Sourdough Wheat Bread Enriched with Grass Pea and Lupine Seed Flour: Physicochemical and Sensory Properties
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Baking Properties of Wheat Flour and Physical Properties of Dough
2.3. Baking Procedure
2.4. Basic Composition of Raw Materials
2.5. Bread Yield, Volume, and Density
2.6. Crumb Texture
2.7. Color of Raw Materials and Bread Samples
2.8. Total Phenolic Content and Antioxidant Capacity
2.8.1. Extract Preparation
2.8.2. Total Phenolic Content
2.8.3. Antiradical Activity against DPPH Free Radicals
2.8.4. Antiradical Activity against ABTS•+ Free Radicals
2.9. Sensory Evaluation of Bread
2.10. Statistical Analysis of Results
3. Results and Discussion
3.1. Water Absorption and Physical Properties of Dough
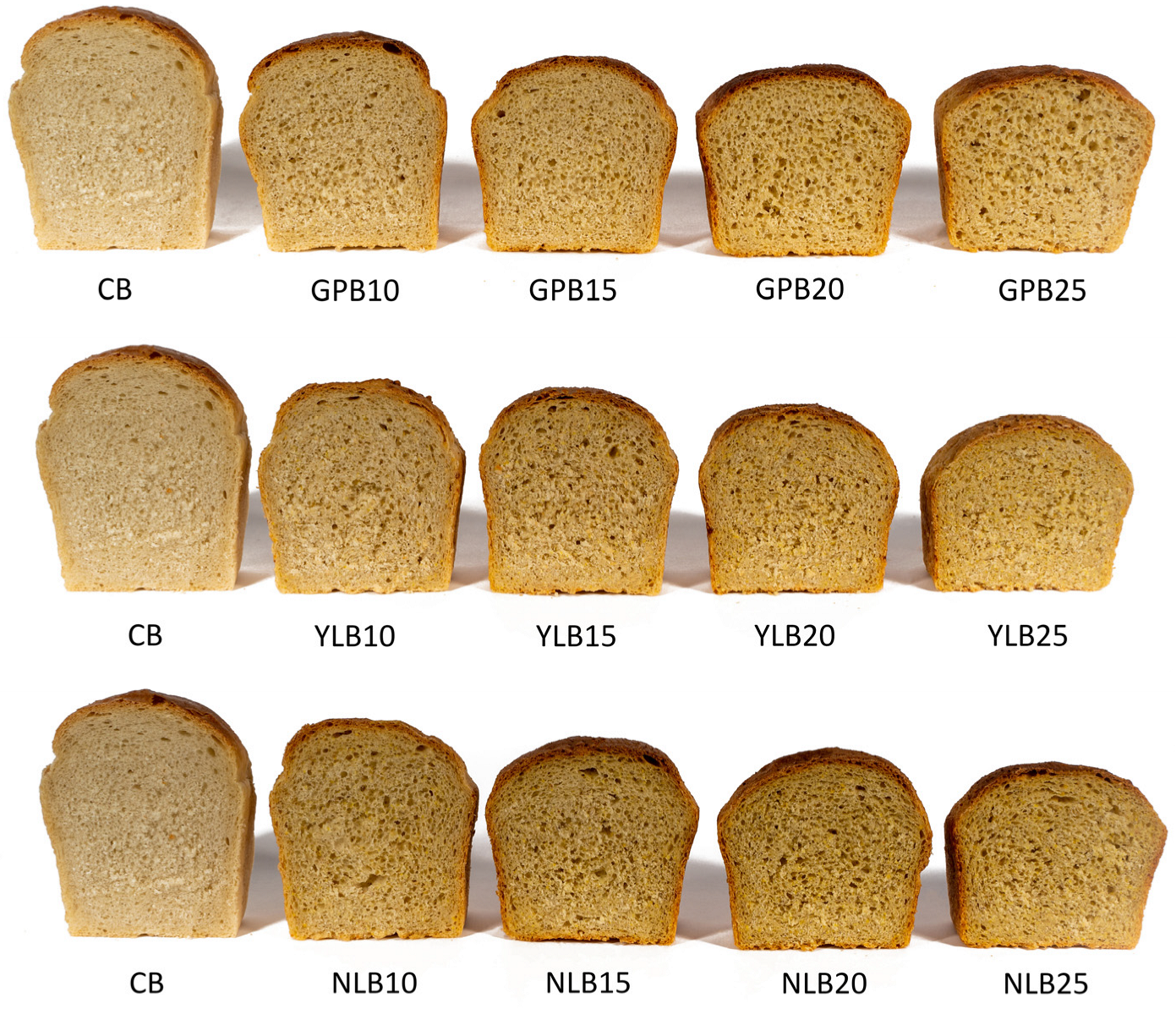
3.2. Basic Properties of Bread Samples
3.3. Crumb Texture
3.4. Color of Raw Materials and Bread
3.5. Basic Chemical Composition of Raw Materials and Bread
3.6. Phenolic Content and Antioxidant Capacity
3.7. Sensory Evaluation Results
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- López, E.P.; Goldner, M.C. Influence of storage time for the acceptability of bread formulated with lupine protein isolate and added brea gum. LWT—Food Sci. Technol. 2015, 64, 1171–1178. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Wójcik, M.; Krajewska, A. Dandelion Flowers as an Additive to Wheat Bread: Physical Properties of Dough and Bread Quality. Appl. Sci. 2023, 13, 477. [Google Scholar] [CrossRef]
- Shewry, P.R.; Hey, S.J. The contribution of wheat to human diet and health. Food Energy Secur. 2015, 4, 178–202. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S. Nutritional, Health, and Technological Functionality of Lupin Flour Additionto Bread and Other Baked Products: Benefits and Challenges. Crit. Rev. Food Sci. Nutr. 2016, 56, 835–857. [Google Scholar] [CrossRef] [PubMed]
- Rawat, M.; Varshney, A.; Rai, M.; Chikara, A.; Pohty, A.L.; Joshi, A.; Binjola, A.; Singh, C.P.; Rawat, K.; Rather, M.A.; et al. A comprehensive review on nutraceutical potential of underutilized cereals and cereal-based products. J. Agric. Food Res. 2023, 12, 100619. [Google Scholar] [CrossRef]
- Sułek, A.; Cacak-Pietrzak, G.; Różewicz, M.; Nieróbca, A.; Grabiński, J.; Studnicki, M.; Sujka, K.; Dziki, D. Effect of Production Technology Intensity on the Grain Yield, Protein Content and Amino Acid Profile in Common and Durum Wheat Grain. Plants 2023, 12, 364. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Różyło, R.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Biernacka, B. Cistus incanus L. as an Innovative Functional Additive to Wheat Bread. Foods 2019, 8, 349. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Sułek, A.; Kalisz, S.; Sujka, K. Effect of the Addition of Dried Dandelion Roots (Taraxacum officinale F. H. Wigg.) on Wheat Dough and Bread Properties. Molecules 2021, 26, 7564. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Hassonn, W.H.; Gawlik-Dziki, U.; Sułek, A.; Różyło, R.; Suger, D. The fruit of sumac (Rhus coriaria L.) as a functional additive and salt replacement to wheat bread. LWT—Food Sci. Technol. 2021, 136, 110346. [Google Scholar] [CrossRef]
