Neural Mechanisms Related to the Enhanced Auditory Selective Attention Following Neurofeedback Training: Focusing on Cortical Oscillations
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
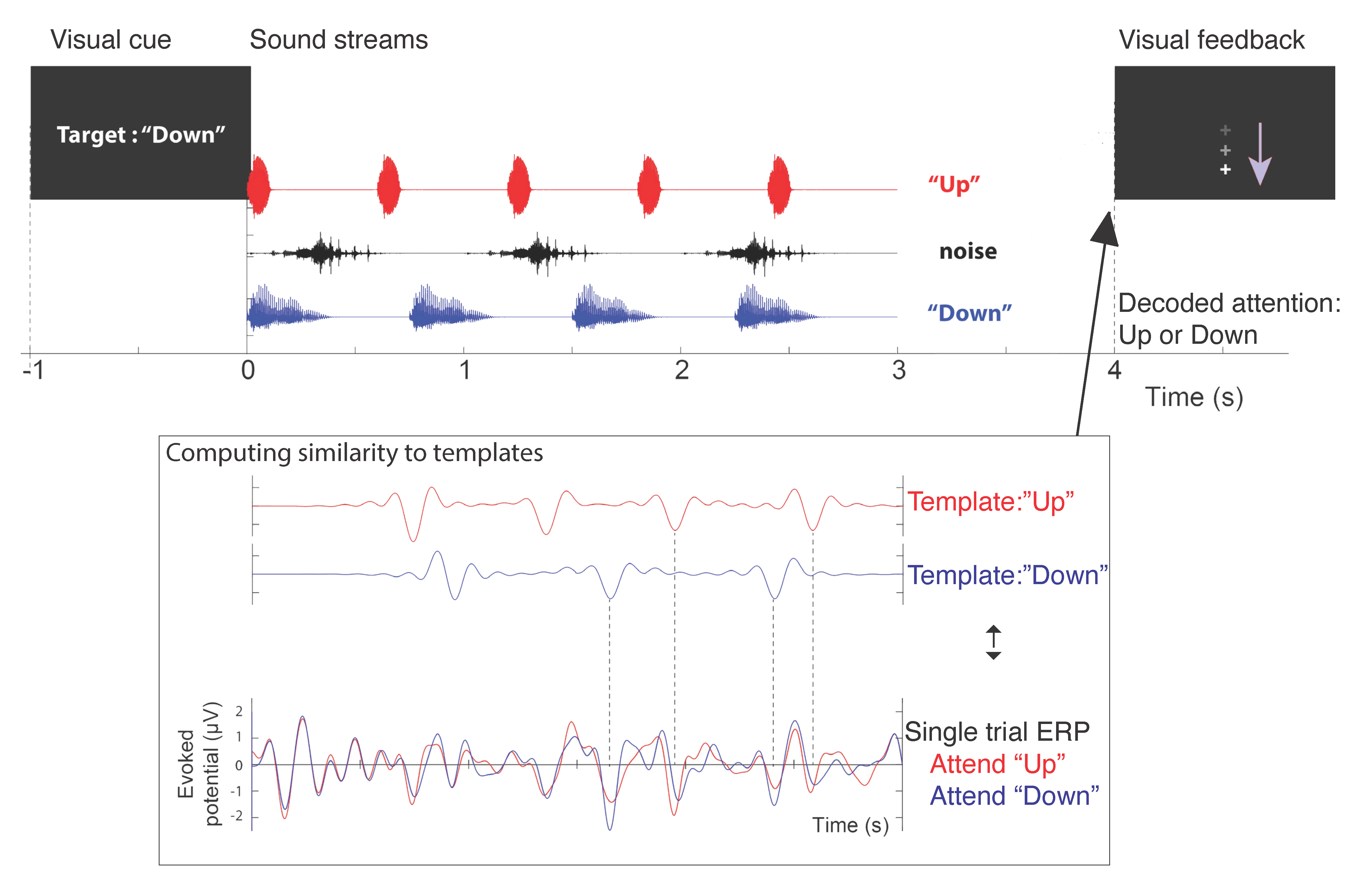
2.2. Task Design and Procedures
2.2.1. Attention Training Procedure: Experimental Group
2.2.2. Attention Training Procedure: Placebo Group
2.3. Induced Oscillatory Activity Analysis
3. Results
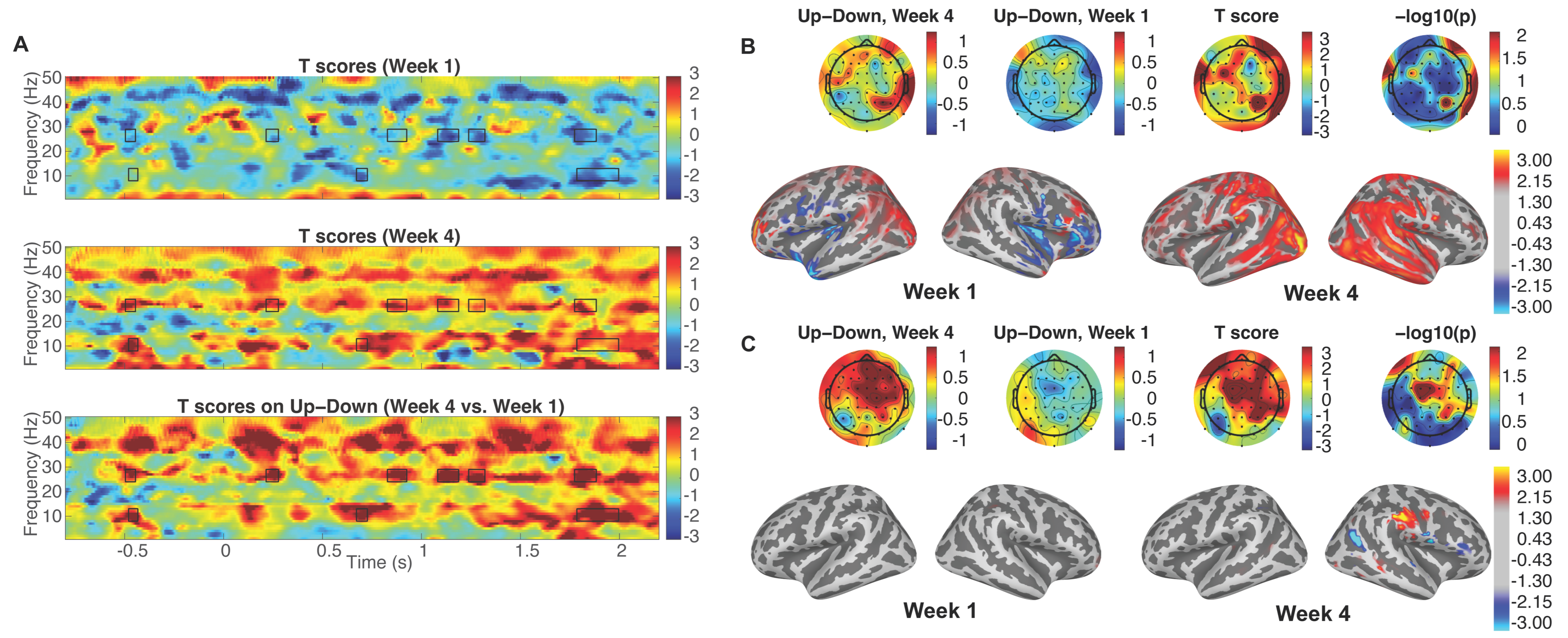
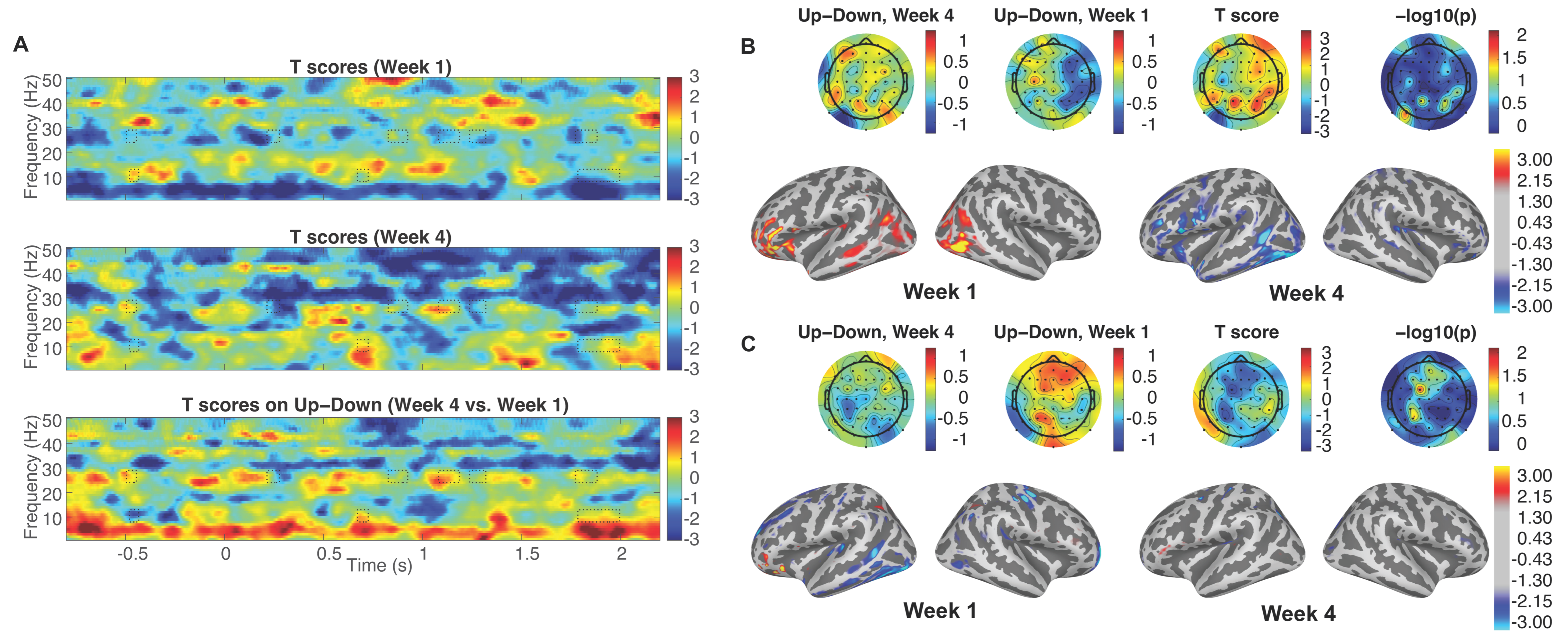
3.1. Enhanced Attentional Modulation
3.2. Induced Cortical Activity Changes in Source Space Topography to Selective Attention
4. Discussion
4.1. Conclusions
4.2. Limitations of the Study
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kumar, G.; Amen, F.; Roy, D. Normal Hearing Tests: Is a Further Appointment Really Necessary? J. R. Soc. Med. 2007, 100, 66. [Google Scholar] [CrossRef]
- Moore, D.R.; Rosen, S.; Bamiou, D.E.; Campbell, N.G.; Sirimanna, T.; James Bellis, T.; Chermak, G.; Weihing, J.; Musiek, F.; Dillon, H.; et al. Evolving Concepts of Developmental Auditory Processing Disorder (APD): A British Society of Audiology APD Special Interest Group “White Paper”. Int. J. Audiol. 2013, 52, 3–13. [Google Scholar] [CrossRef]
- Bressler, S.; Goldberg, H.; Shinn-Cunningham, B. Sensory Coding and Cognitive Processing of Sound in Veterans with Blast Exposure. Hear. Res. 2017, 349, 98. [Google Scholar] [CrossRef]
- Kim, S.; Schwalje, A.T.; Liu, A.S.; Gander, P.E.; McMurray, B.; Griffiths, T.D.; Choi, I. Pre- and Post-Target Cortical Processes Predict Speech-in-Noise Performance. Neuroimage 2021, 228, 117699. [Google Scholar] [CrossRef]
- Hillyard, S.A.; Hink, R.F.; Schwent, V.L.; Picton, T.W. Electrical Signs of Selective Attention in the Human Brain. Science 1973, 182, 177–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hillyard, S.A.; Vogel, E.K.; Luck, S.J. Sensory Gain Control (Amplification) as a Mechanism of Selective Attention: