1. Introduction
The sensations that accompany a person during their lifetime, especially superficial (sensory) feelings, i.e., tactile perception and the sensations referred to as pain, are associated with different factors that can affect their interpretation, modification, and level of feeling [
1,
2,
3,
4]. The receptors located in the skin, muscles, ligaments, and joints receive somatosensory information to be processed by the brain, specifically in the cerebellum, thalamus, posterior parietal cortex, insula, and primary and secondary somatosensory cortex regions. A distinction is made between exteroceptive and proprioceptive somatosensation, with the former including touch and pain and the latter sensing movement and body position [
5].
The sensory threshold is the point at which sensation is consciously perceived, and it is always the first effect of a stimulus, recognized by a human, such as the electrical impulse applied during standard electrotherapy procedures in clinics and hospitals. A sensory impression is based on a subjective assessment of a stimulus acting on the human body, which therefore differs from person to person [
6,
7,
8].
The human skin, the largest sensory organ, is innervated by complex combinations of low- and high-threshold mechanoreceptors (LTMRs and HTMRs, respectively; approximately 17,000), each with its unique physiological profile and response properties elicited by distinct tactile stimuli [
2]. Tactile sensations and their perception rely on mechanosensitive sensory neurons: LTMRs that react to innocuous mechanical stimulation and HTMRs that respond to harmful (nociceptive) mechanical stimuli. Thus, the perception of stimulation that does not damage tissues initiates the activation of cutaneous LTMR mechanoreceptors, and then the sensory neurons are activated, the cell bodies of which are located in the dorsal root ganglia and the sensory ganglia of the brain [
1,
2,
3,
4].
Therefore, an external stimulus, including an electrical impulse, is felt differently depending on the treatment parameters applied, particularly the frequency and the duration of the applied electrical impulse, the physical properties of the skin [
9] (how warm, cool or moisturized it is), and changing external conditions, e.g., air humidity [
10,
11,
12,
13]. The perception of the stimulus may also be influenced by the current somatic state of a person (health or illness), their mental state (e.g., happy, anxious, or depressed), and the physiological properties of the peripheral and central nervous system [
14,
15,
16]. In humans, other factors that may contribute to the modification of somatosensory impressions and differences in the degree of external stimuli perception include age, sex, changing hormone levels such as during the monthly cycle in women (especially estrogen E2 and progesterone P2) [
17], personality traits, and effects of endogenous opioids or medication taken, including painkillers. The perception of stimuli from the environment may also depend on genetic determinants, as well as a system of sociological and cultural beliefs in which a given person functions [
18,
19,
20].
The standard electrical stimulation used for electrotherapeutic procedures performed in health centers and often used in scientific research is transcutaneous electrical nerve stimulation (TENS) [
8]. This stimulation is non-invasive and widely accepted, and its pulse time is short—in the range of 50–350 µs depending on the aperture, which makes it safe for patients’ skin. The amplitude of the current is variable and individually selected, depending on the person’s neurosensory threshold. Each person needs a different electrical current intensity value to reach the sensory threshold. Thus, the aim of this research was to assess the sensory threshold in response to TENS, taking into account selected personal factors, such as participant’s sex, body composition, and the phase of the monthly cycle. The results of this type of research may contribute to a better understanding of an individual’s sensitivity to TENS stimuli.
2. Materials and Methods
2.1. Participants
The study involved 205 women aged 19–33 years (mean 21.13 ± 1.45 years) and 49 men aged 20–27 years (mean 21.57 ± 1.65 years). The participants in the research were students (volunteers) from the Physiotherapy Department at one of the universities in Krakow, where all students had been invited to take part in the research. The final number of participants was dictated by practical considerations, i.e., the number of people willing to participate in the study during a particular month. The disproportion between the number of men and women participating in the study is related to the fact that the physiotherapy faculty is numerically dominated by women. In addition, proportionally more women than men volunteered to participate in the study.
