93 | 0.87 | 0.90 | |
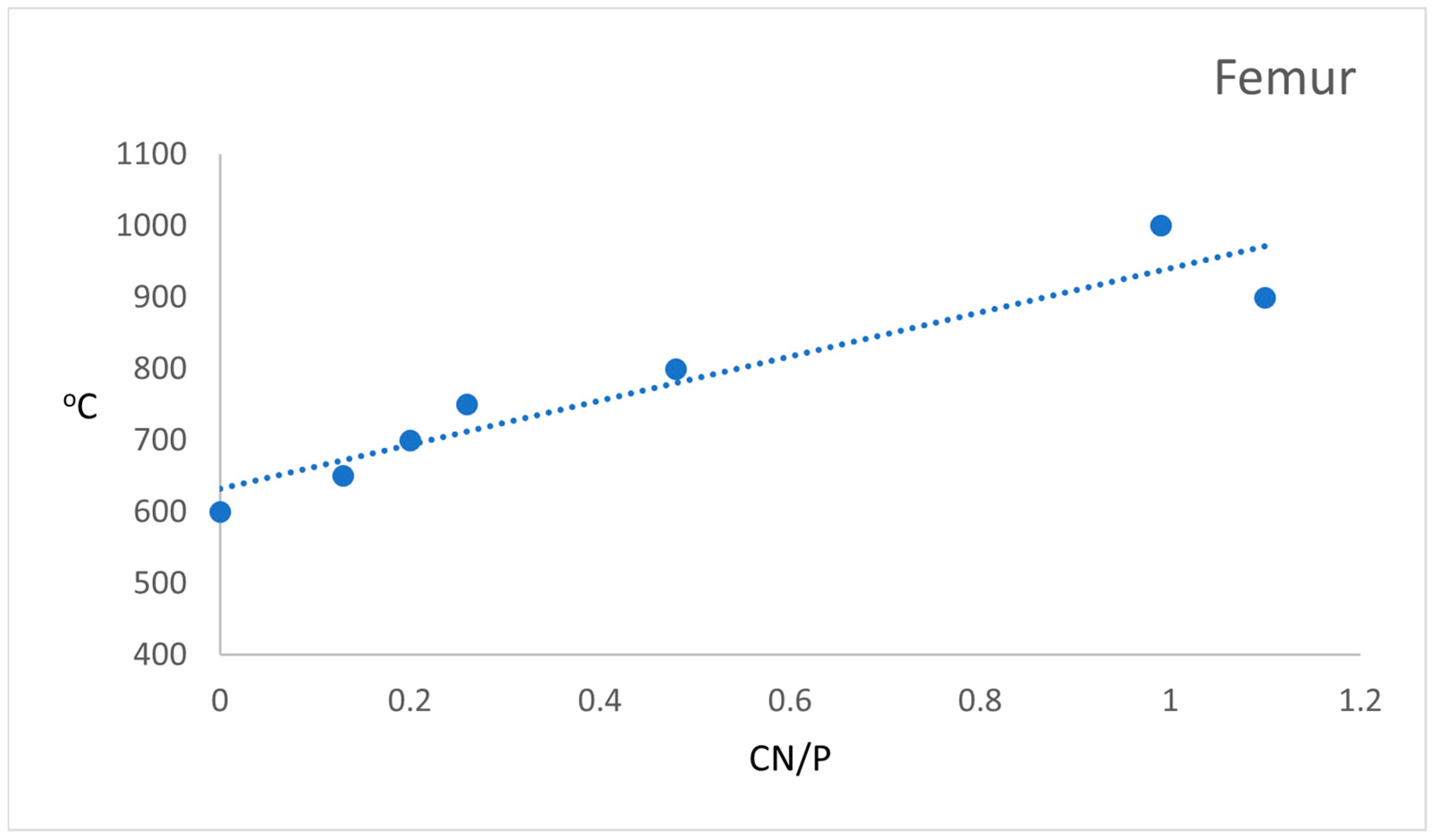
| Cn/P | An F | - | - | - | - | 0.13 | 0.20 | 0.26 | 0.45 | 1.10 | 0.99 | - | - | - | - | - |
| An T | - | - | - | - | - | 0.20 | - | - | 1.16 | 0.96 | ||||||
| Ae * | - | 0.13 0.17 | - | 0.10 0.17 | - | 0.10 0.23 | - | 0.09 0.54 | 0.10 0.18 | - | - | - | - | - | - | |
| Ar | - | - | - | - | - | - | - | - | - | - | Abs | Abs | Abs | Abs | Abs | |
| 630 cm−1 | An F | Abs | Abs | Abs. | Abs. | Abs. | Abs | Abs | Abs | Abs | Abs | - | - | - | - | - |
| An T | Abs | Abs | Abs. | Abs. | Abs. | Abs | Abs | Abs | Abs | Abs | - | - | - | - | - | |
| Ae | Abs | Abs | - | Abs | Pres | Pres | Pres | Pres | - | - | - | - | - | |||
| Ar | - | - | - | - | - | - | - | - | - | - | Possib | Pres | Possib | Abs | Abs | |
| 3572 cm−1 | An F | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | - | - | - | - | - |
| An T | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | Pres | - | - | - | - | - | |
| Ae | Possib | Possib | - | Possib | - | Pres | - | Pres | Pres | Pres | - | - | - | - | - | |
| Ar | - | - | - | - | - | - | - | - | - | - | Abs | Pres | Pres | Abs | Possib |
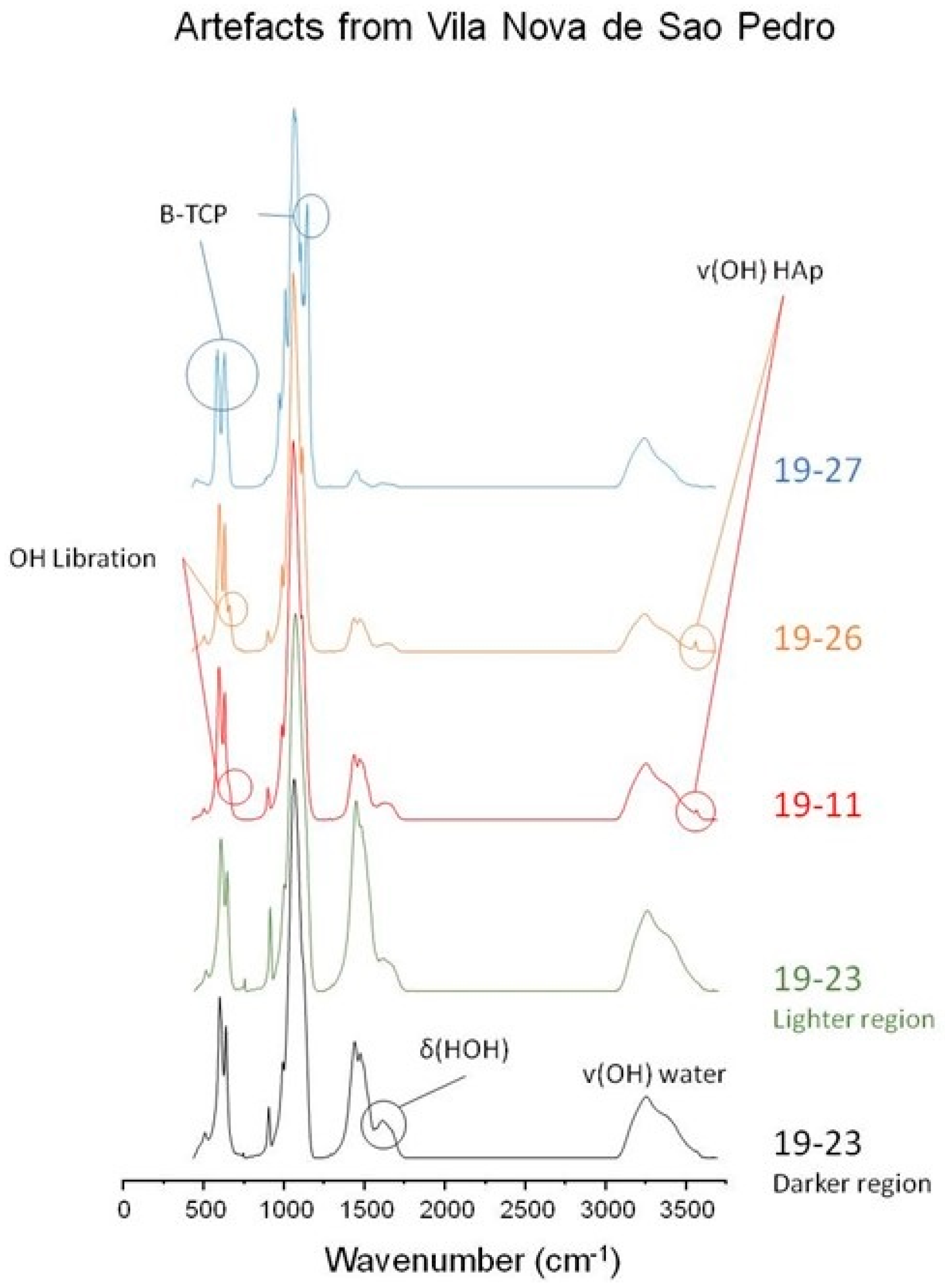
3.2. Analysis of Archaeological Artefacts
3.2.1. Artefact [19-27 AP C16]
3.2.2. Artefact [19-26 AP C16]
3.2.3. Artefact [19-11 AP C16]
3.2.4. Artefact [19-23 AP C16]
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnaud, J.M. Vila Nova de São Pedro revisitada. In Construindo a Memoria—As Colecções do Museu Arqueológico do Carmo; Arnaud, J.M., Fernandes, C.V., Eds.; Associação dos Arqueólogos Portugueses: Lisboa, Portugal, 2005; pp. 141–164. [Google Scholar]
- Müller, R.; Soares, A.M. Traces of Early Copper Prodution at the Chalcolitic Foritification of Vila Nova de São Pedro (Azambuja, Portugal). Madr. Mitt. 2008, 49, 94–114. [Google Scholar]
- Pereira, F.; Silva, R.J.; Soares, A.M.; Araújo, M.F. The role of arsenic in Chalcolithic copper artefacts e insights from Vila Nova de São Pedro (Portugal). J. Archaeol. Sci. 2013, 40, 2045–2056. [Google Scholar] [CrossRef]
- Diniz, M.; Martins, A.; Neves, C.; Arnaud, J.M. Vila Nova de São Pedro (Azambuja), no 3° milénio, um sítio calcolítico no ocidente peninsular–contributos para um debate. In Arqueologia em Portugal–2017, Estado da Questão; Arnaud, J.M., Martins, A.C., Eds.; Associação dos Arqueólogos Portugueses: Lisboa, Portugal, 2017; pp. 591–604. [Google Scholar]