Electrophysiological and Neuroimaging Evidence. Philos. Trans. R. Soc. B Biol. Sci. 1998, 353, 1257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesgarani, N.; Chang, E.F. Selective Cortical Representation of Attended Speaker in Multi-Talker Speech Perception. Nature 2012, 485, 233–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carcea, I.; Insanally, M.N.; Froemke, R.C. Dynamics of Auditory Cortical Activity during Behavioural Engagement and Auditory Perception. Nat. Commun. 2017, 8, 14412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitton, J.P.; Hancock, K.E.; Polley, D.B. Immersive Audiomotor Game Play Enhances Neural and Perceptual Salience of Weak Signals in Noise. Proc. Natl. Acad. Sci. USA 2014, 111, E2606–E2615. [Google Scholar] [CrossRef]
- Whitton, J.P.; Hancock, K.E.; Shannon, J.M.; Polley, D.B. Audiomotor Perceptual Training Enhances Speech Intelligibility in Background Noise. Curr. Biol. 2017, 27, 3237–3247.e6. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Emory, C.; Choi, I. Neurofeedback Training of Auditory Selective Attention Enhances Speech-In-Noise Perception. Front. Hum. Neurosci. 2021, 15. [Google Scholar] [CrossRef] [PubMed]
- Kerlin, J.R.; Shahin, A.J.; Miller, L.M. Attentional Gain Control of Ongoing Cortical Speech Representations in a “Cocktail Party”. J. Neurosci. 2010, 30, 620–628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, I.; Rajaram, S.; Varghese, L.A.; Shinn-Cunningham, B.G. Quantifying Attentional Modulation of Auditory-Evoked Cortical Responses from Single-Trial Electroencephalography. Front. Hum. Neurosci. 2013, 7, 115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Sullivan, J.A.; Power, A.J.; Mesgarani, N.; Rajaram, S.; Foxe, J.J.; Shinn-Cunningham, B.G.; Slaney, M.; Shamma, S.A.; Lalor, E.C. Attentional Selection in a Cocktail Party Environment Can Be Decoded from Single-Trial EEG. Cereb. Cortex 2015, 25, 1697–1706. [Google Scholar] [CrossRef]