Study inclusion and exclusion criteria. The criteria for inclusion in this study were a declaration of good health with no diseases that could in any way affect the results of the study, including sensory disorders, skin diseases, and diseases with acute or chronic pain, e.g., headache, neuralgia, and pain associated with menstrual bleeding. Other conditions for participating in this study were the complete absence of any pain sensation on the day of the examination and not using any over-the-counter medications, including painkillers, anti-inflammatory drugs, and antihistamines on a regular basis, as well as any stimulants during the 24 h before the examination (alcohol, cigarettes, drugs, coffee, and any caffeinated products). Additionally, women had to have had regular monthly cycles (every 28 ± 5 days) each month for at least the last year. The other criteria included no endocrine, neurological, psychiatric, or urogynecological diseases, and no neuromuscular dysfunction of the upper limbs. The exclusion criteria for the study included general contraindications to the use of electrical stimulation, such as cardiovascular diseases, skin diseases, metal implants in the body, and other disorders that can affect pain sensation, as well as the use of painkillers or other medications during the research, sensory disorders, and chronic pain. Additionally, exclusion criteria specifically for women were the use of oral contraceptives, pregnancy, irregular monthly cycles of known or unknown cause, and diseases that could adversely affect the proper course of the menstrual cycle, including hormonal disorders such as thyroid diseases.
2.2. Study Design
In the first stage of the research, participants anonymously completed a proprietary questionnaire containing questions about their demographic data and information that, according to the authors of the study, could be of importance when examining their sensory threshold. The women’s questionnaire additionally contained questions about their monthly cycle, including the day of their monthly cycle at the time they had their examination, the length of their monthly cycles, menarche age, regularity or disorders of their monthly cycles, and the use of oral contraception and other medications, including painkillers, especially on the days preceding the examination and on the day of the examination.
2.2.1. Sensory Threshold Examination
TENS was used, for which an impulse of the amplitude (intensity) increasing from zero mA until the sensory threshold (minimally perceptible tingling) was employed as the sensory stimulus. The point at which a superficial sensation was felt, i.e., the sensory threshold (stimulation of thick Aβ sensory fibers), was defined as the minimally perceptible level of electrical stimulation that the subject was able to feel consciously. Each test subject was informed that the electrical stimulus would resemble a “tingling” sensation.
An electrotherapy device (BTL 5818SLM, Markham, ON, Canada) was used for stimulation, as it allows the current intensity to be read with an accuracy of 0.1 mA. TENS of 100 Hz and 100 µs and of the biphasic current waveform was used. The room in which the tests were performed had a constant ambient temperature of 22 °C. The subject sat on a chair, with the upper limb bent at the elbow joint and placed on a table and the forearm in supination. Surface electrodes with 12 cm
2 pads dipped in warm water were used. The electrodes were placed on the interior surface of the subject’s forearm on the group of flexor muscles of the wrist of the dominant hand and fastened with straps. One of the electrodes was placed 2 cm from the elbow and the other was 2 cm in a straight line from the first one. The upper limb was selected for this study because other studies have shown that the total nerve fiber densities are distinctly lower in the lower limb when compared to the upper limb [
21,
22]. Therefore, it was believed that readings for the upper limb would be more precise than for the lower limb.
A 100 Hz frequency is a prerequisite for effective stimulation of Aβ sensory fibers. Concerning the influence of the pulse duration on the skin sensation, especially in the case of high-frequency (100 Hz) TENS stimulation, the pulse width range should generally be from approximately 100 to 200 µs. An impulse of duration within these limits causes a delicate and pleasant tingling sensation on the skin, while also enabling the effective stimulation of the nerve fibers as planned in this research [
10,
11,
12,
13]. Due to the use of a biphasic current waveform, an anode (a positive current pole) and a cathode (a negative current pole), the location on the above-mentioned group of muscles was not of a great importance. The biphasic waveform of the current prevented both possible errors in the arrangement of the electrodes and a distortion of the results, which can differ when stimulating nerve fibers with currents of opposite polarity.
2.2.2. Body Composition Analysis
The professional body composition analyzer TANITA DC-430MA S was used, which takes measurements using an innovative, non-invasive technology of electrical bioimpedance, operating at two frequencies (dual frequency: 6.25/50 kHz). The measurement was carried out using two-electrode analyzers integrated within the platform. An imperceptible and safe electrical signal flowed through the body of the subject and, based on the impedance of the individual body components, the algorithm in the analyzer calculated their content within 20 s.