- Martins, A.; Diniz, M.; Neves, C.; Arnaud, J.M. The symbolic in Vila Nova de São Pedro: Idols, statues and symbology. In Mobile Images of Ancestral Bodies: A Millennium-Long Perspective from Iberia to Europe–Zona Arqueológica, n° 23; Bueno Ramírez, P., e Soler Diaz, J., Eds.; Museu Arqueológico Regional: Alcala de Henares, Spain, 2021; Volume II, pp. 121–138. [Google Scholar]
- Diniz, M.; Martins, A.; Neves, C.; Arnaud, J.M. Where there is Power, there is Fear. Muralhas calcolíticas, medo, poder e mecanismos exibição–o caso de Vila Nova de São Pedro (Azambuja, Portugal). In Romper Fronteiras, Atravessar Territórios. Identidades e Intercâmbios da Pré-História Recente no Interior da Península Ibérica; Sanches, M.J., Barbosa, M.H., Teixeira, J.C., Eds.; CITCEM: Porto, Portugal, 2022; pp. 109–137. [Google Scholar]
- Mraz, V.; Fisch, M.; Eren, M.I.; Lovejoy, C.O.; Buchanan, B. Thermal engineering of stone increased prehistoric toolmaking skill. Sci. Rep. 2019, 9, 14591. [Google Scholar] [CrossRef]
- Jalhay, E.; Paço, A. El castro de Vilanova de San Pedro. In Actas y Memorias de la Sociedad Espanola de Antropologia: Etnografia y Prehistoria; Museo Antropológico Nacional: Madrid, Spain, 1945; Volume 20, pp. 5–93. [Google Scholar]
- Shahack-Gross, R.; Bar-Yosef, O.; Weiner, S. Black-colored bones in Hayorium Cave, Israel: Differentiating between burning and oxide staining. J. Archaeol. Sci. 1997, 24, 439–446. [Google Scholar] [CrossRef]
- López-González, F.; Grandal-d’Anglade, A.; Vidal-Romaní, J.R. Deciphering bone depositional sequences in caves through the study of manganese coatings. J. Archaeol. Sci. 2006, 33, 707–717. [Google Scholar] [CrossRef]
- Dupras, T.L.; Schultz, J.J. Taphonomic bone changes and color changes in forensic contexts. In Manual of Forensic Taphonomy; Pokines, J.T., Symes, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 315–340. [Google Scholar]
- Junod, C.A.; Pokines, J.T. Subaerial weathering. In Manual of Forensic Taphonomy; Pokines, J.T., Symes, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 287–314. [Google Scholar]
- Pokines, J.T.; Baker, J.E. Effects of burial environment on osseous remains. In Manual of Forensic Taphonomy; Pokines, J.T., Symes, S.A., Eds.; CRC Press: Boca Raton, FL, USA, 2014; pp. 73–114. [Google Scholar]
- Shipman, P.; Foster, G.; Schoeninger, M. Burnt bones and teeth: An experimental study of colour, morphology, crystal structure and shrinkage. J. Archaeol. Sci. 1984, 11, 307–325. [Google Scholar] [CrossRef]
- Etxeberria, F. Aspectos macroscópicos del hueso sometido al fuego: Revisión de las cremaciones descritas en el País Vasco desde la arqueologia. Munibe 1994, 46, 111–116. [Google Scholar]
- Mays, S. The Archaeology of Human Bones, 1st ed.; Routledge: New York, NY, USA, 1998. [Google Scholar]
