Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Modified Biochar Synthesis
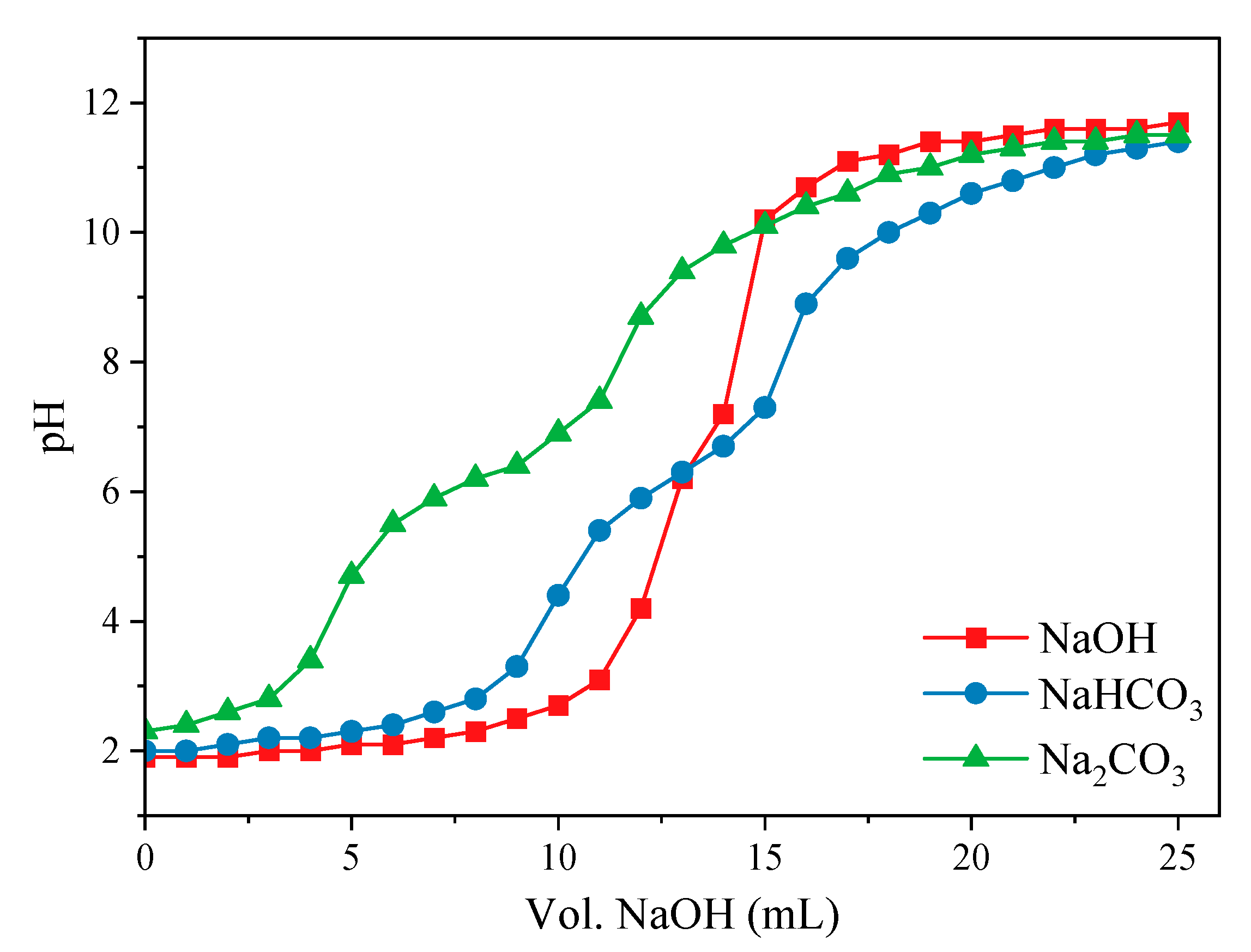
2.3. Characterization Methods
2.4. Adsorption Assays
2.5. Adsorption Isotherms
2.6. Adsorption Kinetics
2.7. Competitive Adsorption Tests
3. Results and Discussion
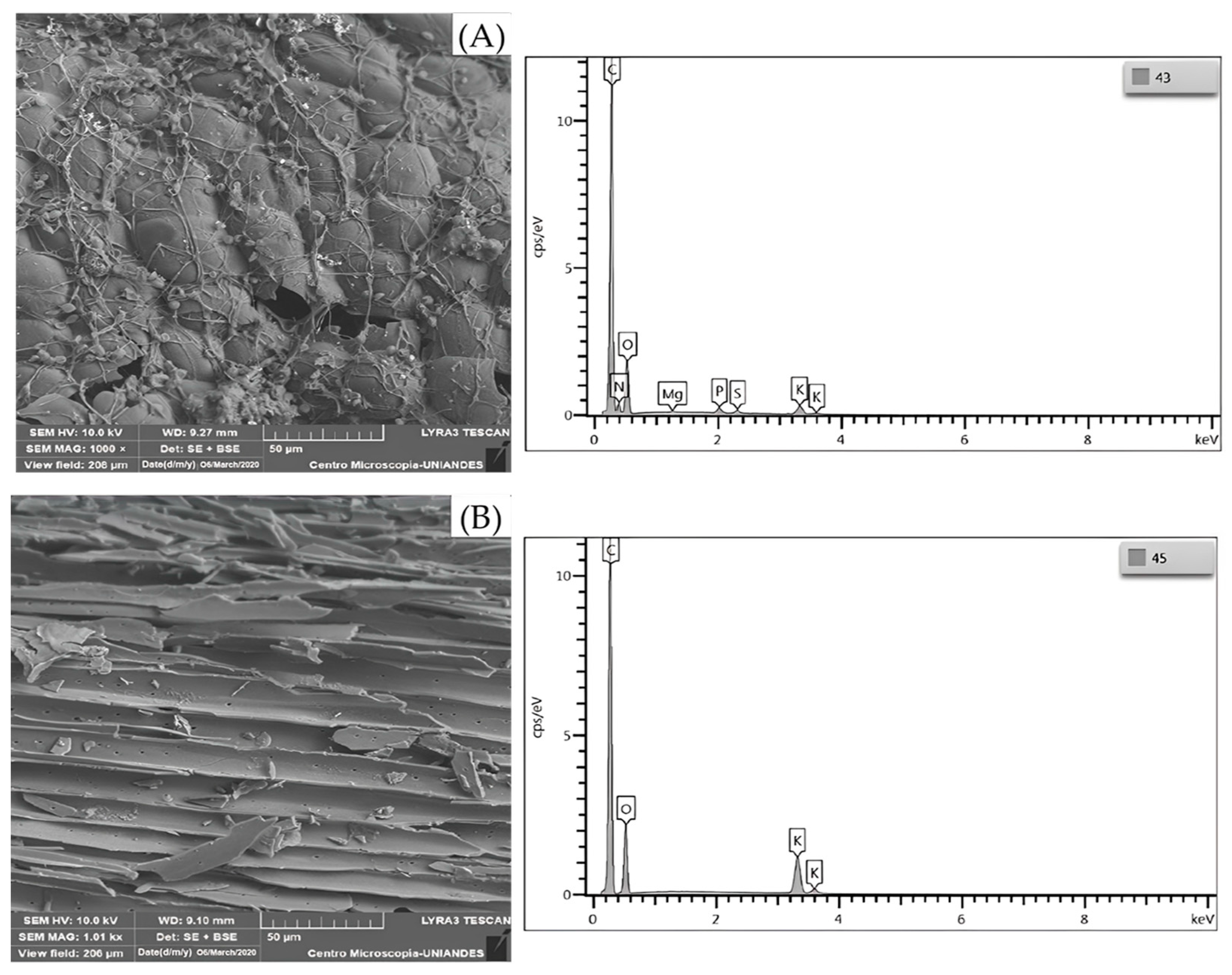
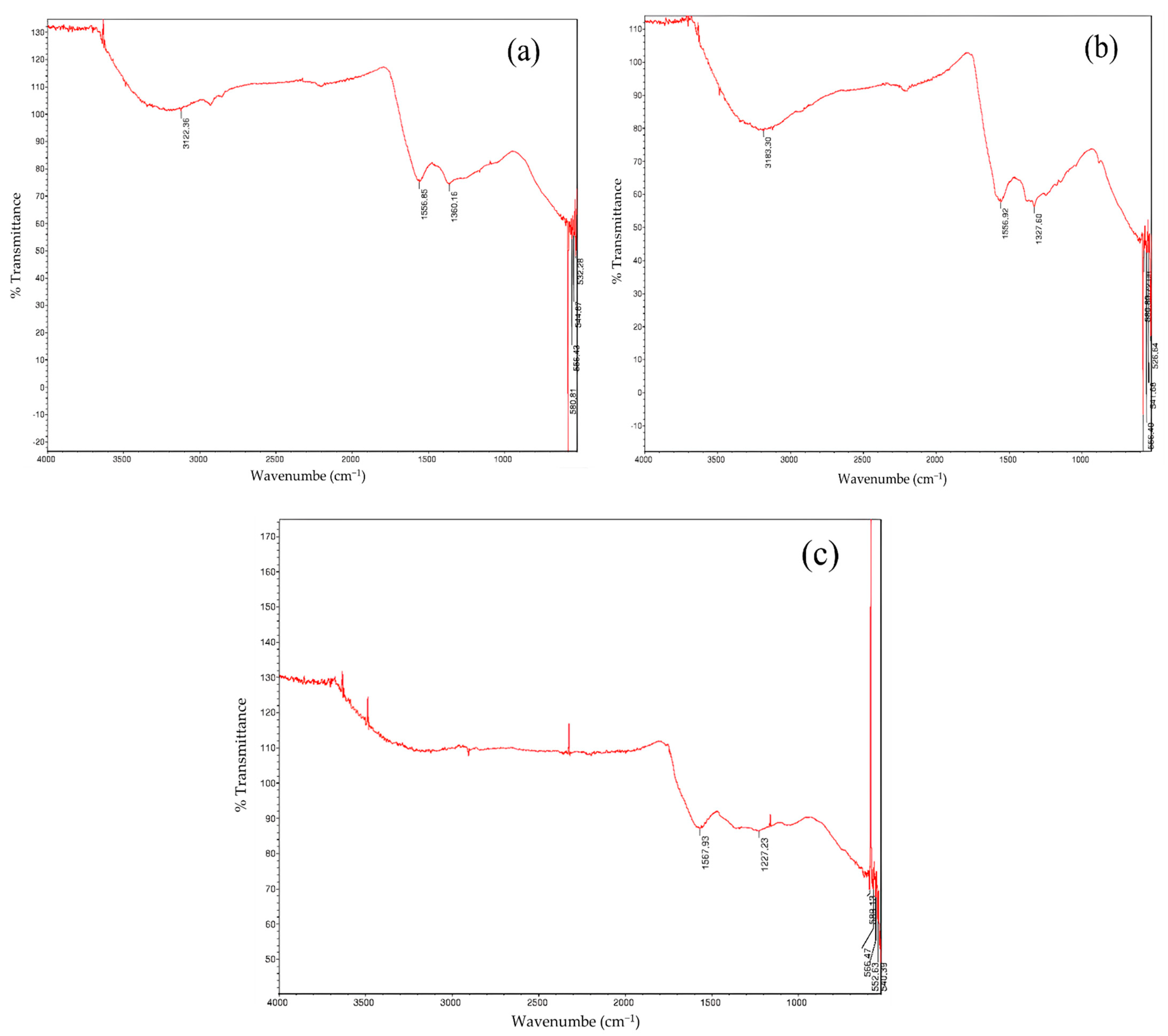
3.1. Characterization of the Biosorbent
3.2. Adsorption Parameters Effect
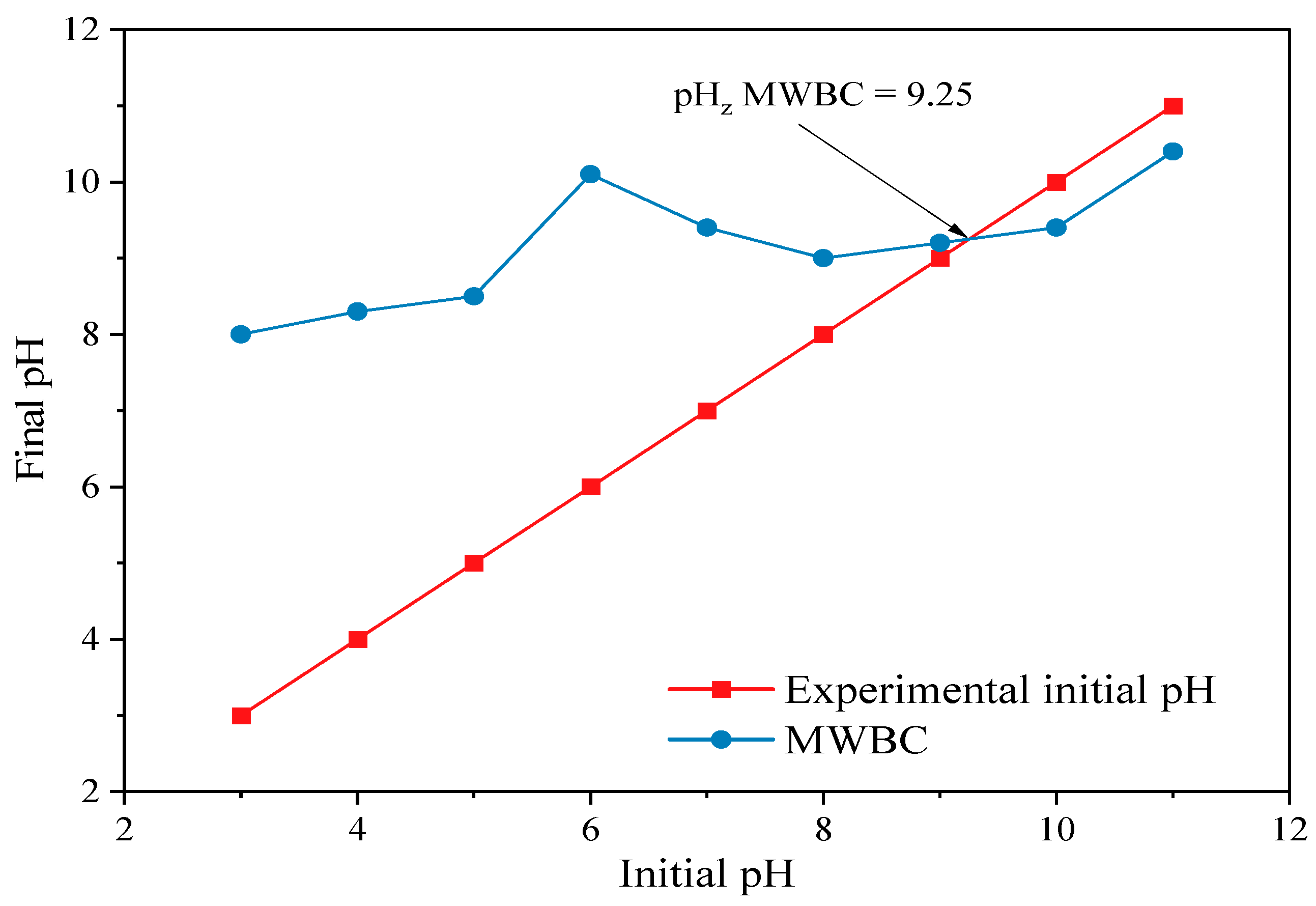
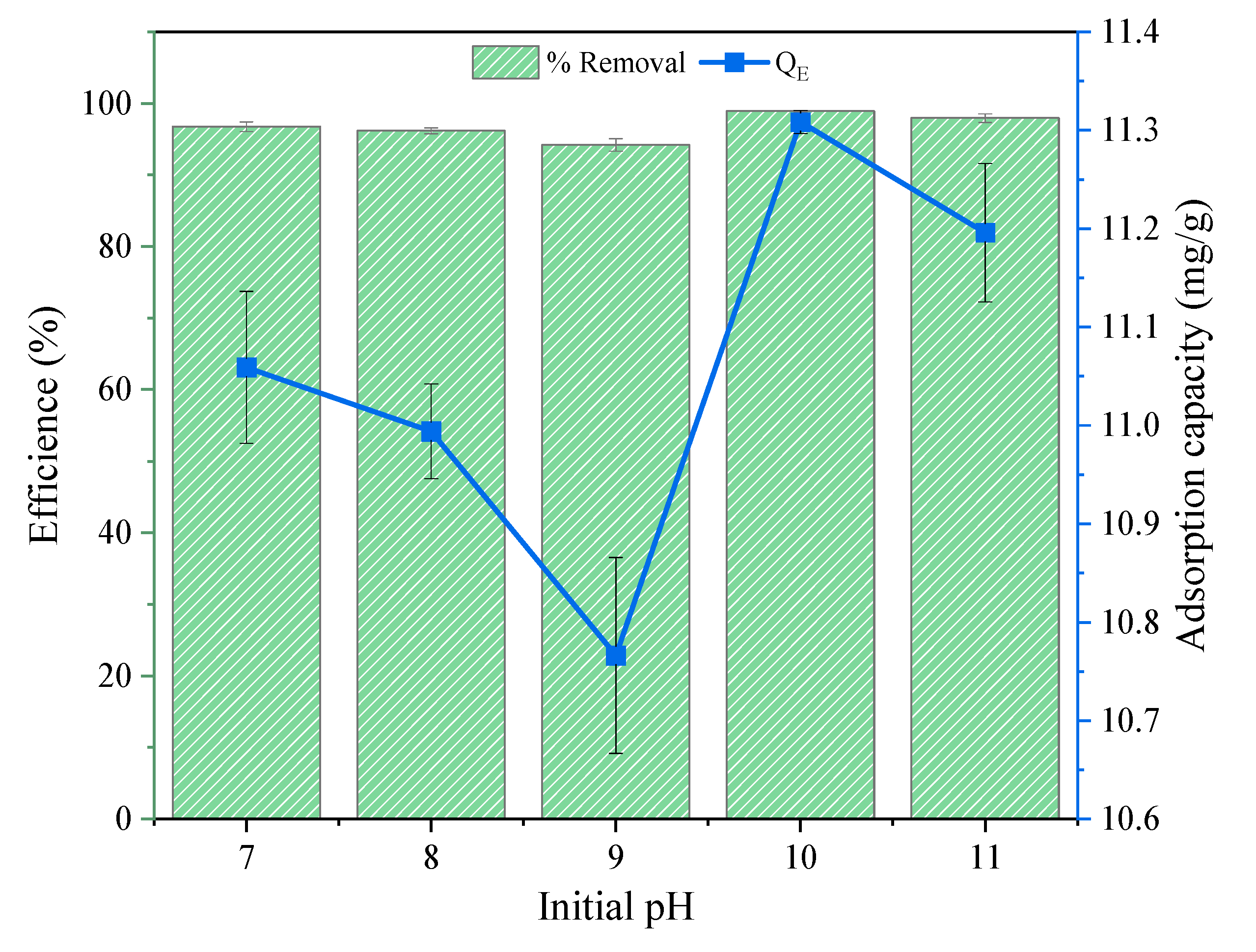
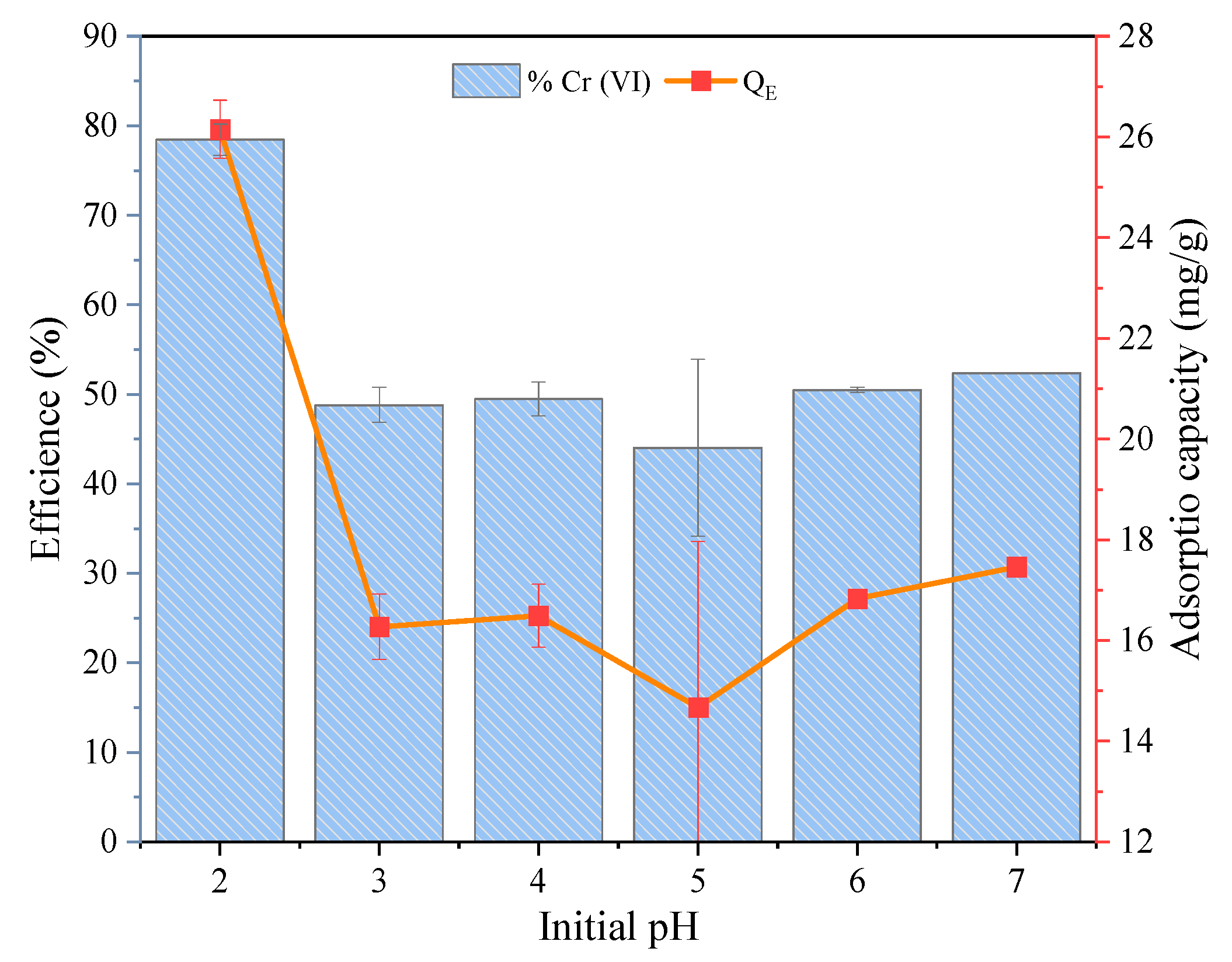
3.2.1. Effect of the pH
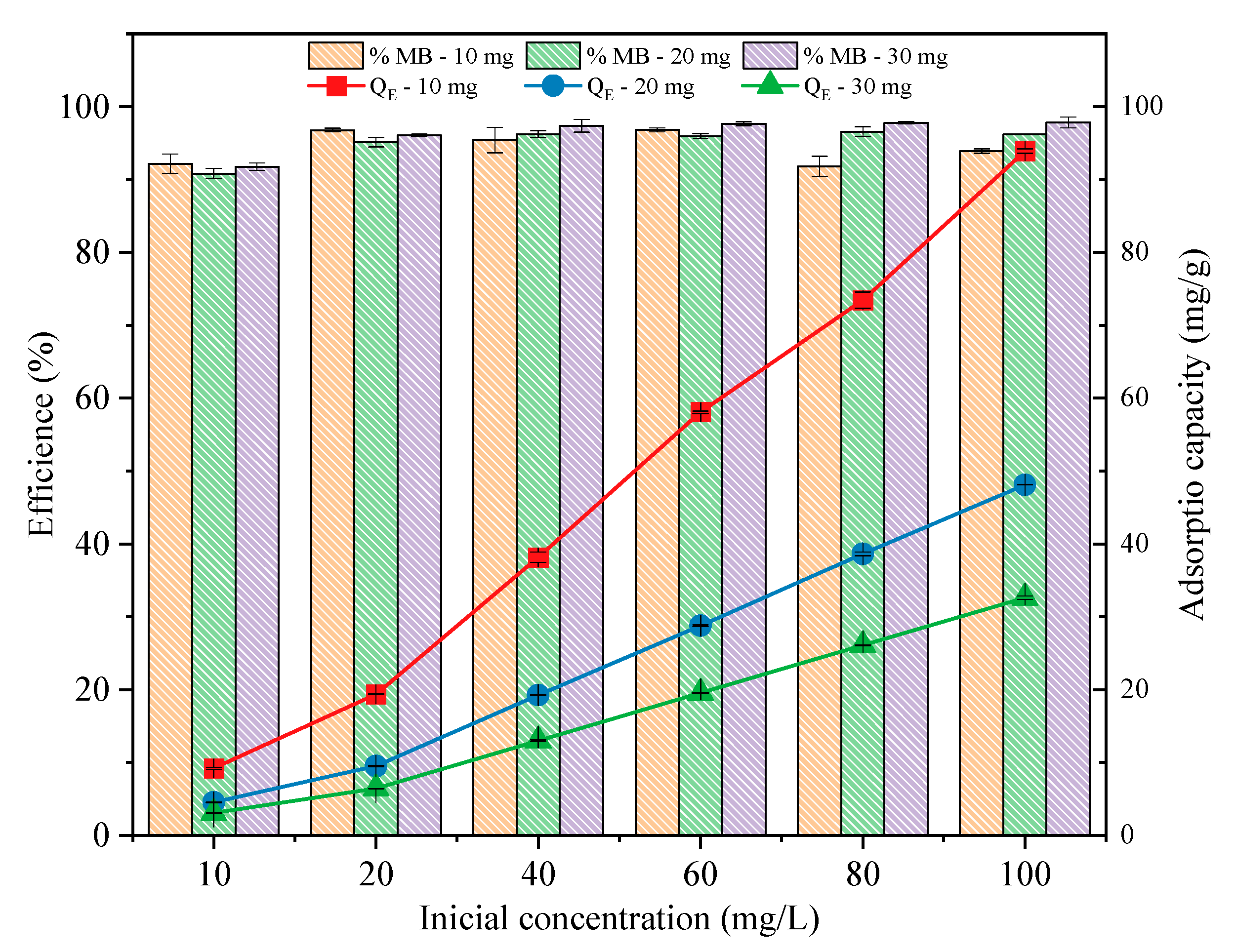
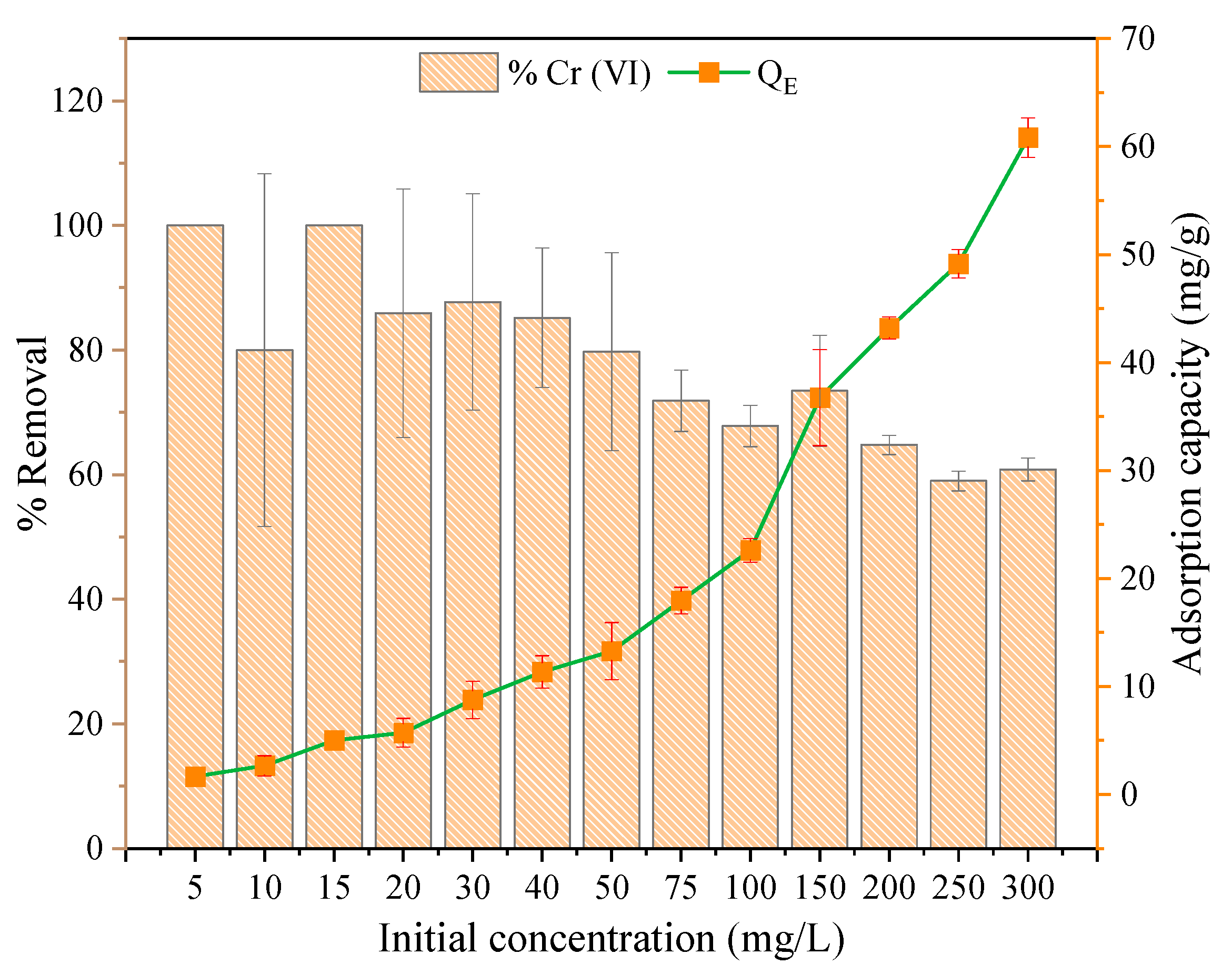
3.2.2. Effect of the Adsorbent Dose
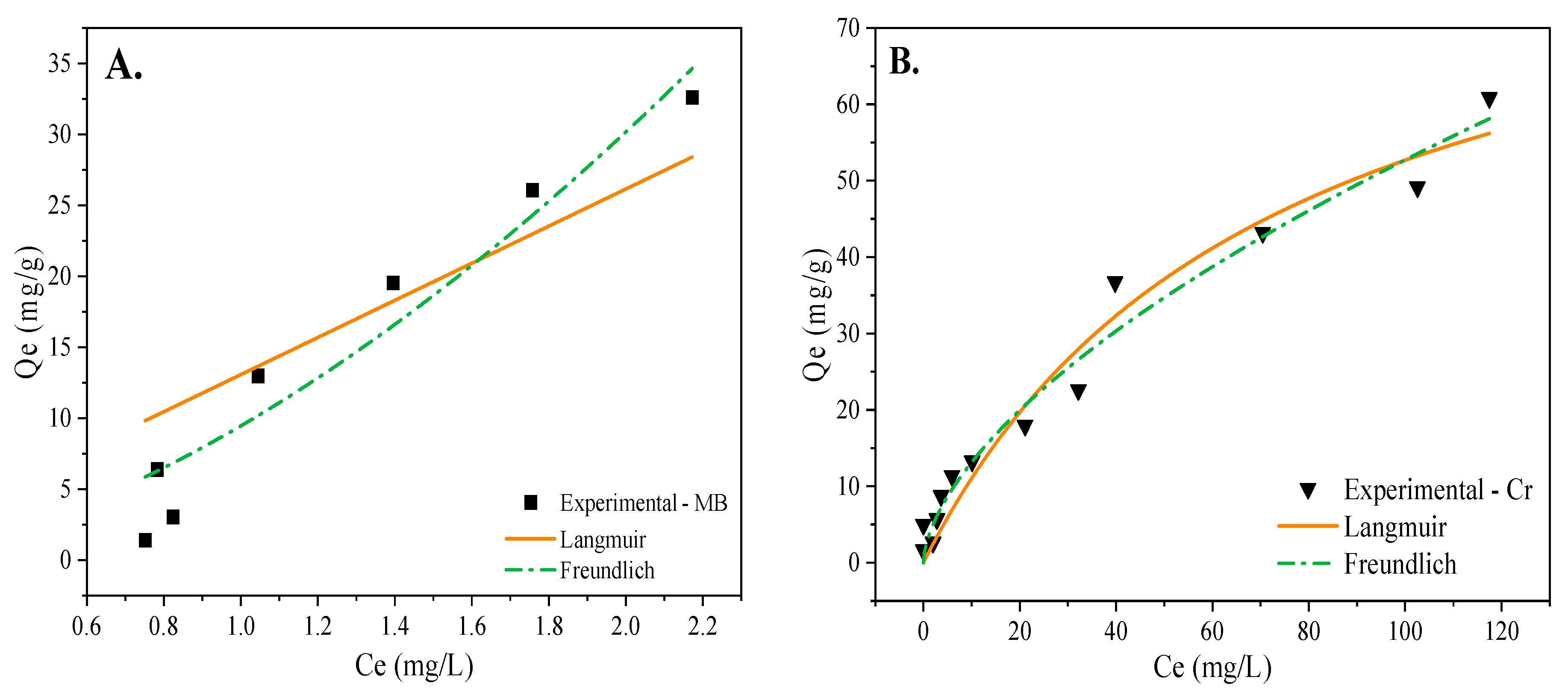
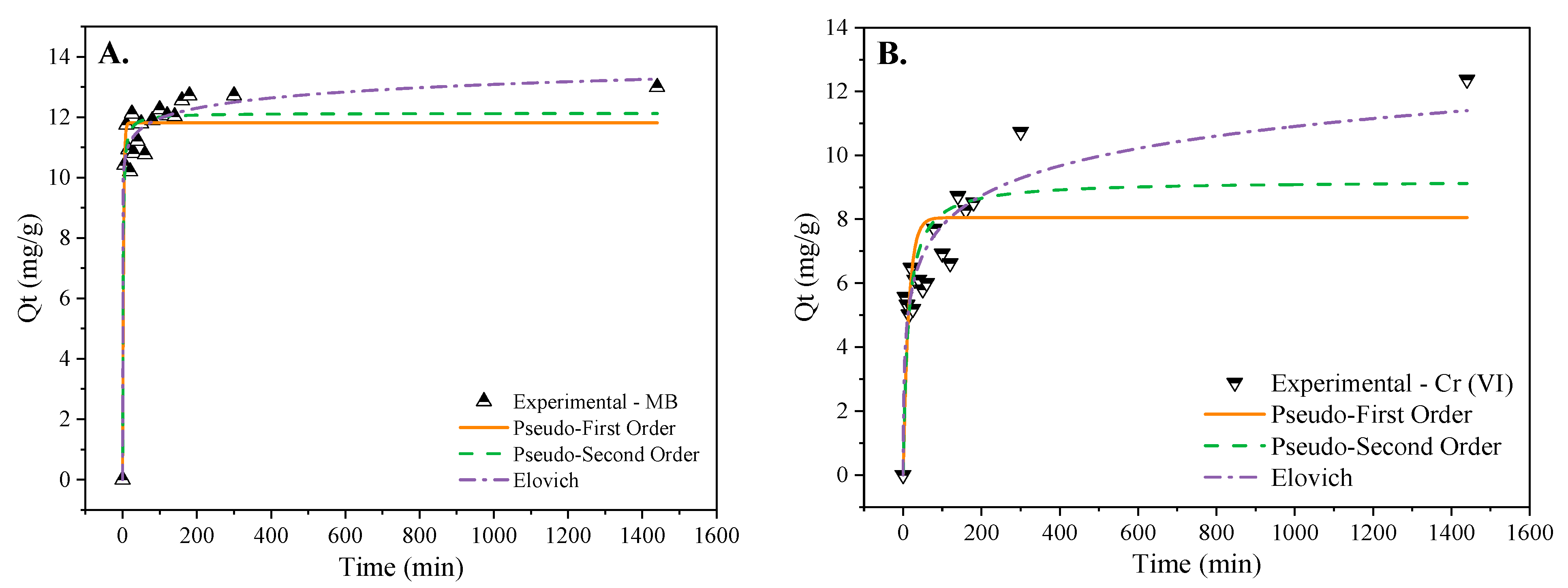
3.3. Adsorption Kinetic and Isotherm
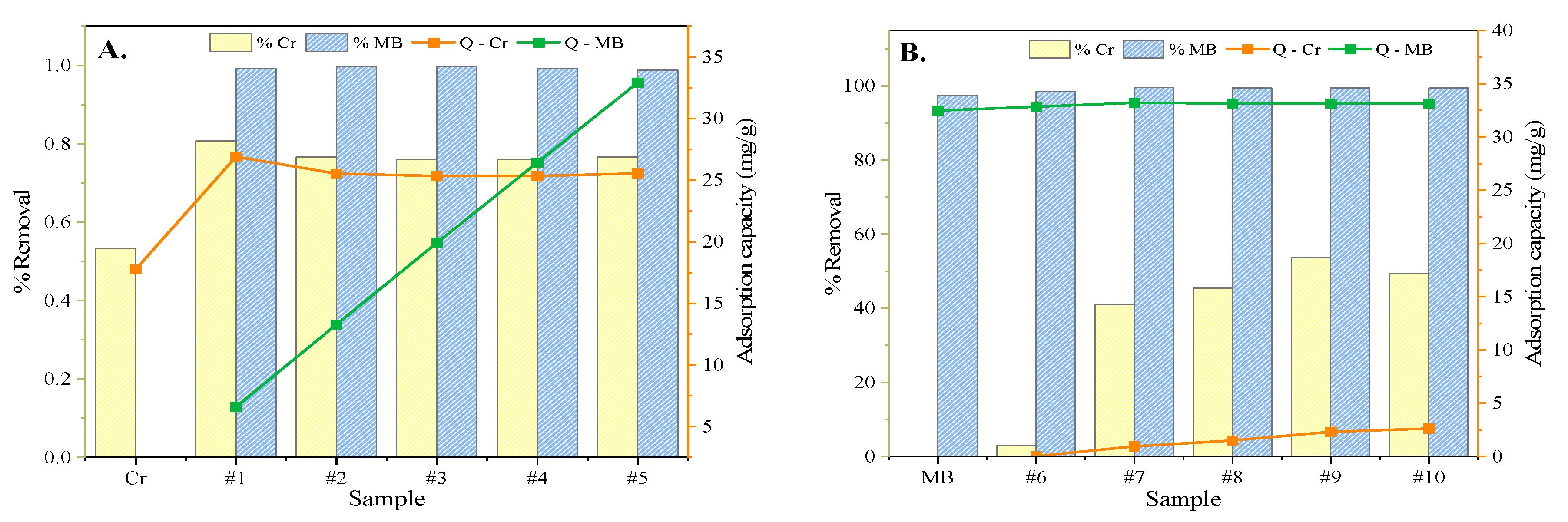
3.4. Competitive Adsorption Tests
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rangabhashiyam, S.; Balasubramanian, P. Adsorption behaviors of hazardous methylene blue and hexavalent chromium on novel materials derived from Pterospermum acerifolium shells. J. Mol. Liq. 2018, 254, 433–445. [Google Scholar] [CrossRef]