In order to ensure maximum reliability of the obtained results, all guidelines recommended by the manufacturer of the device were followed. These included the subjects not drinking water for 2 h before the measurements were taken, because the amount of water drunk has a significant impact on the results obtained. Additionally, the measurements were not taken after the participants had exercised. The subjects stood on the device with their feet bare, with their upper limbs hanging freely by the body and their legs slightly apart. The weight of the subject’s clothes was then entered into the equipment and deducted from the result. All study participants wore a light sports shirt and shorts. The participants did not have mobile phones on them and, due to the equipment’s sensitivity, there were no devices generating an electromagnetic field in the immediate area. After each test, the equipment was disinfected.
The analyzer was approved for professional medical use and met the NAWI and CLASS III standards for scales used for medical measurements. It provided objective results for the body composition parameters, which were important for the project. Of the parameters offered by the manufacturer of the analyzer, the following were selected for this study: height (cm), BMI (kg/m2), fat (%), lean body mass (%), total water (kg), muscle mass (%), and bone mass (%).
2.2.3. Statistical Analysis
The statistical analysis was performed using the Statistica 13 software program (StatSoft, Krakow, Poland). Due to the lack of a normal distribution, the differences between men and women were assessed using the Mann–Whitney U-test. Further analyses were performed in the group of women only. In this group, the effect of different factors on the sensory threshold was assessed using multiple linear regression. Multicollinearity was assessed using Spearman’s Rho. Results where p < 0.05 were considered statistically significant.
4. Discussion
In this study, an attempt was made to determine the factors that may affect the individual variability in the perception of external stimuli. An electrical impulse based on the principles and characteristics of TENS stimulation was used as the sensory stimulus. The use of this type of impulse in the study ensured that the stimulus used was safe, non-invasive, and painless, while being objectively measurable and expressed numerically in milliamperes (mA) [
10,
11,
12,
13].
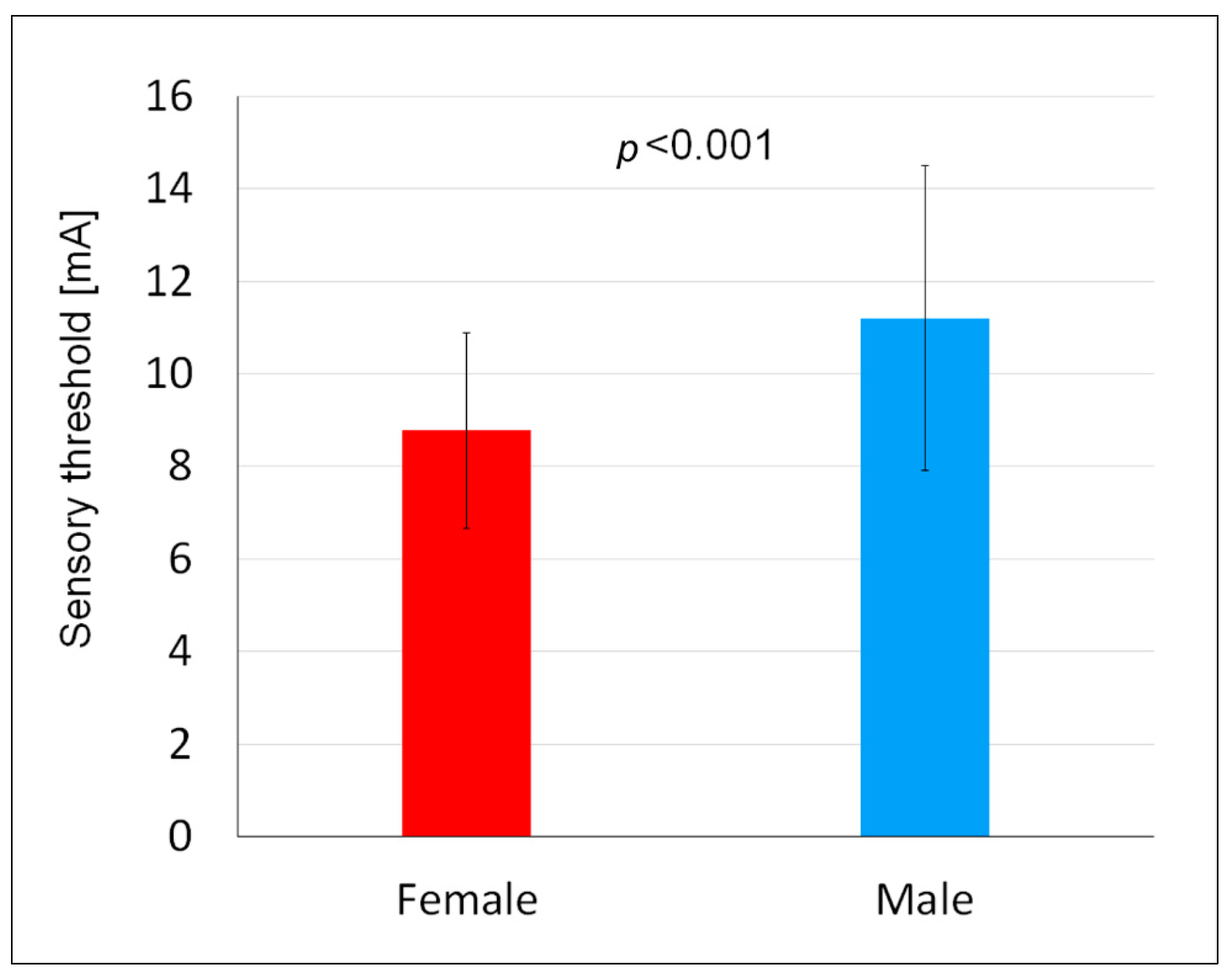
The results obtained showed that the sensory threshold in women was lower than in men. Comparing the results of this study to the results of other authors concerning differences in sensitivity to external stimuli broken down by sex, it was found that women showed a lower perception threshold than men, regardless of changing locations of the electrodes, as demonstrated, for example, in a study in which perception threshold measurements were also carried out using an electric stimulus [