- Sherlin, L.H.; Arns, M.; Lubar, J.; Heinrich, H.; Kerson, C.; Strehl, U.; Sterman, M.B. Neurofeedback and Basic Learning Theory: Implications for Research and Practice. J. Neurother. 2011, 15, 292–304. [Google Scholar] [CrossRef] [Green Version]
- Alho, K.; Salmi, J.; Koistinen, S.; Salonen, O.; Rinne, T. Top-down Controlled and Bottom-up Triggered Orienting of Auditory Attention to Pitch Activate Overlapping Brain Networks. Brain Res. 2015, 1626, 136–145. [Google Scholar] [CrossRef]
- Hill, K.T.; Miller, L.M. Auditory Attentional Control and Selection during Cocktail Party Listening. Cereb. Cortex 2010, 20, 583–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, L.; Michalka, S.W.; Rosen, M.L.; Sheremata, S.L.; Swisher, J.D.; Shinn-Cunningham, B.G.; Somers, D.C. Auditory Spatial Attention Representations in the Human Cerebral Cortex. Cereb. Cortex 2014, 24, 773–784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michalka, S.W.; Kong, L.; Rosen, M.L.; Shinn-Cunningham, B.G.; Somers, D.C. Short-Term Memory for Space and Time Flexibly Recruit Complementary Sensory-Biased Frontal Lobe Attention Networks. Neuron 2015, 87, 882–892. [Google Scholar] [CrossRef] [Green Version]
- Noyce, A.L.; Cestero, N.; Michalka, S.W.; Shinn-Cunningham, B.G.; Somers, D.C. Sensory-Biased and Multiple-Demand Processing in Human Lateral Frontal Cortex. J. Neurosci. 2017, 37, 8755–8766. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Snyder, A.C.; Molholm, S.; Foxe, J.J. Oscillatory Alpha-Band Mechanisms and the Deployment of Spatial Attention to Anticipated Auditory and Visual Target Locations: Supramodal or Sensory-Specific Control Mechanisms? J. Neurosci. 2011, 31, 9923–9932. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Chang, W.-T.; Belliveau, J.W.; Hämäläinen, M.; Ahveninen, J. Lateralized Parietotemporal Oscillatory Phase Synchronization during Auditory Selective Attention. Neuroimage 2014, 86, 461–469. [Google Scholar] [CrossRef] [Green Version]
