Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis
2.3. Specimen Preparation
2.4. Characterization Tests
3. Results and Discussion
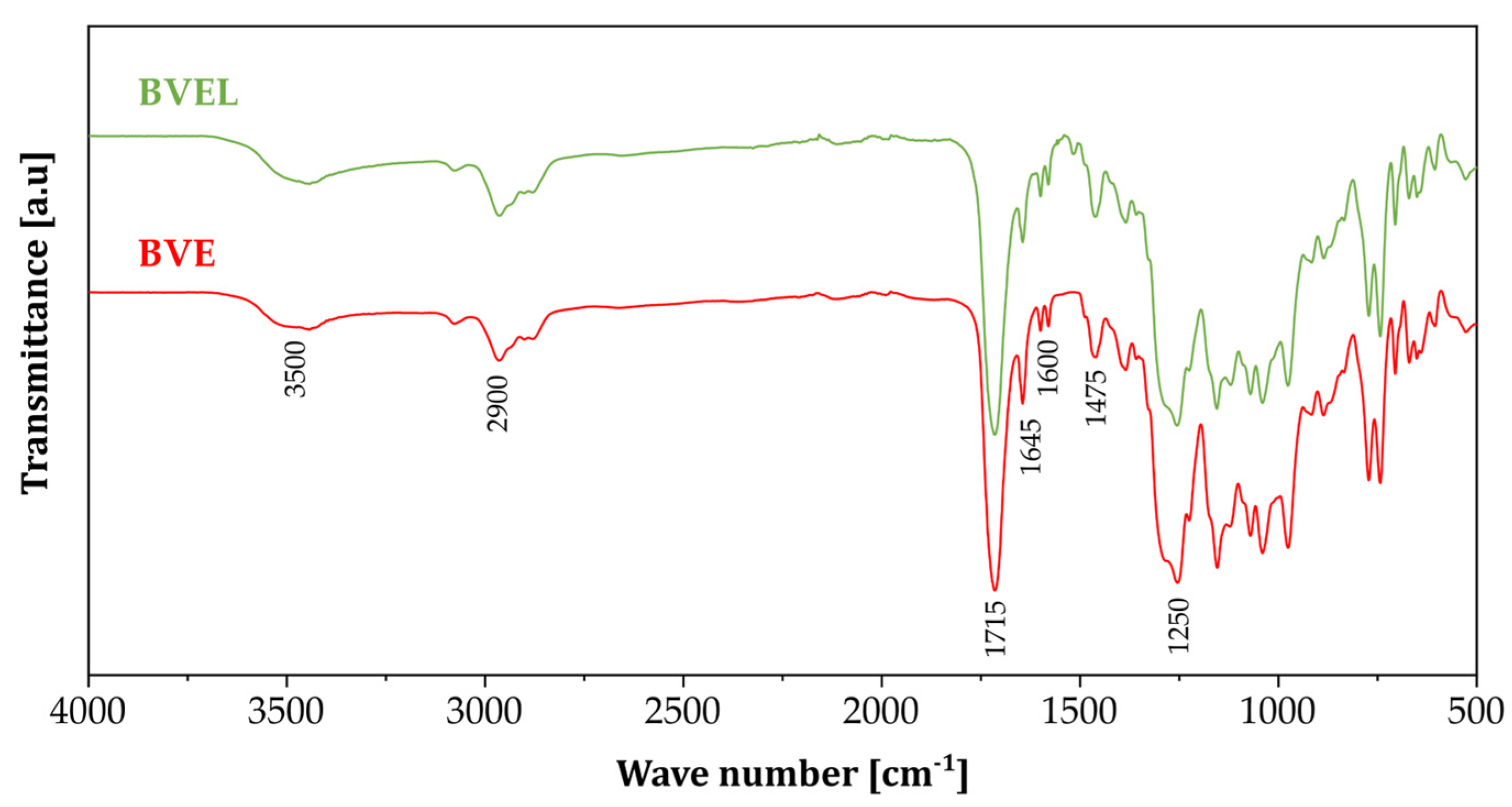
3.1. FTIR
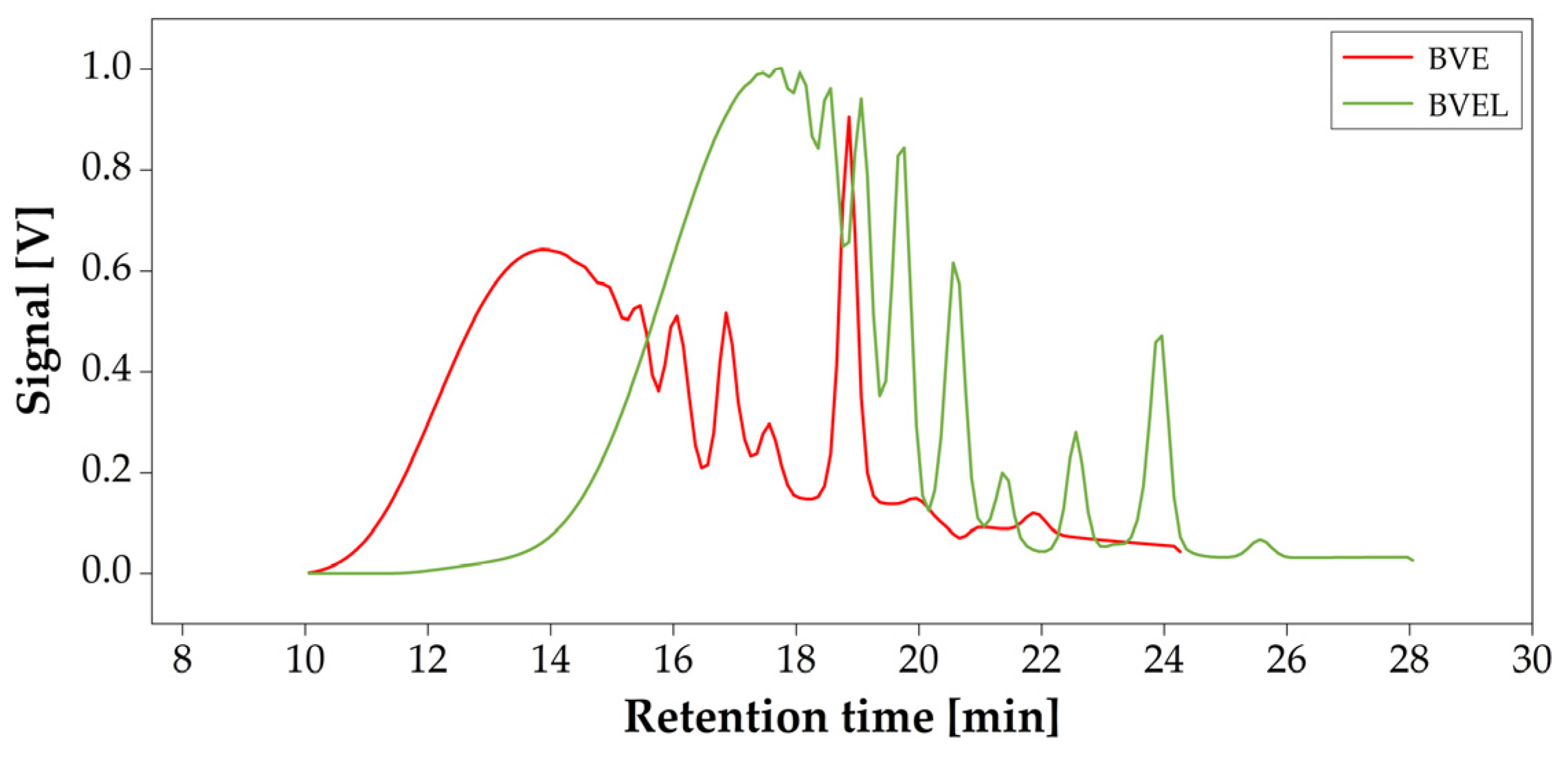
3.2. GPC