8]. The mean differences between male and female subjects were 0.18, 0.22, 0.44, 0.88, and 0.61 mA at five different measurement points [
8]. A study by Saraiva et al. [
23] also found a lower sensory threshold in women compared to men—13.77 vs. 19.57 mA. The authors pointed out the anatomical and physiological discrepancies that can generate differences in sensory response, including size of the neuromotor course and amounts of neurotransmitters [
23]. A study by Guirro et al. [
24] showed that even using two different current frequencies, i.e., 5 and 50 Hz, the threshold of sensory perception in women was lower than that in young and elderly men.
Men and women differ significantly in their body composition, as noted in our own study, so it is difficult to establish unequivocally whether the key factor in the perception of stimuli is sex or the content of individual components of body composition. Seno et al. [
25] proposed selecting men and women with a similar water content (50–55%) for tests on the sensory threshold and to perform a log transformation of the obtained results. After doing this, they showed that there are no statistically significant differences in the sensitivity threshold to electric current between men and women, and the body water content together with the fat content are more important than sex and are strongly correlated with each other. The authors of the discussed article suggest that these factors should be used in sensory research in order to minimize the sex influence. However, the results described above raise some doubts. Perhaps, no statistically significant differences were found because the number of men and women included in the analysis was reduced (initially 35 men and 35 women; afterward, 15 men and 15 women). The group selection and logarithmic transformations also could play a significant role.
Examining the interrelationship of the sensory threshold and the changing phases of the menstrual cycle in women, it was found that the level of the external stimulus perception did not depend on the phase of the menstrual cycle, the time of menarche, the number of days of menstruation, or the declared pain level during menstrual bleeding. It is worth noting, however, that the basic problem and limitation of most scientific studies examining the differences between the sexes in the perception of external stimuli are hormonal changes in a woman’s body during their menstrual cycle. In the available scientific literature, there are ambiguous and sometimes even contradictory reports about the impact of hormonal changes occurring in women during the phases of the menstrual cycle, i.e., the follicular (including the proliferative phase and ovulation), luteal, and menstrual phases, on the level of perception of an external stimulus [
26,
27,
28]. It should be noted, however, that the research of other authors most often focuses on differences in the perception of experimentally induced pain and not the threshold of sensation; therefore, not all of the results of the present study can be compared to such papers.
