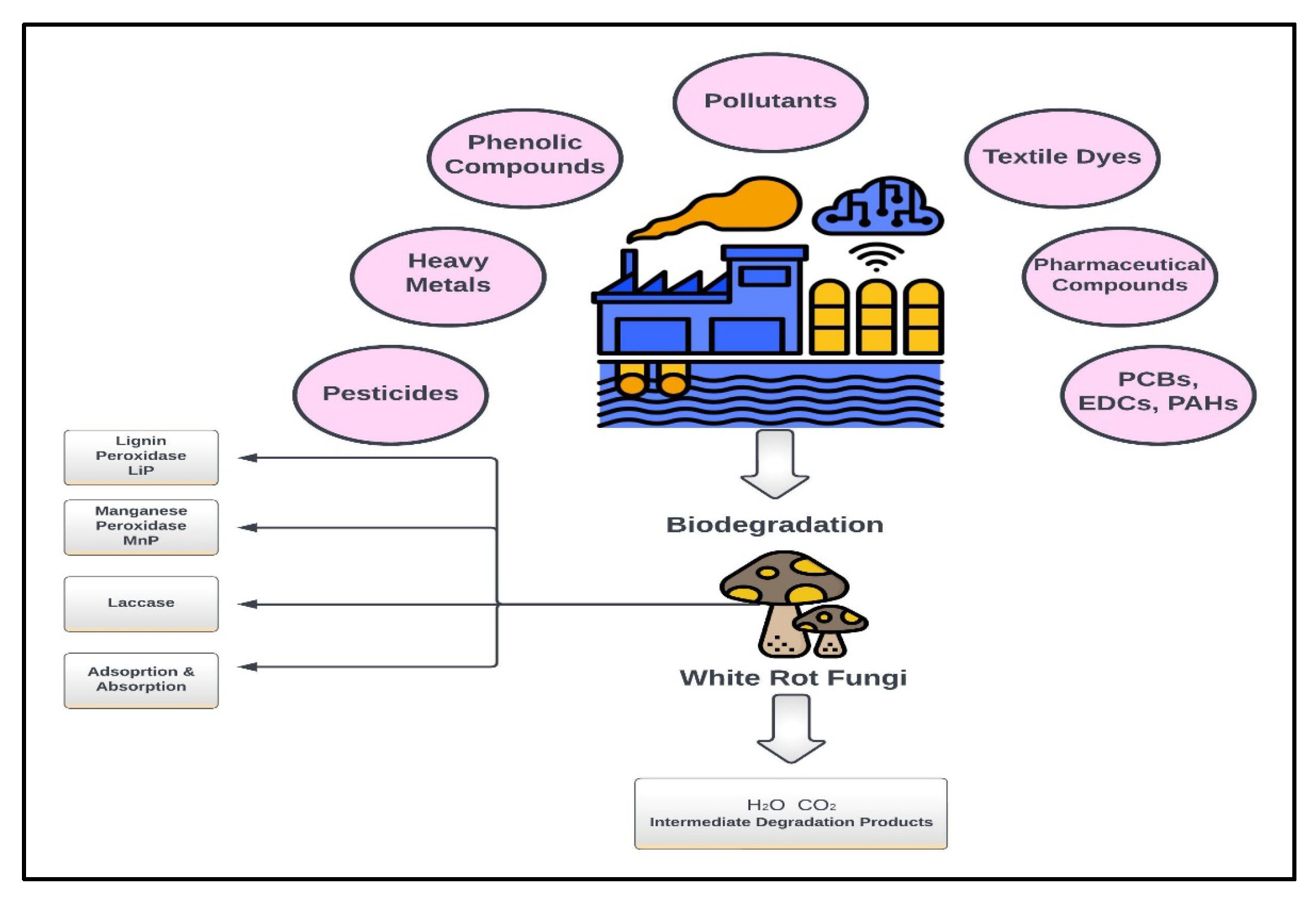
1. Introduction
Although the rapid development of the industrial sector has contributed substantially to the world economy and human welfare, it has consequences in terms of waste generation, resource consumption, and energy use, which required the development of ad hoc strategies and countermeasures to contain their impacts [
1,
2,
3,
4]. Among the most impactful consequences related to human activities is the generation of huge volumes of wastewater, which is rich in a wide variety of pollutants and is liable for serious environmental pollution and public health risks [
5,
6]. The exact nature of pollutants being produced and released into the environment depends on the type of industry. The pollutants may be organic compounds, heavy metals, textile dyes, and any other persistent chemicals of environmental concern [
7]. When these diverse pollutants are discharged into the environment, like in natural water bodies, they cause water pollution, which ultimately poses serious risks to aquatic life and public health [
8,
9,
10].
The currently in-use traditional wastewater treatment methods, such as activated sludge process, coagulation, flocculation, and adsorption, have been used commonly to address issues associated with industrial wastewater pollution [
11,
12,
13]. Although these conventional methods have been proven over time and again to be very effective in treating commonly occurring environmental pollutants, these methods are not effective in degrading industrial pollutants of a complex and recalcitrant nature, such as aromatic dyes and heavy metals. Apart from this bottleneck, these conventional methods produce a wide range of secondary pollutants during the process of degradation that are harmful to the environment [
9].
The limitations of traditional wastewater treatment systems, such as the inability to degrade pollutants of a complex nature and the production of environmentally toxic byproducts, have led to the exploration of novel and innovative treatment methods. Most of the newly developed techniques employ biological methods, involving microorganisms because of their natural ability to biouptake and degrade pollutants of a complex nature [
10,
14,
15,
16,
17,
18]. Among a wide variety of microorganisms, White Rot Fungi (WRF) have recently gained substantial attention from scientists due to their exceptional ability to produce different extracellular lignin-degrading enzymes, such as lignin peroxidase, manganese peroxidase, and laccase. These extracellular enzymes enable WRF to degrade and mineralize industrial/environmental pollutants of a complex nature into less toxic or non-toxic compounds [
19]. The extracellular enzyme-producing capability of WRF makes them an appealing candidate in the field of bioremediation to degrade complex/resistant pollutants emanating from different industries, such as the textile, paper and pulp, pharmaceutical, and/or agrochemical industries, etc. [
20].
In the recent past, researchers have been focusing specifically on WRF’s biodegradation potential and its performance optimization, as well as WRF’s effective integration in already existing wastewater treatment facilities [
21]. Mycoremediation involving White Rot Fungi (WRF) is a growing area of interest for researchers to address challenges associated with industrial wastewater treatment specifically involving complex organic pollutants and heavy metals. WRF fungi can offer effective wastewater treatment solutions at an industrial scale.
Objective and Scope
The overall objective of this literature review is to analyze the role of WRF in degrading industrial wastewater containing complex pollutants. For this purpose, by investigating the current state of research/knowledge in mycoremediation, the following specific objectives are enlisted:
The scope of this review incorporates the following aspects:
Assessing and examining the biodegradation capacity of White Rot Fungi (WRF), with a major focus on the production of extracellular lignin-degrading enzymes of various natures, along with factors influencing the enzymatic capabilities of WRFs.
Capabilities assessment of WRF for degrading complex pollutants arising from different versatile industries, such as textile, paper and pulp, pharmaceutical, etc.
Factors affecting WRF performance in treating industrial pollutants. These factors may include environmental factors, such as pH, temperature, and nutrient availability. Other factors that will be discussed in this review will include fungal strain selection, pollutants characteristics, and the design of the bioreactor.
2. Materials and Methods
To carry out a thorough, balanced, and comprehensive review of existing literature, the below steps were followed:
Various academic databases and web servers, for example, Web of Science, PubMed, and Scopus, were used to extract literature relevant to the topic. Tentatively, articles published in the last 2 decades were considered while extracting data (to report recent advancements in the field), except for a few deemed necessary, but that fell outside the temporal scope of 2 decades. A wide range of keywords were used to identify and download the most relevant research articles. Major keywords and search terms employed were “White Rot Fungi”, “wastewater treatment”, “biodegradation”, “bioremediation”, “lignin-degrading enzymes”, and “industrial wastewater”.
A total of 661 articles were shown in the results based on the keywords used. Articles containing relevant keywords published in English were included, and the rest were excluded.
After inclusion and exclusion criteria, screening of research articles was carried out, primarily involving the removal of duplicate articles and the selection of the remaining articles based on their titles and abstracts being highly relevant to the research question.
Articles were then further shortlisted based on their abstract and relevance to the topic. After exclusion and screening, 121 articles were selected for review. In
Figure 1 and
Figure 2, the geographical and temporal distributions of the selected 121 articles are presented to exhibit the unbiased approach.
By strictly following the above-stated approach, this review paper will provide a thorough and balanced analysis of the existing literature related to the role of WRF in degrading industrial pollutants of a complex nature.
3. Industrial Wastewater Pollution
Recently, wastewater pollution caused by various industrial pollutants has been a major environmental issue. The issue of industrial wastewater pollution is mainly arising from the untreated or inadequately treated chemical compounds being released from different operations/processes. Various industries, like paper and pulp, agro-chemical, textiles, and petrochemical, are producing unprecedented amounts of wastewater containing several toxic and resistant pollutants, such as organic compounds, heavy metals, aromatic dyes, and persistent chemicals. The untreated or inadequate release of these pollutants into natural water bodies and ecosystems may lead to dire environmental and public health consequences [
22].