- Bonacci, L.M.; Bressler, S.; Shinn-Cunningham, B.G. Nonspatial Features Reduce the Reliance on Sustained Spatial Auditory Attention. Ear Hear. 2020, 41, 1635–1647. [Google Scholar] [CrossRef]
- Bonacci, L.M.; Dai, L.; Shinn-Cunningham, B.G. Weak Neural Signatures of Spatial Selective Auditory Attention in Hearing-Impaired Listeners. J. Acoust. Soc. Am. 2019, 146, 2577. [Google Scholar] [CrossRef]
- Tune, S.; Wöstmann, M.; Obleser, J. Probing the Limits of Alpha Power Lateralisation as a Neural Marker of Selective Attention in Middle-Aged and Older Listeners. Eur. J. Neurosci. 2018, 48, 2537–2550. [Google Scholar] [CrossRef]
- Deng, Y.; Choi, I.; Shinn-Cunningham, B. Topographic Specificity of Alpha Power during Auditory Spatial Attention. Neuroimage 2020, 207, 116360. [Google Scholar] [CrossRef]
- Darwin, C.J.; Hukin, R.W. Auditory Objects of Attention: The Role of Interaural Time Differences. J. Exp. Psychol. Hum. Percept. Perform. 1999, 25, 617–629. [Google Scholar] [CrossRef]
- Sach, A.J.; Bailey, P.J. Some Characteristics of Auditory Spatial Attention Revealed Using Rhythmic Masking Release. Percept. Psychophys. 2004, 66, 1379–1387. [Google Scholar] [CrossRef] [Green Version]
- Shinn-Cunningham, B.G. Object-Based Auditory and Visual Attention. Trends Cogn. Sci. 2008, 12, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Strait, D.L.; Kraus, N. Can You Hear Me Now? Musical Training Shapes Functional Brain Networks for Selective Auditory Attention and Hearing Speech in Noise. Front. Psychol. 2011, 2, 113. [Google Scholar] [CrossRef] [Green Version]
- Arnal, L.H.; Giraud, A.L. Cortical Oscillations and Sensory Predictions. Trends Cogn. Sci. 2012, 16, 390–398. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.J. Neurophysiological and Computational Principles of Cortical Rhythms in Cognition. Physiol. Rev. 2010, 90, 1195–1268. [Google Scholar] [CrossRef] [Green Version]