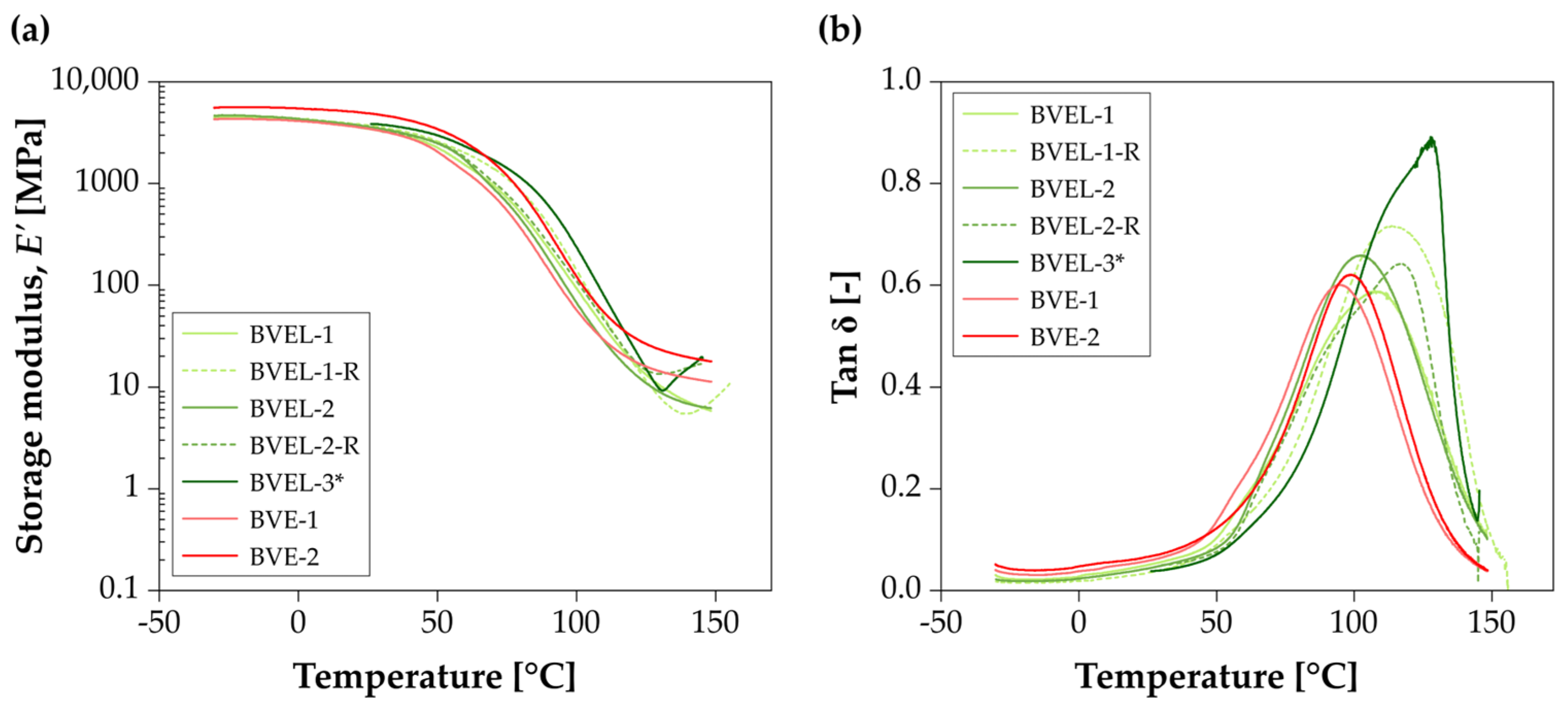
3.3. DMA
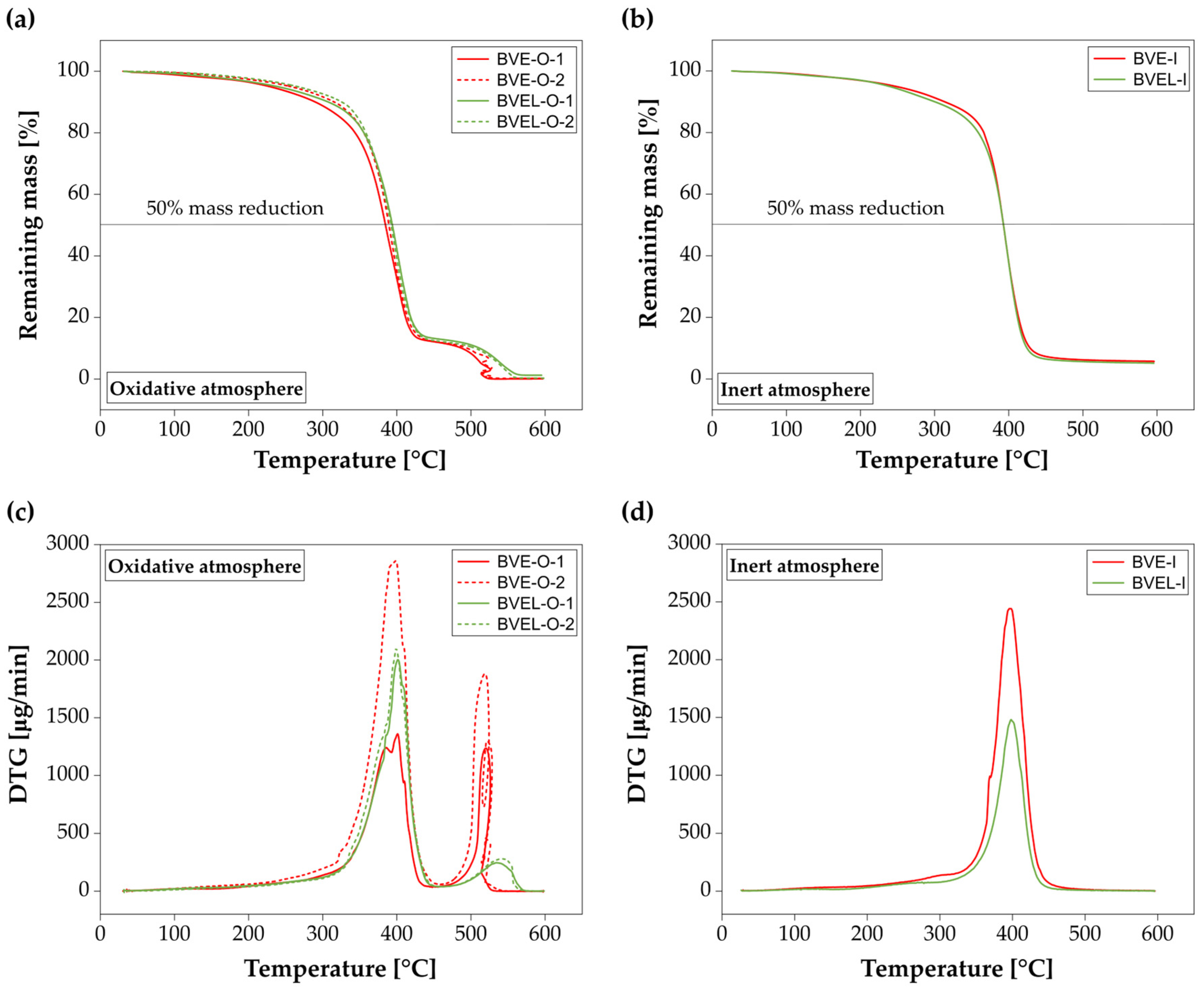
3.4. TGA
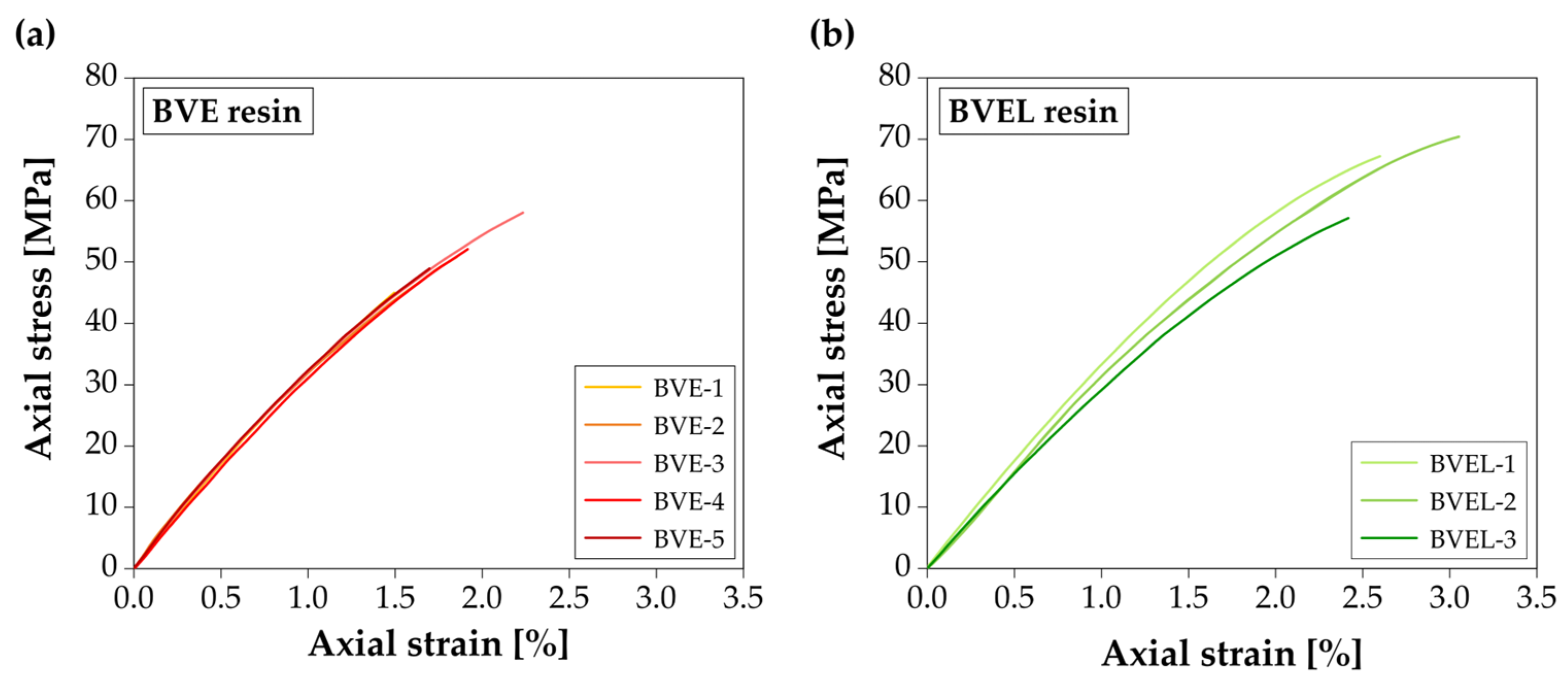
3.5. Tensile Behavior
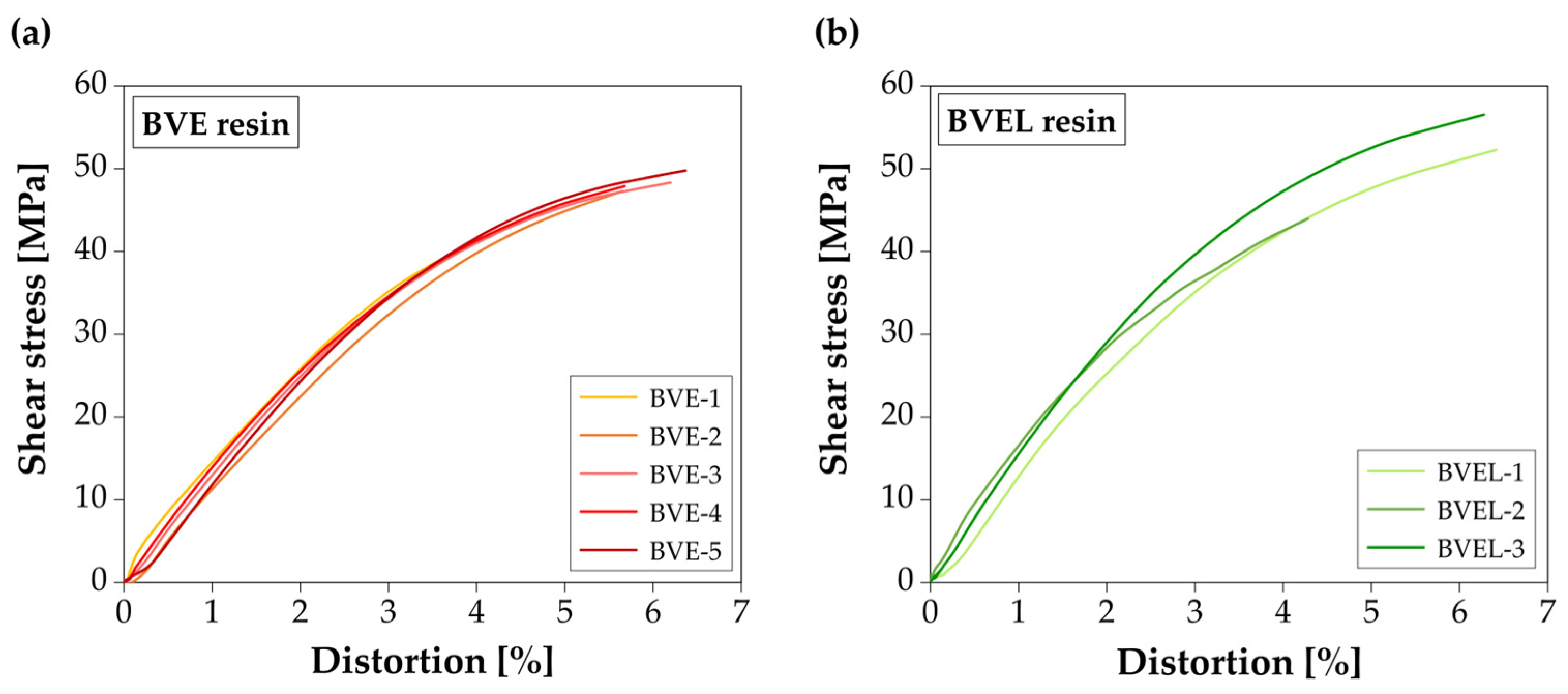
3.6. Shear Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lima, L.; Trindade, E.; Alencar, L.; Alencar, M.; Silva, L. Sustainability in the construction industry: A systematic review of the literature. J. Clean. Prod. 2020, 289, 125730. [Google Scholar] [CrossRef]
- Construction Leadership Council. The Routemap for Zero Avoidable Waste in Construction. 2021. Available online: https://www.constructionleadershipcouncil.co.uk/wp-content/uploads/2021/07/ZAW-Interactive-Routemap-FINAL.pdf (accessed on 1 June 2023).
