A Method for Synthesizing Iron Silicate Slags to Evaluate Their Performance as Supplementary Cementitious Materials
Abstract
1. Introduction
2. Theoretical Considerations
3. Materials and Methods
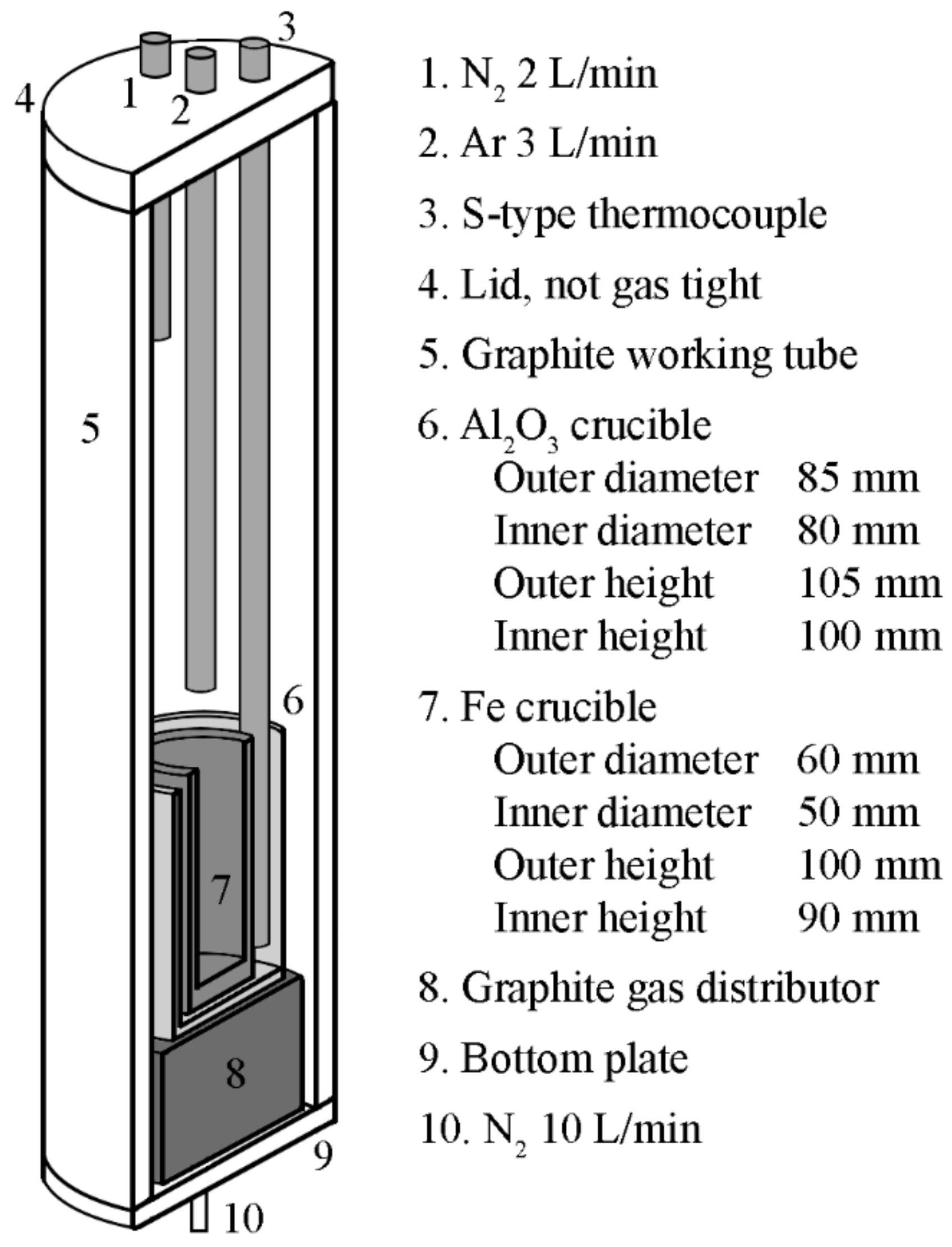
3.1. Synthesizing Iron Silicate Slags
3.2. Relevance of Methodology
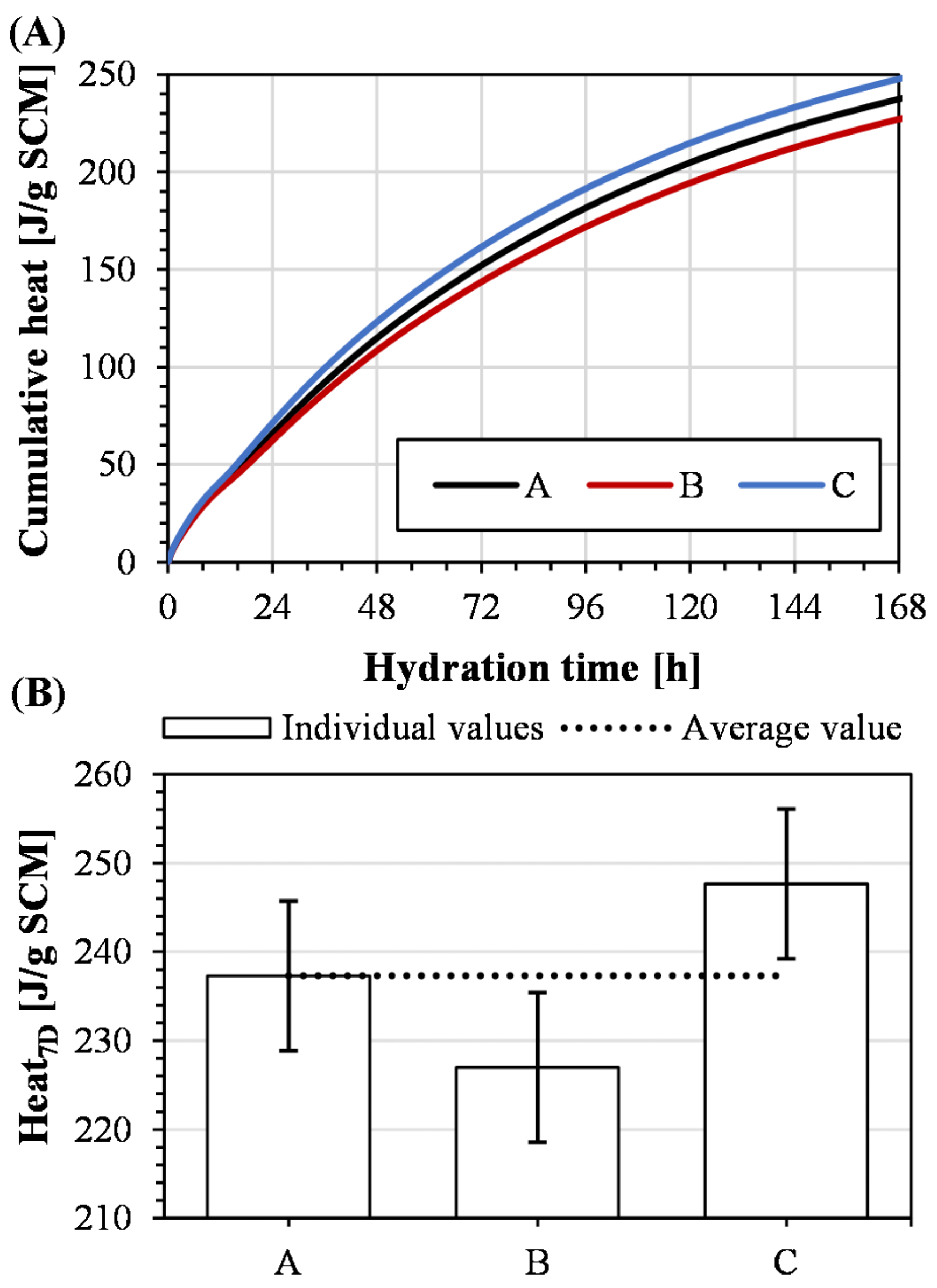
3.3. Robustness of Methodology
4. Results and Discussion
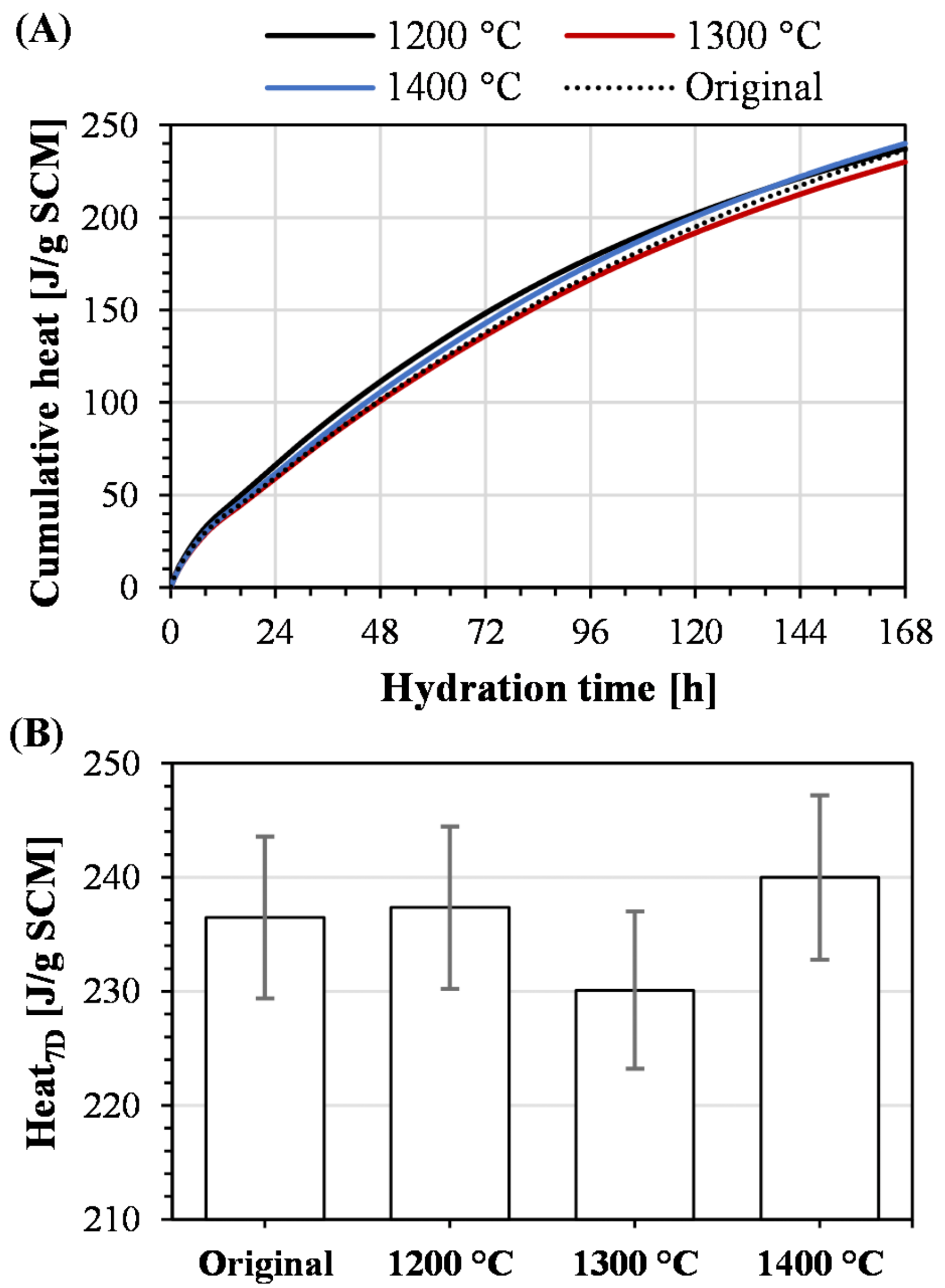
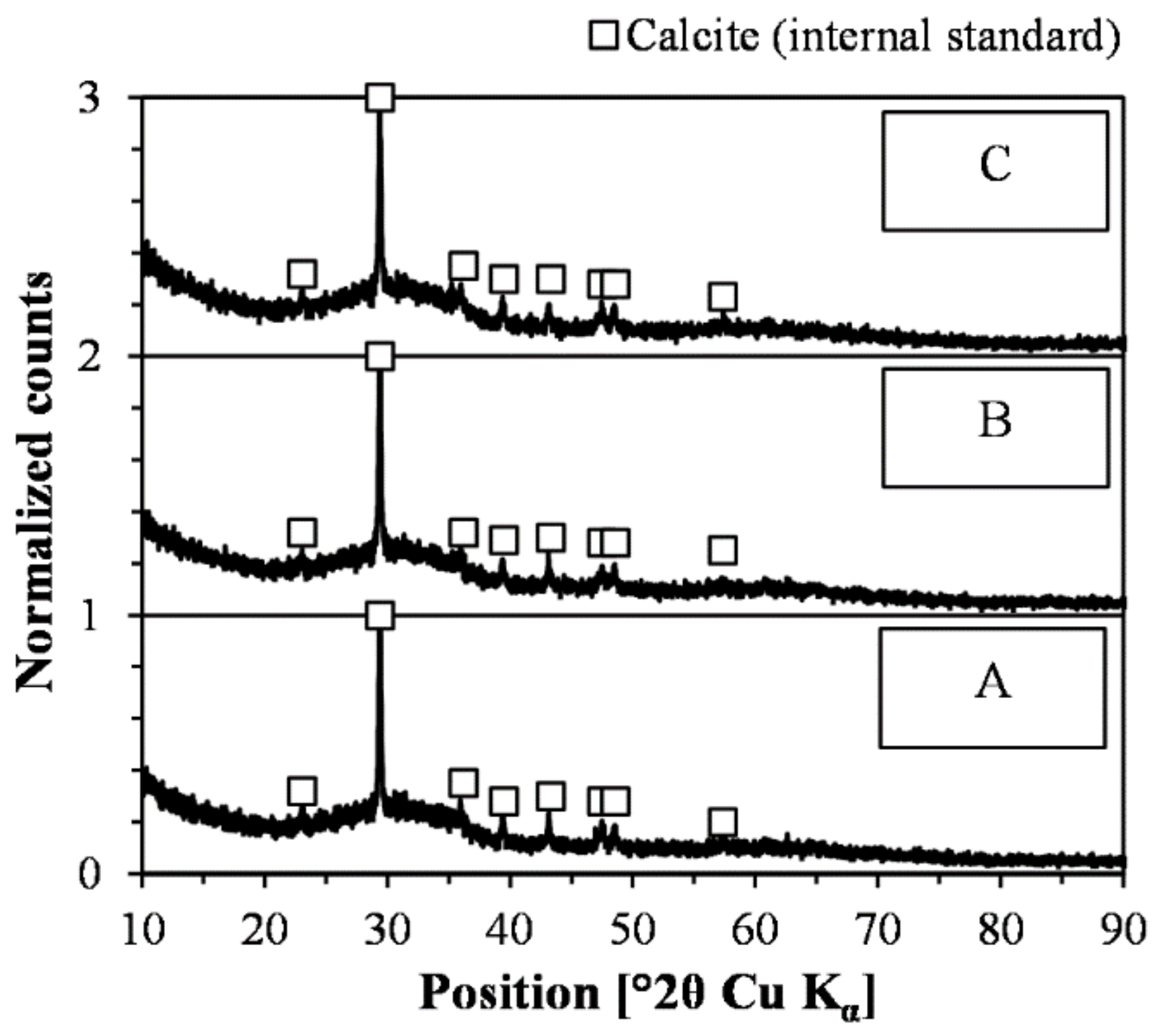
4.1. Relevance of Methodology
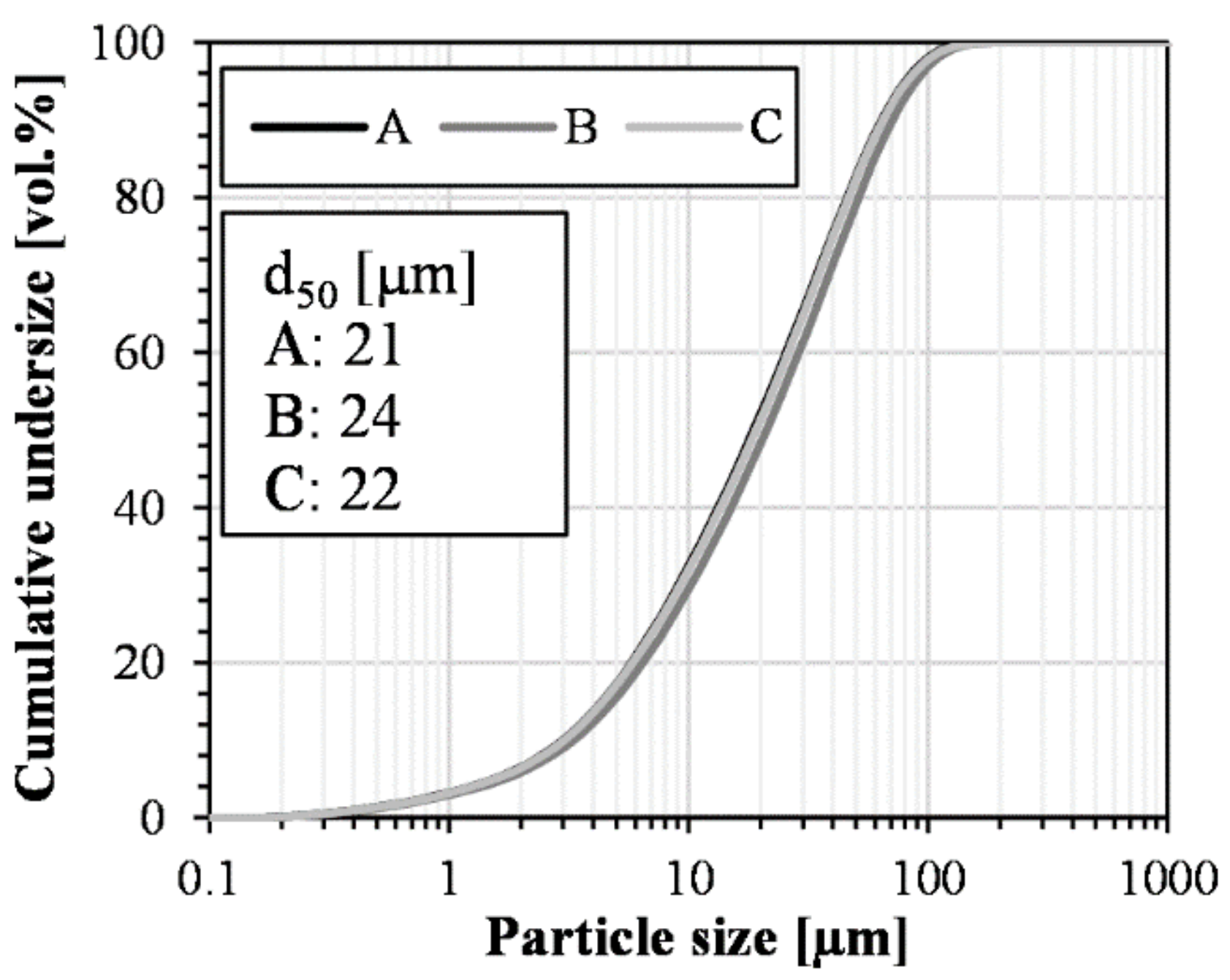
4.2. Robustness of Methodology
5. Conclusions
- The reactivity of an industrial slag granulated on an industrial scale does not differ from the reactivity of the same slag granulated using the method developed in the present study;
- Therefore, results on reactivity achieved using this method are directly applicable to industrial slags.
- The targeted chemical composition can be synthesized with high repeatability, and the constituents are successfully incorporated in an entirely amorphous phase;
- The standard deviation of the developed heat in the R3 isothermal calorimeter experiments was 3.6% of the average value of the triplicate, suggesting that this method is robust.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gabasiane, T.S.; Danha, G.; Mamvura, T.A.; Mashifana, T.; Dzinomwa, G. Environmental and Socioeconomic Impact of Copper Slag—A Review. Crystals 2021, 11, 1504. [Google Scholar] [CrossRef]
- Shi, C.; Meyer, C.; Behnood, A. Utilization of Copper Slag in Cement and Concrete. Resour. Conserv. Recycl. 2008, 52, 1115–1120. [Google Scholar] [CrossRef]
- International Copper Study Group. The World Copper Factbook 2022; International Copper Study Group: Lisbon, Portugal, 2022; p. 11.