- Wójcik, M.; Różyło, R.; Łysiak, G.; Kulig, R.; Cacak-Pietrzak, G. Textural and sensory properties of wheat bread fortified with nettle (Urtica dioica L.) produced by scalded flour method. J. Food Process. Preserv. 2021, 45, e15851. [Google Scholar] [CrossRef]
- Tolve, R.; Simonato, B.; Rainero, G.; Bianchi, F.; Rizzi, C.; Cervini, M.; Giuberti, G. Wheat bread fortification by grape pomace powder: Nutritional, technological, antioxidant, and sensory properties. Foods 2021, 10, 75. [Google Scholar] [CrossRef]
- Cantero, L.; Salmerón, J.; Miranda, J.; Larretxi, I.; Fernández-Gil, M.d.P.; Bustamante, M.Á.; Matias, S.; Navarro, V.; Simón, E.; Martínez, O. Performance of Apple Pomace for Gluten-Free Bread Manufacture: Effect on Physicochemical Characteristics and Nutritional Value. Appl. Sci. 2022, 12, 5934. [Google Scholar] [CrossRef]
- Valková, V.; Ďúranová, H.; Havrlentová, M.; Ivanišová, E.; Mezey, J.; Tóthová, Z.; Gabríny, L.; Kačániová, M. Selected Physico-Chemical, Nutritional, Antioxidant and Sensory Properties of Wheat Bread Supplemented with Apple Pomace Powder as a By-Product from Juice Production. Plants 2022, 11, 1256. [Google Scholar] [CrossRef]
- Cacak-Pietrzak, G.; Dziki, D.; Gawlik-Dziki, U.; Parol-Nadłonek, N.; Kalisz, S.; Krajewska, A.; Stępniewska, S. Wheat Bread Enriched with Black Chokeberry (Aronia melanocarpa L.) Pomace: Physicochemical Properties and Sensory Evaluation. Appl. Sci. 2023, 13, 6936. [Google Scholar] [CrossRef]
- Stanciu, I.; Ungureanu, E.L.; Popa, E.E.; Geicu-Cristea, M.; Draghici, M.; Mitelut, A.C.; Mustatea, G.; Popa, M.E. The Experimental Development of Bread with Enriched Nutritional Properties Using Organic Sea Buckthorn Pomace. Appl. Sci. 2023, 13, 6513. [Google Scholar] [CrossRef]
- Derkanosova, N.M.; Stakhurlova, A.A.; Pshenichnaya, I.A.; Ponomareva, I.N.; Peregonchaya, O.V.; Sokolova, S.A. Amaranth as a bread enriching ingredient. Foods Raw Mater. 2020, 8, 223–231. [Google Scholar] [CrossRef]
- Cotovanu, I.; Ungureanu-Iuga, M.; Mironeasa, S. Investigation of Quinoa Seeds Fractions and Their Application in Wheat Bread Production. Plants 2021, 10, 2150. [Google Scholar] [CrossRef] [PubMed]
- Hall, R.S.; Johnson, S.K. Sensory acceptability of foods containing Australian sweet lupin (Lupinus angustifolius) flour. J. Food Sci. 2004, 69, 92–97. [Google Scholar] [CrossRef]
- Villarino, C.B.J.; Jayasena, V.; Coorey, R.; Chakrabarti-Bell, S.; Johnson, S. Optimization of formulation and process of Australian sweet lupin (ASL)—Wheat bread. LWT—Food Sci. Technol. 2015, 61, 359–367. [Google Scholar] [CrossRef]
- Karamać, M.; Orak, H.H.; Amarowicz, R.; Orak, A.; Piekoszewski, W. Phenolic contents and antioxidant capacities of wild and cultivated white lupin (Lupinus albus L.) seeds. Food Chem. 2018, 258, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Carboni, D.A.; Salinas, V.M.; Puppo, C.M. Production of legume-wheat dough of optimum quality for breadmaking: Essential analyses required. Curr. Opin. Food Sci. 2023, 49, 100970. [Google Scholar] [CrossRef]
- Bessada, S.M.F.; Barreira, J.C.M.; Oliveira, M.B.P.P. Pulses and food security: Dietary protein, digestibility, bioactive and functional properties. Trends Food Sci. Technol. 2019, 93, 53–68. [Google Scholar] [CrossRef]
- FAOSTAT. Available online: https://www.fao.org/faostat/en/#data/QCL (accessed on 24 May 2023).