3.1. Types of Industrial Pollutants
3.1.1. Organic Compounds
Industrial wastewater may contain a variety of organic pollutants, which are not easily degradable. These organic pollutants may include, but are not limited to, Polycyclic Aromatic Hydrocarbons (PAHs), Volatile Organic Compounds (VOCs), and phenols. All these organic compounds and pollutants can pose serious environmental and public health concerns due to their ability to be extremely toxic, carcinogenic, and/or mutagenic. As these compounds/chemicals are organic in nature, they may contribute to depleting the dissolved oxygen content of water bodies by increasing the Biological Oxygen Demand (BOD) and Chemical Oxygen Demand (COD), which may lead to the occurrence of dead zones and disturbance of aquatic ecosystems [
23].
3.1.2. Heavy Metals
Several heavy metals, such as Chromium, Lead, Cadmium, Mercury, Nickel, Copper, and Arsenic, may be present in industrial wastewater discharges. Different heavy metals may originate from different industrial processes, including, but not limited to, metal electroplating, batteries manufacturing, mining, etc. Heavy metals can have serious environmental impacts and substantial public health concerns, as these heavy metals can accumulate in aquatic life through bioaccumulation and may travel towards the top of the food chain up to human beings through the process of biomagnification [
24]. Exposure to heavy metals over a longer period of time may result in various health issues, including, but not limited to, neurological disorders and organ damage [
25].
3.1.3. Dyes
The release of various dyes is a typical characteristic of a textile industry, where these are abundantly used. Almost all types of textile dyes are highly complex, persistent, and resistant and, thus, cannot be treated by conventional wastewater treatment methods. As far as the environmental impacts of dyes are concerned, they can color water bodies, which results in the reduced penetration of light, thus affecting/slowing down the process of photosynthesis. Additionally, many textile dyes are known to be mutagenic, toxic, and carcinogenic in nature; thus, they pose a serious threat to human health, as well [
26].
3.1.4. Persistent Chemicals
Industrial wastewater can comprise various persistent chemicals, such as polychlorinated biphenyls (PCBs), organochlorine pesticides, and per- and polyfluoroalkyl substances (PFAS). These chemicals are tolerant to degradation and can endure in the environment for long periods, resulting in bioaccumulation and potential adverse impacts on human health and ecosystems [
27].
4. Limitations of Conventional Treatment Methods
The currently in-use traditional wastewater treatment methods, such as activated sludge process, coagulation, flocculation, and adsorption, have been used commonly to address issues associated with industrial wastewater pollution. Although these conventional methods have been proven over time and again to be very effective in treating commonly occurring environmental pollutants, these methods are not effective in degrading industrial pollutants of a complex and recalcitrant nature, such as aromatic dyes and heavy metals. Apart from this bottleneck, these conventional methods produce a wide range of secondary pollutants during the process of degradation that are harmful to the environment [
9].
4.1. Incomplete Degradation of Recalcitrant Compounds
The limitation of most of the conventional wastewater treatment systems is their inability to completely treat/degrade, or their incomplete degradation of recalcitrant and toxic compounds, including synthetic dyes, many resistant organic chemicals, and pharmaceuticals. As a result, these persistent and resistant compounds tend to stay in the ecosystem for a very long time, thus posing serious environmental and public health concerns. In many cases, conventional treatment techniques convert these chemicals to other chemical forms/compounds that are still toxic or, in some cases, more toxic to the environment and humans [
28,
29].
4.2. Generation of Secondary Pollution
Traditional wastewater treatment methods involving biological secondary treatment generate large amounts of sludge and chemical byproducts. For example, two common processes of conventional wastewater treatment plants are coagulation and flocculation. Both processes use different chemicals as coagulants or flocculants which are sources of additional pollution to the ecosystem. Furthermore, managing and disposing of sludge in an environmentally safe manner is itself a challenge, as it is hazardous in nature due to the presence of the pathogenic bacteria and heavy metals in it [
30,
31].
4.3. High Energy Consumption
Most of the traditional wastewater treatment plants employ the technique of activated sludge and sometimes advanced oxidation processes. Both these techniques are highly energy intensive. The high energy consumption in conventional wastewater treatment systems not only increases the overall operational cost of the system, but also adds to greenhouse gas emissions and subsequently global warming and climate change [
32].
4.4. Low Heavy Metals Removal Efficiency
Traditional wastewater treatment techniques are usually not suitable for effective removal of heavy metals from industrial wastewater. Although some conventional methodologies, like adsorption and precipitation, can remove some heavy metals, these techniques depend on various external factors, such as pH, the presence of certain ions, and the initial metal concentration. Apart from that, these conventional methods are not cost-effective and may generate large amounts of hazardous sludge, which itself is an environmental risk [
33,
34].
5. Merits of WRF-Based Alternative Treatment Techniques
Considering the limitations associated with conventional wastewater treatment methods, such as the inability of these techniques to degrade pollutants of a complex nature, high operating cost, and generation of secondary pollutants, there is an increasing demand for alternative wastewater treatment methods that can address all these limitations. This has led to the increasing interest of researchers in bioremediation, especially mycoremediation, such as the employment of White Rot Fungi, which provide a promising solution to all these issues.
5.1. Broad-Spectrum Biodegradation Capabilities
White Rot Fungi (WRF) are known for their distinctive capability to produce extracellular lignin-degrading enzymes, for example, manganese peroxidase, lignin peroxidase, and laccase. These extracellular enzymes help WRF oxidize and mineralize/degrade a vast variety of industrial pollutants, such as recalcitrant, resistant, and toxic compounds, which are not easily degradable by traditional wastewater treatment methods. Additionally, WRF can absorb and adsorb various heavy metals from industrial wastewater. These multidimensional and broad-spectrum capabilities of WRF to degrade and adsorb/absorb various industrial pollutants make them an ideal option for treatment [
35].
5.2. Environmentally Sustainable Technology
Industrial pollutants degradation by White Rot Fungi (WRF)-based system is considered as an environmentally safe and sustainable approach, as WRFs rely on the natural process of degradation and adsorption/absorption, rather than intensive use of chemicals (flocculants and coagulants) and energy, which further adds to environmental problems in the form of secondary pollutants and greenhouse gases emissions [
36].
5.3. Adaptability to Different Bioremediation Treatment Technologies
White Rot Fungi (WRF)-based wastewater treatment is very flexible, and it can be used in combination with other state-of-the-art bioremediation techniques, such as solid-state fermentation, submerged fermentation, immobilized fungal systems, and enzymatic treatment using isolated WRF enzymes. This flexibility grants the development of customized treatment systems to address the specialized needs of different industrial sectors and wastewater compositions [
37,
38].
5.4. Potential for Combined Conventional Treatment Approaches
WRF-based wastewater treatment systems can be combined with conventional treatment systems, such as the activated sludge process, Advanced Oxidation Process (AOP), or adsorption, to enhance the already present/designed wastewater treatment system. Combining this comparatively new and innovative technology with conventional methods will help overcome each technique’s limitations, resulting in a more thorough and effective treatment of industrial and otherwise resistant chemical pollutants [
39,
40].
Although WRF-based wastewater treatment systems have many advantages over conventional treatment methods, there are certain challenges that need to be addressed, such as enhancement of the technology for commercial and industrial applications [
41], operational parameters optimization according to the need and type of industry, and assurance of cost-effectiveness. However, rigorous, and widely expanding research is currently being carried out on the degradation capabilities of White Rot Fungi (WRF), which will pave the way for this technology in industrial and commercial wastewater treatment in the near future [
42].
6. White Rot Fungi: An Overview
White Rot Fungi (WRF) belong to the basidiomycetes group. These species are important in the degradation and natural decomposition of lignocellulosic material, like decayed wood and plant residues [
43]. White Rot Fungi have the unique ability to naturally decompose the lignin, which is a complex and recalcitrant biopolymer. This unique ability of WRF has attracted the attention of researchers looking for alternatives to conventional wastewater treatment plants that are incapable of treating complex chemical pollutants [
44].
6.1. Taxonomy and General Characteristics
Agaricomycetes is a class of Fungi within the Phylum Basidomycota, to which White Rot Fungi (WRF) belongs [
45]. The most commonly and vastly studied genera of WRF are Phanerochaete, Trametes, Pleurotus, and Ganoderma. The Fungi from these genera are typically known and characterized by their capability to naturally decompose lignocellulosic material, including lignin, hemicellulose, and cellulose [
43].
White Rot Fungi are filamentous fungi, typically characterized by thread-like structures known as hyphae. Hyphae, when combined, form a structure known as Mycelium. WRF usually exhibit sexual reproduction by producing basidiospores, produced by specialized fungal structures known as basidia [
46]. As far as the habitats of WRF are concerned, these can be commonly found in environments like decaying wood, compost piles, and forest soils. In such environments, WRFs play an important role in nutrient cycling and carbon sequestration [
47].
6.2. Lignin-Degrading Enzyme System
White Rot Fungi (WRF) are capable of producing extracellular lignin-degrading enzymes, like lignin peroxidase (LiP), manganese peroxidase (MnP), and laccase. The excellent natural decomposition (of recalcitrant industrial chemicals) properties of WRF are mainly due to these extracellular lignin-degrading enzymes. These enzymes speed up the process of lignin oxidation and depolymerisation, which further assist WRF to approach the hemicellulose and cellulose components of lignocellulosic materials for the sake of energy and nutrient incorporation [
48,
49].
Lignin Peroxidase (LiP): LiP, a heme-containing enzyme, acts as a catalyst for the oxidation of various aromatic and non-aromatic compounds. LiP has the capability to directly attack the lignin by breaking the C-C and C-O bonds present within the complex structure of lignin. High redox potential is a usual characteristic of the LiP enzyme. This characteristic permits LiP to naturally decompose and oxidize several recalcitrant pollutants, like PAHs, textile dyes, and many phenolic compounds [
50].