- Afshin, S.; Rashtbari, Y.; Shirmardi, M.; Vosoughi, M.; Hamzehzadeh, A. Adsorption of Basic Violet 16 dye from aqueous solution onto mucilaginous seeds of Salvia sclarea: Kinetics and isotherms studies. Desalin. Water Treat. 2019, 161, 365–375. [Google Scholar] [CrossRef]
- Li, L.; Liu, F.; Duan, H.; Wang, X.; Li, J.; Wang, Y.; Luo, C. The preparation of novel adsorbent materials with efficient adsorption performance for both chromium and methylene blue. Colloids Surf. B Biointerfaces 2016, 141, 253–259. [Google Scholar] [CrossRef]
- Dinh, V.P.; Huynh, T.D.T.; Le, H.M.; Nguyen, V.D.; Dao, V.A.; Hung, N.Q.; Tuyen, L.A.; Lee, S.; Yi, J.; Nguyen, T.D.; et al. Insight into the adsorption mechanisms of methylene blue and chromium(iii) from aqueous solution onto pomelo fruit peel. RSC Adv. 2019, 9, 25847–25860. [Google Scholar] [CrossRef]
- Diaconu, M.; Pavel, L.V.; Hlihor, R.M.; Rosca, M.; Fertu, D.I.; Lenz, M.; Corvini, P.X.; Gavrilescu, M. Characterization of heavy metal toxicity in some plants and microorganisms—A preliminary approach for environmental bioremediation. N. Biotechnol. 2020, 56, 130–139. [Google Scholar] [CrossRef]
- Iqbal, F.; Ayub, Q.; Wilson, R.; Song, B.K.; Talei, A.; Yeong, K.Y.; Hermawan, A.; Fahim, M.; Rahman, S. Monitoring of heavy metal pollution in urban and rural environments across Pakistan using House crows (Corvus splendens) as bioindicator. Environ. Monit. Assess. 2021, 193, 237. [Google Scholar] [CrossRef] [PubMed]
- Geng, J.; Yin, Y.; Liang, Q.; Zhu, Z.; Luo, H. Polyethyleneimine cross-linked graphene oxide for removing hazardous hexavalent chromium: Adsorption performance and mechanism. Chem. Eng. J. 2019, 361, 1497–1510. [Google Scholar] [CrossRef]
- Velusamy, S.; Roy, A.; Sundaram, S.; Kumar Mallick, T. A Review on Heavy Metal Ions and Containing Dyes Removal Through Graphene Oxide-Based Adsorption Strategies for Textile Wastewater Treatment. Chem. Rec. 2021, 21, 1570–1610. [Google Scholar] [CrossRef]