- Fenn, K.M.; Nusbaum, H.C.; Margoliash, D. Consolidation during Sleep of Perceptual Learning of Spoken Language. Nature 2003, 425, 614–616. [Google Scholar] [CrossRef]
- Eisner, F.; McQueen, J.M. Perceptual Learning in Speech: Stability over Time. J. Acoust. Soc. Am. 2006, 119, 1950. [Google Scholar] [CrossRef]
- Davis, M.H.; Di Betta, A.M.; Macdonald, M.J.E.; Gaskell, M.G. Learning and Consolidation of Novel Spoken Words. J. Cogn. Neurosci. 2009, 21, 803–820. [Google Scholar] [CrossRef] [Green Version]
- Bentler, R.A. List Equivalency and Test-Retest Reliability of the Speech in Noise Test. Am. J. Audiol. 2000, 9, 84–100. [Google Scholar] [CrossRef]
- Booshardt, S.; Degonda, N.; Schmidt, C.F.; Boesiger, P.; Nitsch, R.M.; Hock, C.; Henke, K. One Month of Human Memory Consolidation Enhances Retrieval-Related Hippocampal Activity. Hippocampus 2005, 15, 1026–1040. [Google Scholar] [CrossRef]
- Maris, E.; Oostenveld, R. Nonparametric Statistical Testing of EEG- and MEG-Data. J. Neurosci. Methods 2007, 164, 177–190. [Google Scholar] [CrossRef]
- Tian, X.; Huber, D.E. Measures of Spatial Similarity and Response Magnitude in MEG and Scalp EEG. Brain Topogr. 2008, 20, 131–141. [Google Scholar] [CrossRef]
- Hämäläinen, M.S.; Sarvas, J. Realistic Conductivity Geometry Model of the Human Head for Interpretation of Neuromagnetic Data. IEEE Trans. Biomed. Eng. 1989, 36, 165–171. [Google Scholar] [CrossRef]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Parkkonen, L.; Hämäläinen, M.S. MNE Software for Processing MEG and EEG Data. Neuroimage 2014, 86, 446–460. [Google Scholar] [CrossRef] [Green Version]
- Gramfort, A.; Luessi, M.; Larson, E.; Engemann, D.A.; Strohmeier, D.; Brodbeck, C.; Goj, R.; Jas, M.; Brooks, T.; Parkkonen, L.; et al. MEG and EEG Data Analysis with MNE-Python. Front. Neurosci. 2013, 7, 267. [Google Scholar] [CrossRef] [Green Version]
- Friston, K.; Harrison, L.; Daunizeau, J.; Kiebel, S.; Phillips, C.; Trujillo-Barreto, N.; Henson, R.; Flandin, G.; Mattout, J. Multiple Sparse Priors for the M/EEG Inverse Problem. Neuroimage 2008, 39, 1104–1120. [Google Scholar] [CrossRef]