Manganese Peroxidase (MnP): Another extracellular enzyme WRF produces is MnP. MnP is also a heme-containing enzyme, which assists in the oxidation of Mn(II) to Mn(III). In return, Mn(III) can degrade a variety of phenolic and non-phenolic compounds. Due to Mn(III) production, MnP can indirectly attack and degrade lignin through oxidation [
51].
Laccase: Another extracellular multicopper oxidase enzyme produced by WRF is laccase. Laccase helps in speeding up the oxidation of phenolic and non-phenolic compounds, with the associated reduction of molecular oxygen to water. Although the redox potential of laccase is lower than the other two enzymes, i.e., MnP and LiP, the enzyme has the capability to degrade and oxidize a range of pollutants, such as phenols, textile dyes, and endocrine-disrupting compounds [
51].
6.3. Non-Lignin-Degrading Enzyme System
Apart from the lignin-degrading enzymes in WRF, other non-lignin-degrading enzymes, such as cellobiose dehydrogenases, versatile peroxidases, and hydrolases, have been found to contribute to the transformation of diverse polluting substances. Cellobiose dehydrogenases play a role in the breakdown of cellulose and lignocellulosic biomass by generating reactive oxygen species. CDHs are capable of oxidizing cellobiose and other cello-oligosaccharides, freeing electrons that can be transported to various electron acceptors, including pollutants, accelerating their degradation [
52]. Moreover, hydrolases are responsible for the hydrolysis of numerous pollutant compounds, such as Esters, Amides, Glycosides, Nitriles, Phosphates, and Sulphates [
53].
Likewise, peroxidases, including versatile peroxidases (VPs) and manganese peroxidases (MnPs), have been identified to influence the degradation of diverse pollutants. VPs are able to oxidize a wide range of aromatic compounds, while MnPs demonstrate degrading ability towards lignin and recalcitrant pollutants [
54]. VP shows a visible substrate specificity and can oxidize both high-redox-potential compounds, such as dyes, and low-redox-potential compounds, such as phenolic compounds and lignin [
55].
6.4. Laccase Isozymes System
During laccase production, White Rot Fungi (WRF) have the notable capability to induce the production of laccase isozymes in the presence of xenobiotic substances/pollutants. These isozymes show similar enzymatic activities on mandatory laccases, but are specifically produced in response to the presence of hazardous substances [
56].
When WRF encounter hazardous pollutants, like xenobiotics, they trigger a signaling cascade that leads to the activation of specific genes responsible for the synthesis of laccase isozymes [
57]. These isozymes support the activity of laccases by increasing the range of substrates that can be treated and enhancing the overall degradation efficiency. The isozymes are involved in the degradation of many recalcitrant pollutants, such as polycyclic aromatic hydrocarbons (PAHs), pharmaceuticals, and other xenobiotics [
58].
6.5. Mechanism of Pollution Degradation by WRF
As already discussed, White Rot Fungi are gaining attention to treat industrial wastewater containing various complex pollutants due to their ability to produce extracellular lignin-degrading enzymes [
51].
Figure 3 shows WRF’s generic pollutants degradation mechanism.
6.5.1. Generation of Reactive Radicals
The extracellular lignin-degrading enzyme production by White Rot Fungi consists mainly of LiP and MnP. These two enzymes are responsible for producing reactive radicals with the assistance of redox reactions. These reactive radicals are non-specific oxidizing agents, which can degrade a range of complex and recalcitrant pollutants present in industrial wastewater. The reactive radicals carry out the oxidation of recalcitrant pollutants through several breakdown processes, including the removal of hydrogen atoms, chemical bond breakdown, and several other oxidative transformations [
59,
60].
6.5.2. Co-Metabolism and Cometabolites
During the process of co-metabolism, extracellular enzymes of WRF, along with the degradation and oxidation of lignin, can oxidize other pollutants without assimilating any energy or nutrients from the process. On the other hand, co-metabolites refer to the auxiliary or secondary products produced during the degradation of primary pollutants by WRF. These co-metabolites can enhance the lignin-degrading capability of WRF, which further facilitates the treatment of industrial wastewater pollutants [
61].
6.5.3. Enzyme-Mediated Oxidation
Laccase is a multicopper oxidase enzyme, which can degrade a range of phenolic and non-phenolic compounds. Laccase has less redox potential than MnP and LiP, but its activity can be enhanced with the help of small molecules mediators, which can expand the range of substrates/pollutants that can be oxidized by the enzyme [
62]. In fact, these mediators can transfer electrons between the enzyme and pollutants, thus facilitating the breakdown and oxidation of the pollutant [
63].
The mediators generally applied in WRF-based bioremediation are the low-molecular-weight redox mediators, such as synthetic dyes and phenolic compounds [
64]. These mediators act as electron shuttles, assisting the transfer of electrons between the enzymes and recalcitrant pollutants. They can accept electrons from the ligninolytic enzymes generated by WRF and transport them to the target pollutants, efficiently starting the degradation process.
The availability of mediators in the reaction medium has numerous valuable effects on enzymatic reactions. Firstly, mediators can directly work together with the enzymes to augment their stability and activity. By acting as electron carriers, mediators can reproduce the active form of enzymes and inhibit their inactivation due to oxidative damage [
65]. This process permits the enzymes to effectively take part in the degradation of pollutants over prolonged periods. Secondly, mediators can broaden the range of pollutants that can be treated by WRF. A few recalcitrant pollutants, like polycyclic aromatic hydrocarbons (PAHs) and chlorinated compounds, are intrinsically resistant to direct enzymatic attack by WRF’s ligninolytic enzymes. However, the addition of mediators can overcome this limitation by allowing indirect degradation pathways. The mediators support the production of reactive radicals, like hydroxyl radicals, which can react with and treat recalcitrant pollutants [
66].
The selection of appropriate mediators is essential for optimizing the bioremediation process. The selection of a mediator relies on the specific pollutants targeted for treatment and the enzymatic systems of the WRF species being employed. Various mediators may exhibit varying efficiencies and selectivity in increasing the degradation of specific pollutants.
6.5.4. Biosorption and Bioprecipitation
Apart from enzyme-based degradation and oxidation of recalcitrant pollutants, White Rot Fungi can employ the processes of biosorption and bioprecipitation to remove certain pollutants from industrial wastewater [
67]. The fungal cell wall is usually filled with chitin and certain other polymers, which help the fungal cell wall to bind with and store metal ions and some other pollutants through several interactions, like electrostatic interactions, complexation, and ion exchange [
68]. Additionally, WRF can alter the pH or redox conditions of the wastewater treatment system, which causes metal ions to precipitate as insoluble salts or hydroxides [
69].
6.5.5. Acid-Based Bioremediation Mechanism
In addition to enzymes, acids, including oxalic acid, play a substantial role in the bioremediation of pollutants and the breakdown of xenobiotic compounds by White Rot Fungi (WRF).
An important characteristic of oxalic acid is its contribution in the chelation of metal ions. WRF, for example,
Phanerochaete chrysosporium, are able to produce oxalic acid as a metabolic byproduct during the degradation of lignocellulosic materials. The produced oxalic acid can chelate with metal ions available in the environment, like iron, manganese, and copper ions [
70]. This chelation process assists in mustering these metal ions and making them available for supplementary enzymatic reactions involved in the degradation of pollutants.
Likewise, the acidic circumstances generated by oxalic acid can accelerate the generation and activity of ligninolytic enzymes in WRF. The increased enzymatic activity under acidic pH conditions can induce the breakdown of complex pollutants, including aromatic hydrocarbons, dyes, and pharmaceuticals [
71,
72]. The mixed action of oxalic acid and ligninolytic enzymes permits the efficient breakdown and detoxification of these hazardous compounds.
6.6. Degradation of Industrial Pollutants by WRF
White Rot Fungi (WRF), due to their exceptional extracellular lignin-degrading enzymes producing ability, coupled with biosorption and bioprecipitation capabilities, have shown promising results in treating industrial wastewater pollutants of a complex nature [
73].
In this section, the role of WRF in degrading various pollutants emanating from different industrial setups will be discussed.
6.6.1. Phenolic Compounds
Phenolic compounds are common pollutants in industrial wastewater originating from various industries, including textile, pulp and paper, and petrochemical [
74]. Typical phenolic compounds are cresols, phenols, and chlorophenols, all of which can be easily degraded by WRF’s extracellular enzymes. These lignin enzymes produce reactive radicals that, in turn, oxidize and degrade phenolic compounds of various types into less toxic and simpler substances, or these enzymes can even mineralize these compounds into CO
2 and water [
75].
Table 1 shows the phenolic compounds removal efficiency WRF.
6.6.2. Dyes and Textile Wastewater
The textile industry is rapidly growing worldwide. The industry generates large amounts of wastewater containing many synthetic dyes, mostly recalcitrant and toxic [
78]. Common dyes in textile industry wastewater are Azo dyes, anthraquinone, and triphenylmethane dyes, which can be easily degraded by WRF’s extracellular enzymes, especially MnP and laccase [
59]. In some cases, redox mediators can augment the enzymatic oxidation of dyes, resulting in quicker and more effective decolorization [
79].
Table 2 higlights WRF removal capabilities of textile dyes.
6.6.3. Pesticides and Agrochemicals
The foremost and major usage point of pesticides is the agricultural sector. These pesticides can contaminate waterbodies through surface water runoff or through percolation and leaching to underground water bodies [
82]. The pesticides used in agricultural sectors are organochlorines, organophosphates, and carbamates. Most of the pesticides can easily be degraded by the WRF’s mechanism of extracellular enzyme production as shown by
Table 3. These enzymes release reactive radicals that can cleave bonds present in pesticide molecules, thus transforming them into non-toxic or less toxic byproducts [
83].