- Kero Andertun, J.; Samuelsson, C.; Peltola, P.; Engström, F. Characterisation and Leaching Behaviour of Granulated Iron Silicate Slag Constituents. Can. Metall. Q. 2022, 61, 14–23. [Google Scholar] [CrossRef]
- Murari, K.; Siddique, R.; Jain, K.K. Use of Waste Copper Slag, a Sustainable Material. J. Mater. Cycles Waste Manag. 2015, 17, 13–26. [Google Scholar] [CrossRef]
- Al-Jabri, K.S.; Taha, R.A.; Al-Hashmi, A.; Al-Harthy, A.S. Effect of Copper Slag and Cement By-Pass Dust Addition on Mechanical Properties of Concrete. Constr. Build. Mater. 2006, 20, 322–331. [Google Scholar] [CrossRef]
- Benhelal, E.; Shamsaei, E.; Rashid, M.I. Challenges against CO2 Abatement Strategies in Cement Industry: A Review. J. Environ. Sci. 2021, 104, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Olivier, J.G.J.; Janssens-Maenhout, G.; Peters, J.A.H.W. Trends in Global CO2 Emissions; 2012 Report; PBL Netherlands Environmental Assessment Agency: The Hauge, The Netherlands, 2012; pp. 16–17. [Google Scholar]
- Lothenbach, B.; Scrivener, K.; Hooton, R.D. Supplementary Cementitious Materials. Cem. Concr. Res. 2011, 41, 1244–1256. [Google Scholar] [CrossRef]
- Tixier, R.; Devaguptapu, R.; Mobasher, B. The Effect of Copper Slag on the Hydration and Mechanical Properties of Cementitious Mixtures. Cem. Concr. Res. 1997, 27, 1569–1580. [Google Scholar] [CrossRef]
- Moura, W.A.; Gonçalves, J.P.; Lima, M.B.L. Copper Slag Waste as a Supplementary Cementing Material to Concrete. J. Mater. Sci. 2007, 42, 2226–2230. [Google Scholar] [CrossRef]
- De Rojas, M.I.S.; Rivera, J.; Frías, M.; Marín, F. Use of Recycled Copper Slag for Blended Cements. J. Chem. Technol. Biotechnol. 2008, 83, 209–217. [Google Scholar] [CrossRef]
- Singh, J.; Singh, J.; Kaur, M. Copper Slag Blended Cement: An Environmental Sustainable Approach for Cement Industry in India. Curr. World Environ. 2016, 11, 186–193. [Google Scholar] [CrossRef]
- Edwin, R.S.; de Schepper, M.; Gruyaert, E.; de Belie, N. Effect of Secondary Copper Slag as Cementitious Material in Ultra-High Performance Mortar. Constr. Build. Mater. 2016, 119, 31–44. [Google Scholar] [CrossRef]
- Sheng, G.; Wang, B.; Wang, S.; Wang, Z. Pozzolanic Reaction of FeO-SiO2 Slag with Portlandite. J. Solid. Waste Technol. Manage 2016, 42, 51–57. [Google Scholar] [CrossRef]
- Sivakumar, P.P.; Gruyaert, E.; de Belie, N.; Matthys, S. Reactivity of Modified Iron Silicate Slag as Sustainable Alternative Binder. In Proceedings of the Fifth International Conference on Sustainable Construction Materials and Technologies, London, UK, 15–17 July 2019. [Google Scholar]