- Wahl, J. Investigations on pre-Roman and Roman cremation remains from southwstern Germany: Results, potentialities and limits. In The Analysis of Burned Remains; Schmidt, C.W., Symes, S.A., Eds.; Academic Press: London, UK, 2008; pp. 145–161. [Google Scholar]
- Walker, P.L.; Miller, K.W.P.; Richman, R. Time, temperature and oxygen availability: An experimental study of the effects of environmental conditions on the color and organic content of cremated bone. In The Analysis of Burned Remains; Schmidt, C.W., Symes, S.A., Eds.; Academic Press: London, UK, 2008; pp. 129–137. [Google Scholar]
- Snoeck, C.; Lee-Thorp, J.A.; Schulting, R.J. From bone to ash: Compositional and structural changes in burned modern and archaeological bone. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2014, 416, 55–68. [Google Scholar] [CrossRef]
- Reidsma, F.H.; van Hoesel, A.; van Os, B.J.H.; Megens, L.; Braadbaart, F. Charred bone: Physical and chemical changes during laboratory simulated heating under reducing conditions and its relevance for the study of fire use in archaeology. J. Archaeol. Sci. Rep. 2016, 10, 282–292. [Google Scholar] [CrossRef]
- Marques, M.P.M.; Gonçalves, D.; Mamede, A.P.; Coutinho, T.; Cunha, E.; Kockelmann, W.; Parker, S.F.; Batista de Carvalho, L.A.E. Profiling of human burned bones: Oxidising versus reducing conditions. Sci. Rep. 2021, 11, 1361. [Google Scholar] [CrossRef]
- Habelitz, S.; Pascuala, L.; Duran, A. Transformation of tricalcium phosphate into apatite by ammonia treatment. J. Mater. Sci. 2001, 36, 4131–4135. [Google Scholar] [CrossRef]
- Marques, M.P.M.; Batista de Carvalho, L.A.E.; Gonçalves, D.; Cunha, E.; Parker, S.F. The impact of moderate heating on human bones: An infrared and neutron spectroscopy study. R. Soc. Open Sci. 2021, 8, 210774. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, D.; Vassalo, A.R.; Mamede, A.P.; Makhoul, C.; Piga, G.; Cunha, E.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Crystal clear: Vibrational spectroscopy reveals intrabone, intraskeleton, and interskeleton variation in human bones. Am. J. Phys. Anthropol. 2018, 166, 296–312. [Google Scholar] [CrossRef] [PubMed]
- Mamede, A.P.; Vassalo, A.R.; Piga, G.; Cunha, E.; Parker, S.F.; Marques, M.P.M.; Batista de Carvalho, L.A.E.; Gonçalves, D. Potential of bioapatite hydroxyls for research on archaeological burned bone. Anal. Chem. 2018, 90, 11556–11563. [Google Scholar] [CrossRef]
- Mamede, A.P.; Gonçalves, D.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Burned bones tell their own stories: A review of methodological approaches to assess heat induced diagenesis. App. Spectrosc. Rev. 2018, 53, 603–635. [Google Scholar] [CrossRef]