6.6.4. Pharmaceuticals and Personal Care Products
Due to their persistence in the environment and association with ecotoxicological impacts, Pharmaceuticals and Personal Care Products (PPCPs) have recently emerged as notable pollutants in industrial wastewater requiring immediate attention [
85]. The most common PPCPs include, but are not limited to, antibiotics, hormones, and anti-inflammatory drugs. All these PPCPs can be degraded by WRF-produced extracellular lignin-degrading enzymes, coupled with the enhancement of the degradation efficiency by the induction of redox mediators [
42].
Table 4 exhibits such removal capabilities of WRF.
6.6.5. Heavy Metals
In addition to degradation of organic pollutants, White Rot Fungi (WRF) play a considerable role in the remediation of heavy metal-contaminated environments.
WRF carry out heavy metals remediation by the process of biosorption. The fungal cell walls contain many functional groups, such as carboxyl, amino, and hydroxyl groups, which exhibit a high affinity for metal ions. These functional groups can bind to heavy metal ions available in the surrounding environment, leading to their sequestration and immobilization on the fungal biomass [
92]. Heavy Metals removal by WRF is also supported by literature as explained in
Table 5.
In addition to biosorption, WRF can employ enzymatic processes for the remediation of heavy metals. Specific enzymes released by WRF, such as laccases and peroxidases, are involved in the oxidation and reduction reactions of metal ions. Laccases participate in the oxidation of metal ions, leading to their precipitation or conversion into less toxic forms [
56].
6.6.6. Other Pollutants
Apart from the industrial pollutants discussed in the previous section, White Rot Fungi can degrade and oxidize many other pollutants of environmental concern that cannot be treated by conventional wastewater treatment plants, including Poly Aromatic Hydrocarbons (PAHs), Poly Chlorinated Biphenyls (PCBs), and Endocrine-Disrupting Compounds (EDCs) [
98]. The unique ability of WRF to produce lignin-degrading extracellular enzymes helps in degrading and oxidizing these compounds [
59,
60].
Table 6 shows degradation of such pollutants by WRF.
White Rot Fungi can degrade several industrial pollutants of a complex nature and can be an excellent option for wastewater treatment in the near future. However, in order to make practical and commercially viable WRF-based wastewater treatment systems, it is important to determine the optimized conditions for WRF and the factors that can affect the performance of these fungal species.
7. Factors Affecting WRF-Based Wastewater Treatment
Various factors can impact the performance and efficacy of White Rot Fungi-based wastewater treatment processes. It is important to understand the positive and/or negative role of these factors in WRF efficiency, so that the WRF-based wastewater treatment systems can be optimized for the enhanced degradation of industrial wastewater pollutants.
7.1. Environmental Factors
Environmental factors, like pH, temperature, Dissolved Oxygen (DO), and nutrient availability, are crucial for White Rot Fungi’s growth and metabolic activities, thus ultimately enhancing the pollutants degrading capabilities of WRFs. Although the optimum temperature and pH ranges may be different for different White Rot Fungi species, the best temperature and pH slots for WRF to work efficiently are 20 °C–3°C and 4–7, respectively [
103]. White Rot Fungi are naturally aerobic; thus, the availability of appropriate Oxygen levels is very important for their enzymatic activities [
104]. Similarly, the availability of nutrients, such as Nitrogen and Phosphorus, can also impact the production of enzymes in fungi, ultimately affecting the overall treatment efficacy [
105].
7.2. Enzyme Induction and Regulation
Industrial wastewater treatment systems contain inducers and inhibitors. Inducers, like aromatic compounds, can facilitate the production of fungal enzymes, leading to more efficient removal of pollutants from wastewater. On the other hand, inhibitors, like heavy metals and certain toxic organic compounds, can slow down or suppress the synthesis of fungal enzymes, reducing or compromising the treatment efficiency of the pollutants. Identification and management of all inducers and inhibitors in an industrial wastewater treatment system are crucial to optimize the degrading capabilities of WRF [
106].
7.3. Fungal Strain Selection
The selection of fungal strains certainly impacts the overall performance of WRF-based wastewater treatment systems because different fungal strains may be able to degrade different classes of pollutants and may contain different sets of enzymatic profiles. White Rot species belonging to Phanerochaete, Trametes, Pleurotus, and Ganoderma are well known for their wastewater treatment traits in general, as per the documented literature and research. However, the selection of certain fungal strains depends on the type of wastewater treatment system, type of pollutants, and objectives of wastewater treatment. The selection of an appropriate strain, or even an engineered strain, will enhance the WRF-based wastewater treatment systems’ efficiency substantially [
107].
7.4. Bioreactor Design and Operating Conditions
WRF-based degradation and oxidation of pollutants can also be affected by the design and operation of the bioreactors in which WRF species are present. Bioreactor conditions, such as mixing, hydraulic retention time, and biomass concentration, can affect the performance of WRF [
108]. Several bioreactor designs, keeping in mind the treatment objectives, have been employed in WRF-based wastewater treatment systems, including, but not limited to, packed-bed [
109], fluidized-bed [
110], and membrane reactors [
111].
7.5. Integration with Other Treatment Technologies
Newly emerged and innovative wastewater treatment techniques, such as WRF-based treatment systems, can be integrated with conventional wastewater treatment methods, such as activated sludge processes [
112], adsorption [
113], or advanced oxidation [
112], to achieve desired objectives of wastewater treatment. A combination of advanced and conventional techniques will assist in addressing the limitations of each individual technique, ultimately enhancing the wastewater treatment efficiency.
8. Bioremediation Technologies Employing WRF
8.1. Solid-State Fermentatioon
Solid-state fermentation can be described as WRF growth on different solid surfaces/substrates, such as agricultural residues, with the purpose of treating wastewater [
114]. The fungal treatment mechanism of wastewater is the same, i.e., the production of extracellular enzymes that lead to the oxidation of pollutants. Solid-state fermentation can be executed in different modes, for example, batch, fed-batch, or continuous mode, considering the specific requirements of treatment objectives. Solid-state fermentation has many merits over other treatment methods because SSF requires a low amount of energy and water, produces less waste, and this type of setup can easily be scaled up [
115].
8.2. Submerged Fermentatioon
Submerged fermentation is defined as the growth of WRF in an aqueous medium, such as wastewater. The pollutants degradation principle is the same, i.e., enzymatic oxidation. Just like solid-state fermentation, submerged fermentation can also be carried out in different modes, like batch, fed-batch, or continuous. SmF can also be combined with conventional treatment technologies, like adsorption and advanced oxidation processes. In submerged fermentation, environmental conditions can be controlled in a more efficient way, and ultimately higher enzymatic activities can be achieved compared to solid-state fermentation [
116].
8.3. Immobilized Systems
Immobilized fungal systems are characterized by fungal strains attached to a solid support, like beads or fibers. Immobilized systems are preferred due to improved stability, a continuous system, and easy biomass separation. Immobilized fungal systems can be configured with a packed bed or fluidized bed bioreactors [
117].
8.4. Fungal Bacterial Co-Cultures Systems
Fungal and bacterial co-cultures are characterized by the combined use of fungal strains and bacterial cultures in the same wastewater treatment system to enhance the degradation of complex industrial pollutants through combined enzymatic actions. However, such types of co-cultures need a careful selection of microbial strains and optimization of operating conditions [
118].
8.5. Enzymatic Treatment with Isolated WRF Enzymes
White Rot Fungi produce different extracellular enzymes, such as manganese peroxidase, lignin peroxidase, and laccase. These enzymes can be isolated from fungal strains through fermentation, further purification, and subsequent addition to wastewater treatment systems. Such wastewater treatment has advantages, like high pollutant specificity and extremely low production of secondary pollutants. However, the treatment comes with challenges, like enzyme stability, recovery, and the associated cost, which need to be addressed before going towards the practical and large-scale applications of the technique [
119].
9. Challenges and Future Perspectives
White Rot Fungi have exhibited enormous potential in treating complex industrial pollutants not treated by conventional wastewater treatment systems. However, there are several challenges associated with WRF-based wastewater treatment systems that need to be addressed to fully utilize the potential of WRF at large industrial and commercial scales.
9.1. Scale-Up and Process Optimization
Scaling up WRF-based wastewater treatment techniques to large industrial and commercial levels is itself a challenge. For this purpose, future researchers must focus on process parameters optimization, like reactor design compatible with WRF, suitable substrate selection, and the determination of optimal operational factors affecting the efficiency of WRFs.
9.2. Economic Feasability
The economic feasibility is another hindrance for WRF-based wastewater treatment systems to enter large industrial and commercial scales. Researchers should determine how to develop cost-effective bioreactors for WRFs, optimize resources, and understand the potential use and fate of byproducts being produced along the process to improve economic viability.
9.3. Regulatory Frameworks and Public Perception
The WRF-based wastewater treatment systems should comply with established regulatory frameworks and must be publicly accepted. Potential future research should focus on the safety and effectiveness of WRF-based treatment systems, coupled with outreach programs to educate stakeholders about the potential merits of the technology.
9.4. Advances in Genetic Engineering and Synthetic Biology
WRF-based treatment systems may still be intolerant of certain types of toxic pollutants. Considering the rapid advancement in genetic engineering and synthetic biology, future research should focus on using these state-of-the-art technologies to improve the performance of WRF-based treatment systems.