- Roland, W.S.U.; Pouvreau, L.; Curran, J.; Van De Velde, F.; De Kok, P.M.T. Flavor aspects of pulse ingredients. Cereal Chem. 2017, 94, 58–65. [Google Scholar] [CrossRef]
- Rajhi, I.; Baccouri, B.; Rajhi, F.; Mhadhbi, H.; Flamini, G. Monitoring the volatile compounds status of whole seeds and flours of legume cultivars. Food Biosci. 2021, 41, 101105. [Google Scholar] [CrossRef]
- Tas, A.A.; Shah, A.U. The replacement of cereals by legumes in extruded snack foods: Science, technology and challenges. Trends Food Sci. Technol. 2021, 116, 701–711. [Google Scholar] [CrossRef]
- Das, G.; Sharma, A.; Sarkar, P.K. Conventional and emerging processing techniques for the post-harvest reduction of antinutrients in edible legumes. Appl. Food Res. 2022, 2, 100112. [Google Scholar] [CrossRef]
- Ulrike, M. Are legumes different? Origins and consequences of evolving nitrogen fixing symbioses. J. Plant Physiol. 2022, 276, 153765. [Google Scholar] [CrossRef]
- Ogbole, O.O.; Akin-Ajani, O.D.; Ajala, T.O.; Ogunniyi, Q.A.; Fettke, J.; Odeku, O.A. Nutritional and pharmacological potentials of orphan legumes: Subfamily faboideae. Heliyon 2023, 9, e15493. [Google Scholar] [CrossRef]
- Kalogeropoulos, N.; Chiou, A.; Ioannou, M.; Karathanos, V.T.; Hassapidou, M.; Andrikopoulos, N.K. Nutritional evaluation and bioactive microconstituents (phytosterols, tocopherols, polyphenols, triterpenic acids) in cooked dry legumes usually consumed in the Mediterranean countries. Food Chem. 2010, 121, 682–690. [Google Scholar] [CrossRef]
- Rebello, C.J.; Greenway, F.L.; Finley, J.W. Whole grains and pulses: A comparison of the nutritional and health benefits. J. Agric. Food Chem. 2014, 62, 7029–7049. [Google Scholar] [CrossRef] [PubMed]
- Temba, M.C.; Njobeh, P.B.; Adebo, O.A.; Olugbile, A.O.; Kayitesi, E. The role of compositing cereals with legumes to alleviate protein energy malnutrition in Africa. Int. J. Food Sci. Technol. 2016, 51, 543–554. [Google Scholar] [CrossRef]
- Li, L.; Yuan, T.Z.; Setia, R.; Raja, R.B.; Zhang, B.; Ai, Y. Characteristics of pea, lentil and faba bean starches isolated from air-classified flours in comparison with commercial starches. Food Chem. 2019, 276, 599–607. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, N.; Reifen, R. The potential of legume-derived proteins in the food industry. Grain Oil Sci. Technol. 2022, 5, 167–178. [Google Scholar] [CrossRef]
- de Almeida Costa, G.E.; da Silva Queiroz-Monici, K.; Reis, S.M.P.M.; de Oliveira, A.C. Chemical composition, dietary fibre and resistant starch contents of raw and cooked pea, common bean, chickpea and lentil legumes. Food Chem. 2006, 94, 327–330. [Google Scholar] [CrossRef]
- Oyeyinka, A.S.; Singh, S.; Amonsou, E.O. A review on structural, digestibility and physicochemical properties of legume starch-lipid complexes. Food Chem. 2021, 349, 129165. [Google Scholar] [CrossRef]
- Hutchins, A.M.; Winham, D.M.; Thompson, S.V. Phaseolus beans: Impact on glycaemic response and chronic disease risk in human subjects. Br. J. Nutr. 2012, 108, S52–S65. [Google Scholar] [CrossRef]
- Samtiya, M.; Aluko, R.E.; Dhewa, T. Plant food anti-nutritional factors and their reduction strategies: An overview. Food Prod. Process. Nutr. 2020, 2, 6. [Google Scholar] [CrossRef]
- Jenkins, D.J.; Kendall, C.W.; Augustin, L.S.; Mitchell, S.; Sahye-Pudaruth, S.; Mejia, S.B.; Chiavaroli, L.; Mirrahimi, A.; Ireland, C.; Bashyam, B.; et al. Effect of legumes as part of a low glycemic index diet on glycemic control and cardiovascular risk factors in type 2 diabetes mellitus: A randomized controlled trial. Arch. Intern. Med. 2012, 172, 1653–1660. [Google Scholar] [CrossRef]
- Mudryj, A.N.; Yu, N.; Aukema, H.M. Nutritional and health benefits of pulses. Appl. Physiol. Nutr. Metab. 2014, 39, 1197–1204. [Google Scholar] [CrossRef]
- Pastor-Cavada, E.; Juan, R.; Pastor, J.E.; Alaiz, M.; Vioque, J. Antioxidant activity of seed polyphenols in fifteen wild Lathyrus species from South Spain. LWT—Food Sci. Technol. 2009, 42, 705–709. [Google Scholar] [CrossRef]