- Dale, A.M.; Liu, A.K.; Fischl, B.R.; Buckner, R.L.; Belliveau, J.W.; Lewine, J.D.; Halgren, E. Dynamic Statistical Parametric Mapping: Combining FMRI and MEG for High-Resolution Imaging of Cortical Activity. Neuron 2000, 26, 55–67. [Google Scholar] [CrossRef] [Green Version]
- Engel, A.K.; Fries, P.; Singer, W. Dynamic Predictions: Oscillations and Synchrony in Top-down Processing. Nat. Rev. Neurosci. 2001, 2, 704–716. [Google Scholar] [CrossRef]
- Bressler, S.L.; Richter, C.G. Interareal Oscillatory Synchronization in Top-down Neocortical Processing. Curr. Opin. Neurobiol. 2015, 31, 62–66. [Google Scholar] [CrossRef]
- Engel, A.K.; Fries, P. Beta-Band Oscillations—Signalling the Status Quo? Curr. Opin. Neurobiol. 2010, 20, 156–165. [Google Scholar] [CrossRef]
- Riddle, J.; Hwang, K.; Cellier, D.; Dhanani, S.; D’esposito, M. Causal Evidence for the Role of Neuronal Oscillations in Top–Down and Bottom–Up Attention. J. Cogn. Neurosci. 2019, 31, 768–779. [Google Scholar] [CrossRef]
- Yuan, Z.; Chen, H.; Ding, Z.; Li, Z.; Song, Y.; Li, X. The Modulating Effect of Top-down Attention on the Optimal Pre-Target Onset Oscillatory States of Bottom-up Attention. Neuroscience 2021, 466, 186–195. [Google Scholar] [CrossRef]
- Theves, S.; Chan, J.S.; Naumer, M.J.; Kaiser, J. Improving Audio-Visual Temporal Perception through Training Enhances Beta-Band Activity. Neuroimage 2020, 206, 116312. [Google Scholar] [CrossRef]
- Viswanathan, V.; Bharadwaj, H.M.; Heinz, M.G.; Shinn-Cunningham, B.G. Induced Alpha And Beta Electroencephalographic Rhythms Covary With Single-Trial Speech Intelligibility In Competition. Sci. Rep. 2023, 13, 10216. [Google Scholar] [CrossRef]
- Price, C.N.; Alain, C.; Bidelman, G.M. Auditory-Frontal Channeling in α and β Bands Is Altered by Age-Related Hearing Loss and Relates to Speech Perception in Noise. Neuroscience 2019, 423, 18–28. [Google Scholar] [CrossRef]