- Kolodziejski, W. Solid-State NMR Studies of Bone. Top. Curr. Chem. 2004, 246, 235–270. [Google Scholar]
- Duer, M.J. The contribution of solid-state NMR spectroscopy to understanding biomineralization: Atomic and molecular structure of bone. J. Magn. Reason. 2015, 253, 98–110. [Google Scholar] [CrossRef]
- Xue, H.; Yin, Y.; He, T.; Song, H.; Li, J.; Kong, X. Solid.state NMR studies on the organic matrix of bone. Nano Res. 2023, 16, 2980–2990. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Coelho, C.; Makhoul, C.; Navega, D.; Gonçalves, D.; Cunha, E.; Curate, F. New data about the 21st Century Identified Skeletal Collection (University of Coimbra, Portugal). Int. J. Leg. Med. 2021, 135, 1087–1094. [Google Scholar] [CrossRef]
- Ferreira, M.T.; Vicente, R.; Navega, D.; Gonçalves, D.; Curate, F.; Cunha, E. A new forensic collection housed at the University of Coimbra, Portugal: The 21st century identified skeletal collection. Forensic Sci. Int. 2014, 245, 202.e1–202.e5. [Google Scholar] [CrossRef]
- Brandão, A.L.C.; Batista de Carvalho, L.A.E.; Gonçalves, D.; Piga, G.; Cunha, E.; Marques, M.P.M. Differentiating Present-day from Ancient Bones by Vibrational Spectroscopy Upon Acetic Acid Treatment. Forensic Sci. Int. 2023, 347, 111690. [Google Scholar] [CrossRef] [PubMed]
- Thompson, T.J.U.; Gauthier, M.; Islam, M. The application of a new method of Fourier Transform Infrared Spectroscopy to the analysis of burned bone. J. Archaeol. Sci. 2009, 36, 910–914. [Google Scholar] [CrossRef]
- Munro, L.E.; Longstaffe, F.J.; White, C.D. Burning and boiling of modern deer bone: Effects on crystallinity and oxygen isotope composition of bioapatite phosphate. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 249, 90–102. [Google Scholar] [CrossRef]
- Thompson, T.J.U.; Islam, M.; Bonniere, M. A new statistical approach for determining the crystallinity of heat-altered bone mineral from FTIR spectra. J. Archaeol. Sci. 2013, 40, 416–422. [Google Scholar] [CrossRef]
- Weiner, S.; Bar-Yosef, O. States of preservation of bones from prehistoric sites in the Near East: A survey. J. Archaeol. Sci. 1990, 17, 187–196. [Google Scholar] [CrossRef]
- Sponheimer, M.; Lee-Thorp, J.A. Alteration of enamel carbonate environments during fossilization. J. Archaeol. Sci. 1999, 26, 143–150. [Google Scholar] [CrossRef]
- Piga, G.; Amarante, A.; Makhoul, C.; Cunha, E.; Malgosa, A.; Enzo, S.; Gonçalves, D. β-Tricalcium phosphate interferes with the assessment of crystallinity in burned skeletal remains. J. Spectrosc. 2018, 2018, 5954146. [Google Scholar] [CrossRef]