- Sonmez, F.O. Optimum Design of Composite Structures: A Literature Survey (1969–2009). J. Reinf. Plast. Compos. 2016, 36, 3–39. [Google Scholar] [CrossRef]
- Aggarwal-Khan, S. How Can a Life-Cycle Approach Curb the Plastic Pollution Crisis; UNEP United Nations Environment Programme: Nairobi, Kenya, 2022; Available online: https://policycommons.net/artifacts/2618339/how-can-a-life-cycle-approach-curb-the-plastic-pollution-crisis/3640927 (accessed on 1 June 2023).
- Hofmann, M.A.; Shahid, A.T.; Garrido, M.; Ferreira, M.J.; Correia, J.R.; Bordado, J.C. Biobased Thermosetting Polyester Resin for High-Performance Applications. ACS Sustain. Chem. Eng. 2022, 10, 3442–3454. [Google Scholar] [CrossRef]
- Hofmann, M.; Garrido, M.; Machado, M.; Correia, J.R.; Bordado, J.C. Development of high-performance partially biobased thermoset polyester using renewable building blocks from isosorbide, 1,3-propanediol, and fumaric acid. J. Appl. Polym. Sci. 2022, 139, e53029. [Google Scholar] [CrossRef]
- Alzagameem, A.; El Khaldi-Hansen, B.; Kamm, B.; Schulze, M. Lignocellulosic biomass for energy, biofuels, biomaterials and chemicals. In Biomass and Green Chemistry; Sílvio, V., Jr., Ed.; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Collins, M.N.; Nechifor, M.; Tanasă, F.; Zănoagă, M.; McLoughlin, A.; Stróżyk, M.A.; Culebras, M.; Teacă, C.-A. Valorization of lignin in polymer and composite systems for advanced engineering applications—A review. Int. J. Biol. Macromol. 2019, 131, 828–849. [Google Scholar] [CrossRef] [PubMed]
- Asim, M.; Saba, N.; Jawaid, M.; Nasir, M.; Pervaiz, M.; Alothman, O.Y. A review on phenolic resin and its composites. Curr. Anal. Chem. 2017, 13, 185–197. [Google Scholar] [CrossRef]
- Pan, H. Synthesis of polymers from organic solvent liquefied biomass: A review. Renew. Sustain. Energy Rev. 2011, 15, 3454–3463. [Google Scholar] [CrossRef]
- Kun, D.; Pukánszky, B. Polymer/lignin blends: Interactions, properties, applications. Eur. Polym. J. 2017, 93, 618–641. [Google Scholar] [CrossRef] [Green Version]
- Bakis, C.E.; Bank, L.C.; Brown, V.L.; Cosenza, E.; Davalos, J.F.; Lesko, J.J.; Machida, A.; Rizkalla, S.H.; Triantafillou, T.C. Fiber-Reinforced Polymer Composites for Construction—State-of-the-Art Review. J. Compos. Constr. 2002, 6, 73–87. [Google Scholar] [CrossRef] [Green Version]
- Davis, K.M.; Rover, M.; Brown, R.C.; Bai, X.; Wen, Z.; Jarboe, L.R. Recovery and Utilization of Lignin Monomers as Part of the Biorefinery Approach. Energies 2016, 9, 808. [Google Scholar] [CrossRef] [Green Version]
- Frollini, E.; Silva, C.; Ramires, E. Phenolic resins as a matrix material in advanced fiber-reinforced polymer (FRP) composites. In Advanced Fibre-Reinforced Polymer (FRP) Composites for Structural Applications; Bai, J., Ed.; Woodhead Publishing Series in Civil and Structural Engineering: Sawston, UK, 2013; pp. 7–43. [Google Scholar] [CrossRef]
- Trinowski, D.M. Foundry. In Phenolic Resins: A Century of Progress; Louis, P., Ed.; Springer: Berlin/Heidelberg, Germany, 2010; pp. 451–502. [Google Scholar]
- dos Santos, R.G.; Acero, N.F.; Matos, S.; Carvalho, R.; Vale, M.; Marques, A.C.; Bordado, J.C.; Mateus, M.M. One-Component Spray Polyurethane Foam from Liquefied Pinewood Polyols: Pursuing Eco-Friendly Materials. J. Polym. Environ. 2017, 26, 91–100. [Google Scholar] [CrossRef]
- dos Santos, R.G.; Carvalho, R.; Silva, E.R.; Bordado, J.C.; Cardoso, A.C.; Costa, M.D.R.; Mateus, M.M. Natural polymeric water-based adhesive from cork liquefaction. Ind. Crop. Prod. 2016, 84, 314–319. [Google Scholar] [CrossRef]
- Domínguez, J.C.; Oliet, M.; Alonso, M.V.; Rojo, E.; Rodríguez, F. Structural, thermal and rheological behavior of a bio-based phenolic resin in relation to a commercial resol resin. Ind. Crops Prod. 2013, 42, 308–314. [Google Scholar] [CrossRef]
- van Haveren, J.; Oostveen, E.A.; Miccichè, F.; Noordover, B.A.J.; Koning, C.E.; van Benthem, R.A.T.M.; Frissen, A.E.; Weijnen, J.G.J. Resins and additives for powder coatings and alkyd paints, based on renewable resources. J. Coat. Technol. Res. 2007, 4, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Solt, P.; Rößiger, B.; Konnerth, J.; Van Herwijnen, H.W.G. Lignin Phenol Formaldehyde Resoles Using Base-Catalysed Depolymerized Kraft Lignin. Polymers 2018, 10, 1162. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Li, N.; Chen, Z.; Ding, C.; Zheng, Q.; Xu, J.; Meng, Q. Synthesis of High-Water-Resistance Lignin-Phenol Resin Adhesive with Furfural as a Crosslinking Agent. Polymers 2020, 12, 2805. [Google Scholar] [CrossRef]