10. Conclusions
White Rot Fungi (WRF) have recently emerged as a reliable candidate for the biodegradation of industrial pollutants, especially when recalcitrant and complex. Their ability to produce extracellular lignin-degrading enzymes, such as manganese peroxidase (MnP), lignin peroxidase (LiP), and laccase, make WRF unique and stand out in breaking down complex organic and inorganic pollutants into less toxic byproducts, or even complete mineralization.
Several WRF-based bioremediation technologies, such as solid-state fermentation, submerged fermentation, immobilized fungal systems, fungal–bacterial co-cultures, and enzymatic treatment with isolated WRF enzymes, have been established and explored to treat industrial pollutants. Each of these technologies has its merits and demerits, and the adoption of certain technology rests on the specific conditions/requirements of the wastewater treatment system.
Despite the numerous advantages of utilizing WRF-based wastewater treatment systems, certain challenges and issues need to be addressed to incorporate the technology at an industrial and commercial scale. These challenges include appropriate fungal strains selection, strains improvement through genetic engineering, enzymes isolation, stability and activity, design of bioreactors and subsequent optimization, combination with other treatment technologies, economic feasibility, and understanding remediation pathways. Future extensive and rigorous research in these specified areas must be carried out to provide stakeholders with an efficient WRF-based wastewater treatment system that can degrade industrial pollutants not degraded by conventional treatment plants.
In conclusion, WRF-based wastewater treatment systems provide a great solution to industrial water pollution, especially after the emergence of novel recalcitrant and complex pollutants that conventional technologies do not treat. In order to bring WRF-based wastewater treatment systems from the lab scale to the industrial scale, overcoming existing challenges is extremely necessary.
Author Contributions
Conceptualization, W.L. and S.P.; methodology, W.L.; formal analysis, W.L.; resources, W.L. and M.S.; data curation, M.I., C.C., M.S. and S.P.; writing—original draft preparation, W.L.; writing—review and editing, W.L., M.I., C.C., S.P. and M.S.; supervision, S.P. and C.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Osman, A.I.; Chen, L.; Yang, M.; Msigwa, G.; Farghali, M.; Fawzy, S.; Rooney, D.W.; Yap, P.-S. Cost, Environmental Impact, and Resilience of Renewable Energy under a Changing Climate: A Review. Environ. Chem. Lett. 2023, 21, 741–764. [Google Scholar] [CrossRef]
- Spagnuolo, A.; De Santo, G.; Vetromile, C.; Masiello, A.; Di Costanzo, P.; Esposito, S.; Buono, U.; Di Cicco, M.R.; Lubritto, C. Characterizing Passenger-Ship Emissions: Towards Improved Sustainability for MedMar Fleet (Gulf of Naples). Energy Effic. 2022, 15, 55. [Google Scholar] [CrossRef] [PubMed]
- Vetromile, C.; Spagnuolo, A.; Petraglia, A.; Masiello, A.; Di Cicco, M.R.; Lubritto, C. Pre- and Post-Operam Comparison of the Energy Consumption of a Radio Base Station under Energy Efficiency Actions. Energy Build. 2021, 236, 110772. [Google Scholar] [CrossRef]
- Udugama, I.A.; Petersen, L.A.H.; Falco, F.C.; Junicke, H.; Mitic, A.; Alsina, X.F.; Mansouri, S.S.; Gernaey, K.V. Resource Recovery from Waste Streams in a Water-Energy-Food Nexus Perspective: Toward More Sustainable Food Processing. Food Bioprod. Process. 2020, 119, 133–147. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Lubritto, C. Energetic and Environmental Analysis of a Wastewater Treatment Plant through Static and Dynamic Monitoring Activities. Int. J. Environ. Sci. Technol. 2020, 17, 4299–4312. [Google Scholar] [CrossRef]
- Roy, S.; Garg, A.; Garg, S.; Tran, T.A. (Eds.) Advanced Industrial Wastewater Treatment and Reclamation of Water: Comparative Study of Water Pollution Index during Pre-Industrial, Industrial Period and Prospect of Wastewater Treatment for Water Resource Conservation; Springer: Cham, Switzerland, 2022. [Google Scholar]
- Bugajski, P.M.; Nowobilska-Majewska, E.; Kurek, K. The Variability of Pollution Load of Organic, Biogenic and Chromium Ions in Wastewater Inflow to the Treatment Plant in Nowy Targ. J. Water Land Dev. 2017, 35, 11–17. [Google Scholar] [CrossRef]
- Lam, S.-M.; Choong, M.-K.; Sin, J.-C.; Zeng, H. Synchronous Organics Removal and Copper Reduction in Semiconductor Wastewater with Energy Recuperation via Photocatalytic Fuel Cell. E3S Web Conf. 2020, 167, 01002. [Google Scholar] [CrossRef]
- Kow, S.-H.; Fahmi, M.R.; Azner Abidin, C.Z.; Ong, S.-A.; Ibrahim, A.H.; Sabri, S.N.; Razali, N.A. Oxidation of P-Cresol by Ozonation. Sains Malays. 2018, 47, 1085–1091. [Google Scholar] [CrossRef]
- Maallah, R.; Chtaini, A. Phenol Removal from Wastewaters by Electrochemical Biosensor. Am. Int. J. Biol. Life Sci. 2019, 1, 24–32. [Google Scholar] [CrossRef]
- Di Cicco, M.; Masiello, A.; Spagnuolo, A.; Vetromile, C.; Borea, L.; Giannella, G.; Iovinella, M.; Lubritto, C. Real-Time Monitoring and Static Data Analysis to Assess Energetic and Environmental Performances in the Wastewater Sector: A Case Study. Energies 2021, 14, 6948. [Google Scholar] [CrossRef]
- Di Cicco, M.R.; Iovinella, M.; Palmieri, M.; Lubritto, C.; Ciniglia, C. Extremophilic Microalgae Galdieria Gen. for Urban Wastewater Treatment: Current State, the Case of “POWER” System, and Future Prospects. Plants 2021, 10, 2343. [Google Scholar] [CrossRef] [PubMed]
- Di Cicco, M.R.; Spagnuolo, A.; Masiello, A.; Vetromile, C.; Nappa, M.; Corbo, G.; Lubritto, C. Energy monitoring of a wastewater treatment plant in Salerno, Campania Region (southern Italy). In Proceedings of the Frontiers in Water-Energy-Nexus—Nature-Based Solutions, Advanced Technologies and Best Practices for Environmental Sustainability: Proceedings of the 2nd WaterEnergyNEXUS Conference, November 2018, Salerno, Italy, 19 September 2019; Springer: Berlin/Heidelberg, Germany, 2020. [Google Scholar]
- Lovinella, M.; Lombardo, F.; Ciniglia, C.; Palmieri, M.; Di Cicco, M.R.; Trifuoggi, M.; Race, M.; Manfredi, C.; Lubritto, C.; Fabbricino, M.; et al. Bioremoval of Yttrium (III), Cerium (III), Europium (III), and Terbium (III) from Single and Quaternary Aqueous Solutions Using the Extremophile Galdieria Sulphuraria (Galdieriaceae, Rhodophyta). Plants 2022, 11, 1376. [Google Scholar] [CrossRef] [PubMed]
- Sirakov, M.; Palmieri, M.; Iovinella, M.; Davis, S.J.; Petriccione, M.; Di Cicco, M.R.; De Stefano, M.; Ciniglia, C. Cyanidiophyceae (Rhodophyta) Tolerance to Precious Metals: Metabolic Response to Palladium and Gold. Plants 2021, 10, 2367. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, M.; Iovinella, M.; Davis, S.J.; Di Cicco, M.R.; Lubritto, C.; Race, M.; Papa, S.; Fabbricino, M.; Ciniglia, C. Galdieria Sulphuraria ACUF427 Freeze-Dried Biomass as Novel Biosorbent for Rare Earth Elements. Microorganisms 2022, 10, 2138. [Google Scholar] [CrossRef] [PubMed]
- Iovinella, M.; Carbone, D.A.; Cioppa, D.; Davis, S.J.; Innangi, M.; Esposito, S.; Ciniglia, C. Prevalent PH Controls the Capacity of Galdieria Maxima to Use Ammonia and Nitrate as a Nitrogen Source. Plants 2020, 9, 232. [Google Scholar] [CrossRef]
- Manfredi, C.; Amoruso, A.J.; Ciniglia, C.; Iovinella, M.; Palmieri, M.; Lubritto, C.; El Hassanin, A.; Davis, S.J.; Trifuoggi, M. Selective Biosorption of Lanthanides onto Galdieria Sulphuraria. Chemosphere 2023, 317, 137818. [Google Scholar] [CrossRef]
- Li, Z.; Wang, X.; Ni, Z.; Bao, J.; Zhang, H. In-Situ Remediation of Carbofuran-ContaminatedSoil by Immobilized White-Rot Fungi. Pol. J. Environ. Stud. 2020, 29, 1237–1243. [Google Scholar] [CrossRef]