- Deshpande, S.S.; Campbell, C.G. Genotype variation in BOAA, condensed tannins, phenolics and enzyme-inhibitors of grass pea (Lathyrus sativus). Can. J. Plant Sci. 1992, 72, 1037–1047. [Google Scholar] [CrossRef]
- Wang, X.F.; Warkentin, T.D.; Briggs, C.J.; Oomah, B.D.; Campbell, C.G.; Woods, S. Total phenolics and condensed tannins in field pea (Pisum sativum L.) and grass pea (Lathyrus sativus L.). Euphytica 1998, 101, 97–102. [Google Scholar] [CrossRef]
- Chavan, U.D.; Amarowicz, R.; Shahidi, F. Antioxidant activity of phenolic fractions of beach pea (Lathyrus maritimus L.). J. Food Lipids 1999, 6, 1–11. [Google Scholar] [CrossRef]
- Chavan, U.D.; McKenzie, D.B.; Amarowicz, R.; Shahidi, F. Phytochemical components of beach pea (Lathyrus maritimus L.). Food Chem. 2003, 81, 61–71. [Google Scholar] [CrossRef]
- Shahidi, F.; Chavan, U.D.; Naczk, M.; Amarowicz, R. Nutrient distribution and phenolic antioxidants in air-classified fractions of beach pea (Lathyrus maritimus L.). J. Agric. Food Chem. 2001, 49, 926–933. [Google Scholar] [CrossRef]
- Dixit, G.P.; Parihar, A.K.; Abhishek Bohra, A.; Singh, N.P. Achievements and prospects of grass pea (Lathyrus sativus L.) improvement for sustainable food production. Crop J. 2006, 4, 407–416. [Google Scholar] [CrossRef]
- Łabuda, S.; Chwil, S. Rhythm of biomass and macroelements accumulation in grass pea (Lathyrus sativus L.). Rocz. Glebozn. 1996, 47, 79–87. (In Polish) [Google Scholar]
- Rao, S.L. A look at the brighter facets of β-N-oxalyl-L-α,β-diaminopropionic acid, homoarginine and the grass pea. Food Chem. Toxicol. 2011, 49, 620–622. [Google Scholar] [CrossRef]
- Singh, S.S.; Rao, S.L.N. Lessons from neurolathyrism: A disease of the past & the future of Lathyrus sativus (Khesari dal). Indian J. Med. Res. 2013, 138, 32–37. [Google Scholar]
- Khandare, A.L.; Babu, J.J.; Ankulu, M.; Aparna, N.; Shirfule, A.; Rao, G.S. Grass pea consumption & present scenario of neurolathyrism in Maharashtra state of India. Indian J. Med. Res. 2014, 140, 96–101. [Google Scholar] [PubMed]
- Cowling, W.A.; Buirchell, B.J.; Tapia, M.E. Lupin. Promoting the Conservation and Use of Underutilized and Neglected Crops 23; Institute of Plant Genetics and Crop Plant Research, Gatersleben/International Plant Genetic Resources Institute: Rome, Italy, 1998; pp. 1–100. [Google Scholar]
- Gladstones, J.S. Distribution, origin, taxonomy, history and importance. In Lupins as Crop Plants: Biology, Production and Utilisation; Gladstones, J.S., Atkins, C.A., Hamblin, J., Eds.; CAB International: Wallingford, UK, 1998; pp. 1–37. [Google Scholar]
- Lampart-Szczapa, E.; Korczak, J.; Nogala-Kalucka, M.; Zawirska-Wojtasiak, R. Antioxidant properties of lupin seed products. Food Chem. 2003, 83, 279–285. [Google Scholar] [CrossRef]
- Khan, M.K.; Karnpanit, W.; Nasar-Abbas, S.M.; Zill-e-Huma; Jayasena, V. Phytochemical composition and bioactivities of lupin: A review. Int. J. Food Sci. Technol. 2015, 50, 2004–2012. [Google Scholar] [CrossRef]
- Arnoldi, A.; Boschin, G.; Zanoni, C.; Lammi, C. The health benefits of sweet lupin seed flours and isolated proteins. J. Funct. Foods 2015, 18, 550–563. [Google Scholar] [CrossRef]
- Starzyńska-Janiszewska, A.; Stodolak, B.; Jamróz, M. Antioxidant properties of extracts from fermented and cooked seeds of Polish cultivars of Lathyrus sativus. Food Chem. 2008, 109, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Bartkiene, E.; Juodeikiene, G.; Vidmantiene, D.; Viskelis, P.; Urbonaviciene, D. Nutritional and quality aspects of wheat sourdough bread using L. luteus and L. angustifolius flours fermented by Pedioccocus acidilactici. Int. J. Food Sci. Technol. 2011, 46, 1724–1733. [Google Scholar] [CrossRef]
- AACC. American Association of Cereal Chemistry Approved Methods, 10th ed.; AACC: St. Paul, MN, USA, 2000; Available online: http://methods.aaccnet.org/toc.aspx (accessed on 10 May 2023).