- De Sousa, C.M.; Cardoso, V.L.; Batista, F.R.X. A coupled photocatalytic system using niobium oxide and microalga: Cr (VI)-contaminated wastewater treatment. J. Photochem. Photobiol. A Chem. 2023, 439, 114602. [Google Scholar] [CrossRef]
- Banerjee, S.; Kamila, B.; Barman, S.; Joshi, S.R.; Mandal, T.; Halder, G. Interlining Cr(VI) remediation mechanism by a novel bacterium Pseudomonas brenneri isolated from coalmine wastewater. J. Environ. Manag. 2019, 233, 271–282. [Google Scholar] [CrossRef]
- Jiang, Y.; Martinez-Guerra, E.; Gnaneswar Gude, V.; Magbanua, B.; Truax, D.D.; Martin, J.L. Wetlands for Wastewater Treatment. Water Environ. Res. 2016, 88, 1160–1191. [Google Scholar] [CrossRef] [PubMed]
- Magoling, B.J.A.; Macalalad, A.A. Optimization and Response Surface Modelling of Activated Carbon Production from Mahogany Fruit Husk for Removal of Chromium (VI) from Aqueous Solution. BioResources 2017, 12, 3001–3016. Available online: https://jtatm.textiles.ncsu.edu/index.php/BioRes/article/view/BioRes_12_2_3001_Magoling_Optimization_Response_Surface_Modelling_Fruit_Husk (accessed on 10 June 2023). [CrossRef]