- Honda, Y.; Tanigawa, E.; Tsukihara, T.; Nguyen, D.X.; Kawabe, H.; Sakatoku, N.; Watari, J.; Sato, H.; Yano, S.; Tachiki, T.; et al. Stable and Transient Transformation, and a Promoter Assay in the Selective Lignin-Degrading Fungus, Ceriporiopsis Subvermispora. AMB Express 2019, 9, 92. [Google Scholar] [CrossRef]
- Zhang, J.; Chi, Y.; Feng, L. The Mechanism of Degradation of Alizarin Red by a White-Rot Fungus Trametes Gibbosa. BMC Biotechnol. 2021, 21, 64. [Google Scholar] [CrossRef]
- Kuramae, E.E.; Dimitrov, M.R.; Da Silva, G.H.R.; Lucheta, A.R.; Mendes, L.W.; Luz, R.L.; Vet, L.E.M.; Fernandes, T.V. On-Site Blackwater Treatment Fosters Microbial Groups and Functions to Efficiently and Robustly Recover Carbon and Nutrients. Microorganisms 2020, 9, 75. [Google Scholar] [CrossRef]
- Singh, A.K.; Raj, A. Emerging and Eco-Friendly Approaches for Waste Management: A Book Review. Environ. Sci. Eur. 2020, 32, 107. [Google Scholar] [CrossRef]
- Danila, V.; Januševičius, T. Removal of Cd, Cu, Ni, and Pb from Nanoscale Zero-Valent Iron Amended Soil Using 0.1 M Acetic Acid Solution. Environ. Clim. Technol. 2022, 26, 406–414. [Google Scholar] [CrossRef]
- Rudzionis, Z.; Navickas, A.A.; Stelmokaitis, G.; Ivanauskas, R. Immobilization of Hexavalent Chromium Using Self-Compacting Soil Technology. Materials 2022, 15, 2335. [Google Scholar] [CrossRef] [PubMed]
- Al-Tohamy, R.; Ali, S.S.; Li, F.; Okasha, K.M.; Mahmoud, Y.A.-G.; Elsamahy, T.; Jiao, H.; Fu, Y.; Sun, J. A Critical Review on the Treatment of Dye-Containing Wastewater: Ecotoxicological and Health Concerns of Textile Dyes and Possible Remediation Approaches for Environmental Safety. Ecotoxicol. Environ. Saf. 2022, 231, 113160. [Google Scholar] [CrossRef] [PubMed]
- Drábová, L.; Dvořáková, D.; Urbancová, K.; Gramblička, T.; Hajšlová, J.; Pulkrabová, J. Critical Assessment of Clean-Up Techniques Employed in Simultaneous Analysis of Persistent Organic Pollutants and Polycyclic Aromatic Hydrocarbons in Fatty Samples. Toxics 2022, 10, 12. [Google Scholar] [CrossRef]
- Pham, V.H.T.; Kim, J.; Chang, S.; Bang, D. Investigating Bio-Inspired Degradation of Toxic Dyes Using Potential Multi-Enzyme Producing Extremophiles. Microorganisms 2023, 11, 1273. [Google Scholar] [CrossRef]
- Cuerda-Correa, E.M.; Alexandre-Franco, M.F.; Fernández-González, C. Advanced Oxidation Processes for the Removal of Antibiotics from Water. An Overview. Water 2019, 12, 102. [Google Scholar] [CrossRef]
- Wang, B.; Shang, C.; Xie, H.; Sun, H.; Zhang, Q.; Xue, L.; Tack, F.M.G.; Hou, D.; Feng, Y.; Rinklebe, J. Unraveling Natural Aging-Induced Properties Change of Sludge-Derived Hydrochar and Enhanced Cadmium Sorption Site Heterogeneity. Biochar 2022, 4, 34. [Google Scholar] [CrossRef]
- Crini, G.; Lichtfouse, E. Advantages and Disadvantages of Techniques Used for Wastewater Treatment. Environ. Chem. Lett. 2019, 17, 145–155. [Google Scholar] [CrossRef]
- Yu, Y.-Y.; Zhai, D.-D.; Si, R.-W.; Sun, J.-Z.; Liu, X.; Yong, Y.-C. Three-Dimensional Electrodes for High-Performance Bioelectrochemical Systems. Int. J. Mol. Sci. 2017, 18, 90. [Google Scholar] [CrossRef]
- Stevens, M.G.F.; Batlokwa, B.S. Environmentally Friendly and Cheap Removal of Lead (II) and Zinc (II) from Wastewater with Fish Scales Waste Remains. Int. J. Chem. 2017, 9, 22. [Google Scholar] [CrossRef]
- Barakat, M.A. New Trends in Removing Heavy Metals from Industrial Wastewater. Arab. J. Chem. 2011, 4, 361–377. [Google Scholar] [CrossRef]
- Duran-Rivera, B.; Moreno-Suarez, J.R.; García-Ramírez, S.I. Decolorization of a Textile Effluent and Methylene Blue by Three White Rot Fungi (WRF), at Pilot and Laboratory Scale. Bionatura 2018, 3, 709–714. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of Fungi in Bioremediation of Emerging Pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Guillaume, A.; Thorigné, A.; Carré, Y.; Vinh, J.; Levavasseur, L. Contribution of Proteases and Cellulases Produced by Solid-State Fermentation to the Improvement of Corn Ethanol Production. Bioresour. Bioprocess. 2019, 6, 7. [Google Scholar] [CrossRef]
- Mérillon, J.-M.; Ramawat, K.G. (Eds.) Fungal Metabolites; Springer International Publishing: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Stoller, M.; Sacco, O.; Sannino, D.; Chianese, A. Successful Integration of Membrane Technologies in a Conventional Purification Process of Tannery Wastewater Streams. Membranes 2013, 3, 126–135. [Google Scholar] [CrossRef]
- Grandclément, C.; Seyssiecq, I.; Piram, A.; Wong-Wah-Chung, P.; Vanot, G.; Tiliacos, N.; Roche, N.; Doumenq, P. From the Conventional Biological Wastewater Treatment to Hybrid Processes, the Evaluation of Organic Micropollutant Removal: A Review. Water Res. 2017, 111, 297–317. [Google Scholar] [CrossRef]
- Sá, H.; Michelin, M.; Tavares, T.; Silva, B. Current Challenges for Biological Treatment of Pharmaceutical-Based Contaminants with Oxidoreductase Enzymes: Immobilization Processes, Real Aqueous Matrices and Hybrid Techniques. Biomolecules 2022, 12, 1489. [Google Scholar] [CrossRef]
- Zhuo, R.; Fan, F. A Comprehensive Insight into the Application of White Rot Fungi and Their Lignocellulolytic Enzymes in the Removal of Organic Pollutants. Sci. Total Environ. 2021, 778, 146132. [Google Scholar] [CrossRef]
- Suryadi, H.; Judono, J.J.; Putri, M.R.; Eclessia, A.D.; Ulhaq, J.M.; Agustina, D.N.; Sumiati, T. Biodelignification of Lignocellulose Using Ligninolytic Enzymes from White-Rot Fungi. Heliyon 2022, 8, e08865. [Google Scholar] [CrossRef]
- Kulshreshtha, S.; Onyedinma, U.; Siddhant, P. (Eds.) Recent Advances in Mushroom Cultivation Technology and Its Application (Volume 2), 1st ed.; Bright Sky Publications: Delhi, India, 2022. [Google Scholar] [CrossRef]
- Lundell, T.K.; Mäkelä, M.R.; De Vries, R.P.; Hildén, K.S. Genomics, lifestyles and future prospects of wood-decay and litter-decomposing Basidiomycota. In Advances in Botanical Research; Elsevier: Amsterdam, The Netherlands, 2014; Volume 70, pp. 329–370. [Google Scholar] [CrossRef]
- Taherzadah, M.J.; Ferreira, J.; Pandey, A. (Eds.) Current Developments in Biotechnology and Bioengineering Filamentous Fungi Biorefinery, 1st ed.; Elsevier Publications: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Purchase, D. (Ed.) Fungal Applications in Sustainable Environmental Biotechnology; Fungal Biology; Springer International Publishing: Cham, Switzerland, 2016. [Google Scholar] [CrossRef]
- Ntwampe, S.; Chowdhury, F.; Sheldon, M.; Volschenk, H. Overview of Parameters Influencing Biomass and Bioreactor Performance Used for Extracellular Ligninase Production from Phanerochaete Chrysosporium. Braz. Arch. Biol. Technol. 2010, 53, 1057–1066. [Google Scholar] [CrossRef]
- Souza, D.F.D.; Costa, S.C.D.; Dacome, A.S.; Souza, C.G.M.D.; Bracht, A.; Peralta, R.M. Pentachlorophenol Removal by Pleurotus Pulmonarius in Submerged Cultures. Braz. Arch. Biol. Technol. 2011, 54, 357–362. [Google Scholar] [CrossRef]
- Ullrich, R.; Hofrichter, M. Enzymatic Hydroxylation of Aromatic Compounds. Cell. Mol. Life Sci. 2007, 64, 271–293. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chandra, R. Ligninolytic Enzymes and Its Mechanisms for Degradation of Lignocellulosic Waste in Environment. Heliyon 2020, 6, e03170. [Google Scholar] [CrossRef] [PubMed]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Springer: Berlin/Heidelberg, Germany; Chemical Industry Press: Dordrecht, The Netherlands; New York, NY, USA, 2014. [Google Scholar]
- Saini, J.K.; Himanshu; Hemansi; Kaur, A.; Mathur, A. Strategies to Enhance Enzymatic Hydrolysis of Lignocellulosic Biomass for Biorefinery Applications: A Review. Bioresour. Technol. 2022, 360, 127517. [Google Scholar] [CrossRef]