- Galdino, D.S.; Kondo, M.Y.; De Araujo, V.A.; Ferrufino, G.L.A.A.; Faustino, E.; dos Santos, H.F.; Christoforo, A.L.; Luna, C.M.R.; de Campos, C.I. Thermal and Gluing Properties of Phenol-Based Resin with Lignin for Potential Application in Structural Composites. Polymers 2023, 15, 357. [Google Scholar] [CrossRef]
- Aristri, M.A.; Lubis, M.A.R.; Yadav, S.M.; Antov, P.; Papadopoulos, A.N.; Pizzi, A.; Fatriasari, W.; Ismayati, M.; Iswanto, A.H. Recent Developments in Lignin- and Tannin-Based Non-Isocyanate Polyurethane Resins for Wood Adhesives—A Review. Appl. Sci. 2021, 11, 4242. [Google Scholar] [CrossRef]
- Guigo, N.; Mija, A.; Vincent, L.; Sbirrazzuoli, N. Eco-friendly composite resins based on renewable biomass resources: Polyfurfuryl alcohol/lignin thermosets. Eur. Polym. J. 2010, 46, 1016–1023. [Google Scholar] [CrossRef]
- Asada, C.; Basnet, S.; Otsuka, M.; Sasaki, C.; Nakamura, Y. Epoxy resin synthesis using low mo-lecular weight lignin separated from various lignocellulosic materials. Int. J. Biol. Macromol. 2015, 74, 413–419. [Google Scholar] [CrossRef]
- Li, R.J.; Gutierrez, J.; Chung, Y.-L.; Frank, C.W.; Billington, S.L.; Sattely, E.S. A lignin-epoxy resin derived from biomass as an alternative to formaldehyde-based wood adhesives. Green Chem. 2018, 20, 1459–1466. [Google Scholar] [CrossRef]
- Li, W.X.; Xiao, L.P.; Li, X.Y.; Xiao, W.Z.; Yang, Y.Q.; Sun, R.C. Renewable and flexible thermosetting epoxies based on func-tionalized biorefinery lignin fractions. Mater. Today Sustain. 2021, 15, 100083. [Google Scholar] [CrossRef]
- Kai, D.; Zhang, K.; Jiang, L.; Wong, H.Z.; Li, Z.; Zhang, Z.; Loh, X.J. Sustainable and Antioxidant Lignin–Polyester Copolymers and Nanofibers for Potential Healthcare Applications. ACS Sustain. Chem. Eng. 2017, 5, 6016–6025. [Google Scholar] [CrossRef]
- Wang, X.; Jia, Y.; Liu, Z.; Miao, J. Influence of the Lignin Content on the Properties of Poly(Lactic Acid)/lignin-Containing Cellulose Nanofibrils Composite Films. Polymers 2018, 10, 1013. [Google Scholar] [CrossRef] [Green Version]
- Pączkowski, P.; Puszka, A.; Gawdzik, B. Investigation of Degradation of Composites Based on Unsaturated Polyester Resin and Vinyl Ester Resin. Materials 2022, 15, 1286. [Google Scholar] [CrossRef]
- Rogers, D. Lignin-Derived Thermosetting Vinyl Ester Resins for High Performance Applications. Master’s Thesis, Rowan University, Glassboro, NJ, USA, 2015. [Google Scholar]
- Podkościelna, B.; Wnuczek, K.; Goliszek, M.; Klepka, T.; Dziuba, K. Flammability Tests and Investigations of Properties of Lignin-Containing Polymer Composites Based on Acrylates. Molecules 2020, 25, 5947. [Google Scholar] [CrossRef]
- Goliszek, M.; Podkościelna, B.; Klepka, T.; Sevastyanova, O. Preparation, Thermal, and Mechanical Characterization of UV-Cured Polymer Biocomposites with Lignin. Polymers 2020, 12, 1159. [Google Scholar] [CrossRef]
- Pilato, L. Phenolic resins: 100Years and still going strong. React. Funct. Polym. 2013, 73, 270–277. [Google Scholar] [CrossRef]
- Tang, K.; Zhang, A.; Ge, T.; Liu, X.; Tang, X.; Li, Y. Research progress on modification of phenolic resin. Mater. Today Commun. 2020, 26, 101879. [Google Scholar] [CrossRef]
- Gardziella, A.; Pilato, L.A.; Knop, A. Phenolic Resins—Chemistry, Applications, Standardization, Safety and Technology; Springer: Berlin/Heidelberg, Germany, 2000. [Google Scholar]
- Pilato, L. Future Aspects. In Phenolic Resins: A Century of Progress; Springer: Berlin/Heidelberg, Germany, 2010. [Google Scholar]
- Notley, S.M.; Norgren, M. Lignin: Functional biomaterial with potential in surface chemistry and nanoscience. In The Nanoscience and Technology of Renewable Biomaterials; Lucia, L.A., Rojas, O.J., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2009; pp. 173–205. [Google Scholar]
- Noh, Y.; Odimayomi, T.; Sendesi, S.M.T.; Youngblood, J.P.; Whelton, A.J. Environmental and human health risks of plastic composites can be reduced by optimizing manufacturing conditions. J. Clean. Prod. 2022, 356, 131803. [Google Scholar] [CrossRef]
- ASTM E1640-09; Standard Test Method for Assignment of the Glass Transition Temperature by Dynamic Mechanical Analysis. American Society for Testing and Materials: West Conshohocken, PA, USA, 2009.