When considering studies on experimentally induced pain, it was found that some authors [
22] have claimed that they did not observe the impact of hormonal changes in individual phases of the menstrual cycle on the pain stimuli perception in women, while others [
21,
27] have clearly stated that the changing hormonal balance has an impact on the stimuli perception, especially in the ovulatory phase. There are also studies [
26] showing that in healthy women, pain is felt more strongly in the luteal phase, which is just before the onset of menstrual bleeding. In [
28], in which the phase of the menstrual cycle was confirmed by examining sex hormones from women’s blood serum, it was found that although women are more sensitive to various harmful stimuli than men, the menstrual cycle does not affect pain sensitivity to cold, heat, or ischemic stimuli, nor does it have an impact on the differences between the sexes in sensitivity to these pain stimuli. In [
26], the authors unequivocally stated that the higher incidence of clinical pain and the greater experimental pain sensitivity in women suggest a clearly significant role of female sex hormones. This is despite the consensus of previous literature reviews that the menstrual cycle influences the perception of pain in women, with the greatest sensitivity occurring in the ovulatory or luteal phases [
28]. A thematic literature review conducted by LeResche et al. [
29] again brought a contradictory conclusion. It stated that there was no consistent evidence for the effects of the menstrual cycle on the perception of pain and various noxious stimuli, with the possible exception of higher pain thresholds in the postovulatory phase. Clearly, the issue of research on the external stimuli perception being affected by the hormonal changes taking place in a woman’s body during their menstrual cycle still remains open, given that it is clearly visible that in clinical settings, women report more painful symptoms and higher pain scores than men and symptoms often differ from the stated hormonal status. The differences between the sexes in the clinical pain experienced begin to manifest themselves particularly strongly in women during puberty, followed by pain disorders predominating in the reproductive years and in the severity of symptoms often reflecting cyclical changes in the menstrual cycle [
29].
This study showed that women with a higher total body water content have a lower current perception threshold. The electrical current flows through the human body, “selecting” tissues that are most hydrated and that offer the least resistance to the flow [
20]. It seems logical that the higher the water content in a woman’s body, the lower the resistance to the flow of current; therefore, women feels electrical stimuli at lower current values and her sensory threshold is lower. Similarly to the total body water content, the level of skin hydration, especially in the stratum corneum, plays a significant role in the perception of electrical stimuli. When performing electrotherapeutic procedures, it is obligatory to use pads soaked in water that separate the electrodes from the skin. This water then moisturizes the stratum corneum, thus changing its properties and reducing the resistance to the flow of current. In this study, in each case, care was taken to soak the pads in water of the same temperature and to ensure the same level of saturation, so that the level of stratum corneum hydration was similar in all subjects tested. It is also known that the stratum corneum layer is thicker in men than in women [
30,
31], which may be one of the reasons for the higher current sensation threshold in men. In contrast to the above, when the electrical current encounters resistance while flowing through, for example, the adipose tissue of much lower electrical conductivity, it may “return” to the moisturized skin, because it “chooses” to flow through the area with the lowest resistance. In this situation, the nerve fibers in the skin are stimulated more and then the phenomenon of increased current flow through the skin appears, i.e., the dose of electricity is higher in the skin. It should be noted that even within the skin located under the electrode, the electric current density is unevenly distributed due to uneven skin resistance [
25,
30,
31]. This is probably why, in our study, it was shown that women with a higher body fat percentage had a lower current perception threshold. Similar results were obtained by Maffiuletti et al. [
31], who studied 30 obese women and compared them with their non-obese peers. They found that obese individuals had lower excitability. According to Geng et al. [
8], the mechanism described above results in a lowered perception threshold in people with a higher body fat percentage compared to people with a low body fat percentage, and this does not hinder the flow of electricity deep into the body. Therefore, a source of the variability in the perception threshold observed between individuals may be the variability in body fat percentage. It is well known that women have a higher percentage of body fat than men, and studies have shown that the perception threshold in women is lower than in men, which may mean that the reported sensations are related to an individual’s body fat percentage [