- Khalil, U.; Shakoor, M.B.; Ali, S.; Rizwan, M.; Alyemeni, M.N.; Wijaya, L. Adsorption-reduction performance of tea waste and rice husk biochars for Cr(VI) elimination from wastewater. J. Saudi Chem. Soc. 2020, 24, 799–810. [Google Scholar] [CrossRef]
- Shakya, A.; Agarwal, T. Removal of Cr(VI) from water using pineapple peel derived biochars: Adsorption potential and re-usability assessment. J. Mol. Liq. 2019, 293, 111497. [Google Scholar] [CrossRef]
- Al-Mokhalelati, K.; Al-Bakri, I.; Wattar, N.A.S.A. Adsorption of methylene blue onto sugarcane bagasse-based adsorbent materials. J. Phys. Org. Chem. 2021, 34, e4193. [Google Scholar] [CrossRef]
- Chen, J.; Yang, J.; Hu, G.; Hu, X.; Li, Z.; Shen, S.; Radosz, M.; Fan, M. Enhanced CO2 Capture Capacity of Nitrogen-Doped Biomass-Derived Porous Carbons. ACS Sustain. Chem. Eng. 2016, 4, 1439–1445. [Google Scholar] [CrossRef]
- Liu, P.; Liu, W.J.; Jiang, H.; Chen, J.J.; Li, W.W.; Yu, H.Q. Modification of bio-char derived from fast pyrolysis of biomass and its application in removal of tetracycline from aqueous solution. Bioresour. Technol. 2012, 121, 235–240. [Google Scholar] [CrossRef] [PubMed]
- Guy, M.; Mathieu, M.; Anastopoulos, I.P.; Martínez, M.G.; Rousseau, F.; Dotto, G.L.; de Oliveira, H.P.; Lima, E.C.; Thyrel, M.; Larsson, S.H.; et al. Process Parameters Optimization, Characterization, and Application of KOH-Activated Norway Spruce Bark Graphitic Biochars for Efficient Azo Dye Adsorption. Molecules 2022, 27, 456. [Google Scholar] [CrossRef]
- Kaya, N.; Uzun, Z.Y. Investigation of effectiveness of pine cone biochar activated with KOH for methyl orange adsorption and CO2 capture. Biomass Convers. Biorefinery 2020, 11, 1067–1083. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm titration. Part I. CO2 expulsion and endpoint determination. Carbon 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Alberto, F.; Villa, A.; Anaguano, A.H. Determinación del punto de carga cero y punto isoeléctrico de dos residuos agrícolas y su aplicación en la remoción de colorantes. RIAA 2013, 4, 27–36. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=5344979&info=resumen&idioma=ENG (accessed on 10 June 2023).