- ASTM E2550-21; Standard Test Method for Thermal Stability by Thermogravimetry. American Society for Testing and Materials: West Conshohocken, PA, USA, 2021.
- ASTM D638-14; Standard Test Method for Tensile Properties of Plastics. American Society for Testing and Materials: West Conshohocken, PA, USA, 2014.
- ASTM D5379/D5379M-12; Standard Test Method for Shear Properties of Composite Materials by the V-Notched Beam Method. American Society for Testing and Materials: West Conshohocken, PA, USA, 2010.
- Costa, C.S.M.F.; Fonseca, A.C.; Moniz, J.; Godinho, M.; Coelho, J.; Serra, A.C. Going greener: Synthesis of fully biobased unsaturated polyesters for styrene crosslinked resins with enhanced thermomechanical properties. Express Polym. Lett. 2017, 11, 885–898. [Google Scholar] [CrossRef]
- Ferreira, G.R.; Braquehais, J.R.; da Silva, W.N.; Machado, F. Synthesis of soybean oil-based polymer lattices via emulsion polymerization process. Ind. Crop. Prod. 2015, 65, 14–20. [Google Scholar] [CrossRef]
- Papadopoulos, L.; Malletzidou, L.; Patsiaoura, D.; Magaziotis, A.; Psochia, E.; Terzopoulou, Z.; Chrissafis, K.; Markessini, C.; Papadopoulou, E.; Bikiaris, D.N. Synthesis and Characterization of Unsaturated Succinic Acid Biobased Polyester Resins. Appl. Sci. 2021, 11, 896. [Google Scholar] [CrossRef]
- Kang, H.; Li, M.; Tang, Z.; Xue, J.; Hu, X.; Zhang, L.; Guo, B. Synthesis and characterization of biobased isosorbide-containing copolyesters as shape memory polymers for biomedical applications. J. Mater. Chem. B 2014, 2, 7877–7886. [Google Scholar] [CrossRef]
- Trino, A.; Costa, C.; Fonseca, A.; Barata, I.; Júlio, E.; Serra, A.; Coelho, J. Novel composites from green unsaturated polyesters and fly ashes: Preparation and characterization. React. Funct. Polym. 2016, 106, 24–31. [Google Scholar] [CrossRef]
- Xue, B.-L.; Wen, J.-L.; Zhu, M.-Q.; Sun, R.-C. Lignin-based polyurethane film reinforced with cellulose nanocrystals. RSC Adv. 2014, 4, 36089–36096. [Google Scholar] [CrossRef]
- Jia, Z.; Lu, C.; Zhou, P.; Wang, L. Preparation and characterization of high boiling solvent lignin-based polyurethane film with lignin as the only hydroxyl group provider. RSC Adv. 2015, 5, 53949–53955. [Google Scholar] [CrossRef]
- Sarkar, S.; Adhikari, B. Synthesis and characterization of lignin–HTPB copolyurethane. Eur. Polym. J. 2001, 37, 1391–1401. [Google Scholar] [CrossRef]
- Jeong, H.; Park, J.; Kim, S.; Lee, J.; Cho, J.W. Use of acetylated softwood kraft lignin as filler in synthetic polymers. Fibers Polym. 2012, 13, 1310–1318. [Google Scholar] [CrossRef]
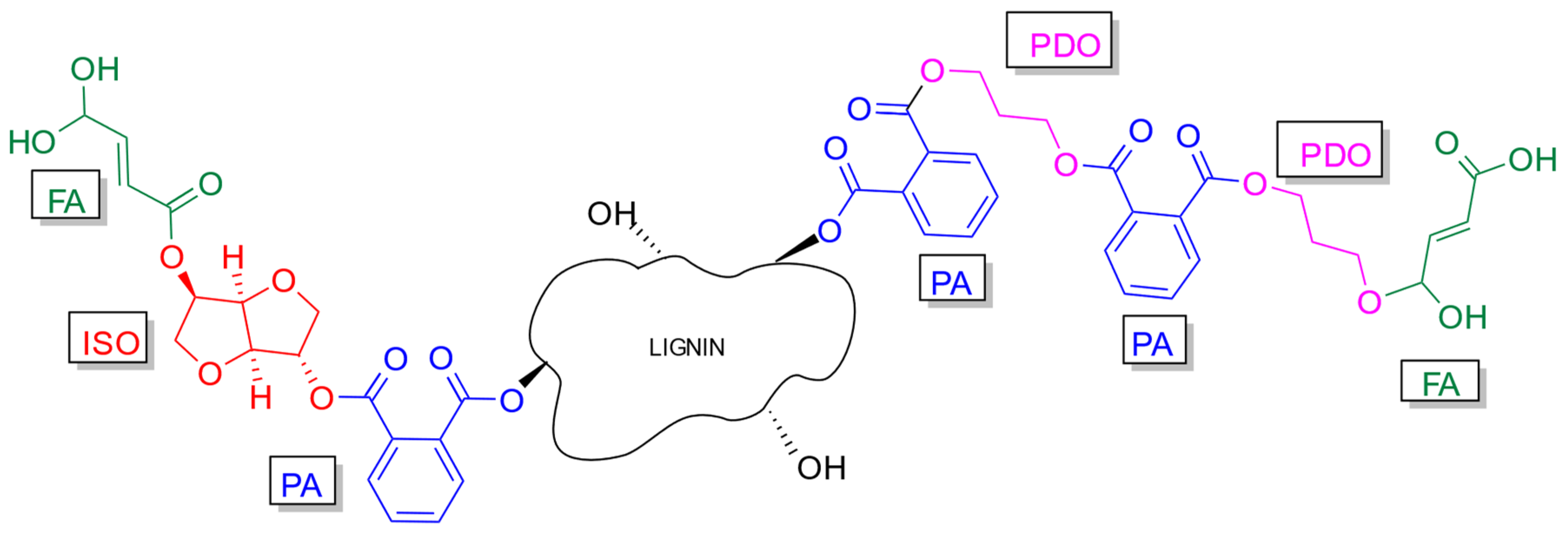
| Monomer | BVE Resin | BVEL Resin |
|---|---|---|
| Dry Mass Content (g) | Dry Mass Content (g) | |
| Ortho-phthalic anhydride (PA) | 148.11 | 148.11 |
| Fumaric acid (FA) | 292.18 | 292.18 |
| 1,3-propanediol (PDO) | 187.99 | 197.27 |
| Isosorbide (ISO) | 200.58 | 188.29 |
| Lignin | - | 35.74 |
| Resin | Specimen | Post-Curing | Runs | |
|---|---|---|---|---|
| Temperature (°C) | Time (h) | |||
| BVE | BVE-1 | 100 | 4 | 1 |