- Romankiewicz, D.; Hassoon, W.H.; Cacak-Pietrzak, G.; Sobczyk, M.; Wirkowska-Wojdyła, M.; Ceglińska, A.; Dziki, D. The effect of chia seeds (Salvia hispanica L.) addition on quality and nutritional value of wheat bread. J. Food Qual. 2017, 2017, 7352631. [Google Scholar] [CrossRef]
- Różyło, R.; Wójcik, M.; Dziki, D.; Biernacka, B.; Cacak-Pietrzak, G.; Gawłowski, S.; Zdybel, A. Freeze-dried elderberry and chokeberry as natural colorants for gluten-free wafer sheets. Int. Agrophys. 2018, 33, 217–225. [Google Scholar] [CrossRef]
- Brand-Williams, W.; Cuvelier, E.; Berset, C. Use of a free radical method to evaluate antioxidant activity. LWT—Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- García-Gómez, B.; Fernández-Canto, N.; Vázquez-Odériz, M.L.; Quiroga-García, M.; Muñoz-Ferreiro, N.; Romero-Rodríguez, M.Á. Sensory descriptive analysis and hedonic consumer test for Galician type breads. Food Control 2022, 134, 108765. [Google Scholar] [CrossRef]
- Dziki, D.; Cacak-Pietrzak, G.; Gawlik-Dziki, U.; Sułek, A.; Kocira, S.; Biernacka, B. Effect of Moldavian dragonhead (Dracocephalum moldavica L.) leaves on the baking properties of wheat flour and quality of bread. CyTA—J. Food 2019, 17, 536–543. [Google Scholar] [CrossRef]
- Feledyn-Szewczyk, B.; Cacak-Pietrzak, G.; Lenc, L.; Gromadzka, K.; Dziki, D. Milling and Baking Quality of Spring Wheat (Triticum aestivum L.) from Organic Farming. Agriculture 2021, 11, 765. [Google Scholar] [CrossRef]
- Rehman, S.; Alistair Paterson, A.; Hussain, S.; Murtaza, M.A.; Mehmood, S. Influence of partial substitution of wheat flour with vetch (Lathyrus sativus L.) flour on quality characteristics of doughnuts. LWT—Food Sci. Technol. 2007, 40, 73–82. [Google Scholar] [CrossRef]
- Wandersleben, T.; Morales, E.; Burgos-Díaz, C.; Barahona, T.; Labra, E.; Rubilar, M.; Salvo-Garrido, H. Enhancement of functional and nutritional properties of bread using a mix of natural ingredients from novel varieties of flaxseed and lupine. LWT—Food Sci. Technol. 2018, 91, 48–54. [Google Scholar] [CrossRef]
- Jaskulska, I.; Jaskulski, D.; Gałȩzewski, L.; Knapowski, T.; Kozera, W.; Wacławowicz, R. Mineral Composition and Baking Value of the Winter Wheat Grain under Varied Environmental and Agronomic Conditions. J. Chem. 2018, 2018, 5013825. [Google Scholar] [CrossRef]
- Steffolani, E.; Martinez, M.M.; León, A.E.; Gómez, M. Effect of pre-hydration of chia (Salvia hispanica L.) seed and flour on the quality of wheat flour breads. LWT—Food Sci. Technol. 2015, 61, 401–406. [Google Scholar] [CrossRef]
- Klupsaite, D.; Juodeikiene, G.; Zadeike, D.; Bartkiene, E.; Maknickiene, Z.; Liutkute, G. The influence of lactic acid fermentation on functional properties of narrow-leaved lupine protein as functional additive for higher value wheat bread. LWT—Food Sci. Technol. 2017, 75, 180–186. [Google Scholar] [CrossRef]
- Cappelli, A.; Lupori, L.; Cini, E. Baking technology: A systematic review of machines and plants and their effect on final products, including improvement strategies. Trends Food Sci. Technol. 2021, 115, 275–284. [Google Scholar] [CrossRef]

| Sample Code | Wheat Flour (%) | Grass Pea Flour (%) | Yellow Lupine Flour (%) | Narrow-Leaf Lupine Flour (%) |
|---|---|---|---|---|
| CD | 100 | - | - | - |