- ASTM C1777-20; Standard Test Method for Rapid Determination of the Methylene Blue Value for Fine Aggregate or Mineral Filler Using a Colorimeter. ASTM International: West Conshohocken, PA, USA, 2020. Available online: https://www.astm.org/c1777-20.html (accessed on 10 June 2023).
- Liu, X.J.; Li, M.F.; Singh, S.K. Manganese-modified lignin biochar as adsorbent for removal of methylene blue. J. Mater. Res. Technol. 2021, 12, 1434–1445. [Google Scholar] [CrossRef]
- Somsesta, N.; Piyamawadee, C.; Sricharoenchaikul, V.; Aht-Ong, D. Adsorption isotherms and kinetics for the removal of cationic dye by Cellulose-based adsorbent biocomposite films. Korean J. Chem. Eng. 2020, 37, 1999–2010. [Google Scholar] [CrossRef]
- Altun, T.; Pehlivan, E. Removal of Cr(VI) from aqueous solutions by modified walnut shells. Food Chem. 2012, 132, 693–700. [Google Scholar] [CrossRef]
- ASTM D1687-17; Standard Test Methods for Chromium in Water. ASTM International: West Conshohocken, PA, USA, 2017. Available online: https://www.astm.org/d1687-17.html (accessed on 12 June 2023).
- Patel, H. Comparison of batch and fixed bed column adsorption: A critical review. Int. J. Environ. Sci. Technol. 2021, 19, 10409–10426. [Google Scholar] [CrossRef]
- Ul-Islam, S. Advanced Materials for Wastewater Treatment; Wiley: Hoboken, NJ, USA, 2017; ISBN 978-1-119-40780-5. [Google Scholar]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, P.; Zhang, S.; Zhou, Q. Comparative Study on the Adsorption of Tartrazine and Indigo Carmine onto Maize Cob Carbon. Sep. Sci. Technol. 2014, 49, 877–886. [Google Scholar] [CrossRef]
- Edet, U.A.; Ifelebuegu, A.O. Kinetics, Isotherms, and Thermodynamic Modeling of the Adsorption of Phosphates from Model Wastewater Using Recycled Brick Waste. Processes 2020, 8, 665. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef]
- Qu, J.; Wang, Y.; Tian, X.; Jiang, Z.; Deng, F.; Tao, Y.; Jiang, Q.; Wang, L.; Zhang, Y. KOH-activated porous biochar with high specific surface area for adsorptive removal of chromium (VI) and naphthalene from water: Affecting factors, mechanisms and reusability exploration. J. Hazard. Mater. 2021, 401, 123292. [Google Scholar] [CrossRef]
- Aigbe, U.O.; Osibote, O.A. Carbon derived nanomaterials for the sorption of heavy metals from aqueous solution: A review. Environ. Nanotechnol. Monit. Manag. 2021, 16, 100578. [Google Scholar] [CrossRef]
- Rodríguez, A.; Ovejero, G.; Sotelo, J.L.; Mestanza, M.; García, J. Adsorption of dyes on carbon nanomaterials from aqueous solutions. J. Environ. Sci. Health Part A 2010, 45, 1642–1653. [Google Scholar] [CrossRef] [PubMed]
- Borba, L.L.; Cuba, R.M.F.; Cuba Terán, F.J.; Castro, M.N.; Mendes, T.A. Use of adsorbent biochar from pequi (Caryocar Brasiliense) husks for the removal of commercial formulation of glyphosate from aqueous media. Braz. Arch. Biol. Technol. 2019, 62, e19180450. [Google Scholar] [CrossRef]
- Labied, R.; Physico-chimiques, A.; Oumessaad Benturki, A.; Poincaré-Nancy, H.I. Adsorption of hexavalent chromium by activated carbon obtained from a waste lignocellulosic material (Ziziphus jujuba cores): Kinetic, equilibrium, and thermodynamic study Adh’ Ya Eddine Hamitouche André Donnot. Adsorpt. Sci. Technol. 2018, 36, 1066–1099. [Google Scholar] [CrossRef]
- Suhaimi, N.; Kooh, M.R.R.; Lim, C.M.; Chou Chao, C.T.; Chou Chau, Y.F.; Mahadi, A.H.; Chiang, H.P.; Haji Hassan, N.H.; Thotagamuge, R. The Use of Gigantochloa Bamboo-Derived Biochar for the Removal of Methylene Blue from Aqueous Solution. Adsorpt. Sci. Technol. 2022, 2022, 8245797. [Google Scholar] [CrossRef]
- Siqueira, C.; da Silva, I.; Rubio, A.; Bergamasco, R.; Gasparotto, F.; Paccola, E.; Yamaguchi, N. Sugarcane Bagasse as an Efficient Biosorbent for Methylene Blue Removal: Kinetics, Isotherms and Thermodynamics. Int. J. Environ. Res. Public Health 2020, 17, 526. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, J.S.; Marley, N.A.; Jones, D.E. Fourier Transform Infrared (FTIR) Spectroscopy. In Characterization of Materials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2012; pp. 1–33. [Google Scholar] [CrossRef]
- Grams, J. Surface analysis of solid products of thermal treatment of lignocellulosic biomass. J. Anal. Appl. Pyrolysis 2022, 161, 105429. [Google Scholar] [CrossRef]
- Park, D.; Yun, Y.S.; Jong, M.P. Studies on hexavalent chromium biosorption by chemically-treated biomass of Ecklonia sp. Chemosphere 2005, 60, 1356–1364. [Google Scholar] [CrossRef]
- Seung Kim, Y.; Rae Park, C. Titration Method for the Identification of Surface Functional Groups. In Materials Science and Engineering of Carbon: Characterization; Butterworth-Heinemann: Oxford, UK, 2016; pp. 273–286. ISBN 9780128052563. [Google Scholar]
- Titchou, F.E.; Zazou, H.; Afanga, H.; El Gaayda, J.; Akbour, R.A.; Hamdani, M. Removal of Persistent Organic Pollutants (POPs) from water and wastewater by adsorption and electrocoagulation process. Groundw. Sustain. Dev. 2021, 13, 100575. [Google Scholar] [CrossRef]
- Yagub, M.T.; Sen, T.K.; Afroze, S.; Ang, H.M. Dye and its removal from aqueous solution by adsorption: A review. Adv. Colloid Interface Sci. 2014, 209, 172–184. [Google Scholar] [CrossRef]