| BVE-2 | 1 | |||
| BVEL | BVEL-1 | 2 | ||
| BVEL-2 | 2 | |||
| BVEL-3 | 110 | 24 | 1 | |
| Parameter | BVE | BVEL |
|---|---|---|
| Mn | 1555 | 1483 |
| Mw | 2956 | 3561 |
| PDI | 1.9 | 2.4 |
| Resin | Specimen | 1st Run | 2nd Run | ||||
|---|---|---|---|---|---|---|---|
| Tg, E’ | Tg, tan δ | Failure | Tg, E’ | Tg, tan δ | Failure | ||
| BVE | BVE-1 | 54.9 | 94.9 | No | (-) | (-) | (-) |
| BVE-2 | 62.1 | 98.3 | No | (-) | (-) | (-) | |
| BVEL | BVEL-1 | 57.6 | 107.2 | No | 66.2 | 116.8 | Yes |
| BVEL-2 | 58.3 | 102.3 | No | 69.2 | 114.3 | Yes | |
| BVEL-3 * | 79.7 | 145.7 | Yes | (-) | (-) | (-) | |
| Atmosphere | Resin | Specimen | T5% (°C) | T10% (°C) | T50% (°C) | Residue (%) |
|---|---|---|---|---|---|---|
| Oxidative | BVE | BVE-O-1 | 229 | 290 | 385 | 0.1 |
| BVE-O-2 | 257 | 316 | 389 | 0.2 | ||
| BVEL | BVEL-O-1 | 239 | 308 | 394 | 1.2 | |
| BVEL-O-2 | 266 | 326 | 392 | 0.2 | ||
| Inert | BVE | BVE-I | 247 | 313 | 393 | 5.7 |
| BVEL | BVEL-I | 239 | 300 | 393 | 5.1 |
| Resin | Specimen | Tensile Strength (MPa) | Tensile Modulus (GPa) | Ultimate Strain (%) |
|---|---|---|---|---|
| BVE | 1 | 45.0 | 3.40 | 1.49 |
| 2 | 50.9 | 3.31 | 1.86 | |
| 3 | 57.9 | 3.39 | 2.21 | |
| 4 | 52.1 | 3.25 | 1.92 | |
| 5 | 48.9 | 3.44 | 1.70 | |
| Average | 51.0 ± 4.7 (CoV = 9%) | 3.36 ± 0.08 (CoV = 2%) | 1.84 ± 0.27 (CoV = 15%) | |
| BVEL | 1 | 67.4 | 3.43 | 2.56 |
| 2 | 70.4 | 3.18 | 3.05 | |
| 3 | 57.2 | 3.08 | 2.41 | |
| Average | 65.0 ± 6.9 (CoV = 11%) | 3.23 ± 0.18 (CoV = 6%) | 2.67 ± 0.33 (CoV = 13%) |
| Resin | Specimen | Shear Strength (MPa) | Shear Modulus (GPa) | Ultimate Distortion (%) |
|---|---|---|---|---|
| BVE | 1 | 49.6 | 1.37 | 4.31 |
| 2 | 47.2 | 1.51 | 5.65 | |
| 3 | 48.2 | 1.57 | 6.19 | |
| 4 | 47.8 | 1.45 | 5.67 | |
| 5 | 57.4 | 1.67 | 6.61 | |
| Average | 50.0 ± 4.2 (CoV = 8%) | 1.51 ± 0.11 (CoV = 8%) | 5.69 ± 0.87 (CoV = 15%) | |
| BVEL | 1 | 52.1 | 1.51 | 6.41 |
| 2 | 47.5 | 1.68 | 5.02 | |
| 3 | 56.3 | 1.86 | 6.27 | |
| Average | 52.0 ± 4.4 (CoV = 8%) | 1.68 ± 0.18 (CoV = 10%) | 5.90 ± 0.77 (CoV = 13%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Machado, M.; Hofmann, M.; Garrido, M.; Correia, J.R.; Bordado, J.C.; Rosa, I.C. Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites. Appl. Sci. 2023, 13, 8342. https://doi.org/10.3390/app13148342
Machado M, Hofmann M, Garrido M, Correia JR, Bordado JC, Rosa IC. Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites. Applied Sciences. 2023; 13(14):8342. https://doi.org/10.3390/app13148342
Chicago/Turabian StyleMachado, Marina, Mateus Hofmann, Mário Garrido, João R. Correia, João C. Bordado, and Inês C. Rosa. 2023. "Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites" Applied Sciences 13, no. 14: 8342. https://doi.org/10.3390/app13148342
APA StyleMachado, M., Hofmann, M., Garrido, M., Correia, J. R., Bordado, J. C., & Rosa, I. C. (2023). Incorporation of Lignin in Bio-Based Resins for Potential Application in Fiber–Polymer Composites. Applied Sciences, 13(14), 8342. https://doi.org/10.3390/app13148342