| GPD10 | 90 | 10 | - | - |
| GPD15 | 85 | 15 | - | - |
| GPD20 | 80 | 20 | - | - |
| GPD25 | 75 | 25 | - | - |
| YLD10 | 90 | - | 10 | - |
| YLD15 | 85 | - | 15 | - |
| YLD20 | 80 | - | 20 | - |
| YLD25 | 75 | - | 25 | - |
| NLD10 | 90 | - | - | 10 |
| NLD15 | 85 | - | - | 15 |
| NLD20 | 80 | - | - | 20 |
| NLD25 | 75 | - | - | 25 |
| Sample | Water Absorption (%) | Development Time (min) | Stability of Dough (min) | Degree of Softening (BU) |
|---|---|---|---|---|
| CD | 54.1 ± 0.1 g | 2.0 ± 0.1 h | 9.1 ± 0.7 c | 35 ± 4.6 bc |
| GPD10 | 55.1 ± 0.1 f | 6.8 ± 0.2 e | 9.7 ± 0.1 c | 19 ± 1 fg |
| GPD15 | 55.0 ± 0.1 f | 6.2 ± 0.3 efg | 7.7 ± 0.3 d | 28 ± 5 de |
| GPD20 | 55.1 ± 0.1 f | 5.6 ± 0.2 fg | 6.1 ± 0.1 ef | 35 ± 0 bc |
| GPD25 | 55.0 ± 0.1 f | 5.7 ± 0.0 fg | 4.0 ± 0.1 g | 51 ± 2 a |
| YLD10 | 56.9 ± 0.1 d | 10.7 ± 0.1 c | 14.0 ± 0.1 a | 4 ± 1 i |
| YLD15 | 59.2 ± 0.2 c | 13.7 ± 0.1 a | 12.5 ± 0.1 b | 12 ± 2 gh |
| YLD20 | 62.0 ± 0.2 b | 12.6 ± 0.1 b | 9.5 ± 0.2 c | 8 ± 1 hi |
| YLD25 | 63.5 ± 0.1 a | 8.6 ± 0.6 d | 9.0 ± 0.1 c | 3 ± 0 i |
| NLD10 | 56.1 ± 0.1 e | 6.4 ± 0.1 de | 9.6 ± 0.3 c | 7 ± 0 hi |
| NLD15 | 55.9 ± 0.1 e | 6.4 ± 0.1 de | 6.9 ± 0.1 e | 22 ± 2 ef |
| NLD20 | 55.9 ± 0.1 e | 5.5 ± 0.1 g | 5.8 ± 0.1 f | 32 ± 2 cd |
| NLD25 | 55.9 ± 0.1 e | 5.6 ± 0.2 g | 3.8 ± 0.2 g | 41 ± 2 b |
| Sample | Baking Loss (%) | Bread Yield (%) | Volume (cm3 100−1 g) | Crumb Density (g cm−3) |
|---|---|---|---|---|
| CB | 11.6 ± 0.1 bc | 139.0 ± 0.7 de | 365 ± 3.7 a | 0.26 ± 0.01 f |
| GPB10 | 11.9 ± 1.3 ab | 138.4 ± 0.5 de | 312 ± 2.1 b | 0.32 ± 0.00 e |
| GPB15 | 12.2 ± 0.1 a | 139.3 ± 0.4 d | 289 ± 5.8 c | 0.34 ± 0.01 de |
| GPB20 | 12.1 ±1.3 a | 139.1 ± 0.3 de | 272 ± 0.9 d | 0.37 ± 0.01 d |
| GPB25 | 11.3 ± 0.2 cd | 140.1 ± 0.8 cd | 262 ± 0.8 ef | 0.41 ± 0.01 c |
| YLB10 | 10.9 ± 0.2 def | 139.7 ± 0.5 d | 292 ± 2.6 c | 0.35 ± 0.01 e |
| YLB15 | 10.8 ± 0.1 ef | 142.0 ± 0.5 c | 273 ± 2.2 d | 0.37 ± 0.00 d |
| YLB20 | 11.1 ± 0.1 de | 144.0 ± 0.2 b | 257 ± 2.9 f | 0.42 ± 0.00 bc |
| YLB25 | 10.1 ± 0.2 e | 147.1 ± 0.3 a | 228 ± 1.7 h | 0.48 ± 0.01 a |
| NLB10 | 10.8 ± 0.1 ef | 137.3 ± 0.6 e | 311 ± 1.3 b | 0.33 ± 0.01 e |
| NLB15 | 10.6 ± 0.1 gh | 139.1 ± 0.3 de | 285 ± 0.5 c | 0.38 ± 0.01 d |
| NLB20 | 10.5 ± 0.2 gh | 139.5 ± 0.9 d | 270 ± 2.5 de | 0.39 ± 0.01 d |
| NLB25 | 10.3 ± 0.1 h | 139.8 ± 0.3 d | 245 ± 1.3 g | 0.44 ± 0.01 b |
| Sample | Hardness (N) | Elasticity (-) | Springiness (-) | Cohesiveness (-) |
|---|---|---|---|---|
| CB | 8.37 ± 0.15 h | 0.23 ± 0.01 a | 0.87 ± 0.01 a | 0.65 ± 0.01 a |
| GPB10 | 11.35 ± 0.41 g | 0.22 ± 0.02 ab | 0.84 ± 0.01 ab | 0.56 ± 0.01 bc |
| GPB15 | 11.73 ± 1.10 g | 0.19 ± 0.01 cde | 0.82 ± 0.02 abc | 0.48 ± 0.02 def |
| GPB20 | 12.08 ± 0.44 fg | 0.17 ± 0.01 efg | 0.82 ± 0.04 abc | 0.45 ± 0.02 ef |
| GPB25 | 13.04 ± 0.28 f | 0.14 ± 0.00 h | 0.79 ± 0.01 bcd | 0.41 ± 0.04 f |
| YLB10 | 15.38 ± 0.31 e | 0.18 ± 0.00 def | 0.82 ± 0.01 abc | 0.49 ± 0.01 cde |
| YLB15 | 17.21 ± 0.42 d | 0.18 ± 0.01 def | 0.81 ± 0.01 bcd | 0.51 ± 0.02 cde |
| YLB20 | 19.42 ± 0.40 c | 0.17 ± 0.01 efg | 0.81 ± 0.02 bcd | 0.52 ± 0.02 bcd |
| YLB25 | 21.19 ± 0.72 b | 0.15 ± 0.02 gh | 0.78 ± 0.02 cd | 0.49 ± 0.03 cde |
| NLB10 | 11.69 ± 0.07 g | 0.21 ± 0.01 bc | 0.84 ± 0.02 ab | 0.59 ± 0.07 ab |