- Rakhunde, R.; Deshpande, L.; Juneja, H.D. Chemical Speciation of Chromium in Water: A Review. Crit. Rev. Environ. Sci. Technol. 2012, 42, 776–810. [Google Scholar] [CrossRef]
- Lian, G.; Wang, B.; Lee, X.; Li, L.; Liu, T.; Lyu, W. Enhanced removal of hexavalent chromium by engineered biochar composite fabricated from phosphogypsum and distillers grains. Sci. Total Environ. 2019, 697, 134119. [Google Scholar] [CrossRef]
- Nguyen, T.A.H.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef] [PubMed]
- Soliman, N.K.; Moustafa, A.F. Industrial solid waste for heavy metals adsorption features and challenges; a review. J. Mater. Res. Technol. 2020, 9, 10235–10253. [Google Scholar] [CrossRef]
- Zhou, L.; Liu, Y.; Liu, S.; Yin, Y.; Zeng, G.; Tan, X.; Hu, X.; Hu, X.; Jiang, L.; Ding, Y.; et al. Investigation of the adsorption-reduction mechanisms of hexavalent chromium by ramie biochars of different pyrolytic temperatures. Bioresour. Technol. 2016, 218, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, B.; Paul, D. Isotherms, kinetics and thermodynamics of hexavalent chromium removal using biochar. J. Environ. Chem. Eng. 2018, 6, 2335–2343. [Google Scholar] [CrossRef]
- Tytłak, A.; Oleszczuk, P.; Dobrowolski, R. Sorption and desorption of Cr(VI) ions from water by biochars in different environmental conditions. Environ. Sci. Pollut. Res. 2014, 22, 5985–5994. [Google Scholar] [CrossRef] [PubMed]
- William Kajjumba, G.; Emik, S.; Öngen, A.; Kurtulus Özcan, H.; Aydın, S. Modelling of Adsorption Kinetic Processes—Errors, Theory and Application. In Advanced Sorption Process Applications; IntechOpen: London, UK, 2019; pp. 187–198. ISBN 978-1-78984-819-9. [Google Scholar] [CrossRef]
- Amaku, J.F.; Ngwu, C.M.; Nnaji, J.C.; Odoemelam, S.A.; Ifeanyi, U.B.; Okorie, H.; Zubairu, S.M. Adsorption of Cr(VI) onto Azadirachta indica stem bark extract modified dolerite composite adsorbent. Int. J. Environ. Anal. Chem. 2021. [Google Scholar] [CrossRef]
- Villabona-Ortíz, A.; Tejada-Tovar, C.N.; Ortega-Toro, R. Modelling of the adsorption kinetics of chromium (VI) using waste biomaterials. Rev. Mex. Ing. Química 2020, 19, 401–408. [Google Scholar] [CrossRef]
- Sherugar, P.; Padaki, M.; Naik, N.S.; George, S.D.; Murthy, D.H.K. Biomass-derived versatile activated carbon removes both heavy metals and dye molecules from wastewater with near-unity efficiency: Mechanism and kinetics. Chemosphere 2022, 287, 132085. [Google Scholar] [CrossRef]
- Jamil, U.; Zeeshan, M.; Khan, S.R.; Saeed, S. Synthesis and two-step KOH based activation of porous biochar of wheat straw and waste tire for adsorptive exclusion of chromium (VI) from aqueous solution: Thermodynamic and regeneration study. J. Water Process Eng. 2023, 53, 103892. [Google Scholar] [CrossRef]

| Cr (VI) isotherms | MB concentration, mg/L | 100 | ||||
| Cr (VI) concentration, mg/L | 3 | 7 | 10 | 13 | 16 | |
| MB isotherms | Cr (VI) concentration, mg/L | 100 | ||||
| MB concentration, mg/L | 20 | 40 | 60 | 80 | 100 | |
| Component | Unmodified Biochar (Wt.%) | Biochar Modified with KOH (Wt.%) |
|---|---|---|
| C | 64.11 | 59.87 |
| O | 19.48 | 24.89 |
| S | 0.71 | - |
| K | 3.46 | 15.25 |
| P | 1.01 | - |
| N | 10.99 | - |
| Mg | 0.24 | - |
| Carboxylic, µ mol | Lactonic, µ mol | Phenolic, µ mol |
|---|---|---|
| 2175 | −630 | 495 |
| Model | Parameters | MWBC—MB | MWBC—Cr (VI) |
|---|---|---|---|
| Langmuir | 186,375.375 | 90.723 | |
| KL | 7.015 × 10−5 | 1.385 × 10−2 | |
| R2 | 0.756 | 0.959 | |
| Freundlich | KF | 9.460 | 3.2698 |
| n | 0.598 | 1.6562 | |
| R2 | 0.918 | 0.969 |
| Model | Parameter | MWBC—MB | MWBC—Cr (VI) |
|---|---|---|---|
| Pseudo-first order | qe | 11.812 | 8.05363 |
| K1 | 0.424 | 0.07054 | |
| R2 | 0.926 | 0.49151 | |
| Pseudo-second order | qe | 12.129 | 9.20214 |
| K2 | 0.078 | 0.00865 | |
| R2 | 0.945 | 0.66108 | |
| Elovich | α | 2.172 × 108 | 4.3733 |
| β | 2.050 | 0.74117 | |
| R2 | 0.967 | 0.87572 |
| Material | Synthesis Parameters | Adsorption Capacity (% Efficiency) | References |
|---|---|---|---|
| Commercial activated carbon | Temperature: 200 °C Time: 45 min Activator: KHO 1:1 | MB: 99 ± 0.5 Pb (II): 99 ± 0.5 Cd (II): 99 ± 0.4 In 12 h | [56] |
| Temperature: 550 °C Time: 1 h Activator: KOH (1 M y 3 M) | Cr (VI): 87 ± 5 in 60 min | [57] | |
| MWBC | Temperature: 200 °C Time: 30 min Activator: KOH 3 M | MB: 98.94 Cr (VI): 85.15 In 24 h | Present study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tejada-Tovar, C.; Villabona-Ortíz, Á.; Ortega-Toro, R. Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials. Appl. Sci. 2023, 13, 8481. https://doi.org/10.3390/app13148481
Tejada-Tovar C, Villabona-Ortíz Á, Ortega-Toro R. Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials. Applied Sciences. 2023; 13(14):8481. https://doi.org/10.3390/app13148481
Chicago/Turabian StyleTejada-Tovar, Candelaria, Ángel Villabona-Ortíz, and Rodrigo Ortega-Toro. 2023. "Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials" Applied Sciences 13, no. 14: 8481. https://doi.org/10.3390/app13148481
APA StyleTejada-Tovar, C., Villabona-Ortíz, Á., & Ortega-Toro, R. (2023). Removal of Metals and Dyes in Water Using Low-Cost Agro-Industrial Waste Materials. Applied Sciences, 13(14), 8481. https://doi.org/10.3390/app13148481