- Tuomela, M. Biodegradation of Lignin in a Compost Environment: A Review. Bioresour. Technol. 2000, 72, 169–183. [Google Scholar] [CrossRef]
- Ruiz-Dueñas, F.J.; Lundell, T.; Floudas, D.; Nagy, L.G.; Barrasa, J.M.; Hibbett, D.S.; Martínez, A.T. Lignin-Degrading Peroxidases in Polyporales: An Evolutionary Survey Based on 10 Sequenced Genomes. Mycologia 2013, 105, 1428–1444. [Google Scholar] [CrossRef]
- Baldrian, P. Fungal Laccases—Occurrence and Properties. FEMS Microbiol. Rev. 2006, 30, 215–242. [Google Scholar] [CrossRef]
- Eggert, C.; Temp, U.; Eriksson, K.E. The Ligninolytic System of the White Rot Fungus Pycnoporus Cinnabarinus: Purification and Characterization of the Laccase. Appl. Environ. Microbiol. 1996, 62, 1151–1158. [Google Scholar] [CrossRef]
- Abdel-Azeem, A.M.; Gherbawy, Y.A.; Sabry, A.M. Enzyme Profiles and Genotyping of Chaetomium Globosum Isolates from Various Substrates. Plant Biosyst. Int. J. Deal. All Asp. Plant Biol. 2016, 150, 420–428. [Google Scholar] [CrossRef]
- Sun, S.; Liu, P.; Ullah, M. Efficient Azo Dye Biodecolorization System Using Lignin-Co-Cultured White-Rot Fungus. J. Fungi 2023, 9, 91. [Google Scholar] [CrossRef] [PubMed]
- Morsi, R.; Bilal, M.; Iqbal, H.M.N.; Ashraf, S.S. Laccases and Peroxidases: The Smart, Greener and Futuristic Biocatalytic Tools to Mitigate Recalcitrant Emerging Pollutants. Sci. Total Environ. 2020, 714, 136572. [Google Scholar] [CrossRef]
- Hadibarata, T.; Kristanti, R.A. Fate and Cometabolic Degradation of Benzo[a]Pyrene by White-Rot Fungus Armillaria Sp. F022. Bioresour. Technol. 2012, 107, 314–318. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, P.; Shrivastava, R.; Agrawal, P.K. Bioprospecting and Biotechnological Applications of Fungal Laccase. 3 Biotech 2016, 6, 15. [Google Scholar] [CrossRef]
- Mester, T.; Tien, M. Oxidation Mechanism of Ligninolytic Enzymes Involved in the Degradation of Environmental Pollutants. Int. Biodeterior. Biodegrad. 2000, 46, 51–59. [Google Scholar] [CrossRef]
- Gianfreda, L.; Xu, F.; Bollag, J.-M. Laccases: A Useful Group of Oxidoreductive Enzymes. Bioremediat. J. 1999, 3, 1–26. [Google Scholar] [CrossRef]
- Singh, G.; Dwivedi, S.K.; Mishra, J. Role of fungal enzymes in the removal of Azo Dyes. In Microbial Enzymes: Roles and Applications in Industries; Arora, N.K., Mishra, J., Mishra, V., Eds.; Microorganisms for Sustainability; Springer: Singapore, 2020; Volume 11, pp. 231–257. [Google Scholar] [CrossRef]
- Gunawardana, B.; Singhal, N.; Swedlund, P. Degradation of Chlorinated Phenols by Zero Valent Iron and Bimetals of Iron: A Review. Environ. Eng. Res. 2011, 16, 187–203. [Google Scholar] [CrossRef]
- Silva, A.; Delerue-Matos, C.; Figueiredo, S.; Freitas, O. The Use of Algae and Fungi for Removal of Pharmaceuticals by Bioremediation and Biosorption Processes: A Review. Water 2019, 11, 1555. [Google Scholar] [CrossRef]
- Javanbakht, V.; Alavi, S.A.; Zilouei, H. Mechanisms of Heavy Metal Removal Using Microorganisms as Biosorbent. Water Sci. Technol. 2014, 69, 1775–1787. [Google Scholar] [CrossRef]
- Chandra, P.; Enespa. Mycoremediation of environmental pollutants from contaminated soil. In Mycorrhizosphere and Pedogenesis; Varma, A., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 239–274. [Google Scholar] [CrossRef]
- Pointing, S. Feasibility of Bioremediation by White-Rot Fungi. Appl. Microbiol. Biotechnol. 2001, 57, 20–33. [Google Scholar] [CrossRef]
- Cullen, D.; Kersten, P.J. Enzymology and molecular biology of lignin degradation. In Biochemistry and Molecular Biology; Brambl, R., Marzluf, G.A., Eds.; Springer: Berlin/Heidelberg, Germany, 2004; pp. 249–273. [Google Scholar] [CrossRef]
- Kellner, H.; Luis, P.; Zimdars, B.; Kiesel, B.; Buscot, F. Diversity of Bacterial Laccase-like Multicopper Oxidase Genes in Forest and Grassland Cambisol Soil Samples. Soil Biol. Biochem. 2008, 40, 638–648. [Google Scholar] [CrossRef]
- Asgher, M.; Bhatti, H.N.; Ashraf, M.; Legge, R.L. Recent Developments in Biodegradation of Industrial Pollutants by White Rot Fungi and Their Enzyme System. Biodegradation 2008, 19, 771–783. [Google Scholar] [CrossRef]
- Farhan Hanafi, M.; Sapawe, N. A Review on the Water Problem Associate with Organic Pollutants Derived from Phenol, Methyl Orange, and Remazol Brilliant Blue Dyes. Mater. Today Proc. 2020, 31, A141–A150. [Google Scholar] [CrossRef]
- Bilal, M.; Adeel, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Emerging Contaminants of High Concern and Their Enzyme-Assisted Biodegradation—A Review. Environ. Int. 2019, 124, 336–353. [Google Scholar] [CrossRef] [PubMed]
- McGrath, R.; Singleton, I. Pentachlorophenol Transformation in Soil: A Toxicological Assessment. Soil Biol. Biochem. 2000, 32, 1311–1314. [Google Scholar] [CrossRef]
- Sedarati, M.R.; Keshavarz, T.; Leontievsky, A.A.; Evans, C.S. Transformation of High Concentrations of Chlorophenols by the White-Rot Basidiomycete Trametes Versicolor Immobilized on Nylon Mesh. Electron. J. Biotechnol. 2003, 6, 104–114. [Google Scholar]
- Islam, T.; Islam, T.; Repon, R. Synthetic Dyes for Textile Colouration: Process, Factors and Environmental Impact. Text. Leather Rev. 2022, 5, 327–373. [Google Scholar] [CrossRef]
- Dafale, N.; Rao, N.N.; Meshram, S.U.; Wate, S.R. Decolorization of Azo Dyes and Simulated Dye Bath Wastewater Using Acclimatized Microbial Consortium—Biostimulation and Halo Tolerance. Bioresour. Technol. 2008, 99, 2552–2558. [Google Scholar] [CrossRef]
- Molla, A.H.; Khan, H.I. Detoxification of Textile Effluent by Fungal Treatment and Its Performance in Agronomic Usages. Environ. Sci. Pollut. Res. 2018, 25, 10820–10828. [Google Scholar] [CrossRef]
- Wikee, S.; Hatton, J.; Turbé-Doan, A.; Mathieu, Y.; Daou, M.; Lomascolo, A.; Kumar, A.; Lumyong, S.; Sciara, G.; Faulds, C.B.; et al. Characterization and Dye Decolorization Potential of Two Laccases from the Marine-Derived Fungus Pestalotiopsis Sp. Int. J. Mol. Sci. 2019, 20, 1864. [Google Scholar] [CrossRef]
- Tudi, M.; Daniel Ruan, H.; Wang, L.; Lyu, J.; Sadler, R.; Connell, D.; Chu, C.; Phung, D.T. Agriculture Development, Pesticide Application and Its Impact on the Environment. Int. J. Environ. Res. Public Health 2021, 18, 1112. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, A.; Kumar, P.S.; Vo, D.-V.N.; Jeevanantham, S.; Karishma, S.; Yaashikaa, P.R. A Review on Catalytic-Enzyme Degradation of Toxic Environmental Pollutants: Microbial Enzymes. J. Hazard. Mater. 2021, 419, 126451. [Google Scholar] [CrossRef] [PubMed]
- Bending, G.D.; Friloux, M.; Walker, A. Degradation of Contrasting Pesticides by White Rot Fungi and Its Relationship with Ligninolytic Potential. FEMS Microbiol. Lett. 2002, 212, 59–63. [Google Scholar] [CrossRef]
- Hanay, Ö.; Türk, H. An Overview on Usage of Nanoscale Zero Valent Iron for Pharmaceuticals Elimination. Eskişehir Tek. Üniversitesi Bilim Teknol. Derg. B Teor. Bilim. 2019, 7, 222–239. [Google Scholar] [CrossRef]
- Blánquez, P.; Guieysse, B. Continuous Biodegradation of 17β-Estradiol and 17α-Ethynylestradiol by Trametes Versicolor. J. Hazard. Mater. 2008, 150, 459–462. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morató, C.; Ferrando-Climent, L.; Rodriguez-Mozaz, S.; Barceló, D.; Marco-Urrea, E.; Vicent, T.; Sarrà, M. Degradation of Pharmaceuticals in Non-Sterile Urban Wastewater by Trametes Versicolor in a Fluidized Bed Bioreactor. Water Res. 2013, 47, 5200–5210. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Morató, C.; Lucas, D.; Llorca, M.; Rodriguez-Mozaz, S.; Gorga, M.; Petrovic, M.; Barceló, D.; Vicent, T.; Sarrà, M.; Marco-Urrea, E. Hospital Wastewater Treatment by Fungal Bioreactor: Removal Efficiency for Pharmaceuticals and Endocrine Disruptor Compounds. Sci. Total Environ. 2014, 493, 365–376. [Google Scholar] [CrossRef]