| NLB15 | 15.77 ± 0.39 e | 0.20 ± 0.00 bcd | 0.78 ± 0.03 cd | 0.55 ± 0.02 bc |
| NLB20 | 17.97 ± 0.52 d | 0.19 ± 0.01 cde | 0.75 ± 0.02 d | 0.54 ± 0.04 bcd |
| NLB25 | 22.54 ± 0.27 a | 0.16 ± 0.01 fgh | 0.69 ± 0.01 e | 0.49 ± 0.00 cde |
| Sample | Lightness | Redness | Yellowness | ΔE |
|---|---|---|---|---|
| WF | 90.91 ± 0.11 A | 0.46 ± 0.03 D | 10.23 ± 0.25 D | - |
| GP | 86.68 ± 0.04 B | 0.76 ± 0.03 C | 17.18 ± 0.06 C | - |
| YL | 82.84 ± 0.21 D | 3.77 ± 0.12 A | 27.45 ± 0.17 A | - |
| NL | 83.80 ± 0.21 C | 1.86 ± 0.04 B | 24.25 ± 0.07 B | - |
| CB | 70.38 ± 0.78 a | 0.14 ± 0.02 h | 14.22 ± 0.24 f | - |
| GPB10 | 63.77 ± 0.42 e | 0.28 ± 0.04 gh | 18.73 ± 0.68 e | 8.0 |
| GPB15 | 62.90 ± 0.23 ef | 0.40 ± 0.02 g | 18.98 ± 0.09 e | 8.9 |
| GPB20 | 61.73 ± 0.15 gh | 0.65 ± 0.04 f | 22.28 ± 0.83 cd | 11.8 |
| GPB25 | 60.71 ± 0.17 hi | 0.98 ± 0.08 e | 23.46 ± 0.32 c | 13.4 |
| YLB10 | 69.44 ± 0.46 a | 1.30 ± 0.02 d | 23.20 ± 0.20 c | 9.1 |
| YLB15 | 67.78 ± 0.22 b | 1.73 ± 0.04 c | 26.13 ± 0.59 b | 12.3 |
| YLB20 | 66.68 ± 0.39 c | 2.17 ± 0.05 b | 26.78 ± 0.27 b | 13.3 |
| YLB25 | 65.23 ± 0.24 d | 3.07 ± 0.05 a | 29.17 ± 0.11 a | 21.3 |
| NLB10 | 65.62 ± 0.05 cd | 0.79 ± 0.06 ef | 21.52 ± 0.29 d | 8.7 |
| NLB15 | 62.62 ± 0.31 fg | 1.28 ± 0.03 d | 25.98 ± 0.19 b | 14.2 |
| NLB20 | 60.90 ± 0.06 hi | 1.62 ± 0.03 c | 26.78 ± 0.76 b | 15.9 |
| NLB25 | 60.23 ± 0.17 i | 2.32 ± 0.15 b | 29.36 ± 0.50 a | 18.4 |
| Sample | Protein (% DM) | Ash (% DM) | Fiber (% DM) | Fat (% DM) | Carbohydrates (% DM) |
|---|---|---|---|---|---|
| WF | 11.13 ± 0.13 D | 0.59 ± 0.02 D | 1.83 ± 0.01 D | 1.22 ± 0.02 C | 85.24 ± 0.08 A |
| GP | 31.99 ± 0.04 C | 3.31 ± 0.02 C | 6.09 ± 0.02 C | 0.32 ± 0.02 D | 58.29 ± 0.05 B |
| YL | 34.01 ± 0.03 B | 3.66 ± 0.00 B | 19.31 ± 0.02 A | 6.42 ± 0.03 A | 36.60 ± 0.05 C |
| NL | 48.18 ± 0.12 A | 3.83 ± 0.01 A | 16.68 ± 0.04 B | 4.73 ± 0.01 B | 26.58 ± 0.13 D |
| CB | 11.45 ± 0.02 k | 0.87 ± 0.02 h | 1.90 ± 0.01 l | 1.29 ± 0.02 h | 84.49 ± 0.08 a |
| GPB10 | 13.70 ± 0.04 i | 1.41 ± 0.01 f | 2.21 ± 0.02 l | 1.20 ± 0,02 i | 81.48 ± 0.04 b |
| GPB15 | 14.42 ± 0.00 h | 1.49 ± 0.01 d | 2.50 ± 0.00 k | 1.10 ± 0.01 j | 80.59 ± 0.08 c |
| GPB20 | 15.12 ± 0.08 f | 1.53 ± 0.00 c | 2.72 ± 0.04 j | 1.08 ± 0.04 j | 79.55 ± 0.12 e |
| GPB25 | 16.04 ± 0.02 d | 1.60 ± 0.02 b | 3.59 ± 0.02 g | 0.99 ± 0.01 k | 77.78 ± 0.03 g |
| YLB10 | 13.42 ± 0.04 j | 1.34 ± 0.01 g | 3.41 ± 0.01 h | 1.82 ± 0.04 e | 80.01 ± 0.04 d |
| YLB15 | 14.95 ± 0.03 fg | 1.45 ± 0.02 e | 4.20 ± 0.02 e | 2.11 ± 0.02 c | 77.24 ± 0.02 h |
| YLB20 | 15.52 ± 0.04 e | 1.50 ± 0.01 d | 5.30 ± 0.04 b | 2.31 ± 0.04 b | 75.37 ± 0.04 i |
| YLB25 | 16.89 ± 0.12 c | 1.63 ± 0.00 b | 6.10 ± 0,00 a | 2.51 ± 0.02 a | 72.87 ± 0.04 k |
| NLB10 | 14.86 ± 0.06 g | 1.41 ± 0.02 f | 3.09 ± 0.06 i | 1.50 ± 0.05 g | 79.14 ± 0.05 f |
| NLB15 | 16.72 ± 0.02 c | 1.48 ± 0.01 d | 4.01 ± 0.00 f | 1.70 ± 0.02 f | 76.00 ± 0.01 h |
| NLB20 | 18.20 ± 0.03 b | 1.54 ± 0.02 c | 4.90 ± 0.05 d | 2.02 ± 0.04 d | 73.34 ± 0.04 j |
| NLB25 | 20.00 ± 0.08 a | 1.72 ± 0.00 a | 5.08 ± 0.02 c | 2.11 ± 0.05 c | 71.09 ± 0.05 l |
| Sample | TPC (mg GAE g DM−1) | EC50 DPPH (mg DM mL−1) | EC50 ABTS (mg DM mL−1) |
|---|---|---|---|
| WF | 0.86 ± 0.02 A | 217 ± 5 C | 188 ± 2 B |