8]. In other studies using TENS stimulation [
26,
31,
32], in which factors that could affect changes in the perception threshold were studied, a relationship was demonstrated between the subcutaneous fat mass and sensation, suggesting that one of the factors influencing sex differences in the perception of electric current is the skin fold thickness. The perception of the current is also influenced by the thickness of the skin itself, as it affects the distance between the source of the stimulus and the nerve endings in the skin [
8]. Generally speaking, the greater the distance between the current source and the activated nerve endings, the greater the loss of free flow of an electric charge and the greater the impedance. Hence, a higher amperage is needed to activate the receptor or afferent nerve fibers, resulting in a higher perception threshold. Some authors [
26,
31,
32] have also suggested that the number of epidermal nerve fibers and the intra-epidermal nerve fiber density may affect individual sensitivity to electrical stimulation, as well as the differences between the sexes in the perception of stimuli. Changes in the density of the nerve endings located under the electrodes may be the source of variability in the perception threshold; however, in this research, it seems that errors related to this factor were avoided, because all of the subjects had the tests performed on the same area of the forearm. During the test, the electrodes were placed on the interior surface of the forearm. The consistent and precisely defined position of the electrodes also minimized errors that may have resulted from the different perceptions of electrical stimuli in different parts of the human body, entirely due to the differences in skin thickness and innervation density in a given area of the body.
In this study, taller women had higher current perception threshold values compared to shorter women. This result can probably be explained by the longer axons found in the bodies of taller women, and the possible lower density of receptors in the skin of taller people, as has been found with thermoreceptors and which may also apply to other skin sensory receptors [
32]. In another study [
33], in which the authors studied the relationship between finger and toe vibrotactile thresholds and age and height, it was stated that the length of the peripheral nerve axons may be a predictor of changes in vibration sensitivity, i.e., the taller the examined person, the longer the axons and the lower the sensitivity to external stimuli. The authors [
30], however, only showed differences in the perception of stimuli in subjects’ lower limbs, but no differences were found in the upper limbs. According to the authors, the length of the subjects’ forearms and arms did not differ significantly enough to assume that there was a difference in the length of axons. Additionally, it was shown that the person’s age also is an important factor for the perception of vibration stimuli in the lower limbs. When compared to the axons extending to the fingers in the upper limbs, it was suggested that longer axons extending to the toes in the lower limbs may be more prone to age-induced effects than shorter axons in the upper limbs. In this study, age did not influence the sensory threshold of the electrical stimulus, as the age of the respondents was within the range of 19–33 years. It can be assumed, therefore, that in young healthy people in this age range, it is not yet possible to talk about, e.g., degenerative changes in axons associated with the subjects’ age.
The authors of this project are aware that research carried out in young and healthy people does not represent a reference to the entire population; however, in order to be able to study further differentiation in terms of age and clinical problems in a specific group of patients and draw conclusions about a direct clinical significance, research results are needed to form a (comparative) base for further research.
The limitations of this study include enrollment of only young and healthy people; therefore the obtained results cannot be directly extrapolated to a group of elderly people, especially those suffering from specific diseases. Due to the limited number of men, the influence of body composition was only analyzed for women. In the analysis of the effects of monthly cycle phases on the sensory threshold, some increase can be observed in the ovulatory and luteal phases, but the differences did not reach the level of statistical significance. The lack of statistically significant differences may be related to individual variability. Perhaps with a different experimental design, where the measurement for each woman is performed five times to obtain the sensory threshold result in each phase of the cycle, the observed changes would be large enough to reach the level of statistical significance. The tests were performed only at one measurement point and only on hairless skin. Only one type of a sensory stimulus and current of constant parameters were used for the research. Despite the aforementioned limitations, the results of this research contribute to the development of knowledge about the factors influencing the perception of electric impulse in people and may constitute the basis for further exploration of this issue.