- He, R.; Zhang, S.; Zhang, X.; Zhang, Z.; Zhao, Y.; Ding, H. Copper Slag: The Leaching Behavior of Heavy Metals and Its Applicability as a Supplementary Cementitious Material. J. Environ. Chem. Eng. 2021, 9, 105132. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Q.; Huang, Z. Reuse of Copper Slag as a Supplementary Cementitious Material: Reactivity and Safety. Resour. Conserv. Recycl. 2020, 162, 105037. [Google Scholar] [CrossRef]
- Feng, Y.; Yang, Q.; Chen, Q.; Kero, J.; Andersson, A.; Ahmed, H.; Engström, F.; Samuelsson, C. Characterization and Evaluation of the Pozzolanic Activity of Granulated Copper Slag Modified with CaO. J. Clean. Prod. 2019, 232, 1112–1120. [Google Scholar] [CrossRef]
- Feng, Y.; Kero, J.; Yang, Q.; Chen, Q.; Engström, F.; Samuelsson, C.; Qi, C. Mechanical Activation of Granulated Copper Slag and Its Influence on Hydration Heat and Compressive Strength of Blended Cement. Materials 2019, 12, 772. [Google Scholar] [CrossRef]
- Sivakumar, P.P.; Matthys, S.; de Belie, N.; Gruyaert, E. Reactivity Assessment of Modified Ferro Silicate Slag by R3 Method. Appl. Sci. 2021, 11, 366. [Google Scholar] [CrossRef]
- Snellings, R.; Scrivener, K.L. Rapid Screening Tests for Supplementary Cementitious Materials: Past and Future. Mater. Struct. 2016, 49, 3265–3279. [Google Scholar] [CrossRef]
- Ramanathan, S.; Perumal, P.; Illikainen, M.; Suraneni, P. Mechanically Activated Mine Tailings for Use as Supplementary Cementitious Materials. RILEM Techn. Lett. 2021, 6, 61–69. [Google Scholar] [CrossRef]
- Ramanathan, S.; Tuen, M.; Suraneni, P. Influence of Supplementary Cementitious Material and Filler Fineness on Their Reactivity in Model Systems and Cementitious Pastes. Mater. Struct. 2022, 55, 1–25. [Google Scholar] [CrossRef]
- Zhang, T.S.; Yu, Q.J.; Wei, J.X.; Zhang, P.P. Effect of Size Fraction on Composition and Pozzolanic Activity of High Calcium Fly Ash. Adv. Cem. Res. 2011, 23, 299–307. [Google Scholar] [CrossRef]
- Mirzahosseini, M.; Riding, K.A. Influence of Different Particle Sizes on Reactivity of Finely Ground Glass as Supplementary Cementitious Material (SCM). Cem. Concr. Compos. 2015, 56, 95–105. [Google Scholar] [CrossRef]
- Schöler, A.; Winnefeld, F.; Haha, M.B.; Lothenbach, B. The Effect of Glass Composition on the Reactivity of Synthetic Glasses. J Am. Ceram. Soc. 2017, 100, 2553–2567. [Google Scholar] [CrossRef]
- Snellings, R. Solution-Controlled Dissolution of Supplementary Cementitious Material Glasses at pH 13: The Effect of Solution Composition on Glass Dissolution Rates. J. Am. Ceram. Soc. 2013, 96, 2467–2475. [Google Scholar] [CrossRef]
- Bignozzi, M.C.; Saccani, A.; Barbieri, L.; Lancellotti, I. Glass Waste as Supplementary Cementing Materials: The Effects of Glass Chemical Composition. Cem. Concr. Compos. 2015, 55, 45–52. [Google Scholar] [CrossRef]
- Skibsted, J.; Snellings, R. Reactivity of Supplementary Cementitious Materials (SCMs) in Cement Blends. Cem. Concr. Res. 2019, 124, 105799. [Google Scholar] [CrossRef]