| GP | 1.72 ± 0.04 B | 169 ± 4 B | 178 ± 2 AB |
| YL | 1.88 ± 0.04 C | 160 ± 9 AB | 169 ± 4 AB |
| NL | 2.15 ± 0.05 D | 148 ± 4 A | 164 ± 5 A |
| CB | 0.72 ± 0.02 g | 275 ± 5 k | 213 ± 18 f |
| GPB10 | 0.81 ± 0.02 ef | 241 ± 3 j | 186 ± 1 ac |
| GPB15 | 0.85 ± 0.01 e | 230 ± 2 i | 163 ± 2 e |
| GPB20 | 0.89 ± 0.03 bf | 218 ± 2 h | 148 ± 3 d |
| GPB25 | 0.99 ± 0.02 ad | 204 ± 3 g | 139 ± 3 d |
| YLB10 | 0.92 ± 0.02 bc | 186 ± 4 f | 199 ± 4 b |
| YLB15 | 1.00 ± 0.03 ad | 174 ± 2 e | 192 ± 3 ab |
| YLB20 | 1.03 ± 0.03 ag | 165 ± 4 d | 193 ± 3 ab |
| YLB25 | 1.09 ± 0.03 h | 154 ± 4 c | 191 ± 5 ab |
| NLB10 | 0.93 ± 0.02 bc | 158 ± 1 cd | 183 ± 7 ac |
| NLB15 | 0.97 ± 0.01 cd | 145 ± 3 b | 181 ± 3 c |
| NLB20 | 1.04 ± 0.01 ag | 140 ± 2 ab | 189 ± 2 abc |
| NLB25 | 1.12 ± 0.02 h | 136 ± 2 a | 168 ± 3 e |
| Sample | Appearance | Smell | Taste | Texture | Color | OA |
|---|---|---|---|---|---|---|
| CB | 8.5 ± 0.5 a | 8.6 ± 1.0 a | 8.7 ± 0.7 a | 8.7 ± 0.7 a | 8.1 ± 0.9 a | 8.5 ± 0.6 a |
| GPB10 | 7.5 ± 0.5 abc | 8.0 ± 0.8 a | 8.3 ± 0.7 ab | 8.0 ± 0.5 ab | 7.7 ± 0.7 a | 7.9 ± 0.5 abc |
| GPB15 | 7.0 ± 0.5 bcd | 7.6 ± 0.7 a | 7.6 ± 0.7 ab | 7.7 ± 0.7 ab | 7.4 ± 0.7 a | 7.5 ± 0.4 bc |
| GPB20 | 6.5 ± 0.5 cde | 5.2 ± 1.4 h | 4.7 ± 2.0 de | 4.3 ± 1.3 cd | 3.8 ± 1.6 bc | 4.8 ± 1.1 de |
| GPB25 | 5.1 ± 1.0 f | 4.8 ± 1.8 bc | 3.1 ± 1.5 f | 1.9 ± 1.0 e | 2.8 ± 1.1 c | 3.2 ± 0.7 f |
| YLB10 | 8.3 ± 0.5 a | 8.6 ± 1.0 a | 8.2 ± 0.6 ab | 8.6 ± 0.7 a | 8.1 ± 0.9 a | 8.4 ± 0.5 ab |
| YLB15 | 7.6 ± 0.5 ab | 8.2 ± 0.9 a | 7.0 ± 0.7 bc | 8.4 ± 0.7 ab | 8.0 ± 0.9 a | 7.3 ± 0.5 c |
| YLB20 | 6.5 ± 0.5 cde | 5.1 ± 1.1 b | 5.6 ± 1.1 cd | 5.3 ± 1.3 c | 5.1 ± 1.3 b | 5.5 ± 0.7 d |
| YLB25 | 6.2 ± 1.2 e | 2.9 ± 1.7 d | 3.4 ± 1.3 ef | 2.3 ± 1.1 e | 3.1 ± 0.7 c | 3.4 ± 0.7 f |
| NLB10 | 8.3 ± 0.5 a | 8.4 ± 0.8 a | 7.5 ± 0.7 ab | 8.3 ± 0.9 ab | 7.5 ± 1.0 a | 8.2 ± 0.4 abc |
| NLB15 | 7.5 ± 0.5 abc | 7.6 ± 1.0 a | 7.5 ± 0.5 ab | 7.0 ± 0.9 b | 6.8 ± 0.8 a | 7.4 ± 0.5 d |
| NLB20 | 6.0 ± 0.5 def | 4.6 ± 1.7 bcd | 4.2 ± 0.8 def | 3.9 ± 1.6 cd | 4.9 ± 1.0 b | 4.7 ± 0.6 de |
| NLB25 | 5.6 ± 1.2 f | 3.6 ± 1.6 bcd | 3.6 ± 1.2 ef | 2.9 ± 0.9 de | 4.2 ± 0.9 bc | 4.0 ± 0.7 ef |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cacak-Pietrzak, G.; Sujka, K.; Księżak, J.; Bojarszczuk, J.; Dziki, D. Sourdough Wheat Bread Enriched with Grass Pea and Lupine Seed Flour: Physicochemical and Sensory Properties. Appl. Sci. 2023, 13, 8664. https://doi.org/10.3390/app13158664
Cacak-Pietrzak G, Sujka K, Księżak J, Bojarszczuk J, Dziki D. Sourdough Wheat Bread Enriched with Grass Pea and Lupine Seed Flour: Physicochemical and Sensory Properties. Applied Sciences. 2023; 13(15):8664. https://doi.org/10.3390/app13158664
Chicago/Turabian StyleCacak-Pietrzak, Grażyna, Katarzyna Sujka, Jerzy Księżak, Jolanta Bojarszczuk, and Dariusz Dziki. 2023. "Sourdough Wheat Bread Enriched with Grass Pea and Lupine Seed Flour: Physicochemical and Sensory Properties" Applied Sciences 13, no. 15: 8664. https://doi.org/10.3390/app13158664
APA StyleCacak-Pietrzak, G., Sujka, K., Księżak, J., Bojarszczuk, J., & Dziki, D. (2023). Sourdough Wheat Bread Enriched with Grass Pea and Lupine Seed Flour: Physicochemical and Sensory Properties. Applied Sciences, 13(15), 8664. https://doi.org/10.3390/app13158664