- Cajthaml, T.; Křesinová, Z.; Svobodová, K.; Möder, M. Biodegradation of Endocrine-Disrupting Compounds and Suppression of Estrogenic Activity by Ligninolytic Fungi. Chemosphere 2009, 75, 745–750. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Biotransformation of Three Pharmaceutical Active Compounds by the Fungus Phanerochaete Chrysosporium in a Fed Batch Stirred Reactor under Air and Oxygen Supply. Biodegradation 2012, 23, 145–156. [Google Scholar] [CrossRef]
- Rodarte-Morales, A.I.; Feijoo, G.; Moreira, M.T.; Lema, J.M. Operation of Stirred Tank Reactors (STRs) and Fixed-Bed Reactors (FBRs) with Free and Immobilized Phanerochaete Chrysosporium for the Continuous Removal of Pharmaceutical Compounds. Biochem. Eng. J. 2012, 66, 38–45. [Google Scholar] [CrossRef]
- Baldrian, P. Interactions of Heavy Metals with White-Rot Fungi. Enzym. Microb. Technol. 2003, 32, 78–91. [Google Scholar] [CrossRef]
- Enayatizamir, N.; Liu, J.; Wang, L.; Lin, X.; Fu, P. Coupling Laccase Production from Trametes Pubescence with Heavy Metal Removal for Economic Waste Water Treatment. J. Water Process Eng. 2020, 37, 101357. [Google Scholar] [CrossRef]
- Sharma, K.R.; Giri, R.; Sharma, R.K. Lead, Cadmium and Nickel Removal Efficiency of White-rot Fungus Phlebia Brevispora. Lett. Appl. Microbiol. 2020, 71, 637–644. [Google Scholar] [CrossRef]
- Lin, C.-W.; Lai, C.-Y.; Liu, S.-H.; Chen, Y.-R.; Alfanti, L.K. Enhancing Bioelectricity Generation and Removal of Copper in Microbial Fuel Cells with a Laccase-Catalyzed Biocathode. J. Clean. Prod. 2021, 298, 126726. [Google Scholar] [CrossRef]
- Kocaoba, S.; Arısoy, M. Biosorption of Cadmium(II) and Lead(II) from Aqueous Solutions Using Pleurotus Ostreatus Immobilized on Bentonite. Sep. Sci. Technol. 2018, 53, 1703–1710. [Google Scholar] [CrossRef]
- Noormohamadi, H.R.; Fat’hi, M.R.; Ghaedi, M.; Ghezelbash, G.R. Potentiality of White-Rot Fungi in Biosorption of Nickel and Cadmium: Modeling Optimization and Kinetics Study. Chemosphere 2019, 216, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Bharati, S.L.; Chaurasia, P.K.; Das, P.S. (Eds.) Research Advancements in Pharmaceutical, Nutritional, and Industrial Enzymology; Advances in Medical Technologies and Clinical Practice; IGI Global: Hershey, PA, USA, 2018. [Google Scholar] [CrossRef]
- Křesinová, Z.; Linhartová, L.; Filipová, A.; Ezechiáš, M.; Mašín, P.; Cajthaml, T. Biodegradation of Endocrine Disruptors in Urban Wastewater Using Pleurotus Ostreatus Bioreactor. N Biotechnol. 2018, 43, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Pezzella, C.; Macellaro, G.; Sannia, G.; Raganati, F.; Olivieri, G.; Marzocchella, A.; Schlosser, D.; Piscitelli, A. Exploitation of Trametes Versicolor for Bioremediation of Endocrine Disrupting Chemicals in Bioreactors. PLoS ONE 2017, 12, e0178758. [Google Scholar] [CrossRef]
- Acevedo, F.; Pizzul, L.; Castillo, M.D.P.; Cuevas, R.; Diez, M.C. Degradation of Polycyclic Aromatic Hydrocarbons by the Chilean White-Rot Fungus Anthracophyllum Discolor. J. Hazard. Mater. 2011, 185, 212–219. [Google Scholar] [CrossRef]
- Benitez, S.F.; Sadañoski, M.A.; Velázquez, J.E.; Zapata, P.D.; Fonseca, M.I. Comparative Study of Single Cultures and a Consortium of White Rot Fungi for Polychlorinated Biphenyls Treatment. J. Appl. Microbiol. 2021, 131, 1775–1786. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Hou, J.; Price, W.E.; Nghiem, L.D. Impact of Wastewater Derived Dissolved Interfering Compounds on Growth, Enzymatic Activity and Trace Organic Contaminant Removal of White Rot Fungi—A Critical Review. J. Environ. Manag. 2017, 201, 89–109. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Geißen, S.-U. Elimination of Carbamazepine in a Non-Sterile Fungal Bioreactor. Bioresour. Technol. 2012, 112, 221–227. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, P.; Zhang, X.; Zhu, X.; Van Lier, J.B.; Spanjers, H. White Rot Fungi Pretreatment to Advance Volatile Fatty Acid Production from Solid-State Fermentation of Solid Digestate: Efficiency and Mechanisms. Energy 2018, 162, 534–541. [Google Scholar] [CrossRef]
- Gomaa, O.M.; Linz, J.E.; Reddy, C.A. Decolorization of Victoria Blue by the White Rot Fungus, Phanerochaete Chrysosporium. World J. Microbiol. Biotechnol. 2008, 24, 2349–2356. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Gerlach, R. Potential Use of Fungal-Bacterial Co-Cultures for the Removal of Organic Pollutants. Crit. Rev. Biotechnol. 2022, 42, 361–383. [Google Scholar] [CrossRef]
- Asif, M.B.; Hai, F.I.; Singh, L.; Price, W.E.; Nghiem, L.D. Degradation of Pharmaceuticals and Personal Care Products by White-Rot Fungi—A Critical Review. Curr. Pollut. Rep. 2017, 3, 88–103. [Google Scholar] [CrossRef]
- Palli, L.; Gullotto, A.; Tilli, S.; Caniani, D.; Gori, R.; Scozzafava, A. Biodegradation of 2-Naphthalensulfonic Acid Polymers by White-Rot Fungi: Scale-up into Non-Sterile Packed Bed Bioreactors. Chemosphere 2016, 164, 120–127. [Google Scholar] [CrossRef]
- Jaganathan, B.; Hossain, S.K.M.; Begum, K.M.M.S.; Anantharaman, N. Aerobic Pollution Abatement of Pulp Mill Effluent with the White Rot Fungus Phanerochaete Chrysosporium in Three-Phase Fluidized Bed Bioreactor. Chem. Eng. Res. Bull. 2009, 13, 13–16. [Google Scholar] [CrossRef]
- Nguyen, L.N.; Hai, F.I.; Yang, S.; Kang, J.; Leusch, F.D.L.; Roddick, F.; Price, W.E.; Nghiem, L.D. Removal of Trace Organic Contaminants by an MBR Comprising a Mixed Culture of Bacteria and White-Rot Fungi. Bioresour. Technol. 2013, 148, 234–241. [Google Scholar] [CrossRef]
- Mir-Tutusaus, J.A.; Jaén-Gil, A.; Barceló, D.; Buttiglieri, G.; Gonzalez-Olmos, R.; Rodriguez-Mozaz, S.; Caminal, G.; Sarrà, M. Prospects on Coupling UV/H2O2 with Activated Sludge or a Fungal Treatment for the Removal of Pharmaceutically Active Compounds in Real Hospital Wastewater. Sci. Total Environ. 2021, 773, 145374. [Google Scholar] [CrossRef]
- He, K.; Chen, G.; Zeng, G.; Huang, Z.; Guo, Z.; Huang, T.; Peng, M.; Shi, J.; Hu, L. Applications of White Rot Fungi in Bioremediation with Nanoparticles and Biosynthesis of Metallic Nanoparticles. Appl. Microbiol. Biotechnol. 2017, 101, 4853–4862. [Google Scholar] [CrossRef] [PubMed]
- Chmelová, D.; Legerská, B.; Kunstová, J.; Ondrejovič, M.; Miertuš, S. The Production of Laccases by White-Rot Fungi under Solid-State Fermentation Conditions. World J. Microbiol. Biotechnol. 2022, 38, 21. [Google Scholar] [CrossRef] [PubMed]
- Mithra, M.G.; Jeeva, M.L.; Sajeev, M.S.; Padmaja, G. Comparison of Ethanol Yield from Pretreated Lignocellulo-Starch Biomass under Fed-Batch SHF or SSF Modes. Heliyon 2018, 4, e00885. [Google Scholar] [CrossRef]
- Bayburt, C.; Karaduman, A.B.; Yenice Gürsu, B.; Tuncel, M.; Yamaç, M. Decolourization and Detoxification of Textile Dyes by Lentinus Arcularius in Immersion Bioreactor Scale. Int. J. Environ. Sci. Technol. 2020, 17, 945–958. [Google Scholar] [CrossRef]
- Przystaś, W.; Zabłocka-Godlewska, E.; Grabińska-Sota, E. Efficiency of Decolorization of Different Dyes Using Fungal Biomass Immobilized on Different Solid Supports. Braz. J. Microbiol. 2018, 49, 285–295. [Google Scholar] [CrossRef] [PubMed]
- Ijoma, G.N.; Tekere, M. Potential Microbial Applications of Co-Cultures Involving Ligninolytic Fungi in the Bioremediation of Recalcitrant Xenobiotic Compounds. Int. J. Environ. Sci. Technol. 2017, 14, 1787–1806. [Google Scholar] [CrossRef]
- Wesenberg, D. White-Rot Fungi and Their Enzymes for the Treatment of Industrial Dye Effluents. Biotechnol. Adv. 2003, 22, 161–187. [Google Scholar] [CrossRef]
| Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).