- Astoveza, J.; Trauchessec, R.; Migot-Choux, S.; Soth, R.; Pontikes, Y. Iron-Rich Slag Addition in Ternary Binders of Portland Cement, Aluminate Cement and Calcium Sulfate. Cem. Concr. Res. 2022, 153, 106689. [Google Scholar] [CrossRef]
- Hallet, V.; de Belie, N.; Pontikes, Y. The Impact of Slag Fineness on the Reactivity of Blended Cements with High-Volume Non-Ferrous Metallurgy Slag. Constr. Build. Mater. 2020, 257, 119400. [Google Scholar] [CrossRef]
- Jak, E.; Hayes, P. Phase Equilibria and Thermodynamics of Zinc Fuming Slags. Can. Metall. Q. 2002, 41, 163–174. [Google Scholar] [CrossRef]
- Sukhomlinov, D.; Klemettinen, L.; Avarmaa, K.; O’Brien, H.; Taskinen, P.; Jokilaakso, A. Distribution of Ni, Co, Precious, and Platinum Group Metals in Copper Making Process. Metall. Mater. Trans. B 2019, 50, 1752–1765. [Google Scholar] [CrossRef]
- Nikolic, S.; Hayes, P.C.; Jak, E. Phase Equilibria in Ferrous Calcium Silicate Slags: Part I. Intermediate Oxygen Partial Pressures in the Temperature Range 1200 °C to 1350 °C. Metall. Mat. Trans. B 2008, 39, 179–188. [Google Scholar] [CrossRef]
- Mysen, B.; Richet, P. Silicate Glasses and Melts, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2019; p. 353. [Google Scholar]
- Yazhenskikh, E.; Jantzen, T.; Hack, K.; Müller, M. A New Multipurpose Thermodynamic Database for Oxide Systems. Rasplawy 2019, 2, 116–124. [Google Scholar] [CrossRef]
- GTT-Technologies. GTOx Version 17 Documentation; GTT-Technologies: Herzogenrath, Germany, 2022. [Google Scholar]
- Bale, C.W.; Bélisle, E.; Chartrand, P.; Decterov, S.A.; Eriksson, G.; Gheribi, A.E.; Hack, K.; Jung, I.H.; Kang, Y.B.; Melançon, J.; et al. Reprint of: FactSage Thermochemical Software and Databases, 2010–2016. Calphad 2016, 55, 1–19. [Google Scholar] [CrossRef]
- Tossavainen, M.; Engstrom, F.; Yang, Q.; Menad, N.; Lidstrom Larsson, M.; Bjorkman, B. Characteristics of Steel Slag under Different Cooling Conditions. Waste Manag. 2007, 27, 1335–1344. [Google Scholar] [CrossRef]
- Lee, C.M.; Fruehan, R.J. Phosphorus Equilibrium between Hot Metal and Slag. Ironmak. Steelmak. 2005, 32, 503–508. [Google Scholar] [CrossRef]
- Allibert, M. Slag Atlas, 2nd ed.; VDEh Verlag Stahleisen: Düsseldorf, Germany, 1995; pp. 142–143. [Google Scholar]
- Anindya, A.; Swinbourne, D.R.; Reuter, M.A.; Matusewicz, R.W. Distribution of Elements between Copper and FeOx-CaO-SiO2 Slags during Pyrometallurgical Processing of WEEE: Part 1—Tin. Min. Process Extr. Metall. 2013, 122, 165–173. [Google Scholar] [CrossRef]
- Lennartsson, A.; Engström, F.; Björkman, B.; Samuelsson, C. Understanding the Bottom Buildup in an Electric Copper Smelting Furnace by Thermodynamic Calculations. Can. Metall. Q. 2019, 58, 89–95. [Google Scholar] [CrossRef]