- Habelitz, S.; Pascuala, L.; Duran, A. Nitrogen-containing apatite. J. Eur. Ceram. Soc. 1999, 19, 2685–2694. [Google Scholar] [CrossRef]
- Marques, M.P.M.; Mamede, A.P.; Vassalo, A.R.; Makhoul, C.; Cunha, E.; Gonçalves, D.; Parker, S.F.; Batista de Carvalho, L.A.E. Heat-induced bone diagenesis probed by vibrational spectroscopy. Sci. Rep. 2018, 8, 15935. [Google Scholar] [CrossRef]
- Amarante, A. Burned Bones vs Unburned Bones: A Pilot Study about the Impact of Differential Post-Depositional Taphonomy on Bioanthropological Research. Master’s Thesis, University of Coimbra, Coimbra, Portugal, 2016. [Google Scholar]
- Amarante, A.; Ferreira, M.T.; Makhoul, C.; Vassalo, A.R.; Cunha, E.; Gonçalves, D. Preliminary results of an investigation on postmortem variations in human skeletal mass of buried bones. Sci. Justice 2019, 59, 52–57. [Google Scholar] [CrossRef]
- Snoeck, C.; Schulting, R.J.; Lee-Thorp, J.A.; Lebon, M.; Zazzo, A. Impact of heating conditions on the carbon and oxygen isotope composition of calcined bone. J. Archaeol. Sci. 2016, 65, 32–43. [Google Scholar] [CrossRef]
- Surovell, T.A.; Stiner, M.C. Standardizing infra-red measures of bone mineral crystallinity: An experimental approach. J. Archaeol. Sci. 2001, 28, 633–642. [Google Scholar] [CrossRef]
- Gonçalves, D.; Vassalo, A.R.; Makhoul, C.; Piga, G.; Mamede, A.P.; Parker, S.F.; Ferreira, M.T.; Cunha, E.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Chemosteometric regression models of heat exposed human bones to determine their pre-burnt metric dimensions. Am. J. Phys. Anthropol. 2020, 173, 734–747. [Google Scholar] [CrossRef]
- Piga, P.; Gonçalves, D.; Thompson, T.J.U.; Brunetti, A.; Malgosa, A.; Enzo, S. Understanding the crystallinity indices behavior of burned bones and teeth by ATR-IR and XRD in the presence of bioapatite mixed with other phosphate and carbonate phases. Int. J. Spectrosc. 2016, 2016, 4810149. [Google Scholar] [CrossRef]
- Ellingham, S.T.D.; Thompson, T.J.U.; Islam, M.; Taylor, G. Estimating temperature exposure of burnt bone: A methodological review. Sci. Justice 2015, 55, 181–188. [Google Scholar] [CrossRef]
- Thompson, T.J.U. Recent advances in the study of burned bone and their implications for forensic anthropology. Forensic Sci. Int. 2004, 146, S203–S205. [Google Scholar] [CrossRef]



| Index | Calculation/Codification | References |
|---|---|---|
| Crystallinity Index (CI) | Weiner and Bar-Yosef [36] | |
| Type B Carbonates (BPI) | Sponheimer and Lee-Thorp [37] Snoeck et al. [19] | |