- Obleser, J.; Weisz, N. Suppressed Alpha Oscillations Predict Intelligibility of Speech and Its Acoustic Details. Cereb. Cortex 2012, 22, 2466–2477. [Google Scholar] [CrossRef] [Green Version]
- Deng, Y.; Reinhart, R.M.G.; Choi, I.; Shinn-Cunningham, B. Causal Links between Parietal Alpha Activity and Spatial Auditory Attention. Elife 2019, 8, e51184. [Google Scholar] [CrossRef]
- Frey, J.N.; Mainy, N.; Lachaux, J.P.; Muller, N.; Bertrand, O.; Weisz, N. Selective Modulation of Auditory Cortical Alpha Activity in an Audiovisual Spatial Attention Task. J. Neurosci. 2014, 34, 6634–6639. [Google Scholar] [CrossRef] [Green Version]
- Shinn-Cunningham, B.G.; Best, V. Selective Attention in Normal and Impaired Hearing. Trends Amplif. 2008, 12, 283–299. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, H.; Gibbs, L.; Rush, K.; Ham, J.; Kim, S.; Kim, S.; Choi, I. Neural Mechanisms Related to the Enhanced Auditory Selective Attention Following Neurofeedback Training: Focusing on Cortical Oscillations. Appl. Sci. 2023, 13, 8499. https://doi.org/10.3390/app13148499
Shim H, Gibbs L, Rush K, Ham J, Kim S, Kim S, Choi I. Neural Mechanisms Related to the Enhanced Auditory Selective Attention Following Neurofeedback Training: Focusing on Cortical Oscillations. Applied Sciences. 2023; 13(14):8499. https://doi.org/10.3390/app13148499
Chicago/Turabian StyleShim, Hwan, Leah Gibbs, Karsyn Rush, Jusung Ham, Subong Kim, Sungyoung Kim, and Inyong Choi. 2023. "Neural Mechanisms Related to the Enhanced Auditory Selective Attention Following Neurofeedback Training: Focusing on Cortical Oscillations" Applied Sciences 13, no. 14: 8499. https://doi.org/10.3390/app13148499
APA StyleShim, H., Gibbs, L., Rush, K., Ham, J., Kim, S., Kim, S., & Choi, I. (2023). Neural Mechanisms Related to the Enhanced Auditory Selective Attention Following Neurofeedback Training: Focusing on Cortical Oscillations. Applied Sciences, 13(14), 8499. https://doi.org/10.3390/app13148499