- Isaksson, J.; Vikström, T.; Lennartsson, A.; Samuelsson, C. Influence of Process Parameters on Copper Content in Reduced Iron Silicate Slag in a Settling Furnace. Metals 2021, 11, 992. [Google Scholar] [CrossRef]
- Kucharczyk, S.; Sitarz, M.; Zajac, M.; Deja, J. The Effect of CaO/SiO2 Molar Ratio of CaO-Al2O3-SiO2 Glasses on Their Structure and Reactivity in Alkali Activated System. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2018, 194, 163–171. [Google Scholar] [CrossRef]
- Glosser, D.; Suraneni, P.; Isgor, O.B.; Weiss, W.J. Using Glass Content to Determine the Reactivity of Fly Ash for Thermodynamic Calculations. Cem. Concr. Compos 2021, 115, 103849. [Google Scholar] [CrossRef]
- Callister, W.D.; Rethwisch, D.G. Materials Science and Engineering: An Introduction, 8th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2010; pp. 350–351. [Google Scholar]
- Isaksson, J.; Vikström, T.; Lennartsson, A.; Andersson, A.; Samuelsson, C. Settling of Copper Phases in Lime Modified Iron Silicate Slag. Metals 2021, 11, 1098. [Google Scholar] [CrossRef]
- Kero Andertun, J.; Vikström, T.; Peltola, P.; Samuelsson, C.; Engström, F. Characterisation and Leaching Behavior of CaO-Modified Iron-Silicate Slag Produced in Laboratory and Industrial Scales. Can. Metall. Q. 2021, 60, 294–305. [Google Scholar] [CrossRef]
- Moon, G.D.; Oh, S.; Choi, Y.C. Effects of the Physicochemical Properties of Fly Ash on the Compressive Strength of High-Volume Fly Ash Mortar. Constr. Build. Mater. 2016, 124, 1072–1080. [Google Scholar] [CrossRef]
- Grazulis, S.; Chateigner, D.; Downs, R.T.; Yokochi, A.F.T.; Quirós, M.; Lutterotti, L.; Manakova, E.; Butkus, J.; Moeck, P.; le Bail, A. Crystallography Open Database—An Open-Access Collection of Crystal Structures. J. Appl. Crystallogr. 2009, 42, 726–729. [Google Scholar] [CrossRef] [PubMed]
- Avet, F.; Snellings, R.; Diaz, A.A.; Haha, M.B.; Scrivener, K. Development of a New Rapid, Relevant and Reliable (R3) Test Method to Evaluate the Pozzolanic Reactivity of Calcined Kaolinitic Clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Li, X.; Snellings, R.; Antoni, M.; Alderete, N.M.; Haha, M.B.; Bishnoi, S.; Cizer, Ö.; Cyr, M.; De Weerdt, K.; Dhandapani, Y.; et al. Reactivity Tests for Supplementary Cementitious Materials: RILEM TC 267-TRM Phase 1. Mater. Struct. 2018, 51, 1–14. [Google Scholar] [CrossRef]
- Romero Sarcos, N.; Hart, D.; Bornhöft, H.; Ehrenberg, A.; Deubener, J. Rejuvenation of Granulated Blast Furnace Slag (GBS) Glass by Ball Milling. J. Non. Cryst. Solids 2021, 556, 120557. [Google Scholar] [CrossRef]
- Ehrenberg, A. Influence of the Granulation Conditions and Performance Potential of Granulated Blastfurnace Slag―Part 2: Chemistry and Physical Properties. ZKG Int. 2013, 3, 60–67. [Google Scholar]