| highest peak of region 1445–1470 cm−1 to type B carbonates (C/C) | Somewhat similar to the C/C ratio proposed by Thompson et al. [33] | |
| Amount of Cyanamide (CN/P) | Snoeck et al. [19] | |
| Amount of Hydroxyl Groups (OH/P 2) | Mamede et al. [25] | |
| Amount of Hydroxyl Groups (OH/P 3) | Mamede et al. [25] |
| AP C16 Artefacts | Preserved Dimensions (mm) | Surface Colour | Surface Appearance | Decoration | ||||
|---|---|---|---|---|---|---|---|---|
| Height | Width | Thickness | External | Internal | External | Internal | ||
| 19-27 | 17.91 | 13.72 | 4.5 | White | White | Eroded | Polished | Four concentric lines; Diamonds in the body |
| 19-26 | 36.15 | 18.93 | 4.6 | Black and white | Black and white | Polished | Polished | Composite diamonds and parallel lines separated by a vertical line |
| 19-11 | 19.60 | 15.01 | 6.28 | Black | Black | Polished | Polished | Four concentric lines above the rim |
| 19-23 | 34.78 | 26.57 | 5.68 | Brownish/yellowish and black | Brownish/yellowish and black | Polished | Polished | No decoration |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, D.; Rosa, J.; Brandão, A.L.; Martins, A.; Neves, C.; Diniz, M.; Arnaud, J.M.; Marques, M.P.M.; Batista de Carvalho, L.A.E. Infrared Spectroscopy to Assess Manufacturing Procedures of Bone Artefacts from the Chalcolithic Settlement of Vila Nova de São Pedro (Portugal). Appl. Sci. 2023, 13, 8280. https://doi.org/10.3390/app13148280
Gonçalves D, Rosa J, Brandão AL, Martins A, Neves C, Diniz M, Arnaud JM, Marques MPM, Batista de Carvalho LAE. Infrared Spectroscopy to Assess Manufacturing Procedures of Bone Artefacts from the Chalcolithic Settlement of Vila Nova de São Pedro (Portugal). Applied Sciences. 2023; 13(14):8280. https://doi.org/10.3390/app13148280
Chicago/Turabian StyleGonçalves, David, Joana Rosa, Ana L. Brandão, Andrea Martins, César Neves, Mariana Diniz, José M. Arnaud, Maria Paula M. Marques, and Luís A. E. Batista de Carvalho. 2023. "Infrared Spectroscopy to Assess Manufacturing Procedures of Bone Artefacts from the Chalcolithic Settlement of Vila Nova de São Pedro (Portugal)" Applied Sciences 13, no. 14: 8280. https://doi.org/10.3390/app13148280
APA StyleGonçalves, D., Rosa, J., Brandão, A. L., Martins, A., Neves, C., Diniz, M., Arnaud, J. M., Marques, M. P. M., & Batista de Carvalho, L. A. E. (2023). Infrared Spectroscopy to Assess Manufacturing Procedures of Bone Artefacts from the Chalcolithic Settlement of Vila Nova de São Pedro (Portugal). Applied Sciences, 13(14), 8280. https://doi.org/10.3390/app13148280