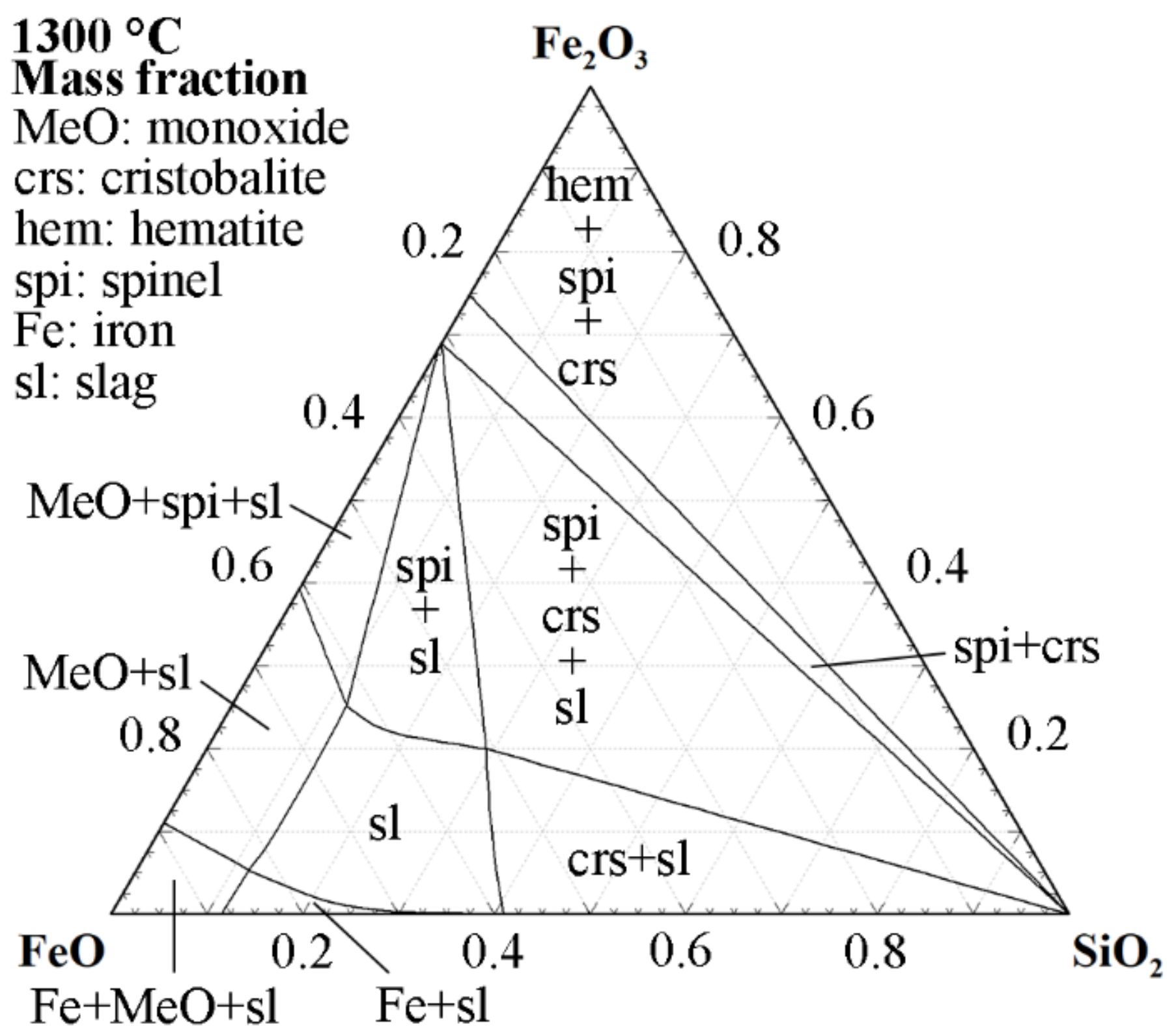
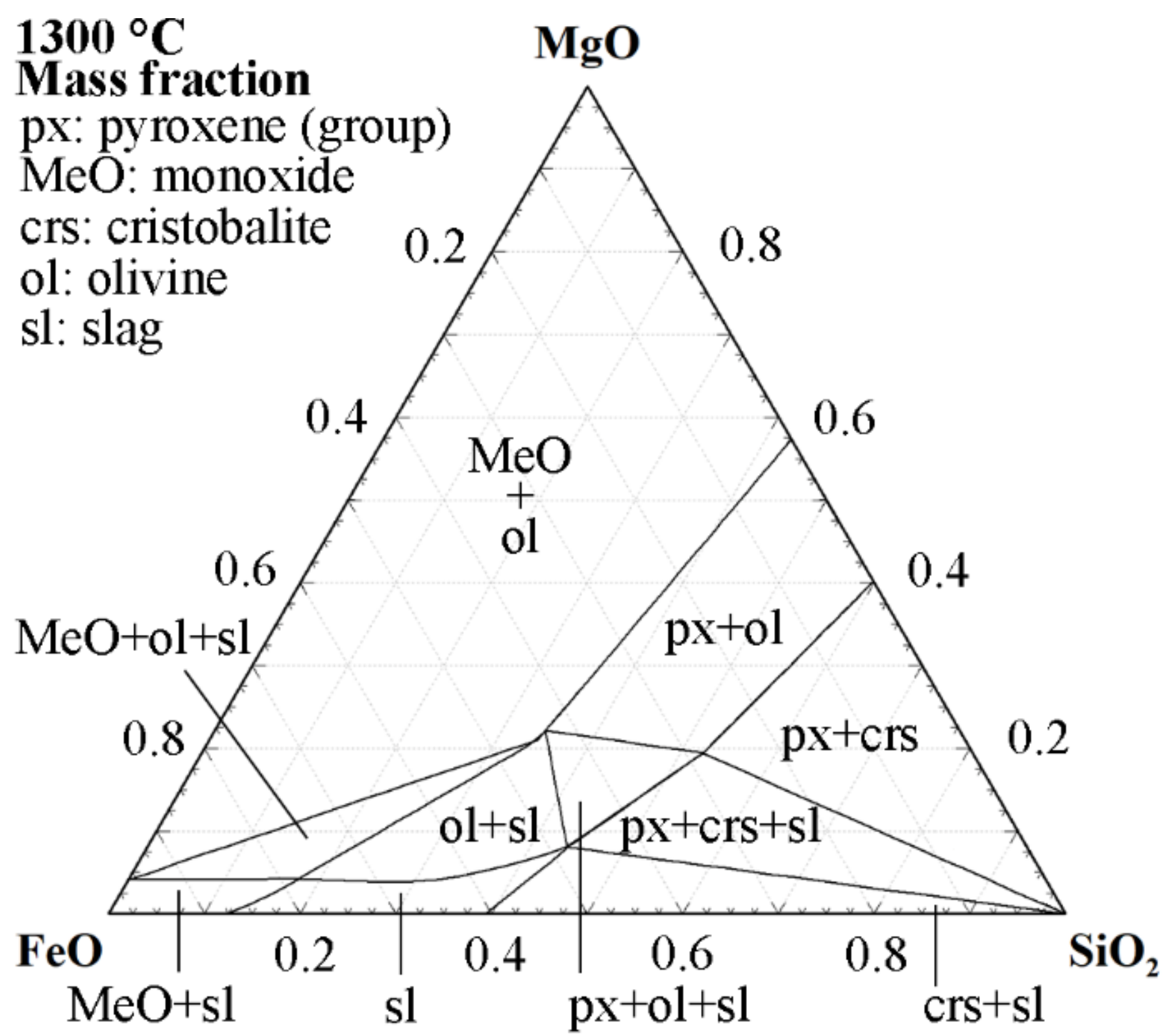

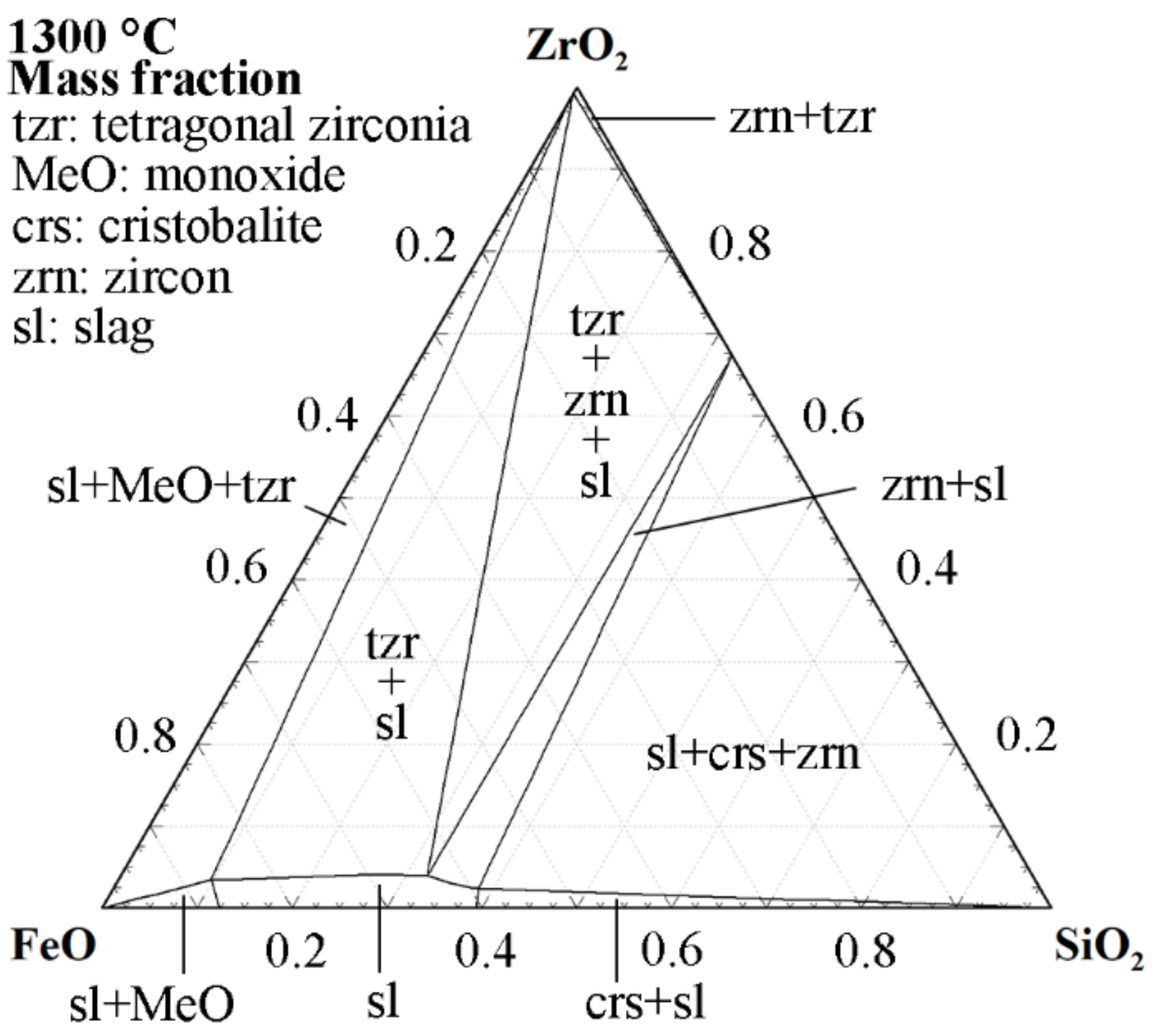
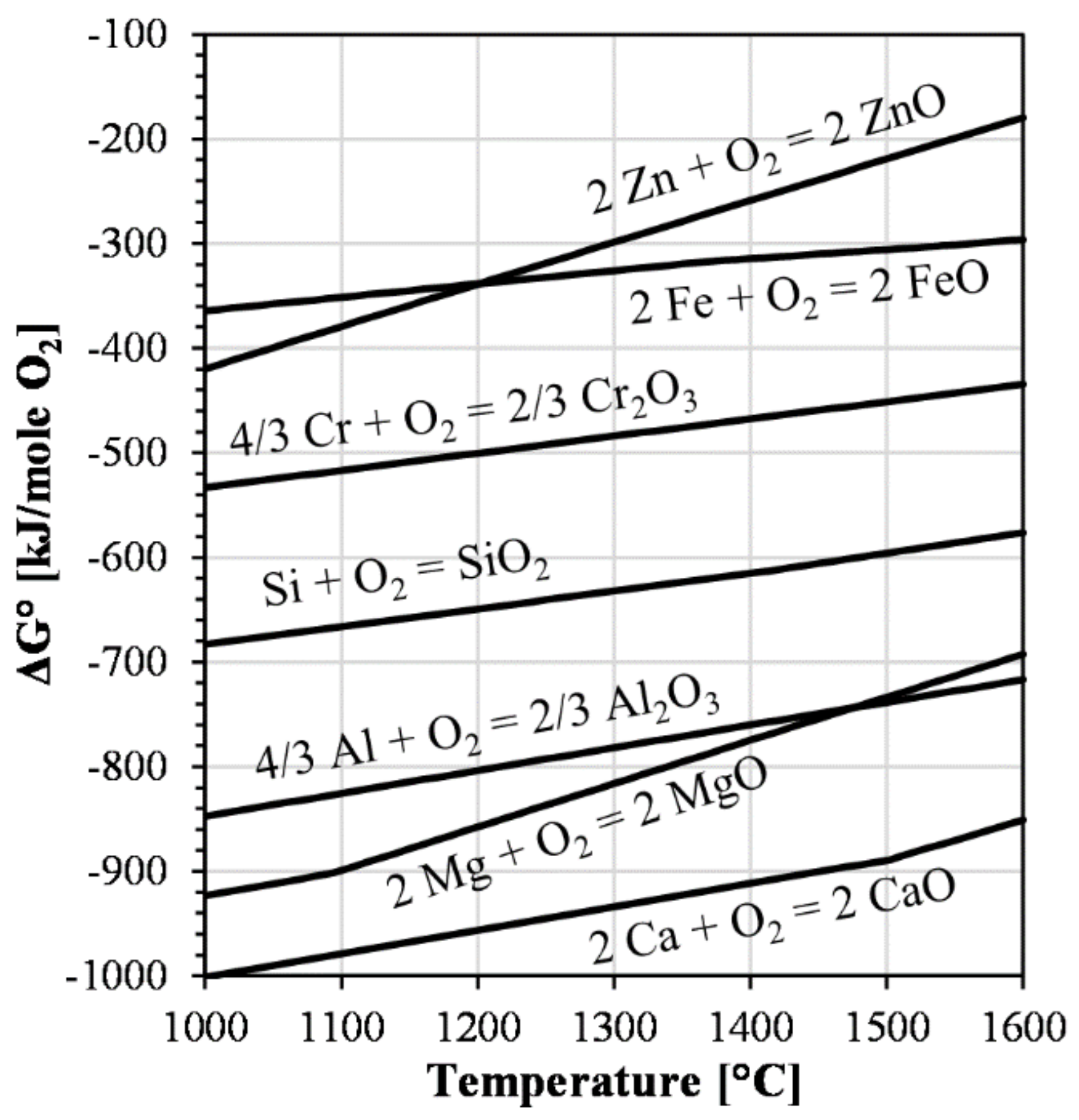



| Ref. | FeO/SiO2 | FeO 1 | SiO2 | CaO | Al2O3 | MgO | ZnO 1 | Cu 1 |
|---|---|---|---|---|---|---|---|---|
| [17] | 1.12 | 37.9 | 33.9 | 12.7 | 4.7 | 0.8 | / | / |
| [19] | 1.14 | 37.9 | 33.3 | 12.3 | 4.6 | 1.1 | 1.3 | 1.4 |
| [19] | 1.14 | 36.9 | 32.3 | 19.5 | 3.9 | 0.9 | 1.2 | 1.2 |
| [16,21] | 1.14 | 36.8 | 32.3 | 3.9 | 11.0 | / | / | / |
| [19,20] | 1.27 | 42.3 | 33.4 | 4.0 | 3.5 | 1.4 | 1.1 | / |
| [10] | 1.35 | 47.5 | 35.2 | 3.3 | 5.0 | 0.6 | / | 0.5 |
| [6] | 1.46 | 48.1 | 33.1 | 6.1 | 2.8 | 1.6 | / | 0.4 |
| [15] | 1.49 | 46.9 | 31.5 | 3.2 | 8.5 | 4.4 | 0.3 | 1.3 |
| [14] | 1.58 | 40.9 | 25.9 | 7.1 | 5.9 | 0.8 | 8.8 | 0.3 |
| [12] | 1.80 | 54.0 | 30.1 | 0.6 | 4.0 | 0.8 | / | / |
| [13] | 1.98 | 55.5 | 28.0 | 2.5 | 4.0 | 1.2 | / | 0.7 |
| [11] | 2.15 | 56.0 | 26.0 | 2.0 | 3.3 | 2.7 | 0.9 | 1.1 |
| Label | Granulation | Temperature [°C] |
|---|---|---|
| Original slag | Industrial scale | Unknown 1 |
| 1200 °C | Laboratory scale | 1200 |
| 1300 °C | Laboratory scale | 1300 |
| 1400 °C | Laboratory scale | 1400 |
| Component | Concentration [wt.%] |
|---|---|
| FeO | 47.50 |
| SiO2 | 39.72 |
| CaO | 5.63 |
| Al2O3 | 4.97 |
| MgO | 1.99 |
| Cr2O3 | 0.19 |
| Component | Concentration [wt.%] |
|---|---|
| FeO | 46.2 |
| SiO2 | 40.7 |
| CaO | 2.9 |
| Al2O3 | 5.2 |
| MgO | 1.3 |
| ZnO | 1.2 |
| Cu | 0.8 |
| Na2O | 0.7 |
| K2O | 0.6 |
| S | 0.3 |
| Cr2O3 | 0.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andersson, A.; Brander, L.; Lennartsson, A.; Roos, Å.; Engström, F. A Method for Synthesizing Iron Silicate Slags to Evaluate Their Performance as Supplementary Cementitious Materials. Appl. Sci. 2023, 13, 8357. https://doi.org/10.3390/app13148357
Andersson A, Brander L, Lennartsson A, Roos Å, Engström F. A Method for Synthesizing Iron Silicate Slags to Evaluate Their Performance as Supplementary Cementitious Materials. Applied Sciences. 2023; 13(14):8357. https://doi.org/10.3390/app13148357
Chicago/Turabian StyleAndersson, Anton, Linus Brander, Andreas Lennartsson, Åke Roos, and Fredrik Engström. 2023. "A Method for Synthesizing Iron Silicate Slags to Evaluate Their Performance as Supplementary Cementitious Materials" Applied Sciences 13, no. 14: 8357. https://doi.org/10.3390/app13148357
APA StyleAndersson, A., Brander, L., Lennartsson, A., Roos, Å., & Engström, F. (2023). A Method for Synthesizing Iron Silicate Slags to Evaluate Their Performance as Supplementary Cementitious Materials. Applied Sciences, 13(14), 8357. https://doi.org/10.3390/app13148357






